Abstract
Thiazolidinediones, a class of drugs for the treatment of type-2 diabetes, are synthetic ligands for peroxisome proliferator-activated receptor-γ. They have been demonstrated to possess cardioprotective effects in humans and anti-atherogenic properties in animal models. However, the question remains whether a peroxisome proliferator-activated receptor-γ ligand can reverse the development of atherosclerosis. In this study, we tested the effects of pioglitazone on the development of established atherosclerosis in low-density lipoprotein receptor-null mice. We observed that atherosclerosis in low-density lipoprotein receptor-null mice progressed when mice were fed a high-fat diet. Pioglitazone treatment of atherogenic mice prevented this progression of atherosclerosis from its middle stages of disease, but was not able to reverse it. Withdrawal of the high-fat diet from mice with advanced atherosclerosis did not result in a reduction in lesion sizes. Pioglitazone treatment also had no effect on advanced atherosclerosis. Levels of high density lipoprotein cholesterol correlated inversely with lesion development when pioglitazone was given during lesion progression. However, pioglitazone had no effect on circulating high density lipoprotein levels in mice in which treatment was initiated following 14 weeks on the high-fat diet. These findings have implications for the analysis of therapeutic agents in murine models of atherosclerosis and the use of pioglitazone in patients with established atherosclerosis.
The low density lipoprotein (LDL) receptor (LDLR) is a cell surface-glycoprotein and is predominantly expressed by hepatocytes. LDLR mediates the binding and endocytosis of excess circulating LDL cholesterol to liver cells where the cholesterol is further catabolized and eventually secreted in the feces by a biliary pathway.1 In humans, mutations in LDLR expression result in a disease of familial hypercholesterolemia, an autosomal dominant disorder. Familial hypercholesterolemia is characterized by elevated plasma LDL cholesterol levels (about twofold in heterozygotes and 4- to 5-fold in homozygotes), deposition of cholesterol in tendons and arteries, and formation of tendon xanthomas and atherosclerotic plaques. Frequently, myocardial infarctions occur in relatively young patients with homozygous LDLR mutations.1,2 In several animal models, genetic deletion of LDLR causes a moderate increase in plasma LDL cholesterol levels when mice are fed normal chow. However, animals have severe elevated plasma LDL cholesterol levels associated with aortic atherosclerotic lesions when fed a high-fat diet.3
Peroxisome proliferator-activated receptors (PPARs) including three isoforms (α, γ, and β/δ) are nuclear transcription factors. They play critical roles in many biological processes, particularly in energy balance.4 Upon ligand activation, these receptors form heterodimers with another nuclear protein, retinoid X receptor (RXR). The PPAR/(RXR) complex binds to PPAR response elements in the regulatory region of target genes, and modulates their transcription. PPARγ is expressed by many cell types, but with a greater abundance in adipocytes and macrophages. PPARγ ligands include natural ligands (prostaglandin J2, linolenic eicosapentaenoic, docohexaenoic, and arachidonic acids) and synthetic ligands (thiazolidinediones, l-tyrosine-based compounds, and several non-steroidal anti-inflammatory drugs). In humans, synthetic PPARγ ligands increase insulin sensitivity and improve glycemic control, thus reducing levels of glycated hemoglobin and triglycerides.5 Three synthetic PPARγ ligands (troglitazone, rosiglitazone, and pioglitazone) were clinically used to treat type 2 diabetic patients. However, troglitazone has been removed from the market because of hepatotoxity. Recently, rosiglitazone also has been shown to cause significant increase in myocardial infarction as well as death.6
Some synthetic PPARγ ligands such as troglitazone, rosiglitazone, and GW7845 have been reported to inhibit the development of atherosclerosis in pro-atherogenic animal models.7,8,9,10 In these reports, ligands were administrated at the beginning of high-fat diet feeding. Thus, these drugs inhibited lesion development at a very early stage. Effects of a PPARγ ligand treatment on advanced atherosclerosis have not been previously evaluated. In addition, it remains unclear if PPARγ ligand treatment, when accompanied by a change of diet (high-fat diet to low-fat diet) can lead to regression of advanced atherosclerotic lesions. In this study, we have addressed these questions. Our studies show that pioglitazone treatment can prevent the progression of atherosclerosis when the treatment is initiated during disease development. However, neither pioglitazone nor diet modification was able to facilitate regression of advanced atherosclerosis.
Materials and Methods
Materials
Pioglitazone was kindly provided by Takeda Chemical Industries, LTD (Osaka, Japan). Rabbit polyclonal antibodies against ATP-binding cassette transporter A1 (ABCA1), ATP-binding cassette transporter G1 (ABCG1), and scavenger receptor class B, type 1 (SR-BI) were obtained from Novus Biologicals (Littleton, CO). High-fat diet food (21% fat, 0.2% cholesterol) was purchased from Harlan Teklad (Madison, WI). Total, LDL, and high density lipoprotein (HDL) cholesterol assay kits were purchased from Wako Chemicals USA, Inc. (Richmond, VA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except as indicated.
Animals
LDLR null mice (males, C57BL/6J background) were purchased from The Jackson Laboratory (Bar Harbor, Maine) and maintained in fully accredited facilities at Weill Cornell Medical College. All mice used in experiments were males beginning at 8 weeks of age.
Collection of serum samples and determination of serum total, LDL, and HDL cholesterol levels: To collect serum samples, mice were starved 24 hours before sacrifice. At the end of experiments, mice were euthanized in a flow regulated carbon dioxide gas chamber. Blood was collected by cardio-puncture, and it was transferred into a 1.5 ml Eppendorf tube. Blood was centrifuged for 5 minutes at 3000 rpm with a Microfuge. Serum was transferred into a new tube and stored in a −80°C freezer until the assay.
Collection of Peritoneal Macrophages
To collect peritoneal macrophages, mice were injected i.p. (3 ml/mouse) with 4% autoclaved thioglycollate solution 1 week before sacrifice. The animals had free access to drinking water and food. Peritoneal macrophages were isolated by lavage of the mouse abdomen with PBS (2 × 8 ml/mouse) following sacrifice. Cells were cultured (density at 300 × 103 cells/cm2) in complete RPMI medium containing 10% fetal calf serum, 50 μg/ml of penicillin and streptomycin, and 2 mmol/L glutamine. After 3 hours culture, floating cells (most are red blood cells) were removed by washing with PBS. Adherent cells (macrophages) were then used to extract total cellular proteins.
Collection of Aortas and Determination of Aortic Lesion Area
Aortas were collected as follows: After euthanasia, the mouse vasculature was perfused with PBS. The entire aorta extending 5 to 10 mm below bifurcation of the iliac arteries and including the subclavian right and left common carotid arteries was removed. The aorta was fixed in PBS containing 3% paraformaldehyde. After removal of outside connecting tissue and fat, the aorta was opened and stained with oil red O solution. The stained aorta was scanned and the total morphometric lesion area was evaluated by using NIH Image software (National Institutes of Health, Bethesda, Maryland). Data were expressed as percent lesion area, and analyzed by comparison of the mean lesion area ± SE by student’s t-test.
Western Analysis of ABAC1, ABCG1, and SR-BI Expression in Peritoneal Macrophages
Peritoneal macrophages were washed twice with cold PBS, then scraped and lysed in ice-cold lysis buffer (50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxychlorate, 1 mmol/L phenylmethylsulfonyl fluoride, 50 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 50 μg/ml aprotinin, and 50 μg/ml leupeptin). The lysate was sonicated for 20 cycles, then microcentrifuged for 15 minutes at 4°C. The supernatants were transferred to a new test tube and stored at −20°C.
After determination of content, proteins extracted from macrophages (40 μg/sample) were loaded on a 7% (for ABCA1) or 12% (for ABCG1 and SR-BI) SDS-polyacrylamide electrophoresis gel. After electrophoresis, proteins were transferred onto nylon enhanced nitrocellulose membrane. The membrane was blocked with a solution of 0.1% Tween 20/PBS containing 5% fat-free milk for 1 hour at room temperature. The membrane was then incubated with rabbit polyclonal anti-ABCA1 or anti-ABCG1 or anti-SR-BI antibody for 2 hours at room temperature, followed by washing for 3 × 10 minutes with 0.1% Tween 20/PBS buffer. The blot was re-blocked with 0.1% Tween 20/PBS containing 5% milk followed by incubation with horseradish peroxidase conjugated with goat anti-rabbit IgG for another hour at room temperature. After washing 3 × 10 minutes with 0.1% Tween 20/PBS, the membrane was incubated for 1 minute in a mixture of equal volumes of Western blot chemiluminescence reagents 1 and 2, and then exposed to film before development.
Results
Pioglitazone Inhibits Progression of Middle Stage Atherosclerosis but Does Not Reverse It
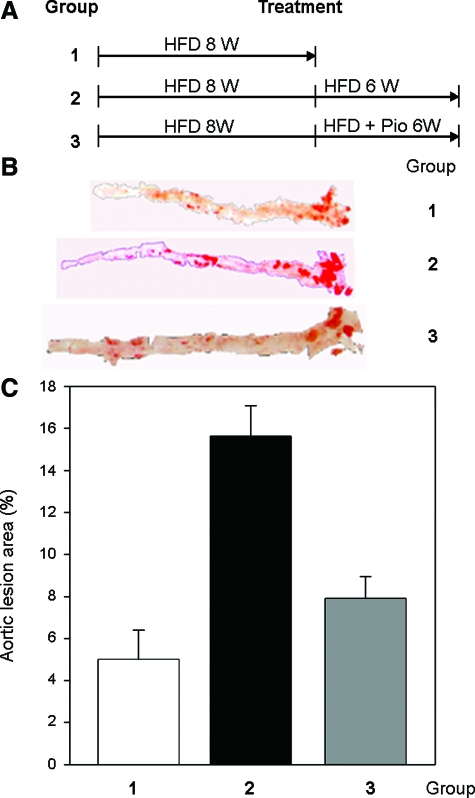
A few PPARγ ligands have been demonstrated to inhibit the development of atherosclerosis in several animal models.7,8,9,10 However, in most of these studies, a PPARγ ligand was added to the pro-atherogenic diet at the beginning of experiments. Since it is unclear if PPARγ ligands also function to inhibit atherosclerosis once the lesions have developed, we fed LDLR null mice with a high-fat diet food (21% fat and 0.2% cholesterol) for 8 weeks, and then divided them into three groups (Figure 1A). The first group was sacrificed for determination of basal lesion development and serum cholesterol levels. The other two groups were both kept on a high-fat diet with or without pioglitazone (15 mg/day/kg) for another 6 weeks. The animals were then sacrificed, and the aortas and serum samples were harvested to analyze lesion development and lipid profiles.
Figure 1.
Pioglitazone inhibits progression of atherosclerosis but does not reverse it in LDLR null mice. A: Experimental designs. LDLR null male mice at 8 weeks of age were fed a high-fat diet for 8 weeks followed by division into 3 groups (15 mice in each group). Group 1 was sacrificed; group 2 was continued to be fed a high-fat diet for an additional 6 weeks; and group 3 was continued to be fed a high-fat diet plus pioglitazone (15 mg/day/kg) for an additional 6 weeks. B: Representative images of aortas. At the end of these experiments, aortas were dissected from mice and used to determine lesion development by oil red O staining as described in the Materials and Methods. Lesion area in the presented image is close to the mean of the corresponding groups. C: Statistical analysis of atherosclerotic lesions in aortas. The determination of atherosclerotic lesion area in each aorta was completed as described in the Materials and Methods. There is a significant difference between group 1 (8 weeks of high-fat diet) or group 3 (8 weeks of high-fat diet plus additional 6 weeks of high-fat diet with pioglitazone) and group 2 (14 weeks of high-fat diet) at P < 0.05 by student’s t-test (n = 15).
The morphometric assay indicated that 8 weeks on a high-fat diet led to 5% of atherosclerotic lesion development in aorta, most occurring in the root area (group1, Figure 1, B and C). Continuation with the high-fat diet feeding for another 6 weeks (total 14 weeks on a high-fat diet, the second group) showed significantly increased lesions in aortas (about 16%, group 2, Figure 1, B and C). However, in the presence of pioglitazone, the third group (total 14 weeks on a high-fat diet but the last 6 weeks on a high-fat diet with pioglitazone) had far less aortic atherosclerosis (∼8%, group 3, Figure 1, B and C). These results suggest that pioglitazone prevented the progression of “middle stage” lesion development. Compared with group 1 (8 weeks on a high-fat diet), group 3 had a slight increase in lesion area. However, the difference was not significant (P = 0.34 by student’s t-test) indicating that pioglitazone was not able to reverse the lesions.
To assess the changes in the lesion with the lipid profiles in circulation system, we collected serum samples from treated mice, and determined total, LDL, and HDL cholesterol levels. As compared with mice on normal chow, total cholesterol levels were increased ∼five-fold (from 235 to 1106 mg/dl, Table 1) after 8 weeks of high-fat diet feeding. The continuation of the high-fat diet feeding further increased total cholesterol levels in the serum (from five- to sixfold, Table 1). The increase in total cholesterol levels (between groups of normal chow and high-fat diet) was attributed mainly to increased LDL cholesterol levels, which were increased about ninefold (from 96 to 837 mg/dl) on the high-fat diet. Thus, the percentage of LDL cholesterol in total cholesterol was elevated (from ∼40% to >60%). Interestingly, pioglitazone did not lower total or LDL cholesterol levels in mice on a high-fat diet. In fact, it slightly increased both (Table 1). Serum HDL cholesterol levels remained unchanged in mice on a high-fat diet for 8 weeks, but it markedly dropped in mice on a high-fat diet for 14 weeks (by 15.5%, Table 1). Treatment with pioglitazone significantly increased serum HDL cholesterol levels (Table 1, 33% higher than the group on a high-fat diet alone for 14 weeks and 12% higher than the group on normal chow) suggesting the effect of HDL cholesterol levels overrides the effect of total or LDL cholesterol levels on the development of atherosclerosis.
Table 1.
Effect of Pioglitazone on Serum Cholesterol Levels in LDLR Null Mice
| Treatment | Total (mg/dl) | LDL
|
HDL
|
|||
|---|---|---|---|---|---|---|
| mg/dl | % of total | mg/dl | % of total | LDL/HDL | ||
| Normal chow | 238 ± 25 | 96 ± 9 | 40 | 82 ± 5 | 34.4 | 1.2 |
| 8W HFD | 1106 ± 119 | 837 ± 71 | 76 | 80 ± 9 | 7.2 | 10.5 |
| 14W HFD | 1411 ± 109 | 893 ± 64 | 63 | 69 ± 5* | 4.9 | 12.9 |
| 8W HFD + 6W HFD/pio | 1593 ± 142 | 970 ± 80 | 61 | 92 ± 8*† | 5.8 | 10.6 |
LDLR null mice (male, 8 weeks old) were divided into four groups (15 mice in each group). Group 1: normal chow; group 2: a high-fat diet (HFD) for 8 weeks; group 3: a high-fat diet for 14 weeks; group 4: a high-fat diet for 8 weeks followed by a high-fat diet plus pioglitazone (15 mg/day/kg) for 6 weeks. Blood was collected after overnight fast at the end of experiment. Serum was isolated from blood and kept at −80°C until assay. Total, LDL, and HDL cholesterol levels were determined by using assay kits from Wako Chemicals.
Significantly different from group of normal chow;
significantly different from group of 14 weeks of HFD alone, at P < 0.05 by student’s t-test (n = 15).
Withdrawal of High-Fat Diet or Addition of Pioglitazone Does Not Influence Advanced Atherosclerosis
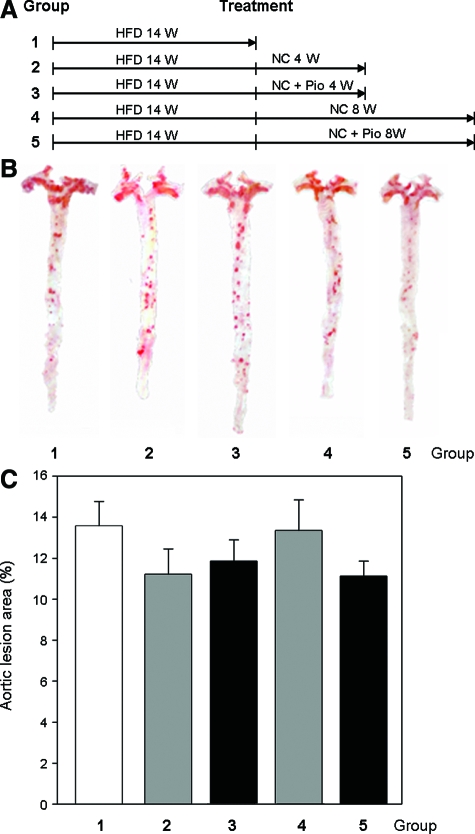
To test if the replacement of a high-fat diet with normal chow with or without pioglitazone is able to reverse advanced atherosclerosis, we initially fed LDLR null mice a high-fat diet for 14 weeks, then switched mice from the high-fat diet to normal chow with or without pioglitazone (15 mg/day/kg) for 4 or 8 weeks. Compared with the control group (group 1 in Figure 2, lesion was ∼14%), these mice with advanced lesions showed little change in lesion area despite the withdrawal of the lipid diet (groups 2 and 4 in Figure 2, lesion sizes are 11% and 13% after 4 and 8 weeks of normal chow, respectively. Decreases are not significant). And, following the addition of pioglitazone to normal chow (groups 3 and 5 in Figure 2), lesion sizes were 12% and 11% after 4 and 8 weeks of treatment. These decreases are not significant compared with groups 1, 2, or 4. Thus, removal of fat from the food or the addition of pioglitazone had little effect on advanced atherosclerosis.
Figure 2.
Withdrawal of high-fat diet and addition of pioglitazone do not influence advanced atherosclerosis. A: Experimental designs. LDLR null mice (8 weeks old) were fed a high-fat diet for 14 weeks, and then were divided into 5 groups (15 male mice in each group). Group 1: mice were sacrificed immediately; and other groups were continued as follows—group 2: 4 more weeks on normal chow; group 3: 4 more weeks on normal chow containing piglitazone (15 mg/day/kg); group 4: 8 more weeks on normal chow; group 5, 8 more weeks on normal chow containing pioglitazone (15 mg/day/kg). B: Representative images of aortas. A typical image in each of above 5 groups (lesion area is close to the mean in the corresponding group) is presented. C: Statistical analysis of atherosclerotic lesions in aortas. All aortas in each group were dissected and stained with oil red O solution as described in the Materials and Methods. There is no significant difference between any two groups by student’s t-test at P < 0.05 (n = 15).
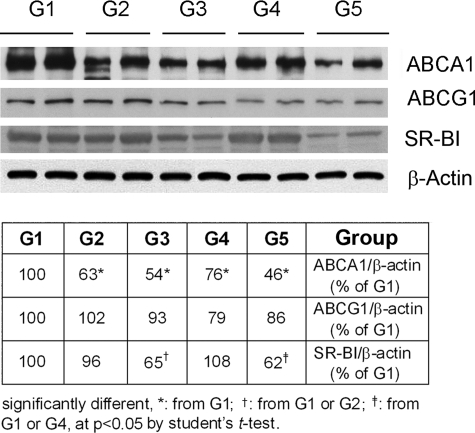
ABCA1, ABCG1, and SR-BI are important molecules mediating macrophage cholesterol homeostasis and development of atherosclerosis.11,12 To test if the withdrawal of high-fat diet or the addition of pioglitazone affects expression of these molecules, we isolated peritoneal macrophages and assessed expression of ABCA1, ABCG1, or SR-BI by Western blot. Results in Figure 3 demonstrate that the switch of atherogenic mice from a high-fat diet to normal chow for 4 and 8 weeks reduced expression of ABCA1 (upper panel, G1 vs G2 or G4). Addition of pioglitazone to normal chow showed the further reduction in ABCA1 protein levels compared with the corresponding groups (upper panel, G2 vs G3 or G4 vs G5). Similar changes in ABCG1 were observed except that ABCG1 was not altered after 4 weeks of normal chow alone (second panel from top in Figure 3). Expression of macrophage SR-BI was not influenced by diet, but was reduced by the addition of pioglitazone in normal chow (third panel from top in Figure 3).
Figure 3.
Withdrawal of high-fat diet reduces expression of ABCA1 and ABCG1 but not SR-BI in peritoneal macrophages. Two mice from each group as described in Figure 2 were i.p. injected with 4% thioglycollate solution (3 ml/mouse) for 1 week before sacrifice. Peritoneal macrophages were collected and used to determine expression of ABCA1 or ABCG1 or SR-BI by Western blot as described in the Materials and Methods.
Total and LDL cholesterol levels were increased about six- and tenfold respectively after 14 weeks of high-fat diet feeding (Table 2). Withdrawal of high-fat diet resulted in a substantial decline in total and LDL cholesterol levels. However, as compared with that in mice fed with normal chow, both total and LDL cholesterol levels are still higher (∼twofold), which may impact on the regression of the advanced lesions. After 4 or 8 weeks on normal chow, the total cholesterol levels decreased 70%. Similarly, LDL cholesterol levels also dropped 75% to 80% (Table 2). Addition of pioglitazone to the normal chow did not help the reduction in total or LDL cholesterol levels (Table 2). Feeding mice a high-fat diet had a greater effect on increased LDL cholesterol levels than total cholesterol levels (Table 1). Correspondingly, withdrawal of fat from the diet lowered LDL cholesterol levels more than total cholesterol levels. Thus, the percentage of LDL cholesterol to the total cholesterol was dramatically reduced by depleting fat or adding pioglitazone to the normal chow. HDL cholesterol levels decreased by 13% on a high-fat diet compared with normal diet. However, the switch to normal chow induced a rebound in HDL cholesterol levels (∼30% and 56% higher than group on a high-fat diet after 4 and 8 weeks, respectively, of normal chow, Table 2). The addition of pioglitazone to the normal chow further increased HDL cholesterol levels after 4 weeks of treatment (by 62%). However, 8 weeks of drug treatment (group 5) did not increase HDL cholesterol levels, as compared with the corresponding controls (group 4, Table 2). Because of a sharp decrease in LDL cholesterol levels as well as the increase in HDL cholesterol levels, the ratio of LDL cholesterol to HDL cholesterol was significantly reduced after the withdrawal of high-fat diet and was close to that in the control group (Table 2).
Table 2.
Effect of Diet and Pioglitazone on Serum Cholesterol Levels in LDLR Null Mice
| Treatment | Total (mg/dl) | LDL
|
HDL
|
|||
|---|---|---|---|---|---|---|
| mg/dl | % of total | mg/dl | % of total | LDL/HDL | ||
| Control (NC) | 238 ± 25 | 96 ± 19 | 40 | 82 | 34 | 1.2 |
| 14W HFD | 1440 ± 101 | 1016 ± 66 | 71 | 72 ± 5 | 5 | 14.2 |
| 14W HFD + 4W NC | 442 ± 45 | 271 ± 16 | 61 | 93 ± 10 | 21 | 2.9 |
| 14W HFD + 4W NC/Pio | 544 ± 45 | 290 ± 24 | 53 | 116 ± 10 | 21 | 2.5 |
| 14W HFD + 8W NC | 450 ± 28 | 219 ± 12 | 49 | 112 ± 1 | 25 | 2.0 |
| 14W HFD + 8W NC/Pio | 427 ± 32 | 201 ± 15 | 47.1 | 105 ± 3 | 25 | 1.9 |
LDLR null mice (8 weeks old, 15 male mice in each group) were fed a high-fat diet (HFD) for 14 weeks followed by 4 or 8 weeks of normal chow (NC) or normal chow plus pioglitazone (NC/Pio, 15 mg/day/kg). The control group was fed normal chow. The lipid profile assay was completed as described in Table 1 (n = 15).
We also examined the changes in serum glucose levels by the various treatment groups (Table 3). High fat diet moderately increased serum glucose levels but the increase was not significant (P = 0.34). Interestingly, the switch from high fat diet to normal chow did not ameliorate serum glucose levels, even in the presence of pioglitazone.
Table 3.
Effect of High Fat Diet and Pioglitazone on Serum Glucose Levels
| Treatment | Glucose (mg/dl) | % of control |
|---|---|---|
| Control (normal chow) | 120 ± 15 | 100 |
| 14W HFD | 135 ± 14 | 113 |
| 14W HFD + 4W NC | 133 ± 17 | 111 |
| 14W HFD + 4W NC/Pio | 155 ± 23 | 129 |
| 14W HFD + 8W NC | 151 ± 24 | 125 |
| 14W HFD + 8W NC/Pio | 145 ± 15 | 121 |
LDLR null mice (8 weeks old, 15 male mice in each group) received treatment as described in Table 2. Serum was collected at the end of experiments and used to assay glucose levels by using the Freestyle Blood Glucose Monitoring System from TheraSense following the manufacturer’s instructions.
Discussion
The effects of thiazolidinediones on the development of atherosclerosis in murine models have been evaluated by several groups.7,8,9,10,13 These studies all had similar designs. Atherosclerosis-prone mice (LDLR null or apoE null) were fed a high-fat diet in the presence or absence of a PPARγ ligand. Li et al reported that PPARγ agonists, rosiglitazone and GW7845, inhibited the development of atherosclerosis in LDLR null male mice.8 Similarly, troglitazone significantly inhibited lesion formation in apoE null mice on a high-fat diet. Troglitazone inhibited atherosclerosis in male LDLR null mice fed either a high-fat diet or a high-fructose diet.13
There are several potential mechanisms that may explain the anti-atherosclerotic effects of thiazolidinediones in mice. Thiazolidinediones and other PPARγ activators are negative regulators of macrophage activation and antagonize the activities of the transcription factors of activator protein 1 (AP-1), signal transducer and activator of transcription (STAT), and nuclear factor-κB.14 Pioglitazone reduces the proliferation and migration of monocytes by inhibiting the expression of monocyte chemoattractant protein (MCP) -1, as well as the MCP family-specific receptor chemokine (C-C motif) receptor 2 (CCR2) in monocytes and other cell types.15,16,17,18 It also reduces monocyte adhesion to vascular endothelium.19 Pioglitazone suppresses the production of inflammatory cytokines, such as interleukin (IL)-1β, IL-2, IL-6, IL-8, and lipopolysaccharaide-induced macrophage colony-stimulating factor (M-CSF), tumor necrosis factor α, and nuclear factor-κB by macrophages and lymphocytes.20,21,22,23 In addition, pioglitazone is able to induce the production of anti-inflammatory cytokine, IL-1 receptor antagonist by monocytes.24 Treatment of macrophages with PPARγ ligands may not induce foam cell formation, despite induction of the oxidized LDL receptor, CD36, because they also induce expression of ABCA1, a transporter that mediates macrophage cholesterol efflux to extracellular apoAI. These effects are likely due to the activation of liver X receptor (LXR), an oxysterol-activated nuclear receptor inducing ABCA1 transcription.25
Our results confirm these earlier studies. However, despite the clear effects of PPARγ ligands on development and progression of atherosclerotic lesions when given concurrently with a high-fat diet, this approach does not accurately mimic a realistic therapeutic modality in humans. We attempted to model a disease process and a therapeutic approach that would more accurately reflect the therapy for a patient with established atherosclerosis. We wanted to determine the effects of pioglitazone administration in mice in which aortic lesions had already developed (following 14 weeks of a high-fat diet). Our results demonstrated that neither return to a normal chow diet or the combination of pioglitazone with a normal chow diet had any significant effect on lesion regression. Our data are in contrast to a recent study showing that pioglitazone (20 mg/day/kg) can slightly reduce advanced atherosclerotic lesions in mice.26 In this study, pioglitazone inhibited expression of matrix metalloproteinases 2, 7, 9, and 13 in lesions. However, HDL cholesterol levels were increased in animals fed a high-fat diet and decreased when pioglitazone was added.26 The reasons for the discrepancy without data are not clear.
A small number of studies have successfully demonstrated lesion regression in murine models of atherosclerosis. LDLR null mice with established fatty streak lesions exhibited regression after administration of an adenoviral vector containing human apoA-I.27 Similarly, infusion of recombinant apoA-IMilano/DPPC (dipalmitoylphosphatidylcholine) complexes decreased arterial lipid in apoE null mice.28 Overexpression of apoE reduced lesion size in LDLR null and apoE null mice.29,30 When atherosclerotic segments of aorta were transplanted from hyperlipidemic apoE null mice to wild-type mice, foam cell content was dramatically reduced.31 These studies demonstrate that raising HDL levels and/or raising HDL while lowering LDL can reverse atherosclerosis.
We show that pioglitazone significantly increased HDL levels in mice during the development of atherosclerosis (Table 1) but had little or no effect on HDL levels in mice with established lesions (Table 2). While it is possible that increased HDL levels may have played a role in inhibiting lesion development, we cannot document a cause and effect. Similarly, we cannot make any conclusions with regard to the lack of an effect of pioglitazone on HDL in mice with established atherosclerosis that had been on a high-fat diet for 14 weeks and the lack of effect on lesion regression. Troglitazone has been previously shown to increase HDL cholesterol levels in apoE null mice fed a high-fat diet.9
As an insulin sensitizer, pioglitazone can significantly reduces glycated hemoglobin and triglycerides in type 2 diabetic patients as a monotherapy or in combination with other drugs, such as sulfonylurea, metformin, and glibenclamide.32,33,34,35,36,37,38,39 While rosiglitazone increases total and LDL cholesterol levels but decreases HDL cholesterol levels in type 2 diabetic patients, pioglitazone on the other hand, can increase HDL cholesterol levels with little to no effect on total and LDL cholesterol levels in these patients.33,34,35,36,40,41 Effects of pioglitazone on HDL cholesterol levels was also observed in non-diabetic patients.36,42,43 Similarly, we observed that pioglitazone increased serum HDL cholesterol levels, which might be attributed its activation of PPARα since it induces production of apoA-I,44,45 the major component of HDL. In contrast, pioglitazone had no effect on total and LDL cholesterol levels in LDLR null mice with atherosclerotic lesions at either middle or advanced stage (Tables 1 and 2).
In addition to improving lipid profiles, pioglitazone has other cardioprotective functions. For instance, it can reduce carotid intima-media thickness, pulse wave velocity, and urinary albumin excretion in normotensive patients with type 2 diabetic nephropathy.46 It can also reduce high-sensitivity C-reactive protein and improve endothelial vasodilator function in patients.32,47 Clinical trial studies demonstrate that pioglitazone treatment results in a significantly lower rate of progression of coronary atherosclerosis compared with glimepiride and a reduction of other macrovascular events in type 2 diabetic patients.48,49 In cell culture, pioglitazone can inhibit arterial smooth muscle cell proliferation and the production of plasminogen activator inhibitor 1 induced by tumor necrosis factor-α in hepatocytes. It can also induce plasma platelet activating factor-acetylhydrolase,47,50,51,52 an enzyme that hydrolyzes platelet-activating factor, as well as structurally similar oxidized phospholipids. In this manner, pioglitazone exerts an anti-inflammatory and an anti-atherogenic effect.53,54,55 In LDLR null mice, pioglitazone can inhibit expression of connective tissue growth factor,56 a molecule that may destabilize plaques by increasing apoptosis and expression of matrix metalloproteinase-2 in vascular smooth muscle cells in advanced atherosclerotic plaques.57 All these functions can be attributed to the anti-atherogenic properties of pioglitazone. Activation of PPARγ has been reported to activate macrophage ABCA1 and ABCG1 expression and cholesterol efflux.58 Although we did not observe an increase in ABCA1, we believe that this is most likely because ABCA1 was already maximally induced by high levels of oxysterols in macrophages derived from mice with elevated lipoprotein levels. Despite these potentially beneficial effects on cardiovascular markers, the risk and benefits of pioglitazone treatment on cardiovascular endpoints in patients with type 2 diabetes is modest. Pioglitazone was able to reduce cardiovascular death and myocardial infarction in one large trial, but the results were not statistically significant. In addition, pioglitazone therapy increased risk of fluid retention and heart failure.59,60
In summary, we demonstrated that pioglitazone, an insulin sensitizing drug used for the treatment of type II diabetes, inhibits progression of atherosclerosis. However, pioglitazone, in combination with the withdrawal of a high-fat diet, was unable to reverse established atherosclerosis. These findings describe, in part, a general paradox of how therapeutic agents are evaluated in common murine models of atherosclerosis and the lack of therapeutic benefit of agents given to mice with established atherosclerotic lesions, and further, may have implications for human therapy.
Footnotes
Address reprint requests to Jihong Han, Ph.D., Center of Vascular Biology, Department of Pathology, Weill Cornell Medical College, 1300 York Avenue, New York, NY 10065. E-mail: jhan@med.cornell.edu.
Supported by a National Institutes of Health Grant NIH P01HL072942 (to D.P.H., A.C.N. and J.H.), the Julia and Seymour Gross Foundation (to D.P.H.), the Abercrombie Foundation (to A.M.G.), and a 973 program in China, No. 2009CB918900 (to J.H).
D.H. and J.H. contributed equally to this work.
D.P.H. is a consultant to the KOWA Pharmaceutical Corp. Japan; and, A.M.G. is a consultant for Genentech, KOWA, Martek, Merck, and Merck/Schering-Plough. He also serves on the board of directors for Aegerion and Arisaph. In addition, he is a member of DuPont’s health advisory board and serves on the data safety monitoring board for Novartis.
References
- Defesche JC. Low-density lipoprotein receptor–its structure, function, and mutations. Semin Vasc Med. 2004;4:5–11. doi: 10.1055/s-2004-822993. [DOI] [PubMed] [Google Scholar]
- van Aalst-Cohen ES, Jansen AC, de JS, de Sauvage Nolting PR, Kastelein JJ. Clinical, diagnostic, and therapeutic aspects of familial hypercholesterolemia. Semin Vasc Med. 2004;4:31–41. doi: 10.1055/s-2004-822984. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- Bhatia V, Viswanathan P. Insulin resistance and PPAR insulin sensitizers. Curr Opin Investig Drugs. 2006;7:891–897. [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Levi Z, Shaish A, Yacov N, Levkovitz H, Trestman S, Gerber Y, Cohen H, Dvir A, Rhachmani R, Ravid M, Harats D. Rosiglitazone (PPARgamma-agonist) attenuates atherogenesis with no effect on hyperglycaemia in a combined diabetes-atherosclerosis mouse model. Diabetes Obes Metab. 2003;5:45–50. doi: 10.1046/j.1463-1326.2003.00240.x. [DOI] [PubMed] [Google Scholar]
- Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ishibashi S, Perrey S, Osuga J, Gotoda T, Kitamine T, Tamura Y, Okazaki H, Yahagi N, Iizuka Y, Shionoiri F, Ohashi K, Harada K, Shimano H, Nagai R, Yamada N. Troglitazone inhibits atherosclerosis in apolipoprotein E-knockout mice: pleiotropic effects on CD36 expression and HDL. Arterioscler Thromb Vasc Biol. 2001;21:372–377. doi: 10.1161/01.atv.21.3.372. [DOI] [PubMed] [Google Scholar]
- Calkin AC, Forbes JM, Smith CM, Lassila M, Cooper ME, Jandeleit-Dahm KA, Allen TJ. Rosiglitazone attenuates atherosclerosis in a model of insulin insufficiency independent of its metabolic effects. Arterioscler Thromb Vasc Biol. 2005;25:1903–1909. doi: 10.1161/01.ATV.0000177813.99577.6b. [DOI] [PubMed] [Google Scholar]
- Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL. ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Collins AR, Meehan WP, Kintscher U, Jackson S, Wakino S, Noh G, Palinski W, Hsueh WA, Law RE. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:365–371. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- Joner M, Farb A, Cheng Q, Finn AV, Acampado E, Burke AP, Skorija K, Creighton W, Kolodgie FD, Gold HK, Virmani R. Pioglitazone inhibits in-stent restenosis in atherosclerotic rabbits by targeting transforming growth factor-beta and MCP-1. Arterioscler Thromb Vasc Biol. 2007;27:182–189. doi: 10.1161/01.ATV.0000251021.28725.e8. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Fukunaga Y, Itoh H, Doi K, Yamashita J, Chun TH, Inoue M, Masatsugu K, Saito T, Sawada N, Sakaguchi S, Arai H, Nakao K. Therapeutic potential of thiazolidinediones in activation of peroxisome proliferator-activated receptor gamma for monocyte recruitment and endothelial regeneration. Eur J Pharmacol. 2005;508:255–265. doi: 10.1016/j.ejphar.2004.10.056. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Egashira K, Hiasa K, Inoue S, Ni W, Zhao Q, Usui M, Kitamoto S, Ichiki T, Takeshita A. Antiinflammatory and antiarteriosclerotic effects of pioglitazone. Hypertension. 2002;40:687–693. doi: 10.1161/01.hyp.0000036396.64769.c2. [DOI] [PubMed] [Google Scholar]
- Toriumi Y, Hiraoka M, Watanabe M, Yoshida M. Pioglitazone reduces monocyte adhesion to vascular endothelium under flow by modulating RhoA GTPase and focal adhesion kinase. FEBS Lett. 2003;553:419–422. doi: 10.1016/s0014-5793(03)01040-8. [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Thomassen MJ, Farver CF, Abraham S, Koloze MT, Zhang X, Mosser DM, Culver DA. Peroxisome proliferator-activated receptor-gamma regulates the expression of alveolar macrophage macrophage colony-stimulating factor. J Immunol. 2008;181:235–242. doi: 10.4049/jimmunol.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WY, Schwartz EA, Permana PA, Reaven PD. Pioglitazone inhibits the expression of inflammatory cytokines from both monocytes and lymphocytes in patients with impaired glucose tolerance. Arterioscler Thromb Vasc Biol. 2008;28:2312–2318. doi: 10.1161/ATVBAHA.108.175687. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Nakamuta M, Enjoji M, Irie T, Sugimoto R, Muta T, Iwamoto H, Nawata H. Activation of retinoic X receptor and peroxisome proliferator-activated receptor-gamma inhibits nitric oxide and tumor necrosis factor-alpha production in rat Kupffer cells. Hepatology. 2001;33:91–99. doi: 10.1053/jhep.2001.21145. [DOI] [PubMed] [Google Scholar]
- Kasai T, Miyauchi K, Yokoyama T, Kajimoto K, Sumiyoshi K, Kubota N, Ikeda E, Daida H. Pioglitazone attenuates neointimal thickening via suppression of the early inflammatory response in a porcine coronary after stenting. Atherosclerosis. 2008;197:612–619. doi: 10.1016/j.atherosclerosis.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Meier CA, Chicheportiche R, Juge-Aubry CE, Dreyer MG, Dayer JM. Regulation of the interleukin-1 receptor antagonist in THP-1 cells by ligands of the peroxisome proliferator-activated receptor gamma. Cytokine. 2002;18:320–328. doi: 10.1006/cyto.2002.1945. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- He L, Game BA, Nareika A, Garvey WT, Huang Y. Administration of pioglitazone in low-density lipoprotein receptor-deficient mice inhibits lesion progression and matrix metalloproteinase expression in advanced atherosclerotic plaques. J Cardiovasc Pharmacol. 2006;48:212–222. doi: 10.1097/01.fjc.0000248831.21973.c4. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- Harris JD, Graham IR, Schepelmann S, Stannard AK, Roberts ML, Hodges BL, Hill V, Amalfitano A, Hassall DG, Owen JS, Dickson G. Acute regression of advanced and retardation of early aortic atheroma in immunocompetent apolipoprotein-E (apoE) deficient mice by administration of a second generation [E1(−), E3(−), polymerase(−)] adenovirus vector expressing human apoE. Hum Mol Genet. 2002;11:43–58. doi: 10.1093/hmg/11.1.43. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Pratico D, FitzGerald GA, Chun S, Tsukamoto K, Maugeais C, Usher DC, Pure E, Rader DJ. Reduction of isoprostanes and regression of advanced atherosclerosis by apolipoprotein E. J Biol Chem. 2001;276:261–266. doi: 10.1074/jbc.M003324200. [DOI] [PubMed] [Google Scholar]
- Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci USA. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanefeld M, Marx N, Pfutzner A, Baurecht W, Lubben G, Karagiannis E, Stier U, Forst T. Anti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: the PIOSTAT Study. J Am Coll Cardiol. 2007;49:290–297. doi: 10.1016/j.jacc.2006.08.054. [DOI] [PubMed] [Google Scholar]
- Herz M, Johns D, Reviriego J, Grossman LD, Godin C, Duran S, Hawkins F, Lochnan H, Escobar-Jimenez F, Hardin PA, Konkoy CS, Tan MH. A randomized, double-blind, placebo-controlled, clinical trial of the effects of pioglitazone on glycemic control and dyslipidemia in oral antihyperglycemic medication-naive patients with type 2 diabetes mellitus. Clin Ther. 2003;25:1074–1095. doi: 10.1016/s0149-2918(03)80068-1. [DOI] [PubMed] [Google Scholar]
- Rosenblatt S, Miskin B, Glazer NB, Prince MJ, Robertson KE. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron Artery Dis. 2001;12:413–423. doi: 10.1097/00019501-200108000-00011. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Einhorn D, Hershon K, Glazer NB, Yu S. Efficacy and safety of pioglitazone in type 2 diabetes: a randomised, placebo-controlled study in patients receiving stable insulin therapy. Int J Clin Pract. 2002;56:251–257. [PubMed] [Google Scholar]
- Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–1611. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- Einhorn D, Rendell M, Rosenzweig J, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The Pioglitazone 027 Study Group. Clin Ther. 2000;22:1395–1409. doi: 10.1016/s0149-2918(00)83039-8. [DOI] [PubMed] [Google Scholar]
- Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111:10–17. doi: 10.1016/s0002-9343(01)00713-6. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Tani S, Anazawa T, Kushiro T, Kanmatsuse K. Effect of pioglitazone on arteriosclerosis in comparison with that of glibenclamide. Diabetes Res Clin Pract. 2005;68:104–110. doi: 10.1016/j.diabres.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Boyle PJ, King AB, Olansky L, Marchetti A, Lau H, Magar R, Martin J. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Ther. 2002;24:378–396. doi: 10.1016/s0149-2918(02)85040-8. [DOI] [PubMed] [Google Scholar]
- Deeg MA, Buse JB, Goldberg RB, Kendall DM, Zagar AJ, Jacober SJ, Khan MA, Perez AT, Tan MH. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2007;30:2458–2464. doi: 10.2337/dc06-1903. [DOI] [PubMed] [Google Scholar]
- Fullert S, Schneider F, Haak E, Rau H, Badenhoop K, Lubben G, Usadel KH, Konrad T. Effects of pioglitazone in nondiabetic patients with arterial hypertension: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2002;87:5503–5506. doi: 10.1210/jc.2002-020963. [DOI] [PubMed] [Google Scholar]
- Szapary PO, Bloedon LT, Samaha FF, Duffy D, Wolfe ML, Soffer D, Reilly MP, Chittams J, Rader DJ. Effects of pioglitazone on lipoproteins, inflammatory markers, and adipokines in nondiabetic patients with metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:182–188. doi: 10.1161/01.ATV.0000195790.24531.4f. [DOI] [PubMed] [Google Scholar]
- Qin S, Liu T, Kamanna VS, Kashyap ML. Pioglitazone stimulates apolipoprotein A-I production without affecting HDL removal in HepG2 cells: involvement of PPAR-alpha. Arterioscler Thromb Vasc Biol. 2007;27:2428–2434. doi: 10.1161/ATVBAHA.107.150193. [DOI] [PubMed] [Google Scholar]
- Orasanu G, Ziouzenkova O, Devchand PR, Nehra V, Hamdy O, Horton ES, Plutzky J. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. J Am Coll Cardiol. 2008;52:869–881. doi: 10.1016/j.jacc.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Matsuda T, Kawagoe Y, Ogawa H, Takahashi Y, Sekizuka K, Koide H. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism. 2004;53:1382–1386. doi: 10.1016/j.metabol.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Campia U, Matuskey LA, Panza JA. Peroxisome proliferator-activated receptor-gamma activation with pioglitazone improves endothelium-dependent dilation in nondiabetic patients with major cardiovascular risk factors. Circulation. 2006;113:867–875. doi: 10.1161/CIRCULATIONAHA.105.549618. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochelliere R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- Peuler JD, Phare SM, Iannucci AR, Hodorek MJ. Differential inhibitory effects of antidiabetic drugs on arterial smooth muscle cell proliferation. Am J Hypertens. 1996;9:188–192. doi: 10.1016/0895-7061(95)00393-2. [DOI] [PubMed] [Google Scholar]
- Takeshita Y, Takamura T, Hamaguchi E, Shimizu A, Ota T, Sakurai M, Kaneko S. Tumor necrosis factor-alpha-induced production of plasminogen activator inhibitor 1 and its regulation by pioglitazone and cerivastatin in a nonmalignant human hepatocyte cell line. Metabolism. 2006;55:1464–1472. doi: 10.1016/j.metabol.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Sumita C, Maeda M, Fujio Y, Kim J, Fujitsu J, Kasayama S, Yamamoto I, Azuma J. Pioglitazone induces plasma platelet activating factor-acetylhydrolase and inhibits platelet activating factor-mediated cytoskeletal reorganization in macrophage. Biochim Biophys Acta. 2004;1673:115–121. doi: 10.1016/j.bbagen.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Tjoelker LW, Wilder C, Eberhardt C, Stafforini DM, Dietsch G, Schimpf B, Hooper S, Le TH, Cousens LS, Zimmerman GA, Yamadat Y, Mclntyre TM, Prescott SM, Gray PW. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature. 1995;374:549–553. doi: 10.1038/374549a0. [DOI] [PubMed] [Google Scholar]
- Quarck R, De GB, Stengel D, Mertens A, Lox M, Theilmeier G, Michiels C, Raes M, Bult H, Collen D, Van VP, Ninio E, Holvoet P. Adenovirus-mediated gene transfer of human platelet-activating factor-acetylhydrolase prevents injury-induced neointima formation and reduces spontaneous atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;103:2495–2500. doi: 10.1161/01.cir.103.20.2495. [DOI] [PubMed] [Google Scholar]
- Stremler KE, Stafforini DM, Prescott SM, McIntyre TM. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J Biol Chem. 1991;266:11095–11103. [PubMed] [Google Scholar]
- Game BA, He L, Jarido V, Nareika A, Jaffa AA, Lopes-Virella MF, Huang Y. Pioglitazone inhibits connective tissue growth factor expression in advanced atherosclerotic plaques in low-density lipoprotein receptor-deficient mice. Atherosclerosis. 2007;192:85–91. doi: 10.1016/j.atherosclerosis.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Oemar BS, Luscher TF. Connective tissue growth factor. Friend or foe? Arterioscler Thromb Vasc Biol. 1997;17:1483–1489. doi: 10.1161/01.atv.17.8.1483. [DOI] [PubMed] [Google Scholar]
- Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, Lee YH, Ricote M, Glass CK, Brewer HB, Jr, Gonzalez FJ. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–2619. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge DJ, DeFronzo RA, Chilton RJ. PROactive: time for a critical appraisal. Eur Heart J. 2008;29:969–983. doi: 10.1093/eurheartj/ehn114. [DOI] [PubMed] [Google Scholar]
- Zinn A, Felson S, Fisher E, Schwartzbard A. Reassessing the cardiovascular risks and benefits of thiazolidinediones. Clin Cardiol. 2008;31:397–403. doi: 10.1002/clc.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]