Abstract
Caveolin-1 has been linked to tumor progression and clinical outcome in breast cancer, but a clear resolution of its role as a prognostic marker is lacking. We assessed caveolin-1 levels in normal breast tissue and two breast cancer cohorts for which outcome data were available. We found that caveolin-1 was not expressed in normal breast luminal epithelium but was present in the epithelial compartment of some tumors. We found no association between caveolin-1 expression in the epithelial compartment and clinical outcome. However, high levels of caveolin-1 in the stromal tissue surrounding the tumor, rather than within tumor cells, associated strongly with reduced metastasis and improved survival (P < 0.0001). The onset of mammary tumors driven by Her2/neu overexpression was accelerated in mice lacking caveolin-1, thereby supporting the observation that the presence of caveolin-1 in the tumor microenvironment modulates tumor development. These studies suggest that stromal caveolin-1 expression may be a potential therapeutic target and a valuable prognostic indicator of breast cancer progression.
In recent years, it has become increasing apparent that stromal components in the tumor microenvironment, including the extracellular matrix and cell types including fibroblasts, vascular endothelial cells, immune and in-flammatory cells, have a profound influence on the growth and metastasis of tumors. The molecular cross talk between tumor cells and these stromal elements plays an important role in defining the phenotype of a tumor.1 Tumor cells can trigger the deposition of a reactive stroma or desmoplasia containing activated fibroblasts, connective tissue, immune and inflammatory cells that may favor invasion and metastasis of the cancer.2 Many cell types in the mammary stroma express caveolin-1. However the role of this protein in molecular cross talk between tumor and stromal cells remains unknown.
Caveolin-1 is an integral plasma membrane protein that resides in specialized lipid rafts called caveolae in terminally differentiated mesenchymal cells including adipocytes, endothelial cells, and fibroblasts.3 Within caveolae, the bilayer of cholesterol and sphingolipids is in an ordered state that restricts the movement of lipids. Caveolin-1 interacts directly with, and organizes cholesterol within caveolae.4 Various receptors and signaling molecules are localized within caveolae, and caveolin-1 interacts with and negatively regulates a number of these through its scaffolding domain.5,6 By ordering lipids and concentrating signaling molecules, caveolae may facilitate cross talk between signaling pathways. Caveolin-1 is also found in the cytosol and has a role in lipid homeostasis and transport.7,8
Caveolin-1 is localized to human chromosome 7q31.1, a site that exhibits loss of heterozygosity in a range of tumor types.9,10 However, the role of caveolin-1 in breast cancer remains unclear. Earlier reports that caveolin-1 is expressed in normal breast epithelium11,12 are contradicted by more recent studies that found that its expression is associated with the myoepithelium and other tissues of mesenchymal origin and not with normal luminal epithelium.13,14,15,16 A number of studies have reported that caveolin-1 is down-regulated in breast cancers compared with normal mammary tissue.14,17,18,19 Similarly, caveolin-1 was absent in 10 invasive breast carcinomas, but present in two breast tumors of myoepithelial origin (basal-like cancers).13 Down-regulation of caveolin-1 may be due to inactivating mutations in the gene, as reported previously,20,21 but not confirmed in another study.18 Caveolin-1 gene expression may also be regulated in breast cancer through methylation.18 New data demonstrate that high caveolin-1 expression in tumor epithelium is associated with basal, metaplastic, and triple negative breast cancers (negative for estrogen receptor [ER], progesterone receptor [PR], and Her2),15,22 as well as with inflammatory breast cancers.23 The role of caveolin-1 in other tumor types appears to be varied, with expression associated with cancer suppression in ovarian, small cell lung, and colon cancers,24,25,26 but with cancer progression in prostate, non-small cell lung, esophageal, and colon cancers.25,27,28,29
To clarify the relationship between caveolin-1 and breast cancer progression or suppression, we have analyzed tissue sections specifically for stromal and tumor epithelial cell expression of caveolin-1 from two cohorts of breast cancer patients. Here we report that caveolin-1 levels in the tumor microenvironment rather than tumor epithelial compartment correlate strongly with clinical outcome. This observation is supported by our studies of Her2/neu driven tumor development in caveolin-1 null mice, where tumor onset is accelerated in the absence of stromal caveolin-1. These findings indicate that stromal caveolin-1 has a potential role as a new prognostic marker for breast cancer progression as well as being a possible therapeutic target.
Materials and Methods
Normal Human Breast and Cancer Tissues
Normal human breast tissue was collected by the Peter Mac Tissue Bank from consenting women undergoing breast reduction surgery. The Breast Prognosis tissue microarray, kindly provided by Dr. O. Kallioniemi, contains one 0.6-mm biopsy from the periphery of each of 612 human primary breast carcinomas with extensive clinical follow-up data.30 The analysis reported here includes 429 samples with interpretable data. The remaining samples did not contain useable information for a number of reasons: (i) samples were not present on the glass slide or were lost from the slide during the immunostaining procedure, (ii) no tumor tissue was present in the sample, (iii) the quality of the sample was too poor to interpret expression, or (iv) survival data were not available. The mean age at diagnosis was 61 years (SD = 12.3; range 33 to 97 years). The stage distribution was TNM stage I, 22%; stage II, 53%; stage III, 17%; and stage IV, 2% of the patients. TNM stage unavailable for 6% of the patients. The samples included 78% ductal, 9% lobular, 3% medullary, and 10% with other tumor subtypes. The median tumor diameter was 23 mm (range, 4 to 130 mm). The 5-year cancer-specific survival rate for all patients was 78% (95% CI: 74% to 83%).
Whole sections were analyzed from archival breast cancer tissues from consecutive patients with stage 1 to 3 operable breast cancer who presented at a single clinic (Dr. F. E. Gago, Mendoza, Argentina) between June 1985 and June 1999. For 173 patients there was sufficient tissue for analysis of both epithelial and stromal caveolin-1 immunostaining, and complete pathology information was available. Informed consent to obtain and analyze tissue was given before surgery, and tissue blocks and clinical data were stored and managed following standard international procedures. The median age of this cohort was 54 years (range, 57 to 81). The median tumor size was 25 mm (range 5 mm to 90 mm). The majority of patients (54%) were lymph node negative. AJCC stage presentation was stage 1, 27%; stage 2a, 38%; stage 2b, 17%; stage 3a, 13%; and stage 3b, 5%. All patients were followed until death or until June 2004 with a median follow up of 11.5 years. Patients were all treated according to approved protocols active at the time of diagnosis, with 28% receiving a mastectomy, 72% receiving chemotherapy, 76% receiving radiotherapy, and 72% being treated with tamoxifen. The diagnosis of metastasis was based on pathological confirmation or on other investigations that confirmed the presence of metastasis. Apart from three, all patients received surgery as the first treatment, and samples for analysis were obtained before chemotherapy. The remaining three samples were obtained after chemotherapy. Parallel analysis that excluded these three patients found substantially similar results to analysis of the entire cohort.
Caveolin-1 Immunostaining
Caveolin-1 immunostaining was completed on 3.5-micron sections of individual tissues or on the tissue microarray using a standard protocol with antigen retrieval in 10 mmol/L sodium citrate, pH 6.0, at 98°C for 15 minutes.31 All tissues were fixed in 10% buffered formalin and paraffin-embedded. Mouse monoclonal and rabbit polyclonal anti-caveolin-1 antibodies (BD Transduction Laboratories, Lexington, KY), were used at 2.5 and 0.5 μg/ml respectively. Non-specific mouse IgG1 antibody and purified rabbit pre-immune serum (DAKO, Kingsgrove, NSW, Australia) were used as isotype controls. Secondary biotin-conjugated goat anti-mouse/rabbit antibodies (Vector Laboratories Homebush, NSW, Australia) were used at 1:250 dilution. Specific primary-secondary antibody complexes were detected using ABC reagent (Vector) and visualized using a diaminobenzidine peroxidase substrate kit (Vector).
For the whole sections, the extent and intensity of caveolin-1 immunostaining was assessed independently by two experienced breast pathologists and in the 22% of the samples where there was some disagreement (often relating to the level of staining intensity), the matter was resolved by majority consensus with a third experienced breast pathologist. The tissue microarray was assessed by one pathologist and two other independent scorers and the majority opinion taken. Caveolin-1 immunostaining in tumor cells or stroma was scored as 0 (no expression), 1 (weak expression), or 2 (strong expression).
Analysis of ER, PR, c-erbB2, proliferating nuclear cell antigen, p53, and p170 on the whole tissue sections has been published previously.32,33
Statistical Analysis
Frequency data were analyzed with the Fisher Exact test and continuous data by the Mann-Whitney U-test. The Kaplan-Meier method was used to estimate overall survival, and differences in outcome for each variable were compared with the log-rank test. Multivariate analysis of factors affecting survival was performed using a Cox proportional hazard model. All statistical tests were two-sided and P < 0.05 was considered to be statistically significant. SPSS software (version 16, Chicago, IL) was used for the statistical analysis of the human data and Graph Pad Prism (version 5.01) for the mouse outcome data.
Animal Studies
Mice lacking stromal caveolin-1 and with mammary-specific expression of Her-2/neu were bred from caveolin-1 null mice (129/Sv/C57Bl/6) obtained from Dr. T. Kurchalia34 through Dr. R. Parton (University of Queensland, Australia) and from mice transgenic for the MMTV-neu oncogene (FVB/N, obtained from Dr. W. Muller through Dr. J. Visrader).35 Matings were performed with male MMTV-neu+/−:caveolin-1+/−, and female caveolin-1+/− mice. Six genotypes, either heterozygote or null for MMTV-neu and with either two, one, or no alleles of caveolin-1, with a minimum of 15 female mice from each genotype were obtained from the same generation of MMTV-neu+/−:caveolin-1+/− male and female breeders. At 8 to 10 weeks and again at 16 to 20 weeks, the mice in each genotype were subjected to pregnancy and 10 days of lactation and were monitored weekly for development of mammary tumors. Once tumors became palpable they were measured weekly using electronic calipers and mice were culled when the primary tumor reached a volume of 1500 mm3. All procedures were performed in a barrier facility under protocols approved by the Peter MacCallum Animal Experimentation Ethics Committee.
Mouse Tissue Analysis
Primary tumors and lungs were removed at autopsy, weighed, fixed in 10% buffered formalin, and processed for paraffin embedding. Primary tumors were sectioned and stained with H&E for general morphology or immunostained to determine caveolin-1 levels as described above. The lungs were ribbon cut into 5-micron sections and every 20th section was H&E-stained and assessed by microscopy for metastatic nodules.
Results
Caveolin-1 Expression in Normal Human Breast Tissue
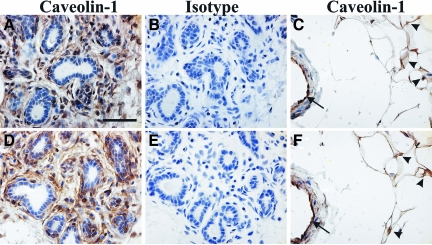
The localization of caveolin-1 in normal breast tissue was assessed by immunostaining of tissue samples from ten healthy individuals using either a monoclonal antibody or a rabbit polyclonal antibody. The same expression pattern was seen with both antibodies. The specificity of the antibodies for caveolin-1 was confirmed previously by western analysis.36 Caveolin-1 immunoreactivity was observed in stromal fibroblasts and in myoepithelial cells underlying the luminal epithelial cells (Figure 1, A, B, D, and E). Adipocytes and vascular endothelial cells were also positive for caveolin-1 (Figure 1, C and F). However, no evidence for caveolin-1 expression was found in luminal epithelial cells. Consistent with our findings, a number of other reports describe the specific localization of caveolin-1 to breast myoepithelial and stromal cells, but not luminal epithelial cells13,14,15 although epithelial cell expression has also been reported.11,12
Figure 1.
Caveolin-1 expression in normal human breast tissue. Normal breast tissue samples were immunostained with rabbit polyclonal (A–C) or mouse monoclonal (D–F) antibodies directed against caveolin-1 (A, C, D, F) or a non-specific protein (isotype) (B and E). Caveolin-1 expression in the stromal tissue surrounding the ductal epithelium and especially in the myoepithelial layer underlining the ducts is clearly visible in panels A and D. Specific reactivity against caveolin-1 in adipose tissue (arrowheads) and vascular endothelial cells (arrows) is shown in panels C and F. Scale bar = 50 μm.
Caveolin-1 Expression in the Tumor Cell Compartment
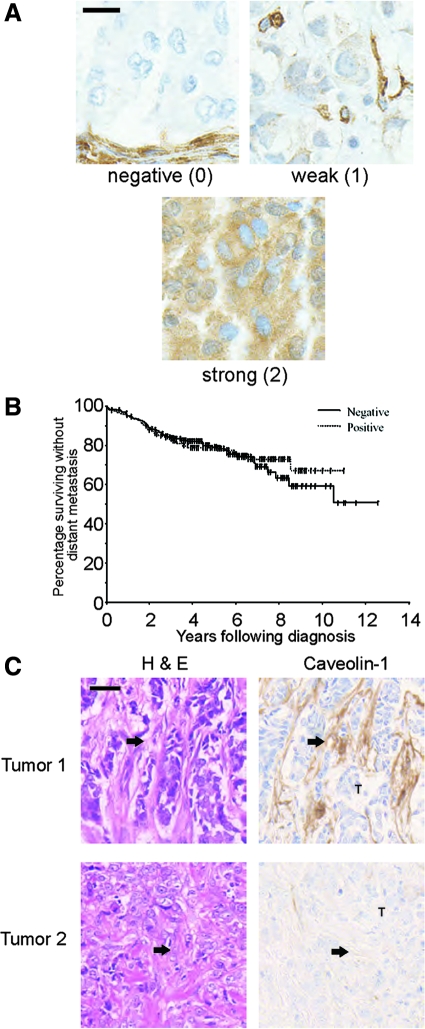
To assess the association between caveolin-1 and clinical outcome in breast cancer, we used a monoclonal anti-caveolin-1 antibody to immunostain 429 arrayed primary human breast cancer samples. Details of the tissue array have been published previously.30 Breast tumor cells in each sample were scored for the intensity of caveolin-1 staining; 268 (63%) of breast tumors were negative for caveolin-1, 130 (30%) had weak caveolin-1 immunoreactivity, and 31 (7%) were strongly positive for caveolin-1. Representative examples are shown in Figure 2A. The level of caveolin-1 expression in primary breast tumor cells was assessed in relation to other factors known to be associated with tumor progression. The presence of caveolin-1 in epithelial tumor cells was positively associated with lower TNM tumor stage (P = 0.05). However, caveolin-1 levels in tumor epithelium did not predict cancer-specific survival over 5 years, 77% (95% CI: 71% to 83%) for patients with caveolin-1 negative primary tumors, 79% (95% CI: 71% to 87%) for patients with weak caveolin-1 immunostaining and 88% (95% CI: 76% to 100%) for patients with strong caveolin-1 immunostaining (P = 0.24) (Figure 2B). Consistent with these findings, caveolin-1 expression in tumor cells was not associated with any of the other standard prognostic factors investigated (data not shown).
Figure 2.
Caveolin-1 expression in human breast cancer. A: Representative images of primary human breast tumors with tumor cells showing negative, weak or strong expression of caveolin-1 in tumor epithelium. Scale bar = 10 μm. B: Metastasis-free survival by caveolin-1 status (positive or negative) in tumor epithelium after adjusting for other prognostic factors. The samples scoring 1 or 2 for expression of caveolin-1 are pooled and shown as positive samples. C: Representative tumors with stroma staining positive or negative for caveolin-1. Stroma indicated by arrows. Scale bar = 40 μm.
Caveolin-1 Levels in the Tumor Microenvironment Predict Survival
During screening of caveolin-1 levels in the arrayed breast tumor samples, we noted significant variation of caveolin-1 levels in stromal tissue in the tumor microenvironment in the few samples where significant amounts of stroma were present. However, further analysis of stromal caveolin-1 levels in the arrayed tumor samples was prevented by insufficient samples containing adequate amounts of stromal tissue. The samples were very small (0.6 mm) and the biopsy location was chosen to maximize tumor cell density. The heterogeneity of breast cancer confounds the analysis of samples with very small amounts of stroma. To explore further the relationship between caveolin-1 levels in the tumor microenvironment and breast cancer progression, we immunostained for caveolin-1 in a consecutive series of 173 primary breast tumors and specifically analyzed the amount of caveolin-1 protein either in tumor cells or in stromal cells.
There were 103 of 173 patients (60%) who had unambiguous staining of the stromal compartment of the tumor. As in normal breast tissue, caveolin-1 was detected in vascular endothelial cells in these tumor sections, but expression in other stromal elements, often fibroblast-rich desmoplastic tissue, was variable. Representative examples of tumors with caveolin-1 positive and negative stromal tissue are shown in Figure 2C. The relationship between standard prognostic factors and other molecular markers with caveolin-1 expression in the stroma is shown in Table 1. Caveolin-1 stromal staining was associated with smaller tumor size (T stage) (p = 0.03) and grade (P = 0.001). There was a trend toward patients expressing stromal caveolin-1 being younger (P = 0.08), having lower AJCC stage tumors at presentation (P = 0.07) and less proliferative tumors (proliferating nuclear cell antigen) (P = 0.07). Caveolin-1 stromal staining was not related to caveolin-1 staining in tumor epithelium.
Table 1.
Correlations between Clinicopathologic Factors and Specific Protein Markers with Caveolin-1 Expression in the Stromal Compartment of 173 Breast Cancers
|
|
All patients n = 173 (%)
|
Caveolin-1 positive stroma n = 103 (60%)
|
Caveolin-1 negative stroma n = 70 (40%)
|
P value
|
|---|---|---|---|---|
| Median age (27–81 years), n = 162 | 54 | 51 | 57 | 0.08 |
| Median tumor size (5–90 mm), n = 164 | 25 | 25 | 25 | 0.7 |
| Tumor stage, n = 165 | 0.03 | |||
| T1 (0–20 mm) | 64 (39) | 41 (42) | 23 (34) | |
| T2 (20–50 mm) | 94 (57) | 54 (56) | 40 (59) | |
| T3 (>50 mm)/T4 | 7 (4) | 2 (2) | 5 (7) | |
| Lymph node status, n = 169 | 0.1 | |||
| N0 (0 nodes) | 92 (54) | 58 (57) | 34 (50) | |
| N1 (1–3 nodes) | 47 (28) | 30 (30) | 17 (25) | |
| N2 (4–9 nodes) | 20 (12) | 10 (10) | 10 (15) | |
| N3 (>9 nodes) | 10 (6) | 3 (3) | 7 (10) | |
| AJCC stage | 0.07 | |||
| I | 45 (27) | 29 (29) | 17 (25) | |
| IIa | 62 (38) | 40 (39) | 34 (23) | |
| IIb | 28 (17) | 18 (19) | 10 (15) | |
| IIIa | 22 (13) | 11 (11) | 11 (16) | |
| IIIc | 8 (5) | 1 (1) | 7 (10) | |
| Age, n = 157 | 0.1 | |||
| <50 years | 63 (40) | 43 (51) | 20 (32) | |
| >50 years | 94 (60) | 51 (54) | 43 (68) | |
| ER status, n = 167 | 0.1 | |||
| Positive | 119 (71) | 73 (74) | 46 (68) | |
| Negative | 48 (29) | 26 (26) | 22 (32) | |
| PR status, n = 168 | 0.1 | |||
| Positive | 107 (64) | 66 (66) | 41 (60) | |
| Negative | 61 (36) | 34 (34) | 27 (40) | |
| Grade, n = 167 | 0.001 | |||
| 1, well differentiated | 48 (29) | 37 (38) | 11 (16) | |
| 2, moderately differentiated | 78 (47) | 44 (45) | 34 (49) | |
| 3, poorly differentiated | 41 (25) | 17 (17) | 25 (36) | |
| CerbB2 status n = 160 | 0.1 | |||
| Negative | 123 (77) | 77 (79) | 46 (73) | |
| positive | 37 (23) | 20 (21) | 17 (27) | |
| PCNA status, n = 159 | 0.07 | |||
| 0–40% staining | 73 (46) | 47 (50) | 26 (41) | |
| >40% staining | 86 (54) | 48 (51) | 38 (60) | |
| P53 status, n = 132 | 0.1 | |||
| Negative | 95 (72) | 61 (77) | 34 (64) | |
| Positive | 37 (28) | 18 (23) | 19 (36) | |
| P170 status, n = 150 | 0.2 | |||
| Negative | 19 (13) | 11 (12) | 8 (13) | |
| Positive | 131 (87) | 78 (88) | 53 (87) | |
| Caveolin-1 tumor expression | 0.3 | |||
| Negative | 153 (88) | 89 (86) | 64 (91) | |
| Positive | 20 (12) | 14 (14) | 6 (9) | |
| Tumor type, n = 162 | N/A | |||
| Invasive ductal | 123 (75) | 69 (72) | 54 (79) | |
| Lobular | 25 (15) | 13 (14) | 12 (18) | |
| Special types | 14 (8) | 13 (14) | 1 (1) | |
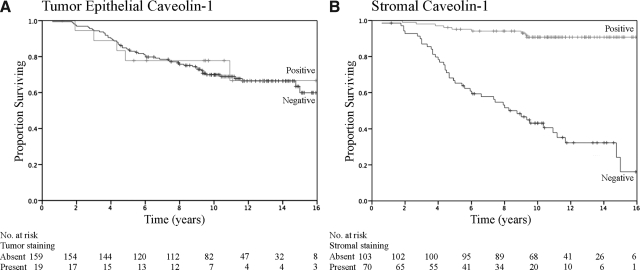
The correlation between prognostic factors and other markers with survival on univariate analysis is shown in Table 2 (log rank test). Small tumor size, lack of nodal involvement, lower AJCC stage, positivity for ER or PR, lower tumor grade, and absence of proliferating nuclear cell antigen or p53 staining were all associated with improved survival (Table 2). Age and expression of p170 were not associated with survival. The association between stromal caveolin-1 expression and survival was also assessed. The 10-year survival rate for patients with tumors that were positive for caveolin-1 expression in the stroma was 91%, as compared with 43% for patients lacking stromal caveolin-1 (P < 0.0001) (Table 2). In contrast, there was no association between caveolin-1 positivity in tumor cells and 10-year survival (P = 0.9) (Figure 3A).
Table 2.
Univariate Survival Analysis of 173 Breast Cancers
| Estimated 10-year survival (%) | P value | |
|---|---|---|
| T stage | 0.05 | |
| T1 (0–20 mm) | 82 | |
| T2 (>20–50 mm) | 67 | |
| T3 (>50 mm) | 56 | |
| Axillary node status | 0.0003 | |
| N0 (0) | 81 | |
| N1 (1–3) | 63 | |
| N2 (3–9) | 48 | |
| N3 (>9) | 51 | |
| AJCC stage | 0.0002 | |
| 1 | 84 | |
| 11a | 80 | |
| 11b | 55 | |
| 111a | 48 | |
| 111c | 5 | |
| Age | 0.1 | |
| <50 years | 76 | |
| ≥50 years | 63 | |
| ER status | 0.04 | |
| Negative | 64 | |
| Positive | 73 | |
| PR status | 0.007 | |
| Negative | 61 | |
| Positive | 76 | |
| Tumor grade | 0.0009 | |
| 1, low grade | 87 | |
| 2, intermediate grade | 67 | |
| 3, high grade | 54 | |
| C erb B2 status | 0.07 | |
| Negative | 74 | |
| Positive | 61 | |
| PCNA status | 0.009 | |
| 0−40% staining | 82 | |
| >40% staining | 60 | |
| P53 status | 0.0002 | |
| Negative staining | 79 | |
| Positive staining | 53 | |
| P170 status | 0.3 | |
| Negative | 69 | |
| Positive | 69 | |
| Caveolin-1 stromal staining | <0.0001 | |
| Negative | 43 | |
| Positive | 91 | |
| Caveolin-1 tumor staining | 0.9 | |
| Negative | 74 | |
| Positive | 71 | |
Estimated 10-year survivals for major prognostic factors with associated P values (log rank test) are shown.
Figure 3.
Stromal expression of caveolin-1 correlates with improved survival. Kaplan-Meier plots for caveolin-1 expression in the tumor epithelium (A) or stroma (B) in a consecutive series of breast cancer patients. The relationship between overall survival and caveolin-1 expression is shown over time (years following diagnosis).
Multivariate analysis (Cox proportional hazards model) was performed to identify factors that were independently associated with survival. Only stromal caveolin-1 staining, tumor size, and p53 status were independently and significantly associated with survival (Table 3). Patients with stromal caveolin-1 expression were twelve times less likely to die, as compared with patients without stromal caveolin-1 expression. Consistent with findings from the arrayed tumor samples, caveolin-1 levels in tumor cells were not associated with clinical outcome (Figure 2B and Figure 3A), while stromal caveolin-1 was strongly associated with increased overall survival (Figure 3B).
Table 3.
Multivariate Survival Results
| RR | 95% CI | P value | |
|---|---|---|---|
| Caveolin stromal staining, positive, negative | 0.088 | 0.03, 0.23 | <0.0001 |
| Tumor size, T1,T2, T3/T4 | 1.28 | 1.01, 1.63 | 0.04 |
| P53 status, negative, positive | 2.80 | 1.39, 5.61 | 0.004 |
Relative risks (RR) with associated 95% confidence levels and P values are shown (Cox Proportional Hazards Model). All factors shown in Table 2 were analyzed but only those that were significant are listed here.
Contribution of Caveolin-1 to Tumor Onset and Progression in Mice Transgenic for MMTV-neu
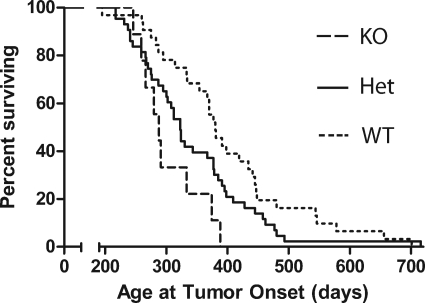
Analysis of the consecutive clinical cohort described above indicates that stromal caveolin-1 expression is a powerful prognostic marker of clinical outcome. To determine whether caveolin-1 expression in stromal cells contributes to tumor growth and progression, we analyzed the onset of spontaneous mammary tumors in mice null for caveolin-1. Latency to tumor onset was measured in mice transgenic for mammary-specific Her-2/neu expression that carried 0, 1, or 2 alleles of caveolin-1. Regardless of their caveolin-1 status, mice lacking the MMTV-neu oncogene did not develop tumors within 3 years, indicating that absence of stromal caveolin-1 is not sufficient to induce tumorigenesis. Tumor onset was more rapid in mice lacking caveolin-1 (mean onset on day 303 from birth) as compared with day 343 for caveolin-1+/− mice or day 404 for caveolin-1 wild-type mice for (Table 4). The decreased latency was statistically significant for both the caveolin-1−/− mice (P = 0.002, compared with +/+ mice) and for caveolin-1+/− mice (P = 0.026, compared with +/+ mice). Kaplan-Meier analysis revealed a significant difference for the trend (P = 0.005) between the three genotypes with tumor onset being most rapid in the caveolin-1−/− mice (Figure 4). The rate of tumor growth was slightly more rapid in mice null for caveolin-1 (Table 4).
Table 4.
Tumor Onset, Growth, and Incidence of Lung Metastases in MMTV-neu Mice by Caveolin-1 Genotype
| Genotype | Cav-1+/+ | Cav-1+/− | Cav-1−/− |
|---|---|---|---|
| Number of mice | 25 | 43 | 9* |
| Age at tumor onset (days ± SEM) | 404 ± 24 | 343 ± 15 | 303 ± 15 |
| Age at harvest (days ± SEM) | 469 ± 26 | 407 ± 16 | 354 ± 17 |
| Tumor growth (days ± SEM) | 65 ± 5 | 64 ± 4 | 51 ± 6 |
| Median survival (days) | 454 | 384 | 361 |
| Incidence of visible lung mets (%) | 28 | 23 | 20 |
Significance differences in tumor onset time were assessed by the Gehan-Breslow-Wilcoxon test. P = 0.002 for the difference between Cav+/+ and Cav−/− mice and P = 0.026 for the difference between Cav+/+ and Cav+/− mice.
Only 9 of the original 15 mice were available for final analysis due to losses from non-tumor related causes during the experiment.
Figure 4.
Loss of stromal expression accelerates tumor onset in the MMTV-neu mouse. Kaplan-Meier plots for tumor onset by caveolin-1 status in mice transgenic for the MMTV-neu gene. Mice were monitored for tumor onset (age at which tumor was first palpable) and tumor growth, and culled when the primary tumor reached 1500 mm3. Analysis of the trend using the log-rank test gave a P value of 0.005.
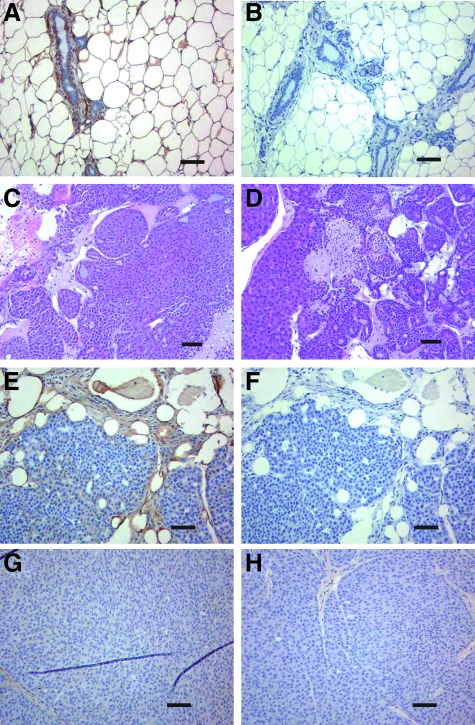
Before tumor onset, caveolin-1 distribution in the mammary glands of MMTV-neu mice that are wild-type for caveolin-1 (Figure 5, A and B) was similar to that in normal human breast tissue (Figure 1, A and D) and in non-transgenic mice (not shown). No differences in the morphology of the tumors that arose in caveolin-1+/+ or caveolin-1−/− mice were evident following examination of H&E stained sections (Figure 5, C and D). As caveolin-1 is lacking from both tumor and stromal cells in MMTV-neu/caveolin-1 null mice, it is possible that the accelerated tumor onset in this model is being driven by the absence of tumor caveolin-1 rather than stromal caveolin-1. However, the absence of caveolin-1 in tumor epithelial cells is unlikely to be responsible for accelerated tumor progression in this model for the following reason. The tumors that arise spontaneously in MMTV-neu transgenic mice that are wild-type at the caveolin-1 locus do not express caveolin-1 in the tumor epithelium (Figure 5, E and F) but have a longer tumor latency compared with MMTV-neu transgenics lacking stromal caveolin-1 (Table 4, Figure 5, G and H). Taken together, the data obtained in the MMTV-neu model are consistent with a role for caveolin-1 in stromal cells in breast cancer growth and strongly indicate that the loss of stromal caveolin-1 contributes to the earlier onset of visible tumors.
Figure 5.
Caveolin-1 expression in the normal mammary gland of MMTV-neu transgenic mice and in MMTV-neu tumors. A: Caveolin-1 expression in the mammary gland of the MMTV-neu mouse before tumor onset. B: isotype matched antibody staining of the mammary gland. C, D: H&E staining to show the morphology of the tumors in caveolin-1 wild-type (C) and null (D) mice. E, G: caveolin-1 expression in tumors from caveolin-1 wild-type mouse (E) and caveolin-1 null mouse (G). The respective isotype control staining is shown in panels (F) and (H). Scale bar = 100 μm.
Lung metastasis was assessed at autopsy and the incidence of visible metastases was found to be similar in all three caveolin-1 genotypes (Table 4). Histological assessment of lung metastasis in H&E stained sections of the lung did not reveal any differences in the number of metastatic nodules present in MMTV-neu/caveolin-1+/+ and in MMTV-neu/caveolin-1−/− mice (data not shown). Visible lung nodules were present in only 20% to 30% of mice, which was insufficient for further analysis of the role of stromal caveolin-1 in metastasis in this model.
Discussion
The studies presented here show that the presence of caveolin-1 positive stromal elements in the primary tumor microenvironment is associated with improved outcome in breast cancer. Caveolin-1 expression in tumor-associated stroma was related to lower tumor grade and improved staging at diagnosis. Consistent with this, 10-year survival rates were significantly better in patients with caveolin-1-positive stromal tissue. Multivariate statistical analyses attributed improved 10-year survival rates to elevated stromal caveolin-1, independent of other established prognostic factors. In the two patient cohorts assessed here, caveolin-1 levels in tumor epithelium were not associated with disease-free or overall survival. These findings suggest that stromal caveolin-1 may be a new prognostic factor for long-term breast cancer survival.
There have been many reports linking caveolin-1 to tumor progression and clinical outcome in different types of cancer, but without a clear resolution of its role as a prognostic marker. Our data demonstrate that loss of caveolin-1 in the stromal cells of breast tumors has a very strong correlation with clinical outcome in samples where the level of expression within the tumor epithelium is not informative of outcome. These results may explain some of the discrepancies in the literature with regard to the proposed pro- or anti-neoplastic roles of caveolin-1 in various cancers in the studies that did not discriminate between stromal and tumor caveolin-1 expression. This clinical finding is supported by our data showing decreased latency in the MMTV-neu mouse model of spontaneous mammary tumor development, indicating a role for stromal caveolin-1 in mammary tumor progression.
Metastasis to distant tissues is one of the key events responsible for reduced survival in patients with breast cancer. Investigation of the role of stromal caveolin-1 in promoting metastasis was limited in the current studies by use of a spontaneous tumor model with low metastatic frequency. However, studies of mammary tumor development driven by the polyoma middle T antigen have shown both a decreased primary tumor latency and an increased frequency of lung metastasis in mice null for caveolin-1.37 While the relative contributions of stromal and epithelium caveolin-1 were not investigated in their study, the data along with ours raise the possibility that stromal caveolin-1, either at the primary site or potentially in distant tissues, may provide a tumor suppressor influence that inhibits growth of metastatic nodules in distant tissues.
While the mechanisms that regulate caveolin-1 production in tumor-associated stromal tissue remain unclear, it is possible that tumor cell signaling is involved in this process. Cross talk between tumor cells and stromal cells of varying lineages within the tumor microenvironment regulates tumor growth and metastasis.38 Tumor cells co-opt stromal cells to produce growth factors and cytokines that the tumor cells use to invade surrounding tissues and metastasize to distant tissues. Loss of caveolin-1 in stromal cells may be regulated by signals originating from the transformed epithelial cells to enable the tumor cells to escape the growth suppressing properties of the stroma. This may promote growth, migration, or invasion of the epithelial tumor cells. Methylation silencing may explain the loss of caveolin-1 expression in stromal tissue18 and is the subject of ongoing studies, as is the nature of the paracrine signaling between stromal and tumor cells that occurs in the presence of caveolin-1.
The mechanism by which stromal caveolin-1 suppresses cancer progression remains to be determined. We hypothesize that caveolin-1 in tumor-associated stroma modulates paracrine signaling with tumor cells, leading to a permissive environment for tumor cell proliferation, migration, and local invasion. Caveolin-1 can act as a negative regulator of several signaling pathways such as the Wnt and Her2/neu pathways.11,39 Its strong expression in the myoepithelial cells of the normal mammary gland has been linked to the tumor suppressive function of these cells.1 This suggests that therapies that target caveolin-regulated processes have the potential to suppress tumor progression and improve survival in relevant subtypes of breast cancer. In addition to the possible development of new therapies, the current studies suggest that analysis of stromal caveolin-1 levels at first diagnosis may be an effective prognostic factor that would allow individualization of patient therapy. In the future, prospective clinical trials will be essential to confirm the prognostic power of stromal caveolin-1 in breast cancer.
Acknowledgments
We thank Dr. Belinda Parker and Brenda Aisbett for technical assistance, Dr. Olli Kallioniemi for providing the breast cancer tissue array, Dr. Teymuras Kurzchalia for providing the caveolin-1 null mice, Dr. John Slavin, Dr. Paul Waring, and Dr. Ken Opeskin for reviewing tumor pathologies, Kally Yuen for assistance with statistical analysis, and Dr. Robert Parton for assistance with obtaining the caveolin-1 null mice and for constructive discussions.
Footnotes
Address reprint requests to Robin L. Anderson, Peter MacCallum Cancer Centre, Locked Bag #1, A’Beckett St., Melbourne, Victoria, Australia, 8006. E-mail: robin.anderson@petermac.org.
See related Commentary on page 1996
Supported by a Susan G. Komen for the Cure Dissertation Award (to E.K.S.) and by grants from the Susan G. Komen for the Cure (BCTR0403075 to R.L.A.), the US Department of Army (DAMD17-98-1-8144, to R.L.A.), the NCI/NIH (ROI CA90291 to R.L.A.) and the National Research Council of Argentina (CONICET to D.R.C.).
E.K.S. and D.R.C. contributed equally to this project.
Current address of E.K.S., Semel Institute, UCLA School of Medicine, Los Angeles, CA 90095-1678.
References
- Bissell MJ, Radisky D. Putting tumors in context. Nature Reviews (Cancer) 2001;1:1–19. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Parton RG, Hanzal-Bayer M, Hancock JF. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci. 2006;119:787–796. doi: 10.1242/jcs.02853. [DOI] [PubMed] [Google Scholar]
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M, Scherer PE, Tang Z, Kubler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA. 1995;92:9407–9411. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uittenbogaard A, Ying Y, Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J Biol Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- Zenklusen JC, Bieche I, Lidereau R, Conti CJ. (C-A)n microsatellite repeat D7S522 is the most commonly deleted region in human primary breast cancer. Proc Natl Acad Sci USA. 1994;91:12155–12158. doi: 10.1073/pnas.91.25.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Lisanti MP. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5′ promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999;448:221–230. doi: 10.1016/s0014-5793(99)00365-8. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP. Reciprocal regulation of neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for human breast cancer. J Biol Chem. 1998;273:20448–20455. doi: 10.1074/jbc.273.32.20448. [DOI] [PubMed] [Google Scholar]
- Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- Hurlstone AF, Reid G, Reeves JR, Fraser J, Strathdee G, Rahilly M, Parkinson EK, Black DM. Analysis of the Caveolin-1 gene at human chromosome 7q31.1 in primary tumours and tumour-derived cell lines. Oncogene. 1999;18:1881–1890. doi: 10.1038/sj.onc.1202491. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Mimori K, Yoshinaga K, Tanaka F, Nishida K, Ohno S, Inoue H, Mori M. Clinical significance of Caveolin-1. Caveolin-2 and HER2/neu mRNA expression in human breast cancer. Br J Cancer. 2004;91:959–965. doi: 10.1038/sj.bjc.6602029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage K, Lambros MB, Robertson D, Jones RL, Jones C, Mackay A, James M, Hornick JL, Pereira EM, Milanezi F, Fletcher CD, Schmitt FC, Ashworth A, Reis-Filho JS. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res. 2007;13:90–101. doi: 10.1158/1078-0432.CCR-06-1371. [DOI] [PubMed] [Google Scholar]
- Pinilla SM, Honrado E, Hardisson D, Benitez J, Palacios J. Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat. 2006;99:85–90. doi: 10.1007/s10549-006-9184-1. [DOI] [PubMed] [Google Scholar]
- Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–1880. [PubMed] [Google Scholar]
- Chen ST, Lin SY, Yeh KT, Kuo SJ, Chan WL, Chu YP, Chang JG. Mutational, epigenetic and expressional analyses of caveolin-1 gene in breast cancers. Int J Mol Med. 2004;14:577–582. [PubMed] [Google Scholar]
- Park SS, Kim JE, Kim YA, Kim YC, Kim SW. Caveolin-1 is down-regulated and inversely correlated with HER2 and EGFR expression status in invasive ductal carcinoma of the breast. Histopathology. 2005;47:625–630. doi: 10.1111/j.1365-2559.2005.02303.x. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, Hayakawa T, Hamaguchi M. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61:2361–2364. [PubMed] [Google Scholar]
- Li T, Sotgia F, Vuolo MA, Li M, Yang WC, Pestell RG, Sparano JA, Lisanti MP. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol. 2006;168:1998–2013. doi: 10.2353/ajpath.2006.051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikh SE, Green AR, Rakha EA, Samaka RM, Ammar AA, Powe D, Reis-Filho JS, Ellis IO. Caveolin 1 and caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br J Cancer. 2008;99:327–334. doi: 10.1038/sj.bjc.6604463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynden GG, Van Laere SJ, Van der Auwera I, Merajver SD, Van Marck EA, van Dam P, Vermeulen PB, Dirix LY, van Golen KL. Overexpression of caveolin-1 and -2 in cell lines and in human samples of inflammatory breast cancer. Breast Cancer Res Treat. 2006;95:219–228. doi: 10.1007/s10549-005-9002-1. [DOI] [PubMed] [Google Scholar]
- Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, Zhumabayeva B, Siebert PD, Dietel M, Schafer R, Sers C. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol. 2001;159:1635–1643. doi: 10.1016/S0002-9440(10)63010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunaga N, Miyajima K, Suzuki M, Sato M, White MA, Ramirez RD, Shay JW, Gazdar AF, Minna JD. Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res. 2004;64:4277–4285. doi: 10.1158/0008-5472.CAN-03-3941. [DOI] [PubMed] [Google Scholar]
- Bender FC, Reymond MA, Bron C, Quest AF. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res. 2000;60:5870–5878. [PubMed] [Google Scholar]
- Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF. Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate. 2007;67:614–622. doi: 10.1002/pros.20557. [DOI] [PubMed] [Google Scholar]
- Fine SW, Lisanti MP, Galbiati F, Li M. Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am J Clin Pathol. 2001;115:719–724. doi: 10.1309/YL54-CCU7-4V0P-FDUT. [DOI] [PubMed] [Google Scholar]
- Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94:929–933. [PubMed] [Google Scholar]
- Barlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, Torhorst J, Haas P, Bucher C, Sauter G, Kallioniemi OP, Kallioniemi A. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst. 2000;92:1252–1259. doi: 10.1093/jnci/92.15.1252. [DOI] [PubMed] [Google Scholar]
- Chia J, Kusuma N, Anderson R, Parker B, Bidwell B, Zamurs L, Nice E, Pouliot N. Evidence for a role of tumor-derived laminin-511 in the metastatic progression of breast cancer, Am J Pathol. 2007;170:2135–2148. doi: 10.2353/ajpath.2007.060709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago FE, Tello OM, Diblasi AM, Ciocca DR. Integration of estrogen and progesterone receptors with pathological and molecular prognostic factors in breast cancer patients. J Steroid Biochem Mol Biol. 1998;67:431–437. doi: 10.1016/s0960-0760(98)00140-x. [DOI] [PubMed] [Google Scholar]
- Gago FE, Fanelli MA, Ciocca DR. Co-expression of steroid hormone receptors (estrogen receptor alpha and/or progesterone receptors) and Her2/neu (c-erbB-2) in breast cancer: clinical outcome following tamoxifen-based adjuvant therapy. J Steroid Biochem Mol Biol. 2006;98:36–40. doi: 10.1016/j.jsbmb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Stanley KL, Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23:7893–7897. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Brown AM, Weinstein DE, Ben-Ze'ev A, Pestell RG, Lisanti MP. Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by recruiting beta-catenin to caveolae membrane domains. J Biol Chem. 2000;275:23368–23377. doi: 10.1074/jbc.M002020200. [DOI] [PubMed] [Google Scholar]