Abstract
Previously, we showed that caveolin-1 (Cav-1) expression is down-regulated in human breast cancer-associated fibroblasts. However, it remains unknown whether loss of Cav-1 occurs in the breast tumor stroma in vivo. Here, we immunostained a well-annotated breast cancer tissue microarray with antibodies against Cav-1 and scored its stromal expression. An absence of stromal Cav-1 was associated with early disease recurrence, advanced tumor stage, and lymph node metastasis, resulting in a 3.6-fold reduction in progression-free survival. When tamoxifen-treated patients were selected, an absence of stromal Cav-1 was a strong predictor of poor clinical outcome, suggestive of tamoxifen resistance. Interestingly, in lymph node-positive patients, an absence of stromal Cav-1 predicted an 11.5-fold reduction in 5-year progression-free survival. Clinical outcomes among patients positive for HER2, and patients triple-negative for estrogen receptor, progesterone receptor and HER2, were also strictly dependent on stromal Cav-1 levels. When our results were adjusted for tumor and nodal staging, an absence of stromal Cav-1 remained an independent predictor of poor outcome. Thus, stromal Cav-1 expression can be used to stratify human breast cancer patients into low-risk and high-risk groups, and to predict their risk of early disease recurrence at diagnosis. Based on related mechanistic studies, we suggest that breast cancer patients lacking stromal Cav-1 might benefit from anti-angiogenic therapy in addition to standard regimens. We conclude that Cav-1 functions as a tumor suppressor in the stromal microenvironment.
Carcinoma cells grow in a complex tumor microenvironment composed of (i) non-epithelial cells (including fibroblasts, pericytes, endothelial, and inflammatory cells), (ii) extracellular matrix, and (iii) secreted diffusible growth factors/cytokines.1,2,3 Although under normal physiological conditions the stroma serves as an important barrier to malignant transformation, its role changes during neoplastic transformation. Instead, the stroma plays a key role in driving cancer cell invasiveness and progression.4 Recently, it was demonstrated that fibroblasts isolated from tumor stroma can promote tumor growth.1,2,3,5 This population of tissue fibroblasts termed “cancer associated fibroblasts” (CAFs) is characterized by a hyperproliferative phenotype, and these cells secrete increased amounts of growth factors, extracellular matrix components, and matrix metalloproteinases.5,6 CAFs also show an ability to prevent cancer cell apoptosis, induce cancer cell proliferation, and stimulate tumor angiogenesis.7 In vitro studies of breast carcinomas showed that CAFs mixed with epithelial carcinoma cells are more proficient than normal fibroblasts at enhancing tumor growth and give rise to highly vascularized tumors.8 To date, the mechanisms that govern the conversion of benign mammary stromal fibroblasts to tumor-associated fibroblasts are poorly understood, and their relationship to disease outcome has not been addressed.
Down-regulation of caveolin-1 (Cav-1) is one of the mechanisms implicated in the oncogenic transformation of fibroblasts. Caveolins are the principal protein component of caveolae, which are located at the cell surface in most cell types.9 One of the caveolins, Cav-1, plays a major role in tumorigenesis through its various functions such as lipid transport, membrane trafficking, gene regulation, and signal transduction.10 In cell culture, the transformation of NIH-3T3 fibroblasts with various activated oncogenes, such as H-Ras (G12V), Bcr-Abl, or v-Abl, causes dramatic reductions in Cav-1 protein expression.11,12
Furthermore, knock-down of endogenous Cav-1 in NIH-3T3 fibroblasts promotes anchorage-independent growth in soft agar and tumor formation in nude mice, which could be reversed by Cav-1 re-expression.13 Finally, Cav-1−/− null fibroblasts have a hyperproliferative phenotype (similar to CAFs) and Cav-1 re-expression drives their arrest in the G0/G1 phase of the cell cycle.14 Taken together, these data suggest that loss of Cav-1 leads to the oncogenic transformation of fibroblasts, where Cav-1 normally functions as a transformation suppressor that prevents cell cycle progression.
Using primary cell cultures established from surgically excised breast tumors, we recently demonstrated that Cav-1 is down-regulated in human breast CAFs when compared with matching normal fibroblasts isolated from the same patient.15 In addition, orthotopic transplantation of Cav-1+/+ tumor tissue into the mammary stroma of Cav-1−/− null mice results in up to a ∼twofold increase in tumor mass, functionally demonstrating that the mammary stroma of Cav-1−/− mice behaves as a tumor promoter.16 However, to date, there is no study addressing the clinical significance of stromal Cav-1 expression in invasive carcinoma of the breast in vivo.
The aim of this study was to evaluate the in vivo stromal expression of Cav-1 in a large series of invasive breast carcinomas and to examine the association between stromal Cav-1 expression, clinicopathological variables, and patient outcome. Our results indicate that loss of stromal caveolin-1 is a novel breast cancer biomarker that predicts early disease recurrence, metastasis, survival, and tamoxifen-resistance. Clinical outcome in HER2(+) and triple-negative (estrogen receptor [ER]−/progesterone receptor [PR]−/HER2−) patients was also strictly dependent on stromal Cav-1 levels. Remarkably, in lymph node-positive [LN(+)] patients, an absence of stromal Cav-1 was associated with an ∼11.5-fold reduction in 5-year progression-free survival. As such, Cav-1 may function as a critical tumor/metastasis suppressor in the mammary stromal compartment.
Materials and Methods
Case Selection and Tissue Microarray Construction
Breast tissues for tissue microarray construction were obtained from the Surgical Pathology files at the University of Michigan with Institutional Review Board approval. The tumor microarray contained tissues derived from 154 largely consecutive patients with invasive carcinomas of the breast, with follow-up information treated at the University of Michigan from 1987 to 1991. Clinical and pathological variables were determined following well-established criteria. All invasive carcinomas were graded according to the method described by Elston and Ellis17; lymphovascular invasion was classified as either present or absent. The tissue microarrays were constructed using a tissue arrayer (Beecher Instruments, Silver Spring, MD). Three tissue cores (0.6 mm diameter) were sampled from each block to account for tumor and tissue heterogeneity and transferred to the recipient block. Only cases with two or three cores containing tumor stromal cells were considered for statistical analysis to address possible heterogeneity of the staining in various tumor portions. Clinical and treatment information was extracted by chart review.
Patients
Our study population consists of 154 women diagnosed with breast cancer, with a median age of 59.5 years (range, 28 to 96 years). 85% of the women were white. The median follow-up time for all survivors was 8.4 years (>30 days to 18.5 years). Forty-five percent of the subjects underwent tamoxifen treatment after diagnosis, and 31% had a recurrence of breast cancer during follow-up. The median time to recurrence or death from any cause was 7.1 years.
Immunohistochemistry
Cav-1 expression in the tumor stroma was assessed by using a standard immunoperoxidase method (DakoCytomation LSAB2 System-HRP, Carpinteria, CA), using rabbit polyclonal anti-Cav-1 IgG (N-20; directed against N-terminal residues 2 to 21 of human Cav-1; Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:500). The staining was scored semiquantitatively as negative (0; no staining), weak (1; either diffuse weak staining or strong staining in less than 30% of stromal cells per core), or strong (2; defined as strong staining of 30% or more of the stromal cells). These were given numerical raw scores of 0, 1 and 2, respectively, and the median score of 2 to 3 cores was taken as the final score of the sample.
Statistical Analysis
For each patient, the date of breast cancer diagnosis, date of last follow-up, vital status at last follow-up, causes of death (breast cancer or other), and breast cancer recurrence, were recorded. Stromal caveolin was scored for each tissue sample based on three cores taken from the sample and given a numeric score of 0, 1, or 2, depending on the degree of stromal Cav-1 staining. The median of the three numeric scores was taken to be the stromal Cav-1 score for the sample. In the event that only two of the cores were scorable, and the median score was fractional, it was rounded upward to reflect the presence of stromal Cav-1. A median score of 0 was interpreted as an absence of stromal Cav-1, and scores of 1 and 2 were interpreted as the presence of stromal Cav-1. For an absence of stromal Cav-1 (final median score = 0), >70% of the patients had a raw score of 0 for all three sample cores (000) and >90% had a raw score of either 000 or 001, indicating strong consistency of this phenotype between all three patient tumor core samples.
Our primary outcome of interest in this study is progression-free survival (PFS) from time of diagnosis to the presence of metastasis, death, or last visit. PFS is evaluated using Kaplan-Meier estimation,18 and comparison of stratified survival curves was done using log-rank tests. Cox proportional hazard regression19 was used to evaluate the association of stromal Cav-1 with PFS, in the presence of various potential prognostic factors for PFS. Associations between the presence of stromal Cav-1 and other factors, including age, race, tumor grade, tumor size, LN status, histological subtype, ER, PR, and HER2, and presence of recurrent disease, were evaluated using Fisher’s exact and Kruskal-Wallis tests, depending on the discrete or continuous nature of the other factors.
The default settings of the recursive partitioning function in R (rpart version 3.1–41; http://mayoresearch.mayo.edu/mayo/research/biostat/splusfunctions.cfm) was used to fit a survival tree model to the data and evaluate prognostic factors for PFS.20,21 All P values are two-sided, and P < 0.05 was considered significant. Statistical analysis was performed, and graphs constructed, using the R statistical analysis software version 2.7.2.22
Results
Clinicopathologic Features of the Specimens
Clinical characteristics of the 154 patients in this study are listed in Table 1. Of the 160 invasive carcinomas used to construct the tumor microarray, 154 had at least 2 cores available for evaluation. Therefore, our study population consists of 154 women with a median age of 59.5 years (range, 28 to 96 years). Eighty-five percent of the women were white. The median follow-up time for all survivors was 8.4 years and the median time to metastasis, death, or last visit was 7.1 years. 45% of the subjects underwent tamoxifen treatment after diagnosis, and 31% had a recurrence of breast cancer during follow-up.
Table 1.
Descriptive Statistics for the Patient Cohort (N = 154)
| N | ||
|---|---|---|
| Age (years) | 147 | 58.6 ± 14.7 (median = 59.5) |
| Race | 146 | |
| White | 85% (124) | |
| Black | 12% (17) | |
| Other | 3% (5) | |
| Tamoxifen treatment | 141 | |
| None | 55% (78) | |
| Tamoxifen | 45% (63) | |
| Menopause status | 139 | |
| Peri | 10% (14) | |
| Post | 69% (96) | |
| Pre | 21% (29) | |
| Tumor size (mm) | 129 | 16.0 ± 7.3 (median = 15) |
| T stage | 148 | |
| T0/T1 | 53% (79) | |
| T2 | 35% (52) | |
| T3/T4 | 11% (17) | |
| N stage | 127 | |
| N0 | 50% (64) | |
| N1 | 29% (37) | |
| N2/N3 | 20% (26) | |
| Grade | 141 | |
| 1, Well-differentiated | 9% (13) | |
| 2, Moderately-differentiated | 45% (63) | |
| 3, Poorly-differentiated | 46% (65) | |
| ER status | 142 | |
| Negative | 34% (48) | |
| Positive | 66% (94) | |
| PR status | 143 | |
| Negative | 46% (66) | |
| Positive | 54% (77) | |
| HER2 status | 142 | |
| 0 | 71% (101) | |
| 1 | 14% (20) | |
| 2 | 2% (3) | |
| 3 | 13% (18) | |
| Triple Negative status | 142 | |
| ER−/PR−/HER2− | 15% (21) | |
| Other | 85% (121) | |
| Recurrence | 154 | |
| No | 69% (106) | |
| Yes | 31% (48) | |
| Number of positive nodes | 110 | 0.290 ± 0.407 |
| Lymphovascular invasion | 144 | |
| Negative | 66% (95) | |
| Positive | 34% (49) |
x ± s represents Mean ± 1 SD.
N is the number of non-missing values.
Numbers after percents are frequencies.
ER, PR, HER2, and Stromal Cav-1 Expression Analysis of the Specimens
One hundred forty patients were evaluated for ER, PR, and HER2, of whom 66% were ER positive (ER+) and 15% were triple-negative (ER−/PR−/HER2−). One hundred and twenty five patients had samples that could be scored for stromal Cav-1. We established a Cav-1 grading scale (0, 1, and 2), with 0 representing an absence of stromal Cav-1 and 2 representing high levels of stromal Cav-1. 37% of the samples showed a loss/or absence of stromal Cav-1 (score = 0).
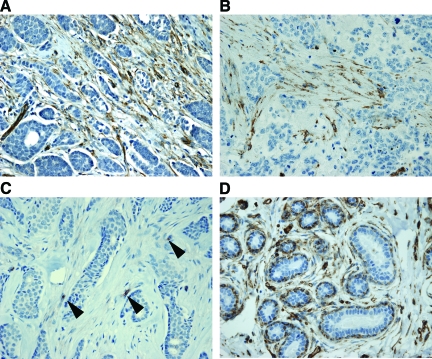
A median score of 0 was interpreted as an absence of stromal Cav-1, and scores of 1 and 2 were interpreted as the presence of stromal Cav-1. Representative examples are shown in Figure 1, A–C. Normal human breast tissue (terminal ductal lobular units) is shown for comparison purposes. Note that the intralobular mammary stroma, the vasculature, and myo-epithelial cells are normally Cav-1 positive (Figure 1D). Tables 2 and 3 show the relation of stromal Cav-1 expression to various clinicopathological variables.
Figure 1.
Stromal caveolin-1 expression in human breast cancers and normal tissue. Breast tumor microarray samples were immunostained with antibodies directed against Cav-1 and subjected to scoring, as detailed in Materials and Methods. Representative examples are shown. Panels A and B show Cav-1 expression in the stroma of invasive ductal carcinomas. Panel C shows an absence of Cav-1 in the neoplastic stroma; however, endothelial cells still remain Cav-1 positive (see arrowheads). Panel D depicts normal human breast tissue (TDLUs; terminal ductal lobular units) for comparison purposes. Note that the mammary intralobular stroma, the vasculature, and myo-epithelial cells are normally Cav-1 positive.
Table 2.
Association of Stromal Cav-1 with Disease Stage, Recurrence, and Lymph Node Metastasis
| N | Stromal Cav-1 Status
|
Combined | P value | ||
|---|---|---|---|---|---|
| Absent | Present | ||||
| N = 47 | N = 78 | N = 125 | |||
| Tumor size (mm) | 129 | 16.97 ± 7.65 | 15.44 ± 6.75 | 15.98 ± 7.30 | 0.338* |
| (median = 16) | (median = 15) | (median = 15) | |||
| T stage | 148 | 0.01† | |||
| T0/T1 | 40% (18) | 61% (46) | 53% (64) | ||
| T2 | 38% (17) | 33% (25) | 35% (42) | ||
| T3/T4 | 22% (10) | 5% (4) | 12% (14) | ||
| N stage | 127 | 0.002† | |||
| N0 | 29% (12) | 60% (38) | 48% (50) | ||
| N1 | 34% (14) | 29% (18) | 31% (32) | ||
| N2/N3 | 37% (15) | 11% (7) | 21% (22) | ||
| Grade | 141 | 0.358† | |||
| 1, Well-differentiated | 7% (3) | 13% (9) | 11% (12) | ||
| 2, Moderately-differentiated | 40% (18) | 46% (31) | 43% (49) | ||
| 3, Poorly-differentiated | 53% (24) | 41% (28) | 46% (52) | ||
| Recurrence | 154 | <0.001† | |||
| No | 43% (20) | 86% (67) | 70% (87) | ||
| Yes | 57% (27) | 14% (11) | 30% (38) | ||
| Number of positive nodes | 110 | 0.4358 ± 0.4502 | 0.2167 ± 0.3468 | 0.2901 ± 0.4067 | 0.001* |
x ± s represents Mean ± 1 SD.
N is the number of non-missing values.
Numbers after percents are frequencies.
Tests used:
Wilcoxon test;
Fisher’s exact test.
The number of positive nodes is reported as a fraction; for example, if 4 out of 10 lymph nodes were positive, then the value would be 0.4.
Table 3.
Association of Stromal Cav-1 with Other Disease-related Parameters and Demographics
| N | Stromal Cav-1 Status
|
Combined | P value | ||
|---|---|---|---|---|---|
| Absent | Present | ||||
| N = 47 | N = 78 | N = 125 | |||
| Age (years) | 147 | 58.4 ± 13.0 | 57.7 ± 15.5 | 58.6 ± 14.7 | 0.678* |
| Race | 146 | 0.314* | |||
| White | 93% (41) | 83% (62) | 87% (103) | ||
| Black | 5% (2) | 12% (9) | 9% (11) | ||
| Other | 2% (1) | 5% (4) | 4% (5) | ||
| Tamoxifen treatment | 141 | 0.02† | |||
| None | 67% (28) | 42% (30) | 51% (58) | ||
| Tamoxifen | 33% (14) | 58% (41) | 49% (55) | ||
| Menopause status | 139 | 0.574† | |||
| Peri | 7% (3) | 14% (10) | 11% (13) | ||
| Post | 72% (31) | 66% (47) | 68% (78) | ||
| Pre | 21% (9) | 20% (14) | 20% (23) | ||
| ER status | 142 | 0.097† | |||
| Negative | 40% (18) | 24% (17) | 30% (35) | ||
| Positive | 60% (27) | 76% (53) | 70% (80) | ||
| PR status | 143 | 0.126† | |||
| Negative | 53% (24) | 38% (27) | 44% (51) | ||
| Positive | 47% (21) | 62% (44) | 56% (65) | ||
| HER2 status | 142 | 0.341† | |||
| 0 | 66% (29) | 73% (52) | 70% (81) | ||
| 1 | 23% (10) | 11% (8) | 16% (18) | ||
| 2 | 0% (0) | 1% (1) | 1% (1) | ||
| 3 | 11% (5) | 14% (10) | 13% (15) | ||
| Triple negative status | 142 | 0.593* | |||
| ER−/PR−/HER2− | 16% (7) | 12% (9) | 14% (16) | ||
| Other | 84% (37) | 88% (63) | 86% (100) | ||
x ± s represents Mean ± 1 SD.
N is the number of non-missing values.
Numbers after percents are frequencies.
Tests used: *Wilcoxon test;
Fisher’s exact test.
Stromal Cav-1 Expression Correlated to Pathological Features
We find that an absence of stromal Cav-1 is strongly associated with tumor stage and nodal stage, as well as with recurrence rate and number of LN metastases (Table 2). Loss of stromal Cav-1 is also significantly associated with lymphovascular invasion (Table 4). In all cases, the absence of Cav-1 is associated with markers of more aggressive disease (higher T-stage, higher N-stage, higher recurrence rate, more positive lymph nodes, and the presence of lymphovascular invasion) (Tables 2 and 4). For example, patients with stromal Cav-1 expression showed a ∼3.6-fold reduction in disease recurrence and a ∼twofold reduction in lymph node metastasis (Table 2). Interestingly, patients with high stromal Cav-1 (score = 2) showed a ∼fivefold reduction in disease recurrence and a ∼2.6-fold reduction in lymph node metastasis (see supplemental Table S1 at http://ajp.amjpathol.org). However, there was no association between stromal Cav-1 expression and tumor grade (Table 2).
Table 4.
Association of Stromal Cav-1 with Lymphovascular Invasion
| N | Stromal Cav-1
|
P value | ||
|---|---|---|---|---|
| Absent | Present | |||
| N = 47 | N = 78 | |||
| Lymphovascular invasion (LVI) | 144 | 0.048 | ||
| Negative | 51% (23) | 70% (50) | ||
| Positive | 49% (22) | 30% (21) | ||
N is the number of non-missing values.
Numbers after percents are frequencies. Test used: Fisher’s exact test.
Stromal Cav-1 was also not associated with ER, PR, HER2, or triple negative (ER−/PR−/HER2−) status, or with demographic parameters (Table 3).
Stromal Cav-1 Expression as a Strong Predictor of Survival
Lack of stromal Cav-1 was also seen to be an important prognostic factor for progression-free survival (PFS). Table 5 gives the median PFS for subjects with and without stromal Cav-1, in the presence of a number of other potential prognostic factors. We find that an absence of stromal Cav-1 results in significantly lower PFS, even in the presence of other prognostic factors, with median survival reduced by several years in many cases—even when adjusted for the same tumor grade. For example, the median PFS was 1.43 years versus 10.84 years in poorly-differentiated breast cancers, depending on the status of stromal Cav-1 (Table 5).
Table 5.
Median Progression-Free Survival (PFS; years) According to Stromal Cav-1 Expression
| Stromal Cav-1
|
P value | ||
|---|---|---|---|
| Absent | Present | ||
| Low T stage (0, 1, or 2) | 2.59 | 14.76 | 6.01 × 10−7 |
| High T stage (3 or 4) | 1.58 | 4.61 | 1.22 × 10−1 |
| No nodes | 10.20 | * | 6.44 × 10−3 |
| Nodes >0 | 1.73 | 10.38 | 1.14 × 10−5 |
| Grade = 1 | 4.21 | 11.86 | 4.89 × 10−2 |
| Grade = 2 | 3.11 | * | 1.17 × 10−4 |
| Grade = 3 | 1.43 | 10.84 | 9.32 × 10−5 |
| ER negative | 1.25 | 10.46 | 9.47 × 10−3 |
| ER positive | 3.23 | * | 5.94 × 10−7 |
| PR negative | 1.53 | 7.58 | 6.73 × 10−4 |
| PR positive | 3.73 | * | 1.18 × 10−5 |
| HER2 negative | 3.16 | * | 1.06 × 10−6 |
| HER2 positive | 1.58 | 9.21 | 7.97 × 10−3 |
| ER−/PR−/HER2− | 1.43 | 14.76 | 2.01 × 10−2 |
| No tamoxifen | 1.66 | 10.84 | 7.74 × 10−5 |
| Tamoxifen | 3.55 | * | 4.61 × 10−5 |
| White | 1.94 | 14.76 | 6.17 × 10−8 |
| Other | 2.04 | * | 1.18 × 10−2 |
| LVI negative | 3.86 | * | 4.71 × 10−6 |
| LVI positive | 1.53 | 6.81 | 7.02 × 10−3 |
ER−/PR−/HER2− represents “triple-negative patients”.
P values are based on log-rank tests on the stratified Kaplan-Meier curves.
Denotes that less than half the at-risk patients had an event, resulting in no estimate of median PFS.
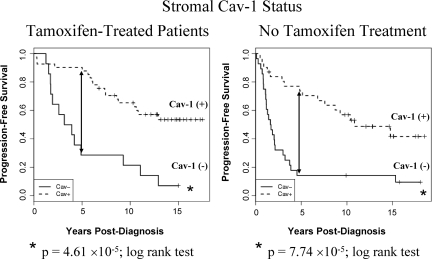
To highlight this, Figure 2 shows the Kaplan-Meier survival curves for patients who did and did not receive tamoxifen therapy. Note that when only patients who underwent tamoxifen-treatment were selected for analysis (Figure 2, Left panel), an absence of Cav-1 in the mammary stroma was a strong predictor of poor clinical outcome, suggestive of an association with tamoxifen-resistance. In direct support of these immunohistochemistry-based observations, virtually identical results were obtained when a “gene-expression signature,” generated using Cav-1−/− null mammary stromal fibroblasts, was used to cluster an independent cohort of ER+ breast cancer patients who underwent tamoxifen mono-therapy.23
Figure 2.
Kaplan-Meier curves of progression-free survival for patients with and without tamoxifen treatment. Left panel: Note that an absence of stromal Cav-1 immunostaining predicts poor clinical outcome in Tamoxifen-treated patients, suggestive of tamoxifen-resistance. Right panel: Virtually identical results were obtained with patients that did not receive tamoxifen. In both panels, 5-year PFS is indicated by an arrow. Tamoxifen-treated (P = 4.61 × 10−5, log-rank test); No tamoxifen (P = 7.74 × 10−5, log-rank test).
Cox regression/multivariate analysis (Table 6) using T stage, N stage, tamoxifen use, and the presence of stromal Cav-1 showed that an absence of stromal Cav-1 conferred significantly reduced PFS, with the adjusted hazard ratio being ∼3.6 (P < 0.0001). We used a survival tree approach to assess the relative importance of the presence of stromal Cav-1 in predicting PFS, using default settings in the R package rpart. Age, race, T stage, N stage, ER, PR, HER2, tamoxifen use, and lymphovascular invasion were also included in this model. We find that an absence of stromal Cav-1 is the strongest factor in predicting PFS, even in the presence of other well-known predictors. Our analyses show that loss or absence of Cav-1 in stromal cells is an important independent predictor of progression-free survival in breast cancer, not associated with ER, PR, or HER2 status.
Table 6.
Cox regression of PFS on T Stage, N stage, Tamoxifen Use, and Cav-1 Score
| N | Coefficient | Hazard ratio | SE(Coef) | Z-score | P value | |
|---|---|---|---|---|---|---|
| T stage | ||||||
| T0/T1 (ref) | 51 | |||||
| T2 | 37 | 0.097 | 1.102 | 0.315 | 0.307 | 7.6 × 10−1 |
| T3/T4 | 13 | 0.789 | 2.202 | 0.401 | 1.966 | 4.9 × 10−2 |
| N stage | ||||||
| N0 (ref) | 48 | |||||
| N1 | 31 | 0.458 | 1.581 | 0.34 | 1.345 | 1.8 × 10−1 |
| N2/N3 | 22 | 1.439 | 4.215 | 0.372 | 3.872 | 1.1 × 10−4 |
| Tamoxifen use | ||||||
| No (ref) | 53 | |||||
| Yes | 48 | −0.476 | 0.621 | 0.274 | −1.738 | 8.2 × 10−2 |
| Stromal Cav-1 | ||||||
| Present (ref) | 62 | |||||
| Absent | 39 | 1.272 | 3.569 | 0.292 | 4.352 | 1.3 × 10−5 |
We find that the Cav-1 score is statistically significant even adjusting for T-stage, N-stage and tamoxifen use. The baseline level has T stage = T0/T1, N stage = N0, no tamoxifen and Cav-1 present. Model is based on 101 observations due to missing data.
Absence of stromal Cav-1 expression was also associated with dramatic reductions in 5-year PFS (see Table 7 for 5-year survival rates). Very similar results were also obtained using overall survival (see supplemental Figure S1 at http://ajp.amjpathol.org). However, PFS is considered more of a cancer-specific measure of clinical outcome.
Table 7.
Association of Stromal Cav-1 with 5-Year PFS
| Patient groups | Stromal Cav-1 Status | 5-Year PFS
|
|||
|---|---|---|---|---|---|
| Patients alive, at risk | Patient death/ recurrence | Percent of patients alive with no recurrence | P value | ||
| 1-Tamoxifen-treated | Absent | 4 | 10 | 28.6% | 2.42 × 10−5 |
| Present | 37 | 4 | 90.2% | ||
| 2-Without tamoxifen treatment | Absent | 4 | 24 | 14.3% | 6.21 × 10−6 |
| Present | 22 | 8 | 73.3% | ||
| Total patients (1 + 2) | Absent | 8 | 34 | 19.1% | 2.10 × 10−11 |
| Present | 59 | 12 | 83.1% | ||
Test used: Fisher’s exact test.
Stromal versus Epithelial Cav-1 as Predictive Breast Cancer Biomarkers
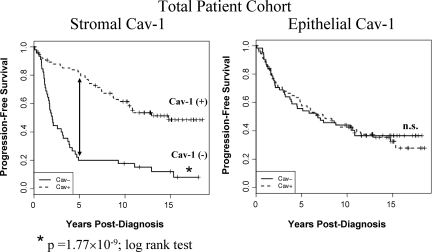
To assess the predictive value of epithelial Cav-1 expression, the same patient population was also scored for the expression of Cav-1 in the epithelial tumor cells, using the same scoring scheme as for stromal Cav-1 (0 = absent; 1 or 2 = present). However, as presented in Figure 3, epithelial Cav-1 did not show any correlation with patient clinical outcome. This is an important internal control for our current studies, and reinforces the idea that stromal expression of Cav-1 is a primary determinant of clinical outcome in breast cancer patients.
Figure 3.
Epithelial Cav-1 expression is not a predictor of progression-Free survival. The status of stromal and epithelial Cav-1 was independently scored in the same total patient population for direct comparison. Note that only stromal Cav-1 is a predictor of clinical outcome (P = 1.77 × 10−9, log-rank test), in a total population of 125 breast cancer patients. 5-year PFS is indicated by an arrow. The status of epithelial Cav-1 is also shown. n.s., denotes not significant.
Status of Stromal Cav-1 in ER+, PR+, and HER2+ Patients
Historically, ER, PR, and HER2 expression have all served as important prognostic and predictive epithelial biomarkers for stratifying breast cancer patients into prognosis and therapy-relevant groups. Thus, we wondered whether stromal Cav-1 would function as a strong predictive biomarker in all three of these patient groups. Figures 4, 5, and 6 show that regardless of epithelial marker status for ER, PR, or HER2, stromal Cav-1 serves as an important predictor of progression-free outcome. Thus, the status of stromal Cav-1 expression appears to be a critical predictor of clinical outcome that is clearly independent of epithelial marker status. The predictive value of epithelial Cav-1 is shown for comparison; it does not behave as a predictive biomarker in any of these patient groups.
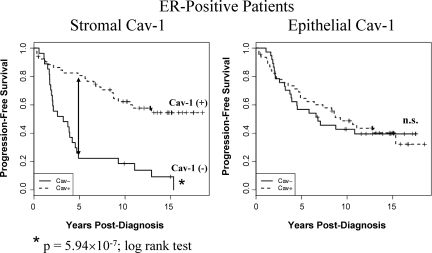
Figure 4.
Kaplan-Meier curves of progression-free survival in ER-positive patients. Note that an absence of stromal Cav-1 immunostaining also predicts poor clinical outcome in ER-positive patients (P = 5.94 × 10−7, log-rank test), which represents a total of 80 breast cancer patients. 5-year PFS is indicated by an arrow. The status of epithelial Cav-1 is shown for comparison. n.s., denotes not significant.
Figure 5.
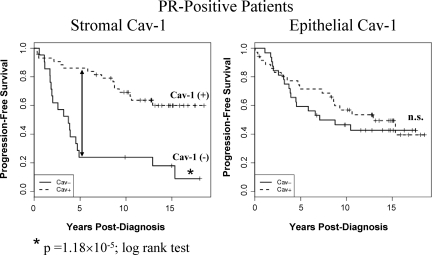
Kaplan-Meier curves of PFS in PR-positive patients. Note that an absence of stromal Cav-1 immunostaining also predicts poor clinical outcome in PR-positive patients (P = 1.18 × 10−5, log-rank test), which represents a total of 65 breast cancer patients. 5-year PFS is indicated by an arrow. The status of epithelial Cav-1 is shown for comparison. n.s., denotes not significant.
Figure 6.
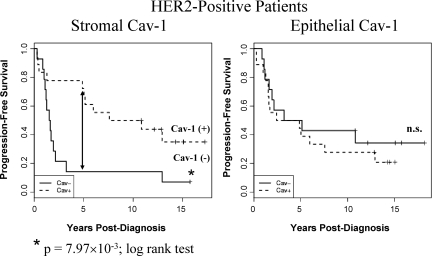
Kaplan-Meier curves of PFS in HER2-positive patients. Note that an absence of stromal Cav-1 immunostaining also predicts poor clinical outcome in HER2-positive patients (P = 7.97 × 10−3, log-rank test), which represents a total of 32 breast cancer patients. 5-year PFS is indicated by an arrow. The status of epithelial Cav-1 is shown for comparison. n.s., denotes not significant.
Status of Stromal Cav-1 in Triple-Negative Patients
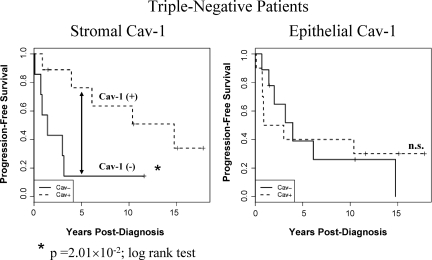
Triple-negative breast cancers lack expression of the three most commonly used epithelial makers (ER−/PR−/HER2−), are generally poorly-differentiated, and are associated with poor clinical outcome. Thus, we examined the predictive value of stromal Cav-1 in triple-negative patients, within our patient population. Interestingly, stromal Cav-1 was also a strong predictor of progression-free outcome in triple negative breast cancer patients (Table 5 and Figure 7). For example, the median PFS was 1.43 years versus 14.76 years in triple-negative patients, depending on the status of stromal Cav-1 (Table 5). However, epithelial Cav-1 did not show any predictive value in triple-negative patients (Figure 7).
Figure 7.
Kaplan-Meier curves of PFS in triple-negative patients. Note that an absence of stromal Cav-1 immunostaining also predicts poor clinical outcome in triple-negative (ER−/PR−/HER2−) patients (P = 2.01 × 10−2, log-rank test), even though this subset of the patient population is small (16 patients). 5-year PFS is indicated by an arrow. The status of epithelial Cav-1 is shown for comparison. n.s., denotes not significant.
Thus, stromal Cav-1 is a powerful predictive biomarker for estimating a patient’s risk of recurrence and survival in all of the four most common classes of breast cancer, which are based on ER, PR, and HER2 expression.
Additional data on ER(−), PR(−), low T stage, and grade 3 patients are provided as supplemental Figures S2, S3, and S4 at http://ajp.amjpathol.org. In all these additional patient subgroups, an absence of stromal Cav-1 also consistently predicts poor clinical outcome.
Status of Stromal Cav-1 in LN(−) and LN(+) Patients
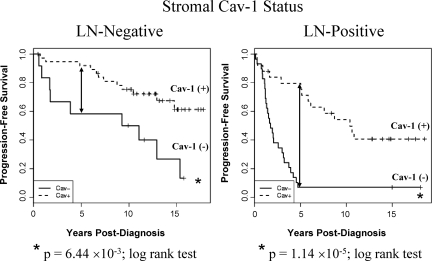
Lymph node (LN) status is often used as a critical predictor of disease recurrence, metastasis, and survival in patients with breast cancer. As an absence of stromal Cav-1 behaves as a predictor of disease recurrence and poor clinical outcome, we also assessed the predictive role of stromal Cav-1 in LN(−) and LN(+) patients. Our results are shown in Figure 8. Note that in both LN(−) and LN(+) patients, an absence of stromal Cav-1 still remains a significant predictor of progression-free outcome. However, our results were most dramatic in LN(+) patients, where an absence of stromal Cav-1 is associated with an ∼11.5-fold reduction in 5-year survival (Table 8).
Figure 8.
Kaplan-Meier curves of PFS in LN-negative and positive patients. Note that in both LN(−) and LN(+) patients, an absence of stromal Cav-1 still remains a significant predictor of progression-free outcome. However, the results were most dramatic in LN(+) patients, where an absence of stromal Cav-1 is associated with an ∼11.5-fold reduction in 5-year progression-free survival. There were 50 patients in the LN(−) group and 54 patients in the LN(+) group. P values are as shown. 5-year PFS is indicated by an arrow.
Table 8.
Association of Stromal Cav-1 with 5-Year PFS in Lymph Node-Positive and -Negative Patients
| Patient groups | Stromal Cav-1 Status | 5-Year PFS
|
|||
|---|---|---|---|---|---|
| Patients alive, at risk | Patient death/ recurrence | Percent of patients alive with no recurrence | P value | ||
| 1-LN-positive | Absent | 2 | 27 | 6.90% | 6.87 × 10−8 |
| Present | 19 | 5 | 79.17% | ||
| 2-LN-negative | Absent | 7 | 5 | 58.33% | 0.015 |
| Present | 34 | 3 | 91.89% | ||
| Total patients (1 + 2) | Absent | 9 | 32 | 21.95% | 5.32 × 10−11 |
| Present | 53 | 8 | 86.89% | ||
Test used: Fisher’s exact test.
Thus, the use of stromal Cav-1 as a predictive biomarker, especially in LN(+) patients, may allow for early interventions with more aggressive therapies.
Discussion
In this study, we have evaluated the expression of Cav-1 in the stroma of invasive breast carcinomas and demonstrated that loss of stromal Cav-1 expression is a strong predictor of tumor recurrence and dramatically lower progression-free survival. Although epithelial Cav-1 expression has been extensively studied in breast carcinomas, there is little or no data on the expression and significance of Cav-1 in the stroma of invasive breast carcinomas.24,25,26 Previous studies demonstrated that epithelial expression of Cav-1 in malignant breast cancer cells correlates with histological grade, loss of ER and PR positivity, and the expression of basal markers including cytokeratins and p63.24 However, in multivariate analysis, epithelial Cav-1 expression was not an independent prognostic factor for patient outcome.24 Consistent with these published findings, we observed here that epithelial Cav-1 expression was not a prognostic factor for clinical outcome in our patient cohort.
Several prior studies investigated the significance of stromal marker expression in invasive carcinomas of the breast. For example, it was shown that stromal CD10 expression was associated with decreased survival in LN(−) patients and a higher risk of developing lymph node metastasis.27,28 Another study showed that stromal expression of matrix metalloproteinase 9 was associated with aggressive tumor behavior and had a negative impact on overall and disease-free survival.29 Gene expression profiling of breast cancers focusing on stroma-related genes demonstrated that tumors with a solitary-fibrous tumor-type or a desmoid-type/fibromatosis-type signature have different clinical outcomes.30 The desmoid-type fibromatosis stromal signature was associated with lower tumor grade, increased expression of ER, and improved survival. Another stromal-related gene signature, termed the “wound-response signature,” predicted poor overall survival and increased risk of metastasis in patients with breast cancer.31
A recent study found a “stroma-derived prognostic predictor” signature that included genes that are involved in the immune responses, angiogenesis, and hypoxia.32 This 26-gene signature was independent of ER and HER2 status, lymph node involvement, tumor grade, age and chemotherapy and hormonal therapy, in identifying a poor-outcome patient group who had a substantially increased risk of disease recurrence or death. Interestingly, in our current study, an absence or loss of stromal Cav-1 expression was associated with increased risk of breast cancer recurrence independent of standard clinicopathological risk factors and treatment regimens. Thus, our new data provide a reliable and powerful single marker to predict important clinical variables independently of other known biomarkers.
Our data showing that Cav-1 reduction in the tumor stroma increases the aggressiveness of breast carcinomas warrant further discussion and investigation. Recently, we showed that CAFs down-regulated Cav-1 protein expression, in conjunction with RB tumor suppressor inactivation.15 Additional studies showed that mammary stromal fibroblasts from Cav-1−/− knock-out mice share a similar gene expression profile with human CAFs,23 and both show the upregulation of RB/E2F responsive genes. Thus, one possibility is that an absence of Cav-1 expression in mammary stromal fibroblasts leads to RB tumor suppressor functional inactivation in vivo, thereby releasing E2F. This, in turn, generates ‘activated stromal fibroblasts’ that can increase the transcription of a number of cell cycle (S-phase) related genes, including target genes that encode growth promoting factors and cytokines. Another possibility is that loss of Cav-1 in stromal cells allows for activation of transforming growth factor-β signaling.33 It has been shown that this activated transforming growth factor-β signaling in CAFs could induce the secretion of growth promoting proteins such as human growth factor, vascular endothelial growth factor, and interleukin-6.34 Yet, the most intriguing possibility is a controversial finding that reported p53 mutations in breast cancer stromal cells.35 Previously, an unrelated study showed a novel mechanism by which p53 transcriptionally up-regulates Cav-1 expression.36 Thus, mutational inactivation of p53 in breast cancer stromal cells could reduce or prevent the expression of Cav-1 in these cells. Future studies will correlate p53 status (ie, loss of heterozygosity, allelic imbalance, and sporadic mutations) with Cav-1 expression in breast cancer stromal cells.
Abundant tumor stroma and formation of the central scar-like area termed a fibrotic focus is a known predictor of aggressive behavior in breast cancers.37 The presence of a fibrotic focus is correlated with larger tumor size, increased tumor proliferation, higher histological grade, and higher pathological stage.38 Furthermore, the presence of a fibrotic focus has been associated with poor short- and long-term survival and has been confirmed as an independent prognostic factor for patients with breast cancer in both retrospective and prospective.38,39,40 Since breast carcinomas with a fibrotic focus have a distinct molecular signature, as determined by gene expression studies, it will be interesting to evaluate stromal Cav-1 expression in this morphological variant of breast cancers.41
It remains unknown what causes the down-regulation of Cav-1 in the mammary tumor stroma. However, in experiments with human breast CAFs, we previously showed that Cav-1 mRNA transcript levels were either increased ∼2.3- to 2.4-fold or not changed, suggesting that the loss of Cav-1 protein expression occurs at a posttranscriptional or posttranslational level.15 Since human breast CAFs show a loss of Cav-1 protein expression, we recently examined the phenotypic behavior of mammary stromal fibroblasts derived from Cav-1−/− null mice. Interestingly, Cav-1−/− mammary stromal fibroblasts assumed many of the characteristics of CAFs and secreted increased levels of proliferative and pro-angiogenic growth factors, including vascular endothelial growth factor.23 In this regard, Cav-1−/− mammary stromal fibroblasts also underwent endothelial-like trans-differentiation in vitro, with the up-regulation of CD31 (Pecam1). In support of the idea that Cav-1−/− mammary stromal fibroblasts may have increased cellular plasticity, genome-wide transcriptional profiling showed that they also up-regulate numerous embryonic stem cell associated genes. Consistent with these findings, the mammary stromal compartment in Cav-1−/− mice shows dramatically increased vascularization (via CD31 staining)23 and promotes tumorigenesis in vivo.16 Thus, based on these mechanistic studies, we suggest that breast cancer patients lacking stromal Cav-1 might benefit from anti-angiogenic therapy (such as bevacizumab, [Avastin]), in addition to the more standard treatment regimens.
Since ER, PR, and HER2 expression have long served as important epithelial biomarkers for stratifying breast cancer patients into different diagnostic and therapeutic groups, we also assessed the status of stromal Cav-1 in these different patient groups within our cohort. Strikingly, we observed that an absence of stromal Cav-1 effectively predicts early tumor recurrence and poor clinical outcome in all four groups: ER+, PR+, HER2+, and triple-negative patients (ER−/PR−/HER2−). Thus, stromal Cav-1 may serve as a new “universal” or “widely-applicable” breast cancer biomarker that can be used to predict early tumor recurrence and clinical outcome across many different “subclasses” of breast cancer. This is a potentially paradigm-shifting notion, and suggests that we should be more actively targeting the tumor stroma in our therapeutic interventions. Thus, the status of the tumor stroma may be a primary determinant of disease recurrence and poor clinical outcome in breast cancer patients.
Although other independent retrospective and prospective studies will be necessary before direct clinical application of the current findings, our data strongly suggest that loss of stromal Cav-1 expression is closely linked to aggressive biological behaviors, including invasion and metastasis of breast carcinomas. This study, along with previously published work, underscores the importance of depicting the molecular changes and other phenotypic aspects of stromal-related tumor cells. Uncovering critical molecular events, such as Cav-1 reduction in the mammary tumor stroma, will allow us to begin to unravel the key features of epithelial-stromal cross talk that are critical for tumor progression and metastasis.
Note Added in Proof
Recently, we observed that loss of stromal Cav-1 also has predictive value for clinical outcome in DCIS patients, regarding progression to invasive breast cancer.42
Supplementary Material
Footnotes
Address reprint requests to Drs. Agnieszka K. Witkiewicz or Michael P. Lisanti, Stem Cell Biology and Regenerative Medicine Center, Thomas Jefferson University, 233 South 10th Street, Philadelphia, PA, 19107. E-mail: agnieszka.witkiewicz@jefferson.edu or michael.lisanti@kimmelcancercenter.org.
See related Commentary on page 1996
Supported by grants from the NIH/NCI (R01-CA-80250; R01-CA-098779; R01-CA-120876, the American Association for Cancer Research, and the Department of Defense-Breast Cancer Research Program (Synergistic Idea Award) (to M.P.L.). A.K.W was supported by a Young Investigator Award from Breast Cancer Alliance, Inc. and a Susan G. Komen Career Catalyst Grant. F.S. was supported by grants from the Elsa U. Pardee Foundation, the W.W. Smith Charitable Trust, and a Research Scholar Grant from the American Cancer Society. C.G.K. was supported by NIH/NCI grants (R01-CA-090876; R01-CA107469) and a grant from the Avon Foundation. This project is funded, in part, under a grant with the Pennsylvania Department of Health (to M.P.L.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. R.G.P. was supported by grants from the NIH/NCI (R01-CA-70896, R01-CA-75503, R01-CA-86072, and R01-CA-107382) and the Dr. Ralph and Marian C. Falk Medical Research Trust. The Kimmel Cancer Center was supported by the NIH/NCI Cancer Center Core grant P30-CA-56036 (to R.G.P.).
A.K.W. and A.D. contributed equally and should be considered co-first authors.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University or Albert Einstein College of Medicine was involved in the peer review process or final disposition for this article.
References
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Kim JB, Stein R, O'Hare MJ. Tumour-stromal interactions in breast cancer: the role of stroma in tumourigenesis. Tumour Biol. 2005;26:173–185. doi: 10.1159/000086950. [DOI] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3:445–464. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–16381. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Minkeu A, Allen KG, Danilo C, Sotgia F, Bonuccelli G, Jasmin JF, Xu H, Bosco E, Aronow B, Witkiewicz A, Pestell RG, Knudsen ES, Lisanti MP. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol Ther. 2008;7:1212–1225. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Sotgia F, Lee H, Hassan G, Di Vizio D, Bonuccelli G, Capozza F, Mercier I, Rui H, Pestell RG, Lisanti MP. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. Am J Pathol. 2006;169:1784–1801. doi: 10.2353/ajpath.2006.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991;19: 403–410, Histopathology. 2002;41:151–152. discussion 152–153. [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- Breiman L, Friedman J, Stone CJ, Olshen RA. Belmont, CA: Chapman and Hall,; Classification and Regression Trees. 1984:pp 216–264. [Google Scholar]
- LeBlanc M, Crowley J. Relative risk trees for censored survival data. Biometrics. 1992;48:411–425. [PubMed] [Google Scholar]
- R Development Core Team Vienna, Austria: R Foundation for Statistical Computing,; RA Language and Environment for Statistical Computing. 2008 [Google Scholar]
- Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, Daumer KM, Zhou J, Wang C, Katiyar S, Xu H, Bosco E, Quong AA, Aronow B, Witkiewicz AK, Minetti C, Frank PG, Jimenez SA, Knudsen ES, Pestell RG, Lisanti MP. Caveolin-1 (−/−) null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts, Am J Pathol. 2008;174:746–761. doi: 10.2353/ajpath.2009.080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikh SE, Green AR, Rakha EA, Samaka RM, Ammar AA, Powe D, Reis-Filho JS, Ellis IO. Caveolin 1 and Caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br J Cancer. 2008;99:327–334. doi: 10.1038/sj.bjc.6604463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Sotgia F, Vuolo MA, Li M, Yang WC, Pestell RG, Sparano JA, Lisanti MP. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol. 2006;168:1998–2013. doi: 10.2353/ajpath.2006.051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y, Mimori K, Yoshinaga K, Tanaka F, Nishida K, Ohno S, Inoue H, Mori M. Clinical significance of Caveolin-1. Caveolin-2 and HER2/neu mRNA expression in human breast cancer. Br J Cancer. 2004;91:959–965. doi: 10.1038/sj.bjc.6602029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya K, Ogawa H, Izumi M, Kuroda M, Mukai K. Stromal expression of CD10 in invasive breast carcinoma: a new predictor of clinical outcome. Virchows Arch. 2002;440:589–593. doi: 10.1007/s00428-002-0639-4. [DOI] [PubMed] [Google Scholar]
- Makretsov NA, Hayes M, Carter BA, Dabiri S, Gilks CB, Huntsman DG. Stromal CD10 expression in invasive breast carcinoma correlates with poor prognosis, estrogen receptor negativity, and high grade. Mod Pathol. 2007;20:84–89. doi: 10.1038/modpathol.3800713. [DOI] [PubMed] [Google Scholar]
- Mylona E, Nomikos A, Magkou C, Kamberou M, Papassideri I, Keramopoulos A, Nakopoulou L. The clinicopathological and prognostic significance of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-9 according to their localization in invasive breast carcinoma. Histopathology. 2007;50:338–347. doi: 10.1111/j.1365-2559.2007.02615.x. [DOI] [PubMed] [Google Scholar]
- West RB, Nuyten DS, Subramanian S, Nielsen TO, Corless CL, Rubin BP, Montgomery K, Zhu S, Patel R, Hernandez-Boussard T, Goldblum JR, Brown PO, van de Vijver M, van de Rijn M. Determination of stromal signatures in breast carcinoma. PLoS Biol. 2005;3:E187. doi: 10.1371/journal.pbio.0030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- Cat B, Stuhlmann D, Steinbrenner H, Alili L, Holtkotter O, Sies H, Brenneisen P. Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. J Cell Sci. 2006;119:2727–2738. doi: 10.1242/jcs.03011. [DOI] [PubMed] [Google Scholar]
- Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL, Platzer P, Eng C. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- Bist A, Fielding CJ, Fielding PE. p53 regulates caveolin gene transcription, cell cholesterol, and growth by a novel mechanism. Biochemistry. 2000;39:1966–1972. doi: 10.1021/bi991721h. [DOI] [PubMed] [Google Scholar]
- Hasebe T, Tsuda H, Hirohashi S, Shimosato Y, Iwai M, Imoto S, Mukai K. Fibrotic focus in invasive ductal carcinoma: an indicator of high tumor aggressiveness. Jpn J Cancer Res. 1996;87:385–394. doi: 10.1111/j.1349-7006.1996.tb00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Tsuda H, Hirohashi S, Shimosato Y, Tsubono Y, Yamamoto H, Mukai K. Fibrotic focus in infiltrating ductal carcinoma of the breast: a significant histopathological prognostic parameter for predicting the long-term survival of the patients. Breast Cancer Res Treat. 1998;49:195–208. doi: 10.1023/a:1006067513634. [DOI] [PubMed] [Google Scholar]
- Baak JP, Colpaert CG, van Diest PJ, Janssen E, van Diermen B, Albernaz E, Vermeulen PB, Van Marck EA. Multivariate prognostic evaluation of the mitotic activity index and fibrotic focus in node-negative invasive breast cancers. Eur J Cancer. 2005;41:2093–2101. doi: 10.1016/j.ejca.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Colpaert C, Vermeulen P, Van Marck E, Dirix L. The presence of a fibrotic focus is an independent predictor of early metastasis in lymph node-negative breast cancer patients. Am J Surg Pathol. 2001;25:1557–1558. doi: 10.1097/00000478-200112000-00016. [DOI] [PubMed] [Google Scholar]
- Van den Eynden GG, Smid M, Van Laere SJ, Colpaert CG, Van der Auwera I, Bich TX, van Dam P, den Bakker MA, Dirix LY, Van Marck EA, Vermeulen PB, Foekens JA. Gene expression profiles associated with the presence of a fibrotic focus and the growth pattern in lymph node-negative breast cancer. Clin Cancer Res. 2008;14:2944–2952. doi: 10.1158/1078-0432.CCR-07-4397. [DOI] [PubMed] [Google Scholar]
- Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, Schwartz GF, Pestell RG, Sotgia F, Rui H, Lisanti MP. Stromal Caveolin-1 Levels Predict Early DCIS Progression to Invasive Breast Cancer, Cancer Biology & Therapy. 2009;8:65–73. doi: 10.4161/cbt.8.11.8874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.