Abstract
Podocytes are a crucial cell type in the kidney and play an important role in the pathology of glomerular kidney diseases like membranous nephropathy (MN). The identification of new factors involved in the progression of glomerular kidney diseases is of great importance to the development of new strategies for the treatment of renal injury. Here we demonstrate that CXCL16 and ADAM10 are constitutively expressed in human podocytes in normal renal tissue. Proinflammatory cytokines like interferon-γ and tumor necrosis factor-α induced the expression of cellular CXCL16 and the release of its soluble form from human podocytes. Using different metalloproteinase inhibitors, we provide evidence that ADAM10 is involved in the interferon-γ- and tumor necrosis factor-α-induced shedding of CXCL16 from human podocytes. In addition, ADAM10 knockdown by siRNA significantly increased both CXCL16 levels and, surprisingly, its ADAM17-mediated release. Notably, targeting of CXCL16 in human podocytes both decreased the chemotaxis of CXCR6-expressing T cells and strongly reduced oxidized low-density lipoprotein uptake in human podocytes. Importantly, in kidney biopsies of patients with MN, increased glomerular CXCL16 expression was accompanied by high levels of oxidized low-density lipoprotein and decreased expression of ADAM10. In addition, we found increased glomerular ADAM17 expression in patients diagnosed with MN. In summary, we presume important roles for CXCL16, ADAM10, and ADAM17 in the development of MN, suggesting these proteins as new therapeutic targets in this glomerular kidney disease.
The podocyte is a highly specialized cell that constitutes a crucial component of the glomerular filtration barrier.1 Podocyte damage leads to retraction of foot processes, resulting in proteinuria.2 In many renal glomerular diseases like diabetic nephropathy, minimal change, focal segmental glomerulosclerosis and membranous nephropathy podocytes are the primary target of injury.3 The precise mechanisms that lead to podocyte damage and proteinuria are only roughly understood. It has been suggested that (as yet unknown) circulating mediators might affect podocyte function and cause the retraction of foot processes and thus proteinuria.4 In this context chemokines are an attractive family of molecules. They are a group of primarily soluble molecules, which were originally characterized by their ability to induce leukocyte migration.5 Unlike the soluble chemokines, two members, CX3CL1 (fractalkine) and CXCL16 are synthesized as surface-expressed molecules.6
Expression of CXCL16 has been reported on immune cells like dendritic cells, macrophages, B cells, T cells, and on smooth muscle cells and endothelial cells.7,8,9,10,11 As a transmembrane molecule CXCL16 can act as an adhesion molecule for CXCR6 expressing immune cells12,13 or as a scavenger receptor for oxidized low-density lipoprotein (oxLDL).14,15 Besides its surface-expressed form, CXCL16 can be released from the cell membrane, a process called ectodomain shedding. Inhibitor studies revealed that the two disintegrin like metalloproteinases ADAM10 and ADAM17 are mainly involved in this release of CXCL16.16,17,18 It has been already described that the soluble form of CXCL16 participates in the recruitment of CXCR6-expressing immune cells to sites of inflammation.19,20,21
Membranous nephropathy (MN) is a glomerular disease, which is characterized by an accumulation of immune deposits on the outer aspect of the glomerular basement membrane. The immune deposits consist of IgG (often IgG4), thus far unidentified antigens, and the membrane attack complex of complement C5b-9. Although spontaneous remission of nephrotic syndrome occurs in about a third of patients, MN ends for about 40% of patients in end-stage renal failure after 10 years from the diagnosis of the disease.22 The treatment of MN is often disappointing; therefore more specific, concept-driven therapies are urgently needed.
Nothing is known about the expression of CXCL16 and ADAM10 in membranous nephropathy and only a few studies investigated the expression of CXCL16 and ADAM10 in the kidney. Interestingly, elevated levels of urinary CXCL16 have been observed in patients with acute tubular necrosis23 or with lupus nephritis,24 which suggest that CXCL16 may be a useful diagnostic biomarker in these kidney diseases. In addition, we have recently characterized the tubular expression of CXCL16 and ADAM10 in the healthy and transplanted human kidney.23 Importantly, a variable CXCL16 expression and an increased ADAM10 expression was observed in biopsies from kidney transplanted patients with the diagnosis of acute interstitial rejection assuming an important role of both molecules in the recruitment of T cells to sites of injury in the kidney.23
In this study, we demonstrate that CXCL16 and ADAM10 are expressed in human podocytes in vitro and in vivo. Treatment with the proinflammatory cytokines tumor necrosis factor (TNF)-α or interferon (IFN)-γ increased the metalloproteinase mediated shedding of CXCL16 from human podocytes. Notably, supernatants obtained from podocytes transfected with CXCL16-specific siRNA significantly reduced the chemotaxis of CXCR6-expressing T cells. By using blocking antibodies against CXCL16 we could show, that CXCL16 acts as a scavenger receptor of oxidized LDL in human podocytes. Importantly, in biopsies of patients with membranous nephropathy increased glomerular CXCL16 expression was accompanied with higher levels of oxLDL. In summary, we assume an important role of CXCL16 and ADAM10 in MN and therefore both proteins may function as new therapeutic targets in the treatment of this glomerular kidney disease.
Materials and Methods
Kidney Sections
Specimens were taken from healthy parts of renal tissue from six different tumor nephrectomies (obtained from two female and four male patients aged from 34 to 66 years), which were originally submitted for diagnostic purposes and studied in accordance with national and local ethical principles. The use of human tissue samples has been approved by the local ethics committee (Ref. No. 11/10/04). Tissue sections of nine patients diagnosed with MN were obtained from the Department of Pathology, University of Erlangen-Nürnberg, Erlangen, Germany.
Sera of Patients and Healthy Volunteers
Nine milliliters of sera from six patients with membranous nephropathy, one patient with lupus nephritis, and seven healthy controls have been collected at the nephrology department at the university hospital Frankfurt am Main. Proteinuria and serum creatinine of the patients have been routinely determined and CXCL16 levels were measured using a CXCL16-specific ELISA (PeproTech, London, UK).
Cell Culture
Human conditionally immortalized podocytes were isolated and cultivated as previously described.25 Cells were grown in flasks either at the permissive temperature of 32°C to promote cell propagation as a cobblestone phenotype (undifferentiated) or at the nonpermissive temperature of 37°C to inactivate the SV40 T antigen and allow the cells to differentiate. The growth medium was RPMI 1640 supplemented with nonessential amino acids, fetal bovine serum (10%), Hepes (10 mmol/L) pH 7.4, penicillin (100 U/ml), streptomycin (100 μg/ml), transferrin (5 μg/ml), and sodium selenite (5 ng/ml). Before stimulation, cells were incubated for 16 hours in RPMI 1640 medium, supplemented with 0.1 mg/ml fatty acid-free bovine serum albumin.
siRNA
For down-regulation of endogenous CXCL16, and ADAM10 and ADAM17 expression in human podocytes the following duplexes (MWG Biotech AG, Ebersberg, Germany) were used: CXCL16 construct, 5′-CAUGAAUCGUCUCCGGAAACATT-3′; ADAM10 construct, 5′-AGACAUUAUGAAGGAUUAUTT-3′ and ADAM17 construct, 5′-GAGAAGCUUGAUUCUUUGCTT-3′. As a negative control an unspecific scrambled siRNA duplex (5′-AGGUAGUGUAAUCGCCUUGTT-3′) was applied.
Transfection of siRNA
Twenty-four hours before transfection 5 × 104 cells were seeded in six-well plates. Transfection of siRNA was performed using Oligofectamine (Invitrogen, Karlsruhe, Germany) and 10 nmol/L siRNA duplexes (MWG Biotech AG, Ebersberg, Germany) per well. All cells were assayed 24 to 48 hours after transfection. Specific silencing of targeted genes was confirmed by at least two independent experiments using Western blot analysis. Conditioned media were harvested and analyzed for the presence of released CXCL16 by enzyme-linked immunosorbent assay (ELISA).
Cytokines, Chemicals, and Antibodies
Recombinant human CXCL16, recombinant human IFN-γ, and the ADAM10 antibody used for immunofluorescence staining were obtained from R&D Systems (Wiesbaden, Germany). Unconjugated rabbit CXCL16 antibody used for immunohistochemistry and double immunofluorescence analysis was from PeproTech EC (London, UK). The ADAM10 antibody (11G2) for double immunofluorescence analysis on tissue sections was purchased from Diaclone (Besancon, France). The WT1 (sc-846) antibody was obtained from Santa Cruz (Heidelberg, Germany). DiI-oxLDL was from Intracel (Frederick, MD). The polyclonal antibody to human CXCL16 used for Western blot analysis was obtained from Acris (Acris Antibodies, Hiddenhausen, Germany). The polyclonal rabbit ADAM10 and oxLDL antibody for immunohistochemistry and immunofluorescence analysis were from Chemicon (Hampshire, UK). The monoclonal antibody (ab57484) against ADAM17 used for immunofluorescence analysis was purchased from Abcam (Cambridge, UK). The polyclonal ADAM17 antibody (14-6202) used for Western blot was from Natutec (Frankfurt, Germany). The metalloproteinase inhibitors GI254023X and GW280264X were kindly provided by Dr. Andreas Ludwig (Aachen, Germany). The broad spectrum metalloproteinase inhibitors GM6001 and TAPI-2 were obtained from Calbiochem (Darmstadt, Germany).
CXCL16 Shedding Assays
Twenty-four hours before stimulation human podocytes were seeded at a density of 4 × 105 cells in six-well plates. Cells were washed with phosphate-buffered saline (PBS) and 1 ml of fetal calf serum-free medium with or without metalloproteinase inhibitors was added. After 15 minutes, the cells were stimulated with IFN-γ (20 ng/ml) or TNF-α (2 ng/ml) or left unstimulated for times indicated. The conditioned media were harvested and cleared by centrifugation. The presence of soluble CXCL16 in the conditioned media was analyzed by ELISA (PeproTech, London, UK) following the manufacturer’s instructions. The expression of endogenous CXCL16 was controlled by Western blot analysis of the cell lysates.
Protein Extraction and Western Blotting
Cell extracts were prepared and processed as described recently at times indicated.26 Western blot membranes were incubated with antibodies against CXCL16 (Acris Antibodies, Hiddenhausen, Germany) and ADAM10 (Chemicon, Hampshire, UK). Blots were developed using the ECL system (Amersham Pharmacia, Buckinghamshire, UK). To confirm equal loading, blots were reprobed with a β-actin antibody (Sigma, Deisenhofen, Germany).
Immunohistochemistry
Paraffin tissue sections were deparaffinized in xylene, rehydrated through a graded ethanol series and washed in 10 mmol/L phosphate-buffered 150 mmol/L saline, pH 7.4. Antigen retrieval was performed by incubating the tissue sections for 20 minutes in 0.01 mol/L sodium citrate buffer, pH 6.0, in a microwave oven (500 W). Tissue sections were treated with 3% H2O2 in methanol for 30 minutes at room temperature. After blocking the sections with PBS containing 10% horse serum, 1% bovine serum albumin for 1 hour, the sections were incubated with an avidin/biotin blocking kit (Linaris, Wertheim, Germany) following the manufacturer’s protocol. The serial sections were incubated overnight at 4°C with primary antibodies as indicated. After washing the slides, the Universal Quick Kit (Linaris) was used to stain the kidney sections. As a substrate the AEC substrate kit from BioGenex (San Ramon, CA) was used to detect the immune complexes. The slides were incubated with hematoxylin (Roth, Karlsruhe, Germany) to counterstain the sections.
For double immunohistochemistry analysis, sections were incubated overnight in a mixture of CXCL16 and WT1 antibodies or ADAM10 and WT1 antibodies, respectively. CXCL16 and ADAM10 staining was developed as described above to detect the WT1 antigen an alkaline phosphatase-conjugated goat anti-rabbit antibody (Dianova, Hamburg, Germany) was added for 1 hour at 37°C. Before adding the substrate, the endogenous alkaline phosphatases were blocked with levamesole (Sigma, Taufkirchen, Germany). Immune complexes were detected by adding the Sigma fast BCIP/NBT substrate (Sigma, Taufkirchen, Germany). The sections were inspected with a Zeiss microscope coupled to a 12-bit digital image camera.
Immunofluorescence Analysis of Tissue Sections
For immunofluorescence analysis, tissue sections were deparaffinized as described above and antigen retrieval was performed incubating the tissue sections for 20 minutes in 0.01 mol/L sodium citrate buffer, pH 6.0, in a microwave oven (500 W). After incubation with blocking buffer (0.1% Triton X-100/PBS containing 1% bovine serum albumin and 10% horse serum) for 1 hour, tissue sections were incubated for two hours at 37°C and then overnight at 4°C with the first antibodies (diluted in 1% bovine serum albumin/10% horse serum/PBS/0.1% Triton X-100) as indicated. Following washing, bound antibodies were detected by Alexa 488 conjugated goat anti-mouse (Molecular Probes, Karlsruhe, Germany) or goat anti-rabbit Cy3 (Molecular Probes, Karlsruhe, Germany) secondary antibodies. Nuclei were stained with 4′-6-diamidino-2-phenylindole (Sigma, Deisenhofen, Germany) and slides were mounted in Fluoromount G (Southern Biotech, Birmingham, AL). Evaluation was performed by fluorescence microscopy and analyzed with an LSM 510 Meta confocal laser-scanning microscope (Carl Zeiss, Jena, Germany).
Uptake of DiI-Labeled Oxidized Human LDL
To analyze the uptake of Dil-labeled oxidized human LDL in human podocytes, cells were grown on coverslips and left unstimulated or treated with IFN-γ in the absence or presence of the metalloproteinase inhibitor GM6001. For antibody blocking experiments, human podocytes were incubated for 1 hour with or without 10 μg/ml CXCL16 antibody (R&D, Wiesbaden, Germany). After 4 hours of incubation with 10 μg/ml DiI-oxLDL, media was gently removed from cells and cells were washed three or four times with serum-free media. Subsequently, cells were fixed with methanol/0.01% EDTA for 20 minutes at −20°C, nuclei were stained with 4′-6-diamidino-2-phenylindole (Sigma, Deisenhofen, Germany) and slides were mounted in Fluoromount G (Biozol, Eching, Germany).
Fluorescence Microscopy
Cells plated on coverslips were fixed with methanol/0.01% EDTA for 20 minutes at −20°C. After fixation, cells were blocked with 0.1% Triton X-100/PBS containing 5% bovine serum albumin for 15 minutes at room temperature. Cells were then washed with PBS and incubated with the primary antibody (diluted in 5% bovine serum albumin/PBS/0.1% Triton X-100) as indicated. Following washing, bound antibodies were detected by Alexa 488 conjugated goat anti-rabbit (Molecular Probes, Karlsruhe, Germany) or goat anti-mouse Cy3 (Sigma, Deisenhofen, Germany) secondary antibodies. To stain the nuclei cells were incubated with 4′-6-diamidino-2-phenylindole (Sigma, Deisenhofen, Germany) and slides were mounted in Fluoromount G (Southern Biotech, Birmingham, AL). Evaluation was performed by fluorescence microscopy (Keyence, Neu-Isenburg, Germany) or with an LSM 510 Meta confocal laser-scanning microscope (Carl Zeiss, Jena, Germany).
Fluorescence-Activated Cell Sorting Analysis
Human podocytes were incubated for 30 minutes with the monoclonal antibody against ADAM10 or the polyclonal antibody against CXCL16 (diluted 1:50). All steps were performed as recently described.27
Chemotaxis Assay
The chemotaxis of Jurkat T cells in response to recombinant CXCL16 or supernatants of siRNA-transfected human podocytes was measured across 8-μm pore size polycarbonate filters (6.5 mm diameter) in 24-well Transwell chambers (Costar Corning Co., Cambridge, MA). Transwells were pretreated with 10 μg/ml fibronectin (Calbiochem, Darmstadt, Germany) and washed with PBS. Jurkat T cells (1 × 105 cells in 100 μl of RPMI 1640) were added to the upper chamber of each Transwell. Recombinant CXCL16 (100 ng/ml) or serum free supernatants of siRNA-transfected human podocytes were added to the lower chamber in a volume of 600 μl. The plates were incubated for 2 hours at 37°C in 5% CO2. Non migrated cells were removed with a cotton swab, the Transwell inserts were fixed with paraformaldehyde and cells were stained with 4′-6-diamidino-2-phenylindole according to the manufacturer’s recommendations. Migrated cells were counted per visual field using inverted microscopy at magnification of ×400, and the experiments were repeated twice in triplicate.
Statistical Analysis
Data were analyzed by unpaired two-tailed t-test or one-way analysis of variance with the Tukey-Kramer test for multiple comparisons using GraphPad InStat-2. A P value of less than 0.05 was considered to show a significant difference between two groups.
Results
CXCL16 and ADAM10 Are Expressed in Human Podocytes
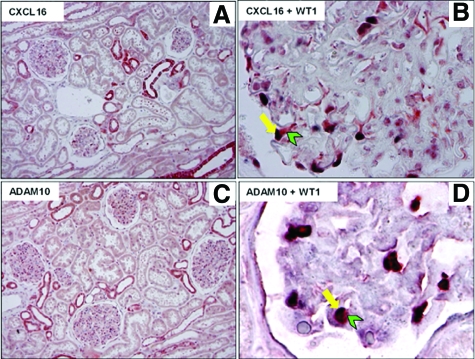
We have recently shown that CXCL16 and ADAM10 are mainly expressed in distal convoluted tubule cells, in connecting tubule cells, and in principal cells of the collecting duct.23 Beside tubular expression of CXCL16 and ADAM10, as also shown in Figure 1, A and C, constitutive expression of both proteins was also detectable in glomeruli of human kidneys. To specify CXCL16- and ADAM10-expressing cells in glomeruli of human kidneys we performed double immunohistochemical analysis in sections of normal renal tissue. As demonstrated in Figure 1, B and D, CXCL16 and ADAM10, respectively (both red), were co-expressed with WT1 (blue), which has been described to be only expressed in the nuclei of podocytes in the human kidney.28 In summary, we can demonstrate that CXCL16 and ADAM10 are constitutively expressed in podocytes of normal renal tissue.
Figure 1.
CXCL16 and ADAM10 are constitutively expressed in podocytes in human kidney sections. A: CXCL16 is constitutively expressed in tubular cells and in the glomeruli of the healthy kidney. Paraffin sections of normal human renal tissue were stained with a polyclonal antibody against CXCL16. B: Double immunohistochemical staining revealed CXCL16 expression in podocytes shown by colocalization of CXCL16 (red) with WT-1 (blue), a specific marker for podocytes. C: In the overview picture, ADAM10 expression is detectable in tubular and glomerular cells. D: Double immunohistochemical staining of ADAM10 (red) and WT-1 (blue) display localization of ADAM10 in podocytes.
IFN-γ and TNF-α Increased the Expression and Release of CXCL16 in Human Podocytes
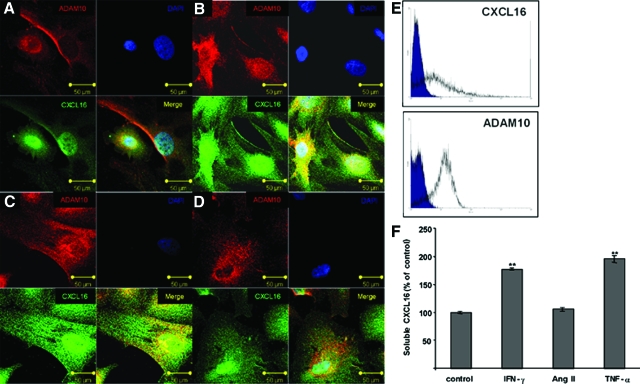
The expression of many chemokines can be regulated through proinflammatory molecules. Therefore we examined the influence of IFN-γ, TNF-α, and angiotensin II on the expression of CXCL16 in human podocytes. As shown by immunofluorescence staining, the application of IFN-γ (Figure 2B) and TNF-α (Figure 2C) induced the expression of CXCL16 compared with unstimulated podocytes (Figure 2A). In contrast, treatment with angiotensin II (Ang II, Figure 2D) did not change the levels of CXCL16 compared with unstimulated cells (Figure 2A). Confocal microscopy (Figure 2, A–D) and fluorescence-activated cell sorting analysis (Figure 2E) demonstrated that beside cytoplasmic CXCL16 and ADAM10 expression, both proteins were also expressed on the cell surface of human podocytes. It is known, that CXCL16 can be released from the cell membrane through proteolytic cleavage. To examine the influence of proinflammatory cytokines on the release of CXCL16 from human podocytes we analyzed supernatants of the cells in the presence or absence of IFN-γ, TNF-α and angiotensin II (Figure 2F). CXCL16 was constitutively released into the medium of human podocytes (Figure 2F). Furthermore, IFN-γ and TNF-α increased the shedding of CXCL16 from human podocytes, whereas angiotensin II again did not change the release of CXCL16 compared with untreated cells (Figure 2F).
Figure 2.
Induction of cellular and soluble CXCL16 by proinflammatory cytokines in human podocytes in vitro. Cultured human podocytes were left unstimulated (A) or stimulated with 20 ng/ml IFN-γ (B), 2 ng/ml TNF-α (C), or 10 nmol/L angiotensin II (D). Immunofluorescence staining of ADAM10 (red fluorescence) and CXCL16 (green fluorescence) was performed and inspected by confocal microscopy. Scale bars = 50 μm. E: Cell surface expression of CXCL16 and ADAM10 was detected by fluorescence-activated cell sorting analysis. F: Conditioned media were harvested and analyzed for the presence of soluble CXCL16 by ELISA. Data are presented as mean ± SE from one representative of three independent experiments (n = 3). **P < 0.01 considered statistically significant compared with cells grown in serum free medium (control).
ADAM10 Is Involved in the Release of CXCL16 under Inflammatory Conditions
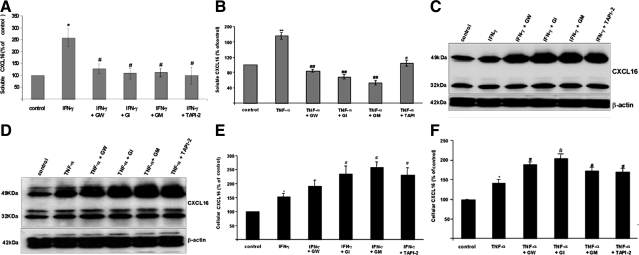
To determine the metalloproteinases involved in the cytokine induced shedding of CXCL16 in human podocytes, we first used different metalloproteinase inhibitors to block the release of CXCL16 from human podocytes. The broad spectrum metalloproteinase inhibitors GM6001 and TAPI-2, the ADAM10-specific inhibitor GI254023X, and the combined ADAM10 and ADAM17 inhibitor GW280264X significantly reduced the amount of soluble CXCL16 in the presence of IFN-γ (Figure 3A) or TNF-α (Figure 3B). To confirm these results, we also investigated cell-associated CXCL16 by Western blot analysis (Figure 3, C and D) and by a CXCL16-specific ELISA (Figure 3, E and F). As expected, the inhibition of metalloproteinases reduced the cleavage of CXCL16 and increased levels of cellular CXCL16 were detectable in INF-γ- (Figure 3, C and E) and TNF-α- (Figure 3, D and F) stimulated cells.
Figure 3.
Influence of metalloproteinase inhibitors on the expression of soluble and cellular CXCL16 in human podocytes in the presence of IFN-γ or TNF-α. A: Effects of different metalloproteinase inhibitors on the IFN-γ release of CXCL16 (18 hours treatment). Fifteen minutes prior to IFN-γ treatment, the cells were preincubated with 3 μmol/L GW280264X (GW), 3 μmol/L GI254023X (GI), 50 μmol/L GM6001 (GM), or 20 μmol/L TAPI-2. All data were reproduced in three independent experiments and are represented as mean ± SE (n = 3). *P < 0.05 considered statistically significant compared with the control; #P < 0.05, considered statistically significant compared with the IFN-γ treated cells. B: Soluble CXCL16 was measured in supernatants of podocytes treated for 18 hours with TNF-α. Fifteen minutes prior to TNF-α treatment, cells were preincubated with 3 μmol/L GW280264X (GW), 3 μmol/L GI254023X (GI), 50 μmol/L GM6001 (GM), or 20 μmol/L TAPI-2. All data were reproduced in three independent experiments and are represented as mean ± SE (n = 3). **P < 0.01 considered statistically significant compared with the control; #P < 0.05, ##P < 0.01 considered statistically significant compared with the TNF-α treated cells. C and D: Western blot analysis of cellular CXCL16 in human podocytes after the application of different metalloproteinase inhibitors in the presence of IFN-γ (C) or TNF-α (D). E and F: Cellular CXCL16 protein levels were determined by ELISA in podocytes treated with different metalloproteinase inhibitors in the presence of IFN-γ (E) or TNF-α (F). All data were reproduced in three independent experiments and are represented as mean ± SE (n = 3). *P < 0.05 considered statistically significant compared with the cells grown in serum-free medium (control). #P < 0.05 considered statistically significant compared with IFN-γ- or TNF-α-treated cells.
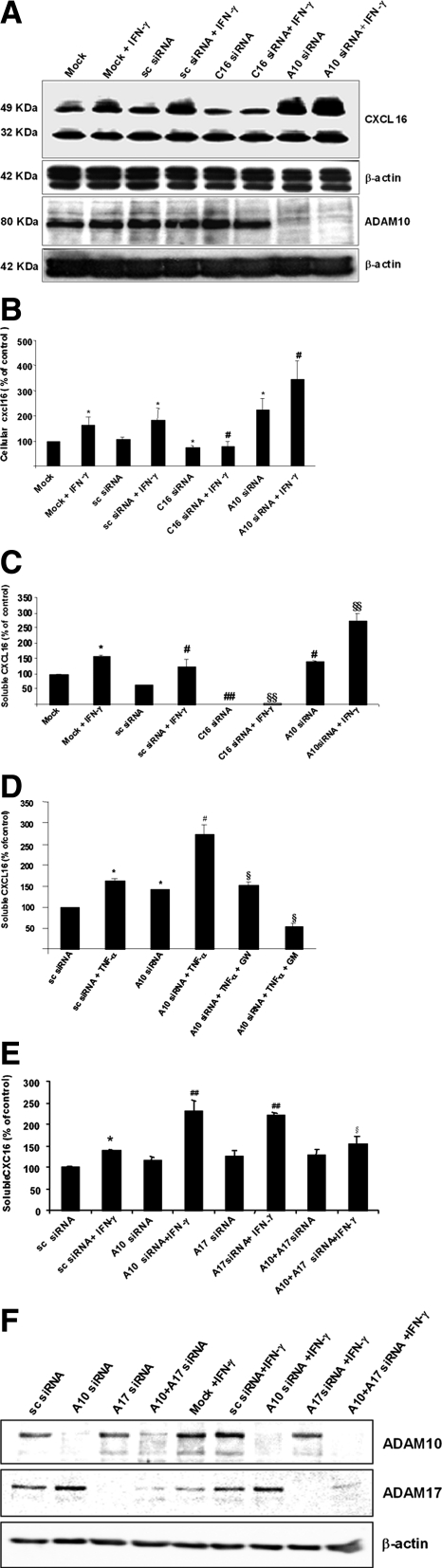
As the metalloproteinase inhibitors GW280264X and GI254023X showed a very similar influence on the processing of CXCL16, we concluded that ADAM17 is not involved in the cytokine-induced CXCL16 shedding.16 To validate the involvement of ADAM10 in the constitutive and cytokine-induced cleavage of CXCL16 we blocked ADAM10 expression by specific siRNAs. As shown by Western blot analysis transfection of ADAM10 siRNA resulted in a full suppression of ADAM10 protein expression (Figure 4A). In addition, the application of ADAM10-specific siRNA increased levels of cell-associated CXCL16 in the presence or absence of IFN-γ as shown by Western blot (Figure 4A) and ELISA (Figure 4B), confirming the results obtained in our inhibitor studies. Unexpectedly, knockdown of the ADAM10 protein further augmented the release of soluble CXCL16 (Figure 4C). In contrast to the metalloproteinase inhibitors, the knockdown of ADAM10 with siRNA prevented ADAM10 protein synthesis. Therefore we speculated that the loss of ADAM10 proteins rather than the inhibition of the ADAM10 activity can be compensated by another metalloproteinase. To investigate this hypothesis, we preincubated podocytes with the metalloproteinase inhibitors GW280264X or GM6001 before the siRNA transfection. In the presence of TNF-α the preincubation with the hydroxamate GW280264X significantly reduced the amount of soluble CXCL16 in comparison with cells treated with ADAM10 siRNA alone (Figure 4D). Moreover, the broad spectrum metalloproteinase inhibitor GM6001 decreased the release of CXCL16 under basal levels (Figure 4D). To further confirm that ADAM17 may be involved in the shedding of CXCL16 in the absence of ADAM10, we performed a double knockdown of ADAM10 and ADAM17 in podocytes and determined the amount of soluble CXCL16 by ELISA. As shown in Figure 4E, the knockdown of ADAM17 alone did not reduce the constitutive as well the IFN-γ-induced shedding of CXCL16. In contrast, the double knockdown of ADAM10 and ADAM17 reduced significantly the IFN-γ-induced shedding of CXCL16, assuming that ADAM17 can cleave CXCL16 in the absence of ADAM10. The efficient knockdown of both ADAM proteases are demonstrated by ADAM10- or ADAM17-specific Western blot (Figure 4F).
Figure 4.
Effect of ADAM10, CXCL16 and ADAM17 knockdown on the constitutive and IFN-γ-induced CXCL16 shedding. A: Western blot analysis of CXCL16 and ADAM10 expression after the transfection of specific siRNAs in the presence or absence of IFN-γ. β-Actin was used as a loading control. B: The amount of cellular CXCL16 was measured after specific siRNA transfection in the presence or absence of IFN-γ by a CXCL16-specific ELISA. All data were reproduced in three independent experiments and are represented as mean ± SE (n = 3). *P < 0.05 considered statistically significant compared with mock or sc-siRNA- transfected cells. #P < 0.05 considered statistically significant compared with sc siRNA transfected and IFN-γ treated cells. C: Human podocytes were transfected with CXCL16 and ADAM10-specific siRNA in the presence or absence of IFN-γ and supernatants were harvested. Supernatants were concentrated by centrifugation and soluble CXCL16 was measured by a CXCL16-specific ELISA. All data were reproduced in three independent experiments and are represented as mean ± SE (n = 3). *P < 0.05 considered statistically significant compared with the mock transfected cells. #P < 0.05, ##P < 0.01 considered statistically significant compared with sc-siRNA-transfected cells. §P < 0.05, §§P < 0.01 considered statistically significant compared with sc siRNA-transfected cells in the presence of IFN-γ. D: Influence of ADAM10 knockdown on the release of CXCL16 in the presence of TNF-α with or without preincubation with 3 μmol/L GW280264X (GW) or 50 μmol/L GM6001(GM). All data were reproduced in three independent experiments and are represented as mean ± SE (n = 3). *P < 0.05 considered statistically significant compared with the sc-siRNA-transfected cells. #P < 0.05 considered statistically significant compared with A10 siRNA-transfected cells. §§P < 0.01 considered statistically significant compared with A10 siRNA-transfected cells in the presence of TNF-α. E: Soluble CXCL16 was measured with a CXCL16-specific ELISA after single or double knockdown of ADAM10 and ADAM17 in the presence or absence of IFN-γ. All data were reproduced in three independent experiments and are represented as mean ± SE (n = 3). *P < 0.05 considered statistically significant compared with the sc-siRNA-transfected cells. ##P < 0.01 considered statistically significant compared with the A10 siRNA-transfected podocytes. §P < 0.05 considered statistically significant to A10 siRNA-transfected cells in the presence of IFN-γ. F: Western blot analysis with ADAM10- and ADAM17-specific antibodies in lysates of podocytes transfected with specific siRNAs in the presence and absence of IFN-γ. ß-Actin Western blot has been performed to demonstrate equal loading. A10, ADAM10; C16, CXCL16; A17, ADAM17; sc, scrambled.
CXCL16 Functions as a Scavenger Receptor for OxLDL in Human Podocytes
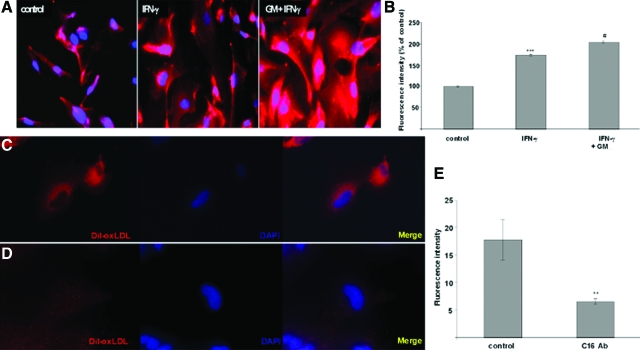
OxLDL plays a major role in the pathogenesis of atherosclerosis and in the development of various glomerular diseases.29,30,31,32 It has been described that CXCL16 functions as a scavenger receptor for oxLDL in macrophages.10 To analyze oxLDL uptake in human podocytes, we treated cells with IFN-γ in the presence or absence of the broad spectrum metalloproteinase inhibitor GM6001 (Figure 5A). As mentioned previously, IFN-γ increased the amount of cellular CXCL16, which could be further potentiated by the application of GM6001 (Figure 5, A and C). This up-regulation of the cell-associated CXCL16 was accompanied with a strong increase of DiI-oxLDL uptake (Figure 5, A and B).
Figure 5.
Involvement of CXCL16 in the uptake of ox-LDL in human podocytes. A: Dil-oxLDL uptake was analyzed in unstimulated (control), IFN-γ (IFN-γ), or 50 μmol/L GM6001 and IFN-γ (GM + IFN-γ) treated cells. After stimulation of human podocytes, cells were incubated for 4 hours with 100 μg/ml Dil-oxLDL and subsequently washed with PBS and fixed in methanol/0.02% EDTA. B: Fluorescence intensities of Dil-oxLDL in untreated (control), IFN-γ, or IFN-γ and GM6001 (GM) treated human podocytes. ***P < 0.001 considered statistically significant compared to untreated cells. #P < 0.05 considered statistically significant compared to the IFN-g and GM6001 (GM) treated cells. Dil-oxLDL uptake in the presence of control antibodies (C) or CXCL16 blocking antibodies (D) was analyzed by immunofluorescence microscopy. E: Fluorescence intensities of Dil-oxLDL in human podocytes treated with control IgG antibodies (control) or CXCL16 blocking antibodies (C16 Ab) were determined and represented in a graph. **P < 0.01 considered statistically significant compared to untreated cells.
To demonstrate that CXCL16 is involved in the uptake of oxLDL in human podocytes, we used antibodies to specifically block CXCL16 function. As shown in Figure 5, D and E, the application of a CXCL16 blocking antibody strongly reduced the amount of DiI-oxLDL uptake in human podocytes.
Knockdown of CXCL16 by siRNA Decreased the Chemotaxis of CXCR6-Expressing Jurkat T Cells
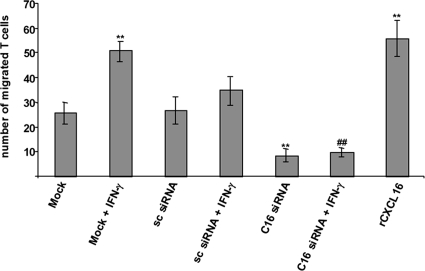
The recruitment of leukocytes into the kidney has emerged as a key event in the development of acute renal failure.33 CXCR6 is the only known receptor of CXCL16 and was first discovered as a coreceptor for HIV/SIV.13,34 It is expressed on CD4 positive effector memory T cell subsets and NKT cells.19,34 We have recently shown that Jurkat T cells express transmembrane CXCR6.23 It is known that CXCR6-expressing cells can be recruited by soluble CXCL16 to sites of inflammation.21,35 To investigate the role of CXCL16 in the process of T cell recruitment, we inhibited CXCL16 expression in human podocytes using the RNA interference technology. As shown in Figure 4C, transfection of CXCL16-specific siRNA completely blocked the release of soluble CXCL16 in the presence or absence of IFN-γ. Applying this CXCL16 deficient supernatant in a chemotaxis assay resulted in a significant reduction of migrated Jurkat T-cells in comparison with the control supernatants (Figure 6). To further underline the role of CXCL16 in the migration of T cells, we used recombinant CXCL16 in the chemotaxis assay, which resulted in an almost twofold induction of T cell migration (Figure 6).
Figure 6.
Silencing of CXCL16 in podocytes significantly decreased the chemotaxis of Jurkat T cells. Loss of CXCL16 correlated with a reduced number of migrated Jurkat T cells. Supernatants presented in Figure 5C were used for chemotaxis assay (n = 4). Recombinant CXCL16 induced the migration of Jurkat T cells approximately twofold. Data are means ± SD. **P < 0.01 considered statistically significant compared with mock treated cells; ##P < 0.01 considered statistically significant compared with the IFN-γ treated cells.
CXCL16 and ADAM17 Expression Is Increased in Glomeruli of Patients with Membranous Nephropathy
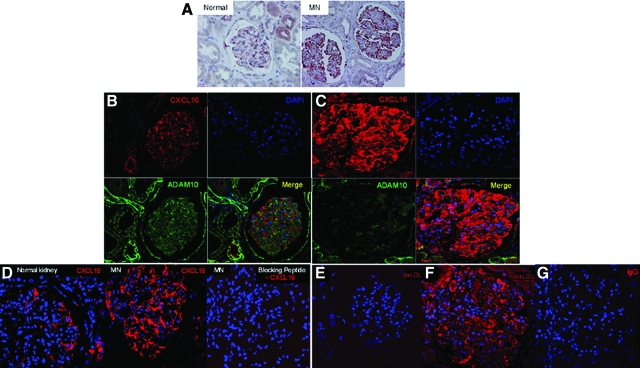
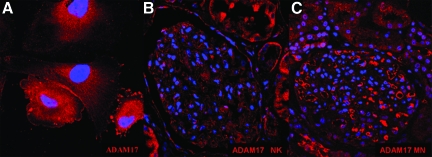
MN is an immune-mediated disease that is characterized by the accumulation of immune deposits leading to complement activation and podocyte injury.36 To study the expression of CXCL16 and ADAM10 in human glomerular diseases we performed immunohistochemical and immunofluorescence analysis on tissue sections of patients with MN. As shown in Figure 7A, immunohistochemical analysis revealed that CXCL16 was dramatically up-regulated in the glomeruli of MN patients compared with normal renal tissue. This up-regulation of CXCL16 was confirmed by immunofluorescence staining (Figure 7C). Interestingly, ADAM10 expression was down-regulated in glomeruli of MN patients in comparison with normal kidneys (Figure 7, B and C). The specificity of CXCL16 staining on biopsies of MN patients was controlled by using CXCL16 peptide blocking experiments (Figure 7D). To investigate if increased glomerular CXCL16 expression correlated with elevated oxLDL levels we performed immunofluorescence analysis on tissue sections of patients with MN. As documented in Figure 7F, glomerular oxLDL levels in MN patients were significantly increased compared with healthy kidneys (Figure 7E). To demonstrate the specificity of our immunofluorescence staining results on biopsies of patients with MN, IgG control antibodies were used for immunofluorescence analysis and did not show any specific fluorescence patterns (Figure 7G). In addition, we investigated the expression of ADAM17 in human podocytes (Figure 8A), in normal kidney (Figure 8B), and in patients with membranous nephropathy (Figure 8C). In contrast to ADAM10, increased ADAM17 expression was detectable in glomeruli of patients diagnosed with MN. The glomerular expression of CXCL16, ADAM10, ADAM17, and oxLDL in biopsies of nine patients with MN are summarized in Table 1. To investigate if increased CXCL16 levels are detectable in sera of patients with membranous nephropathy, we analyzed in a pilot study CXCL16 levels in sera of seven patients and of seven healthy controls. Interestingly, CXCL16 levels were found in controls (mean 1400 pg/ml), but in sera of six patients increased levels of CXCL16 were found (Table 2).
Figure 7.
Expression of CXCL16, ADAM10, and oxLDL in glomeruli of normal renal tissue and in tissue sections of patients with membranous nephropathy. A: Immunohistochemical analysis of CXCL16 expression in tissue sections of healthy kidney (Normal) and in a patient with membranous nephropathy (MN). Results were confirmed by immunofluorescence analysis of CXCL16 (red) and ADAM10 (green) expression in the normal human kidney (B) and in tissue sections of MN patients (C). D: CXCL16 peptide competition assay was performed to demonstrate specificity of glomerular CXCL16 staining in MN patients. Left and middle panels represent CXCL16 expression in normal kidney and in patients with MN, respectively. Right panel demonstrates no specific CXCL16 signal in glomeruli of MN patients, if CXCL16 antibody was preincubated with 10 μg/ml recombinant CXCL16 peptide. Healthy renal tissue (E) and renal biopsies of patients diagnosed with MN (F) were analyzed for oxidized LDL expression with immunofluorescence staining using antibodies against oxLDL followed by Cy3 coupled secondary antibodies. G: Immunofluorescence staining with an IgG control antibody on biopsies of patients with MN did not show any specific immunofluorescence signal.
Figure 8.
ADAM17 is expressed in podocytes and increased glomerular ADAM17 expression is detectable in patients with MN. A: Podocytes were fixed with methanol and immunofluorescence analysis were performed using a monoclonal antibody against ADAM17 and Cy3 coupled secondary antibody (red). B: ADAM17 expression in human normal kidney was visualized by monoclonal antibody against ADAM17 and Cy3 coupled secondary antibody. C: The expression of ADAM17 was visualized in biopsies of patients with MN by monoclonal ADAM17 followed by Cy3 coupled secondary antibody.
Table 1.
Glomerular Expression of ADAM17, ADAM10, CXCL16, and OxLDL in Kidney Biopsies of Patients with Membranous Nephropathy
| MN patient no. | ADAM17 | ADAM10 | CXCL16 | OxLDL |
|---|---|---|---|---|
| 1 | −/+ | − | +++ | ++ |
| 2 | + | − | +++ | ++ |
| 3 | + | − | +++ | ++ |
| 4 | + | − | + | −/+ |
| 5 | ++ | − | ++ | ++ |
| 6 | ++ | − | + | + |
| 7 | ++ | − | +++ | +++ |
| 8 | + | − | ++ | + |
| 9 | ++ | − | ++ | ++ |
−/+, slightly induced expression; +, weak induction; ++, moderate induction; +++, strong induction compared with expression in normal kidney.
Table 2.
CXCL16 Serum Levels and Clinical Parameters of Patients with Membranous Nephropathy or Lupus Nephritis
| Patient no. | Diagnosis | Age | Sex | Proteinuria (g/d) | Serum creatinine (mg/dl) | Immunosuppressive therapy | Antihypertensive therapy | CXCL16 (pg/ml) |
|---|---|---|---|---|---|---|---|---|
| 1 | MN | 41 | F | 0, 4 | 0, 74 | ACEI, BB | 1870, 86 | |
| 2 | MN | 20 | F | 0 | 1, 13 | CSA | ACEI, AT1RB | 2392, 30 |
| 3 | MN | 53 | M | 0, 5 | 1, 15 | CSA, S | 1979, 66 | |
| 4 | MN | 40 | F | 6, 8 | 0, 73 | ACEI, AT1RB | 1915, 83 | |
| 5 | MN | 40 | M | 10 | 1, 21 | ACEI, AT1RB, RI | 1960, 46 | |
| 6 | MN | 30 | F | 0 | 0, 78 | ACEI, AT1RB | 1344, 17 | |
| 7 | LN | 55 | F | 0, 6 | 0, 84 | MMF, S | ACEI, AT1RB | 3230, 50 |
MN, membranous necropathy; LN , lupus nephritis; ACE-I, angiotensin-converting enzyme inhibitor; AT1R-B, angiotensin receptor 1 blocker; BB, β blocker; CSA, cyclosporine A; MMF, mycophenolate mofetil; RI, renin inhibitor; S, steroids.
Discussion
Chemokines are important chemotactic molecules that control leukocyte trafficking and function. Recent studies have shown that these molecules also play an important role in several additional biological functions, such as regulation of lymphocyte development, expression of adhesion molecules, cell proliferation, angiogenesis, virus-target cell interactions, and various aspects of cancer.37 Most of the over 40 known chemokines are dramatically induced under inflammatory conditions and released as soluble molecules.38 CX3CL1 and CXCL16 are two exceptional chemokines that are synthesized as transmembrane molecules and are known to be constitutively expressed in the absence of inflammation.6 Constitutive expression of CXCL16 was already described on keratinocytes, endothelial cells, smooth muscle cells, and some cancer cells.7,16,39,40 CXCL16 does not only exist in a transmembrane form, but can also be processed by members of the ADAM (a disintegrin and metalloproteinase) family, resulting in a soluble CXCL16 form.16,17,18 With immunohistochemistry, Western blot, and immunofluorescence analysis we provide conclusive evidence that human podocytes constitutively express CXCL16 in vitro and in vivo. Furthermore our in vitro results clearly demonstrate that proinflammatory cytokines like TNF-α and IFN-γ can further increase cellular as well as soluble CXCL16 in human podocytes. In addition, with metalloproteinase inhibitor studies we demonstrated that ADAM10 is involved in the induced shedding of CXCL16. Surprisingly, in contrast to the inhibitor studies, the knockdown of ADAM10 by siRNAs increased not only cellular CXCL16 expression but unexpectedly also enhanced the release of CXCL16 from human podocytes. Incubation of human podocytes with the ADAM17/ADAM10-specific metalloproteinase inhibitor GW280264X after ADAM10 siRNA transfection blocked the induced shedding of CXCL16, showing that ADAM17 cleaves CXCL16 in the absence of ADAM10. In addition, the double knockdown of ADAM10 and ADAM17 by specific siRNA in the presence of IFN-γ confirmed that ADAM17 (in the absence of ADAM10) is involved in the release of CXCL16 under inflammatory conditions. At first glance these findings seem to be in conflict with previous publications. In keratinocytes the down-regulation of ADAM10 by siRNAs resulted in a decrease of the constitutive shedding of CXCL16.41 This difference could be a cell-type specific effect, which may depend on the availability of the specific protease. Tissue-specific variations in the molecular size of CXCL16 proteins expressed in spleen and lymph nodes have been already described.8 It has been recently shown that depending on the stimuli used, different metalloproteinase can regulate the release of CXCL16. In COS cells phorbol 12-myristate 13-acetate-induced release of CXCL16 was primarily mediated by ADAM17, whereas shedding induced by ionomycin was independent of the proteolytic activity of ADAM17.42 The discrepancy between the effects of the pharmacological inhibition of ADAM10 versus the siRNA-mediated loss of ADAM10 in human podocytes can be due to differences in the way of action between these two methods. The application of the metalloproteinase inhibitor GI254023X resulted in the inhibition of ADAM10 activity. The protein is still present in the cells in contrast to the treatment with ADAM10-specific siRNA. In human podocytes it seems that the loss of ADAM10 expression induced compensatory effects, whereas the blockage of ADAM10 activity did not.
As mentioned above, an important function of transmembrane CXCL16 is its activity as a scavenger receptor for oxLDL.10,14 Accumulation of oxLDL has been reported in the circulation and in the renal interstitium in both experimental models and patients with chronic kidney disease and end-stage renal disease. Interestingly, in a mouse model of unilateral ureteral obstruction CXCL16 expression was significantly increased in injured tubules and interstitial cells.43 In addition, the authors described a significant increase of renal tubulointerstitial oxLDL in this animal model, but the potential role of CXCL16 in the endocytosis of oxLDL was not further investigated. In our study, treating human podocytes with IFN-γ with or without a metalloproteinase inhibitor significantly increased oxLDL uptake. Furthermore, with blocking antibodies against CXCL16 we significantly reduced the oxLDL uptake in human podocytes, thus defining an important role of CXCL16 as a scavenger receptor for oxLDL on podocytes. Our data are consistent with a recent publication demonstrating a CXCL16-mediated uptake of oxLDL in endothelial cells.44 Importantly, the deposition of atherogenic lipoproteins has been implicated in the progression of glomerular injury.45 Scavenger receptors are known to promote or attenuate atherogenic effects in vivo through the endocytosis of oxLDL and activation of proinflammatory cascades following ligand binding.46,47,48 Furthermore, it has been shown that oxLDL induced apoptosis in mouse podocytes.49 In contrast, treatment of human podocytes with oxLDL did not induce apoptosis (data not shown). Importantly, we found in renal biopsies of MN patients displaying dramatic up-regulation of CXCL16 increased levels of glomerular oxLDL. To further evaluate this important finding, siRNA experiments or blocking antibodies against CXCL16 should be performed in animal models of membranous nephropathy to clarify whether CXCL16 is also in vivo responsible for the uptake of oxLDL in podocytes.
The role of CXCL16 in the progression of kidney diseases has been recently described. So far, in humans an important role of CXCL16 has only been suggested in lupus patients and in kidney transplanted patients with the diagnosis of acute interstitial rejection.23 Elevated urinary CXCL16 amounts were found in several strains of mice with spontaneous lupus nephritis and in patients with lupus nephritis.24 In addition, in sera of lupus nephritis patients with active renal disease elevated levels of CXCL16 were found.24 We therefore investigated CXCL16 levels in sera of patients with MN. Interestingly, in five of six MN patients, elevated levels of serum CXCL16 were detectable. In contrast to CXCL16 levels from the lupus nephritis patient, MN patients had much lower CXCL16 levels. Further investigations should be performed to evaluate whether CXCL16 in sera of MN patients maybe an potential marker molecule to monitor disease progression.
The cellular origin of CXCL16 in lupus patients has been so far not identified. Our study is the first report demonstrating increased CXCL16 expression in podocytes of patients with membranous nephropathy. In the same line, elevated CXCL16 mRNA levels were detected in the glomeruli of a rat model of experimental glomerulonephritis, thus demonstrating the relationship between altered glomerular CXCL16 expression and progression of kidney diseases.50 Furthermore, blocking CXCL16 in the acute inflammatory phase of established glomerulonephritis significantly attenuated monocyte/macrophage infiltration and glomerular injury.50 Our results also displayed a chemoattractant activity of soluble CXCL16 released from human podocytes as CXCL16-deficient supernatants of human podocytes displayed reduced chemotaxis activity for CXCR6-expressing T cells.
To investigate the role of CXCL16 and ADAM10 in patients with membranous nephropathy we performed immunofluorescence analysis on biopsies of patients with MN. Importantly, in patients with MN elevated levels of CXCL16 were accompanied with decreased ADAM10 expression in podocytes. In contrast to ADAM10, we found that ADAM17 was increased in glomeruli of MN patients. An important role of ADAM17 in the progression of chronic kidney diseases has been shown in experimental model of renal injury.51 The authors demonstrated that transforming growth factor-α and its sheddase ADAM17 were induced by angiotensin II treatment.51 In addition, angiotensin II-induced renal lesions were substantially reduced in mice lacking transforming growth factor-α or in mice given a specific TNF-α converting enzyme inhibitor.51 Beside CXCL16 and transforming growth factor-α, ADAM17 has been shown to cleave TNF-α and fractalkine, two molecules known to play important roles in the development of various kidney diseases.52,53
MN is the most common cause of idiopathic nephritic syndrome in white adults, accounting for about 20% of cases.22,54 MN is characterized by an accumulation of immune deposits on the outer aspect of the glomerular basement membrane, which causes a membrane-like thickening. The immune deposits consists of IgG, with a predominance of IgG4,55,56 unidentified antigens, and the membrane attack complex of complement C5b-9. Therefore an applicable strategy to treat MN patients is to target B-lymphocytes as a major cell type to prevent disease progression. Interestingly, both B-lymphocytes and bone marrow plasma cells express CXCR6, the sole receptor for CXCL16.11,57 Therefore, the importance of CXCL16, ADAM10, and ADAM17 needs to be further investigated in an animal model of MN (Heymann nephritis) to evaluate whether these proteins are useful target molecules to overcome the currently existing disappointing treatment strategies of MN.
Footnotes
Address reprint requests to Dr. Paul Gutwein, Pharmazentrum Frankfurt, Klinikum der Johann Wolfgang Goethe-Universität Frankfurt, Theodor-Stern-Kai 7, D-60590 Frankfurt am Main, Germany. E-mail: p.gutwein@med.uni-frankfurt.de.
References
- Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192:385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Yanagida-Asanuma E, Takagi M, Kodama F, Tomino Y. The role of podocytes in proteinuria. Nephrology (Carlton) 2007;12(Suppl 3):S15–S20. doi: 10.1111/j.1440-1797.2007.00876.x. [DOI] [PubMed] [Google Scholar]
- Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Weber C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb Haemost. 2007;97:694–703. [PubMed] [Google Scholar]
- Hofnagel O, Luechtenborg B, Plenz G, Robenek H. Expression of the novel scavenger receptor SR-PSOX in cultured aortic smooth muscle cells and umbilical endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:710–711. doi: 10.1161/01.atv.0000012402.85056.45. [DOI] [PubMed] [Google Scholar]
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- Shashkin P, Simpson D, Mishin V, Chesnutt B, Ley K. Expression of CXCL16 in human T cells. Arterioscler Thromb Vasc Biol. 2003;23:148–149. doi: 10.1161/01.atv.0000043906.61088.4b. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Kume N, Minami M, Hayashida K, Kataoka H, Kita T, Yonehara S. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J Biol Chem. 2000;275:40663–40666. doi: 10.1074/jbc.C000761200. [DOI] [PubMed] [Google Scholar]
- Wilbanks A, Zondlo SC, Murphy K, Mak S, Soler D, Langdon P, Andrew DP, Wu L, Briskin M. Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol. 2001;166:5145–5154. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Nakayama T, Fukumoto N, Kume N, Takahashi S, Yamaguchi J, Minami M, Hayashida K, Kita T, Ohsumi J, Yoshie O, Yonehara S. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J Leukoc Biol. 2004;75:267–274. doi: 10.1189/jlb.1003465. [DOI] [PubMed] [Google Scholar]
- Minami M, Kume N, Shimaoka T, Kataoka H, Hayashida K, Akiyama Y, Nagata I, Ando K, Nobuyoshi M, Hanyuu M, Komeda M, Yonehara S, Kita T. Expression of SR-PSOX, a novel cell-surface scavenger receptor for phosphatidylserine and oxidized LDL in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001;21:1796–1800. doi: 10.1161/hq1001.096652. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Nakayama T, Hieshima K, Kume N, Fukumoto N, Minami M, Hayashida K, Kita T, Yoshie O, Yonehara S. Chemokines generally exhibit scavenger receptor activity through their receptor-binding domain. J Biol Chem. 2004;279:26807–26810. doi: 10.1074/jbc.C400163200. [DOI] [PubMed] [Google Scholar]
- Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, Schuster B, Kallen KJ, Saftig P, Rose-John S, Ludwig A. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–6372. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- Gough PJ, Garton KJ, Wille PT, Rychlewski M, Dempsey PJ, Raines EW. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol. 2004;172:3678–3685. doi: 10.4049/jimmunol.172.6.3678. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, Leesnitzer MA, Becherer JD. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. 2005;8:161–171. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Shimaoka T, Kojo S, Harada M, Watarai H, Wakao H, Ohkohchi N, Yonehara S, Taniguchi M, Seino K. Cutting edge: critical role of CXCL16/CXCR6 in NKT cell trafficking in allograft tolerance. J Immunol. 2005;175:2051–2055. doi: 10.4049/jimmunol.175.4.2051. [DOI] [PubMed] [Google Scholar]
- van d, V, van Lieshout AW, Toonen LW, Sloetjes AW, van den Berg WB, Figdor CG, Radstake TR, Adema GJ. Elevated CXCL16 expression by synovial macrophages recruits memory T cells into rheumatoid joints. Arthritis Rheum. 2005;52:1381–1391. doi: 10.1002/art.21004. [DOI] [PubMed] [Google Scholar]
- Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23:324–332. doi: 10.1016/s0270-9295(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Schramme A, Abdel-Bakky MS, Gutwein P, Obermuller N, Baer PC, Hauser IA, Ludwig A, Gauer S, Schafer L, Sobkowiak E, Altevogt P, Koziolek M, Kiss E, Grone HJ, Tikkanen R, Goren I, Radeke H, Pfeilschifter J. Characterization of CXCL16 and ADAM10 in the normal and transplanted kidney. Kidney Int. 2008;74:328–338. doi: 10.1038/ki.2008.181. [DOI] [PubMed] [Google Scholar]
- Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N, Calixto S, Mackay M, Aranow C, Putterman C, Mohan C. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J Immunol. 2007;179:7166–7175. doi: 10.4049/jimmunol.179.10.7166. [DOI] [PubMed] [Google Scholar]
- Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, Schneider U. Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am J Pathol. 2001;158:833–839. doi: 10.1016/S0002-9440(10)64031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutwein P, Schramme A, Sinke N, Abdel-Bakky MS, Voss B, Obermuller N, Doberstein K, Koziolek M, Fritzsche F, Johannsen M, Jung K, Schaider H, Altevogt P, Ludwig A, Pfeilschifter J, Kristiansen G. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur J Cancer. 2009;45:478–489. doi: 10.1016/j.ejca.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development. 1993;119:1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- Bosmans JL, Holvoet P, Dauwe SE, Ysebaert DK, Chapelle T, Jurgens A, Kovacic V, Van Marck EA, De Broe ME, Verpooten GA. Oxidative modification of low-density lipoproteins and the outcome of renal allografts at 1 1/2 years. Kidney Int. 2001;59:2346–2356. doi: 10.1046/j.1523-1755.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- Tsimikas S. Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr Atheroscler Rep. 2006;8:55–61. doi: 10.1007/s11883-006-0065-1. [DOI] [PubMed] [Google Scholar]
- Singbartl K, Ley K. Leukocyte recruitment and acute renal failure. J Mol Med. 2004;82:91–101. doi: 10.1007/s00109-003-0498-8. [DOI] [PubMed] [Google Scholar]
- Tabata S, Kadowaki N, Kitawaki T, Shimaoka T, Yonehara S, Yoshie O, Uchiyama T. Distribution and kinetics of SR-PSOX/CXCL16 and CXCR6 expression on human dendritic cell subsets and CD4+ T cells. J Leukoc Biol. 2005;77:777–786. doi: 10.1189/jlb.1204733. [DOI] [PubMed] [Google Scholar]
- Nanki T, Shimaoka T, Hayashida K, Taniguchi K, Yonehara S, Miyasaka N. Pathogenic role of the CXCL16-CXCR6 pathway in rheumatoid arthritis. Arthritis Rheum. 2005;52:3004–3014. doi: 10.1002/art.21301. [DOI] [PubMed] [Google Scholar]
- Ponticelli C. Membranous nephropathy. J Nephrol. 2007;20:268–287. [PubMed] [Google Scholar]
- Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–176. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H, Takano Y, Yoshie O, Tsukada K, Saiki I. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67:4725–4731. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Schulte A, Schnack C, Hundhausen C, Reiss K, Brodway N, Held-Feindt J, Mentlein R. Enhanced expression and shedding of the transmembrane chemokine CXCL16 by reactive astrocytes and glioma cells. J Neurochem. 2005;93:1293–1303. doi: 10.1111/j.1471-4159.2005.03123.x. [DOI] [PubMed] [Google Scholar]
- Scholz F, Schulte A, Adamski F, Hundhausen C, Mittag J, Schwarz A, Kruse ML, Proksch E, Ludwig A. Constitutive expression and regulated release of the transmembrane chemokine CXCL16 in human and murine skin. J Invest Dermatol. 2007;127:1444–1455. doi: 10.1038/sj.jid.5700751. [DOI] [PubMed] [Google Scholar]
- Hundhausen C, Schulte A, Schulz B, Andrzejewski MG, Schwarz N, von Hundelshausen P, Winter U, Paliga K, Reiss K, Saftig P, Weber C, Ludwig A. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J Immunol. 2007;178:8064–8072. doi: 10.4049/jimmunol.178.12.8064. [DOI] [PubMed] [Google Scholar]
- Okamura DM, Lopez-Guisa JM, Koelsch K, Collins S, Eddy AA. Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol. 2007;293:F575–F585. doi: 10.1152/ajprenal.00063.2007. [DOI] [PubMed] [Google Scholar]
- Postea O, Koenen RR, Hristov M, Weber C, Ludwig A. Homocysteine upregulates vascular transmembrane chemokine CXCL16 and induces CXCR6(+) lymphocyte recruitment in vitro and in vivo. J Cell Mol Med. 2008;12:1700–1709. doi: 10.1111/j.1582-4934.2008.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle J, Heermeier K, Wanner C. Atherogenic lipoproteins, oxidative stress, and cell death. Kidney Int Suppl. 1999;71:S62–S65. doi: 10.1046/j.1523-1755.1999.07116.x. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman SC, Rateri DL, Szilvassy SJ, Cornicelli JA, Daugherty A. Macrophage-specific expression of class A scavenger receptors in LDL receptor(−/−) mice decreases atherosclerosis and changes spleen morphology. J Lipid Res. 2002;43:1201–1208. [PubMed] [Google Scholar]
- Yoshimi N, Ikura Y, Sugama Y, Kayo S, Ohsawa M, Yamamoto S, Inoue Y, Hirata K, Itabe H, Yoshikawa J, Ueda M. Oxidized phosphatidylcholine in alveolar macrophages in idiopathic interstitial pneumonias. Lung. 2005;183:109–121. doi: 10.1007/s00408-004-2525-0. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, Di Carlo F, Camussi G. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936–1947. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Truong LD, Li P, Zhang P, Johnson RJ, Wilson CB, Feng L. Inhibition of CXCL16 attenuates inflammatory and progressive phases of anti-glomerular basement membrane antibody-associated glomerulonephritis. Am J Pathol. 2007;170:1485–1496. doi: 10.2353/ajpath.2007.060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- Carrero JJ, Yilmaz MI, Lindholm B, Stenvinkel P. Cytokine dysregulation in chronic kidney disease: how can we treat it? Blood Purif. 2008;26:291–299. doi: 10.1159/000126926. [DOI] [PubMed] [Google Scholar]
- Holdsworth SR, Kitching AR, Tipping PG. Chemokines as therapeutic targets in renal disease. Curr Opin Nephrol Hypertens. 2000;9:505–511. doi: 10.1097/00041552-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Wasserstein AG. Membranous glomerulonephritis. J Am Soc Nephrol. 1997;8:664–674. doi: 10.1681/ASN.V84664. [DOI] [PubMed] [Google Scholar]
- Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 1997;51:270–276. doi: 10.1038/ki.1997.32. [DOI] [PubMed] [Google Scholar]
- Noel LH, Aucouturier P, Monteiro RC, Preud'homme JL, Lesavre P. Glomerular and serum immunoglobulin G subclasses in membranous nephropathy and anti-glomerular basement membrane nephritis. Clin Immunol Immunopathol. 1988;46:186–194. doi: 10.1016/0090-1229(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–1140. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]