Abstract
Uterine carcinosarcomas (UCSs) are considered to represent true examples of the epithelial-mesenchymal transition. Akt plays a key role in the induction of epithelial-mesenchymal transition, but little is known about its involvement in tumorigenesis. Here we examined the functional roles of the Akt/β-catenin pathway in UCSs. In clinical samples, phospho-Akt (pAkt) expression was found to be significantly increased in mesenchymal compared with epithelial components, exhibiting both positive and negative correlations with nuclear β-catenin and E-cadherin, respectively. Expression levels of the transcription factor Slug were also significantly up-regulated in the mesenchymal components and strongly correlated with both pAkt and nuclear β-catenin. In endometrial cancer cell lines, active Akt induced the stabilization of nuclear β-catenin through the phosphorylation of GSK-3β, and this, in turn, led to the transactivation of Slug, which was mediated by nuclear β-catenin. Moreover, Slug overexpression itself caused repression of E-cadherin, with subtle changes in cell morphology. In addition, knockdown of the retinoblastoma gene product (Rb) up-regulated pAkt and repressed E-cadherin, consistent with the in vivo finding of significantly decreased Rb expression in mesenchymal components. These findings suggest that changes in the Akt/β-catenin pathway, as well as alterations in Rb expression, may be essential for both the establishment and maintenance of phenotypic characteristics of UCSs, playing key roles in the regulation of E-cadherin through the transactivation of the Slug gene.
Uterine carcinosarcomas (UCSs) are highly aggressive neoplasms with a biphasic histology featuring both epithelial and mesenchymal elements, the latter being generally further subdivided into two subcategories, homologous with tissues normally found in the uterus and heterologous containing nonnormal elements, most commonly malignant cartilage or skeletal muscles.1 Recent studies have revealed that the carcinomatous component is the driving force, while the sarcomatous portions are derived from carcinoma or single stem cells that undergo divergent differentiation, indicating that most, if not all, are monoclonal in origin rather than because of collision.2,3
The epithelial-mesenchymal transition (EMT) is a fundamental feature of development that is responsible for initiating pathological alteration in adult organisms. In the process, cells of epithelial origin lose epithelial characteristics and polarity acquiring a mesenchymal phenotype with increased migratory behavior.4,5,6,7 The molecular mechanisms are characterized by down- and up-regulation of epithelial and mesenchymal markers, respectively, resulting in acquisition of a fibroblast-like morphology with cytoskeleton reorganization and increase in motility, invasiveness, and metastatic capacity.4,5,6,7 Although observation of full EMT in clinical samples of human and animal tumors is rare,8,9 the breast carcinosarcoma is now considered to represent a true example of complete EMT.6,10 In contrast, little is known about the association with tumorigenesis of UCSs.
A hallmark of EMT is lost expression of E-cadherin, a key component of adhesion junctions that plays an important role in maintenance of epithelial integrity.11 A number of specific transcription factors, including Snail, Slug, SIP-1, and Twist, contribute to induction of EMT, acting as transcriptional repressors of the E-cadherin gene.12,13,14,15 In addition, the oncogenic serine/threonine kinase Akt also promotes the process, modulating several signaling and transcriptional networks linking Wnt/β-catenin, nuclear factor (NF)-κB/p65, and the retinoblastoma gene product (Rb).16,17,18,19 We therefore hypothesized that these factors may serve as mediators for establishment and maintenance of phenotypic characteristics of UCSs. To test this, we here investigated changes in expression of Akt, β-catenin, E-cadherin, and Slug, with reference to the Rb and NF-κB/p65 status, using in vivo and in vitro assays.
Materials and Methods
Clinical Cases
We reviewed cases of comprehensively staged high-grade endometrial adenocarcinomas from the patient records of Kitasato University Hospital for the period from 1997 to 2008. According to the criteria of the 2003 World Health Organization classification, tumors were designed as UCS if they had evidence of both malignant epithelial (endometrioid, serous, or clear cell components) and mesenchymal (homologous or heterologous) elements. Endometrioid adenocarcinomas with spindle elements and hyalinized stroma were specifically excluded. Finally, a total of 14 UCSs were investigated (see Supplementary Table S1 at http://ajp.amjpathol.org). Thirteen cases of endometrial carcinomas, including six serous, three clear, and four grade 3 endometrioid lesions, were also examined as controls. All tissues were routinely fixed in 10% formalin and processed for embedding in paraffin wax. Approval for this study was given by the Ethics Committee of the Kitasato University School of Medicine (B07-16).
Antibodies and Reagents
Antibodies to phospho-Akt at Ser473 (pAkt) (Cell Signaling Technology, Beverly, MA), total AKT (Cell Signaling Technology), β-catenin (clone 14; Transduction Laboratories, Lexington, KY), phospho-β-catenin at Ser33/37/Thr41 (pβ-catenin) (Cell Signaling Technology), NF-κB subunit p65 (p65) (clone 20; Transduction Laboratories), phospho-p65 at Ser276 (pp65) (Cell Signaling Technology), E-cadherin (HECD-1; Takara, Shiga, Japan), p53 (DO7; Novocastra Ltd., Newcastle, UK), cytokeratin (clone AE1/AE3; DAKO, Glostrup, Denmark), vimentin (clone V9, DAKO), GKS-3β (clone 7, Transduction Laboratories), phospho-GSK-3β at Ser9 (pGSK-3β) (Cell Signaling Technology), IκBα (Cell Signaling Technology), HA (Y-11; Santa Cruz Biotechnology, Santa Cruz, CA), Slug (ab38551; Abcam, Tokyo, Japan), Snail (ab17732, Abcam), Rb (BD Bioscience Pharmingen, San Jose, CA), phospho-Rb at Ser807/811 (pRb) (Cell Signaling Technology), Ki-67 (DAKO), and β-actin (Sigma Chemical Co., St Louis, MO) were used in this study. Cycloheximide, a specific inhibitor of protein synthesis, was purchased from Calbiochem (Darmstadt, Germany).
Immunohistochemistry
Immunohistochemistry was performed using a combination of the microwave oven heating and polymer immunocomplex (Envision; DAKO, Copenhagen, Denmark) methods. For evaluation of immunostaining, tumor lesions were subdivided into areas containing epithelial and mesenchymal elements. Nuclei immunopositive for β-catenin, pp65, p53, Slug, Snail, Rb, pRb, and Ki-67, were counted in at least 1000 tumor cells in five randomly selected fields each for both components in all cases. Labeling indices (LIs) were then calculated as numbers per 100 cells, as described previously.20,21,22 Cells with nuclear and/or cytoplasmic immunoreactivity for pAkt were also examined in a similar manner. Scoring of membrane and cytoplasmic immunoreactivity for E-cadherin, cytokeratin, and vimentin was performed on the basis of percentages of immunopositive cells and the immunointensity, with multiplication of values for the two parameters, as described previously.20,21,22
Plasmids and Cell Lines
A full-length cDNA for HA-tagged human Slug (GenBank accession number NM003068) was generated by polymerase chain reaction (PCR) and subcloned into pcDNA3.1 (Invitrogen, Carlsbad, CA). The promoter sequence between −2125 and −34 bp relative to the translation start site (AF084243) amplified by PCR was subcloned into the pGL3-basic vector (Promega, Madison, WI). A series of 5′-truncated promoter constructs were also generated by PCR, using a combination of specific 5′-forward and common 3′ primers (R-235 and R-34). The identity of all constructs was confirmed by sequencing before use. pcDNA3.1-HA-β-cat▵S45, pcDNA3.1-mouse p65, pCI-Flag-p300, pcDNA3.1-TCF4▵N30 (dominant-negative form TCF4), pGL3B (−547/+123) E-cadherin-Luc, pNFκB-Luc, and Top and Fop reporter constructs were as described previously.20,21,22 The constitutively active myristylated Akt expression plasmid (pUSEamp-myrAkt1) was purchased from Upstate Biotechnology (Lake Placid, NY). pGL3-HBCP containing a β-catenin promoter (−2760/+21 bp) was provided by Dr. Roderick H. Dashwood (Oregon State University, Corvallis, OR). The sequences of PCR primers used in this study are listed in Table 1.
Table 1.
Primer Sequences Used in This Study
| Rb | mRNA | Forward | 5′-GCAGTTGACCTAGATGAGATG-3′ |
| Reverse | 5′-CAACATGGGAGGTGAGAGTTT-3′ | ||
| E-cadherin | mRNA | Forward | 5′-CAACATGGGAGGTGAGAGTTT-3′ |
| Reverse | 5′-CGAAGAAACAGCAAGAGCAGCAGAATCAGA-3′ | ||
| β-Catenin | mRNA | Forward | 5′-CCAGCGTGGACAATGGCTAC-3′ |
| Reverse | 5′-CTCTGAGCTCGAGTCATTGC-3′ | ||
| P65 | mRNA | Forward | 5′-AATGGCTCGTCTGTAGTGCA-3′ |
| Reverse | 5′-GGTCCTGTGTAGCCATTGAT-3′ | ||
| Fibronectin | mRNA | Forward | 5′-GTGCCTGGGCAACGGA-3′ |
| Reverse | 5′-CCCGACCCTGACCGAAG-3′ | ||
| Snail | mRNA | Forward | 5′-TGCCTCGACCACTATGCCGC-3′ |
| Reverse | 5′-AGCATTGGCAGCGAGGCGGT-3′ | ||
| Slug | Cloning | Forward | 5′-CAAGATGCCGCGCTCCTTCC-3′ |
| Reverse | 5′-CACCTGAGTTCGCGTCTGGC-3′ | ||
| mRNA | Forward | 5′-CAAGATGCCGCGCTCCTTCC-3′ | |
| Reverse | 5′-TGGCATGGGGGTCTGAAAGCT-3′ | ||
| Promoter | |||
| Forward | F-2125 | 5′-CTGGATTATGCCTCTGTGATCC-3′ | |
| F-1895 | 5′-GGAGTGAAAAGCAAGGAGGACT-3′ | ||
| F-1587 | 5′-TAGGAAATCTGTGAGTGCCCCA-3′ | ||
| F-813 | 5′-GTACTTAATTTGCACGCGGCC-3′ | ||
| F-592 | 5′-CCACTTCCAAATATAGGCTCTC-3′ | ||
| F-444 | 5′-TCCTGCGCCCCTCCTAGCTC-3′ | ||
| F-361 | 5′-ACACTTTTTTTCCTCTCCACTGA-3′ | ||
| Reverse | R-235 | 5′-GAGGTTCAGATTTCAGCTCCTC-3′ | |
| R-34 | 5′-GGCGGTCCCTACAGCATCGC-3′ |
Two endometrial cancer cell lines, Ishikawa and Hec251,23,24 were maintained in Eagle’s minimal essential medium with 10% bovine calf serum. Ishikawa cells stably overexpressing HA-tagged β-cat▵S45 (Ish-HA-bcat#25) were also used, as described previously.20,21 To establish cells stably overexpressing HA-Slug, the expression plasmid or empty vector were transfected into Hec251 cells. After 2 weeks of culture in the presence of 1 mg/ml of geneticin (Life Technology, Grand Island, NY), three independent clones were established.
Transfection
Transfection was performed using LipofectAMINE Plus (Invitrogen), in duplicate or triplicate, as described previously.20,21,22 The pRL-TK plasmid (Promega) was used to normalize for transfection efficiency, and luciferase activity was assayed with the dual-luciferase reporter assay system (Promega), as described previously.20,21,22 In addition, siRNAs for Rb, β-catenin, and p65 were transfected using the siPORT NeoFX transfection agent (Ambion, Austin, TX), according to the manufacturer’s instructions. The siRNAs, as well as the negative control, are available from Ambion.
Reverse Transcription (RT)-PCR
cDNA was synthesized from 2 μg of total RNA. Amplification was performed in the exponential phase (20 to 28 cycles) to allow comparison among cDNAs synthesized from identical reactions, using specific primers (Table 1). The GAPDH gene was amplified as an internal control, as described previously.20,21,22
β-Catenin Gene Mutation Analyses
DNA was extracted from paraffin wax sections of UCS cases, using proteinase K/phenol-chloroform methods. Exon 3 of the β-catenin gene was amplified and sequenced as described previously.20
Western Blot Assays
Total cellular proteins were isolated using RIPA buffer [50 mmol/L Tris-HCl (pH7.2), 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate]. Nuclear and cytoplasmic fractions were also prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL). Aliquots of 1 to 10 μg of proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to membranes, and probed with primary antibodies, coupled with the ECL detection system (Amersham Pharmacia Biotechnology, Tokyo, Japan).
Immunofluorescence
After transient transfection of pUSEamp-myr-Akt1 into Hec251 cells, they were incubated with primary antibodies. Fluorescein isothiocyanate- or rhodamine-labeled anti-mouse or rabbit IgGs (Molecular Probes, Leiden, The Netherlands) were used as secondary antibodies, as described previously.20,21,22
Statistics
Comparative data were analyzed using the Mann-Whitney U-test and the Spearman’s correlation coefficient. The cutoff for statistical significance was set as P < 0.05.
Results
Relationships Among Expression of Rb, Akt, and E-Cadherin in UCSs
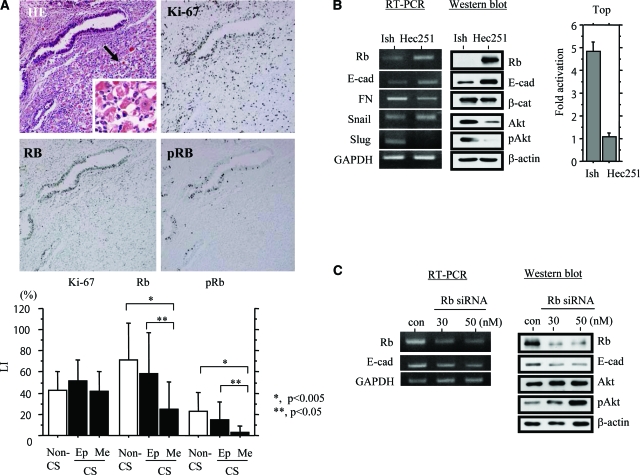
Knowing that Rb plays an important role in maintenance of epithelial phenotype in cells,25 we first examined whether alteration in its expression is associated with genesis of UCSs. As shown in Figure 1A, immunoreactivity for both Rb and its phosphorylated form (pRb) was significantly higher in epithelial than mesenchymal components, independent of cell proliferation determined by Ki-67 immunohistochemistry, suggesting an association between loss of expression and mesenchymal features within UCSs. To verify this hypothesis, we further investigated Hec251 and Ishikawa cells because the former has considerable Rb expression, in contrast to the extremely low levels in the latter.26 As shown in Figure 1B, the Rb status appeared to be closely linked with mRNA and protein expression of several EMT-related molecules, including E-cadherin, fibronectin, Slug, and pAkt, as well as activity of Top (β-catenin/TCF4) and κB (p65) reporter constructs (see Supplementary Figure S1 at http://ajp.amjpathol.org). Furthermore, inhibition of Rb by specific siRNAs caused decreased E-cadherin and increased pAkt expression in Hec251 cells (Figure 1C).
Figure 1.
Expression of Rb and pRb in UCSs. A, Top: H&E staining and immunohistochemistry for Rb, pRb, and Ki-67 are illustrated. The mesenchymal lesion indicated by the arrow is magnified in the inset. Bottom: Nuclear LIs for Rb, pRb, and Ki-67 in epithelial (Ep) and mesenchymal (Me) components of uterine CS (carcinosarcoma), as well as non-CS tumors. B, Left and middle: Comparison of expression levels of several EMT-related molecules for mRNA by RT-PCR (left) and protein by Western blot (middle) between Ishikawa (Ish) and Hec251 cells. E-cad, E-cadherin; FN, fibronectin Right: Ishikawa and Hec251 cells were transfected with Top reporter constructs to determine β-catenin-dependent transcriptional activity. Relative activity was determined based on arbitrary light units of luciferase activity normalized to pRL-TK activity. The activities of the reporter plus the effector relative to that of the reporter plus empty vector are shown as means ± SD. C: Analysis of mRNA (at 24 hours after transfection) (left) and total protein (at 48 hours) (right) in Hec251 cells transfected 30 and 50 nmol/L siRNAs for Rb. A siRNA with no homology to the mammalian genome was used as a negative control (con). Original magnifications: ×100 (A, top); ×400 (A, inset).
Akt Regulates Expression of E-Cadherin and Nuclear β-Catenin in UCSs
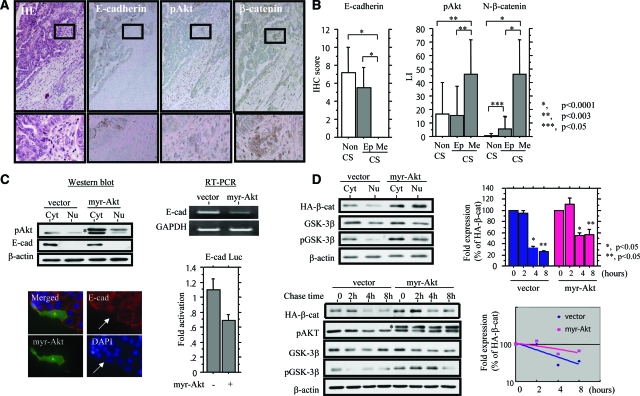
To further show phenotypic characteristics of epithelial and mesenchymal components of UCSs, we conducted immunohistochemistry for pAkt, E-cadherin, and β-catenin, as well as pp65, p53, cytokeratin, and vimentin. Examples of the findings are illustrated in Figure 2A and Supplementary Figure S2 (see http://ajp.amjpathol.org). As shown in Figure 2B, E-cadherin expression was completely lacking in mesenchymal elements, in contrast to moderate levels in epithelial cells, the difference in the scores being significant. Average pAkt and nuclear β-catenin LIs also showed significant differences between the two components. The pAkt LIs and E-cadherin scores, as well as pp65 LIs, did not show any differences between epithelial parts of UCSs and non-UCS tumors, with the exception of nuclear β-catenin LIs, despite a low frequency (7.1%) of mutations in exon 3 of the gene (see Supplementary Table S1 and Supplementary Figure S3 at http://ajp.amjpathol.org). Significant differences in both cytokeratin and vimentin scores were also observed between the components. A similar tendency was also observed in a case of pp65 LIs, but the difference did not reach significance (P = 0.05), and similar values were found for p53 LIs (see Supplementary Figure S2 at http://ajp.amjpathol.org). E-cadherin scores were inversely correlated with LIs of pAkt and nuclear β-catenin, whereas nuclear β-catenin LIs were positively correlated with LIs of pAkt. Moreover, both pAkt and nuclear β-catenin LIs showed negative and positive correlations with cytokeratin and vimentin scores, respectively, whereas Rb and pRb status were positively correlated with E-cadherin scores only. In addition, LIs of pp65, as well as p53, did not show correlations with any of the markers investigated, with the exception of nuclear β-catenin (Table 2 and see Supplementary Table S2 at http://ajp.amjpathol.org).
Figure 2.
Akt regulates expression of E-cadherin and β-catenin. A: Serial sections through a UCS. H&E and immunohistochemistry for E-cadherin, pAkt, and β-catenin are illustrated. The lesions enclosed by boxes in the top panels are magnified in the bottom panels. B: Immunohistochemical (IHC) scores for E-cadherin (left) and LIs for pAkt and nuclear (N) β-catenin (right) in epithelial (Ep) and mesenchymal (Me) components of uterine CS (carcinosarcoma), as well as non-CS tumors. C, Top left: Western blot analysis of E-cadherin (E-cad) was performed with subcellular protein fractions (Cyt, cytoplasmic; Nu, nuclear) extracted from myr-Akt-transfected Hec251 cells. In the pAkt panel, upper bands (indicated by the asterisk) are exogenous myr-Akt, in contrast to the lower bands demonstrating endogenous pAkt. Bottom left: After transfection of myr-Akt into Hec251 cells, staining for pAkt and E-cadherin was performed. Note the lack of E-cadherin expression in cell overexpressing myr-Akt (indicated by arrows). Top right: Analysis of E-cadherin (E-cad) mRNA levels by RT-PCR with total RNA extracted from myr-Akt-transfected Hec251 cells. Bottom right: Hec251 cells were transfected with reporter constructs containing E-cadherin promoter (E-cad Luc), together with expression plasmid for myr-Akt. Relative activity was determined based on arbitrary light units of luciferase activity normalized to pRL-TK activity. The activities of the reporter plus the effector relative to that of the reporter plus empty vector are shown as means ± SD. D, Top left: Western blot analysis of HA-β-catenin, total GSK-3β, and phospho-GSK-3β (pGSK-3β) was performed with subcellular protein fractions (Cyt, cytoplasmic; Nu, nuclear) extracted from myr-Akt-transfected Ish-bcat mo.#25 cells. Bottom left: After transfection of myr-Akt, Ish-bcat#25 cells were treated with cycloheximide (25 μg/ml) for the times shown. Western blotting was performed using anti-HA, anti-pAkt, anti-GSK-3β, and anti-pGSK-3β antibodies. In the pAkt panel, upper bands (indicated by the asterisk) are exogenous myr-Akt, in contrast to lower bands demonstrating endogenous pAkt. Top and bottom right: Relative amounts of HA-β-catenin were calculated by normalization to the signals for β-actin, using NIH Image. Expression levels of HA-β-catenin observed in myr-Akt-transfected cells in the absence of cycloheximide treatment (0 hours) were set as 100%. The experiment was performed in triplicate. Original magnifications: ×100 (A, top); ×400 (A, bottom).
Table 2.
Correlations Among Immunohistochemical Markers Investigated in UCSs
| pAkt ρ* (P) | N-β-cat ρ (P) | pp65 ρ (P) | E-cad ρ (P) | Snail ρ (P) | Slug ρ (P) | Rb ρ (P) | pRb ρ (P) | |
|---|---|---|---|---|---|---|---|---|
| N-β-cat | 0.85 (<0.01) | * | ||||||
| pp65 | 0.2 (0.29) | 0.44 (0.02) | * | |||||
| E-cad | −0.57 (0.003) | −0.68 (0.0004) | −0.21 (0.28) | * | ||||
| Snail | −0.04 (0.82) | 0.15 (0.43) | 0.26 (0.17) | 0.02 (0.9) | * | |||
| Slug | 0.52 (0.007) | 0.75 (<0.0001) | 0.3 (0.08) | −0.61 (0.002) | 0.1 (0.56) | * | ||
| Rb | −0.16 (0.41) | −0.1 (0.59) | −0.05 (0.8) | 0.45 (0.03) | 0.1 (0.57) | −0.3 (0.12) | * | |
| pRb | 0.03 (0.89) | −0.04 (0.83) | −0.19 (0.33) | 0.42 (0.03) | 0.03 (0.88) | −0.2 (0.26) | 0.869 (<.0001) | * |
| Ki-67 | −0.15 (0.44) | −0.24 (0.22) | −0.36 (0.004) | 0.3 (0.12) | 0.11 (0.98) | −0.19 (0.32) | 0.06 (0.76) | 0.06 (0.74) |
ρ, Spearman correlation coefficient.
Labeling indices, pAKT, N-β-catenin (nuclear β-catenin), pp65, Snail, Slug, pRb, ppRb, JHC score, E-cad (E-cadherin), Ki-67.
Based on the above findings and the previous reports that Akt acts as an upstream regulator for several signal pathways,16,17,18,19 we next examined whether Akt is involved in regulation of E-cadherin and β-catenin, as well as p65, using Hec251 and Ish-HA-bcat#25 cells. Transient transfection of a constitutively active form, myristylated Akt (myr-Akt), resulted in a decrease in endogenous E-cadherin expression at both mRNA and protein levels, in line with repression of promoter activity in Hec251 cells (Figure 2C). In Ish-HA-bcat#25 cells, overexpression also caused an increase in both cytoplasmic and nuclear HA-β-catenin levels, along with an increase in phosphorylated GSK-3β (Figure 2D), despite no changes in the endogenous mRNA levels and the promoter activity (see Supplementary Figure S4, A and B, at http://ajp.amjpathol.org). A cycloheximide charge study revealed the half-life of HA-β-catenin protein to be significantly increased in myr-Akt transfected cells as compared with the mock-transfected case (Figure 2D), in line with the finding of considerable decrease in phosphorylated β-catenin in myr-Akt-transfected Hec251 cells (see Supplementary Figure S4C at http://ajp.amjpathol.org). Furthermore, the transfection also caused increase in nuclear p65 levels, as well as inhibition of IκBα expression, resulting in activation of κB-dependent transcription in Hec251 cells (see Supplementary Figure S5A at http://ajp.amjpathol.org).
Up-Regulation of Slug by Nuclear β-Catenin
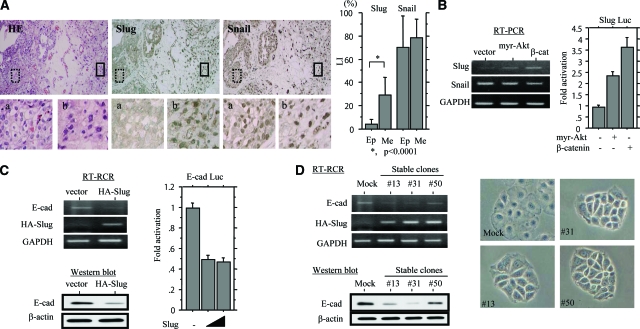
Because transcription of E-cadherin gene is regulated, to a large extent, by Snail/Slug family members that bind to the E-box in the proximal promoter,12,13 we examined whether their expressions are required for development of UCSs. Distinct nuclear Slug staining was predominantly found in mesenchymal rather than epithelial components, the difference in the LIs being significant, in contrast to diffuse Snail immunoreactivity within tumors (Figure 3A). As shown in Table 2, Slug LIs were positively correlated with LIs of pAkt and nuclear β-catenin but not pp65, and inversely with E-cadherin scores. However, such associations were not observed in a case of Snail LIs.
Figure 3.
Up-regulation of Slug in UCSs. A, Left: Serial sections through a UCS. H&E staining and immunohistochemistry for Slug and Snail are illustrated. Boxes with dotted and solid outlines enclose epithelial and mesenchymal lesions magnified in a and b, respectively. Right: Nuclear LIs for Slug and Snail in epithelial (Ep) and mesenchymal (Me) components of UCSs. B, Left: Analysis of mRNA levels for Slug and Snail by RT-PCR with total RNA extracted from Hec251 cells transfected with myr-Akt and β-catenin. Right: Hec251 cells were transfected with reporter constructs containing 1.89 kbp of the Slug promoter (Slug Luc), together with expression plasmids for myr-Akt and β-catenin. Relative activity was determined based on arbitrary light units of luciferase activity normalized to pRL-TK activity. The activities of the reporter plus the effector relative to that of the reporter plus empty vector are shown as means ± SD. C, Left: Analysis of E-cadherin (E-cad) mRNA by RT-PCR (top) and protein by Western blot (bottom) in HA-Slug-transfected Hec251 cells. Detection of HA-Slug mRNA was performed using a combination of 5′-HA forward (5′-TACCCATACGATGTTCCAGATTACGC-3′) and Slug mRNA reverse (Table 1) primers. Right: Hec251 cells were transfected with reporter constructs containing the E-cadherin promoter (E-cad Luc), together with a Slug expression plasmid. D: Hec251 cells stably overexpressing HA-Slug. Left: Analysis of E-cadherin expression levels for mRNA by RT-PCR (top) and protein by Western blot (bottom) in three independent stable cell lines (nos. 13, 31, and 50). Right: The subtle changes in the morphology in the stable cell lines. Original magnifications: ×100 (A, top); ×400 (A, bottom).
Transient transfection of myr-Akt and β-catenin, as well as p65, resulted in considerable increase in mRNA levels for Slug but not Snail, in line with activation of the promoter in Hec251 cells (Figure 3B and see Supplementary Figure S5B at http://ajp.amjpathol.org). However, Akt-mediated up-regulation of Slug expression was not abrogated by inhibition of either β-catenin or p65, respectively, using specific siRNAs (see Supplementary Figure S5C at http://ajp.amjpathol.org), again indicating posttranslational regulation of β-catenin and p65 by myr-Akt. Moreover, overexpression of HA-Slug caused a considerable decrease in E-cadherin at both mRNA and protein levels, along with repression of promoter activity (Figure 3C), although Hec251 cells stably overexpressing exogenous Slug showed subtle changes in morphology, preserving cell-cell adhesion (Figure 3D).
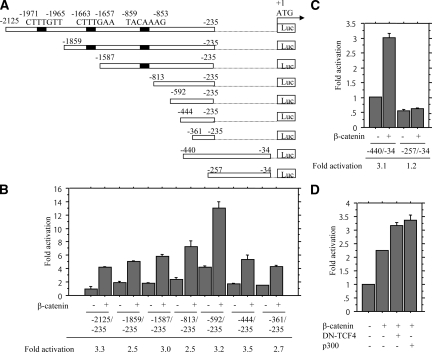
Searching of an ∼2-kbp fragment upstream of the translation start site in the Slug gene (AF084243) revealed three potential TCF-binding sites (CTTTG T/A T/A), but a lack of putative NF-κB-binding elements (GGRNNYYCC) (Figure 4A). With the series of 5′-truncated promoter constructs, deletion from −2125 to −361 bp had little effect on induction of promoter activity by β-catenin (Figure 4B). Whereas the −440/−34-bp and −361/−235 fragments responded very similarly to these activators, −257/−34 bp did so to only a very low levels (Figure 4C), indicating that sequences involved in the response for β-catenin are present between −361 to −257 bp. However, β-catenin-mediated transactivation of the Slug gene was not affected by co-transfection of either dominant-negative TCF4 or transcriptional co-activator p300 (Figure 4D), in contrast to significant inhibition of the transcription activity by the former as determined with Top and Fop reporter constructs (see Supplementary Figure S5D at http://ajp.amjpathol.org). Similar findings were also observed on transfection of p65, and activation of −257/−34-bp fragment was intermediate (see Supplementary Figure S5E at http://ajp.amjpathol.org), suggesting that more than one sequence contribute to the stimulation.
Figure 4.
Transactivation of Slug promoter by β-catenin. A: The various promoter deletion constructs used for evaluating transcriptional regulation of the Slug promoter. Numbers for each construct corresponding to the 5′- and 3′-bp locations within the promoter (relative to the translation start site as +1). B and C: Hec251 cells were transfected with 5′ various deletion constructs of Slug promoter, together with the β-catenin expression plasmid. Relative activity was determined based on arbitrary light units of luciferase activity normalized to pRL-TK activity. The activities of the reporter plus the effector relative to that of the reporter plus empty vector are shown as means ± SD. D: Hec251 cells were transfected with reporter constructs containing 1.89 kbp of the Slug promoter, together with expression plasmids for β-catenin, dominant-negative (DN)-TCF4, and p300.
Discussion
The present study demonstrated that most UCSs in our series showed not only co-expression of cytokeratin and vimentin, but also identical p53 staining patterns in epithelial and mesenchymal components, supporting the idea that carcinosarcomas are best regarded as metaplastic carcinomas.1 The complete shut-down of E-cadherin expression in mesenchymal elements is in line with evidence that cells undergoing EMT down-regulate E-cadherin,11 suggesting that molecular events responsible for triggering EMT may occur during development of this type of tumor.
Akt is considered to be a key mediator for EMT induction in human epithelial malignancies.27,28 In the present study, the expression was up-regulated in mesenchymal components, exhibiting an inverse correlation with E-cadherin. Although transfection of myr-Akt caused transcriptional repression of the E-cadherin gene, it is probable that this is effected in cooperation with other transcription factors, because Akt can phosphorylate many substrates but does not directly regulate gene transcription.19,29 It is well accepted that Akt can inactivate GSK-3β through phosphorylation at Ser9, resulting in stabilization of β-catenin and activation of β-catenin-dependent transcription.30,31 Phosphorylation of β-catenin at Ser552 by Akt also facilitates β-catenin dissociation from cell-cell contacts and accumulation in nuclei but does not alter the protein stability.32 In our study, overexpression of myr-Akt could induce stabilization of cytoplasmic and nuclear β-catenin through phosphorylation of GSK-3β, in line with the in vivo finding of a very strong correlation (ρ = 0.85, P < 0.0001), suggesting that β-catenin is involved in the Akt downstream pathway. In addition, although induction of myr-Akt was also found to activate p65-dependent transcription, probably through repression of IκBα expression,33 we failed to confirm such an association in tumor tissues, suggesting that the in vivo pp65 status may be affected not only by pAkt but also by other factors, such as transforming growth factor-β and tumor necrosis factor-α.34,35
In our results, expression of Slug, rather than Snail, appeared to be closely associated with the mesenchymal phenotype in UCSs, despite both being capable of repressing E-cadherin expression.12,13 A similar finding has also been reported for basal-like breast carcinomas.36 Interestingly, stable overexpression of exogenous Slug inhibited E-cadherin expression at transcriptional levels, although changes in cell morphology were relatively minor. We therefore conclude from the present data that transactivation of the Slug gene may be essential in cells that have lost epithelial characteristics in UCSs, acting as an in vivo transcriptional repressor of the E-cadherin gene. Because cell-cell contacts were still preserved in the stable cell lines, it appears that a critical threshold for E-cadherin must be crossed for complete loss function. Further studies of this point are clearly warranted.
An important finding here is that Slug expression is under transcriptional control of nuclear β-catenin, from the following evidence: i) the very significant correlation (ρ = 0.75, P < 0.0001) between the two in tumor tissues; ii) up-regulation of Slug expression at both mRNA and protein levels induced by transfection of β-catenin in cell lines; iii) activation of the Slug promoter mediated by β-catenin, through the proximal region (−361 to −257 bp). Although most β-catenin targeted genes require TCF/LEF factors for their activation,37,38,39 the observed activation did not require binding sites in the promoter, as evidenced by the failure of deletion or dominant-negative TCF4, in contrast to Xenopus and mouse Slug promoters activated by β-catenin/TCF complexes through the binding sites.40,41 In addition, evidence has been presented that an increase in the cytoplasmic pool of β-catenin occurs after release from interaction with E-cadherin,42,43,44 leading us to speculate on existence of a positive feedback loop of nuclear β-catenin mediated by Slug and E-cadherin. In contrast, p65 might also induce transactivation of the Slug promoter, but such an association was not evident in tumor tissues, suggesting that a signal pathway involving β-catenin rather than p65 may be the primary mechanism underlying regulation of in vivo Slug transcription in UCSs.
There is a rapidly growing body of evidence that Rb plays key roles not only in cell cycle progression but also maintenance of the epithelial phenotype by preventing EMT.18,19,25 For example, Rb binds to an E-cadherin promoter sequence in association with the transcription factor activator protein 2α, suggesting that Rb plays a key role in the EMT/MET (mesenchymal-epithelial transition) switch through regulation of E-cadherin and other EMT-related molecules.25 In agreement with this notion, we demonstrated that expression of Rb, as well as pRb, was decreased in mesenchymal components of UCSs, independent of cell proliferative activity. Further support was provided by the in vitro finding that the knockdown was because of changes in expression of several EMT-related molecules, including Akt and E-cadherin.
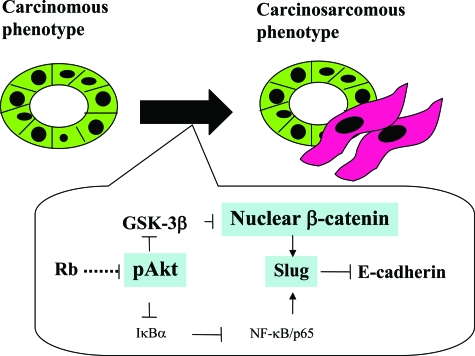
Together, our observations suggest a model for molecular mechanisms determining phenotypic characteristics of UCSs (Figure 5). In most tumors, constitutive expression of Rb may be required for preserving the epithelial phenotype of the tumor cells, but decreased expression is responsible for activation of the Akt/β-catenin pathway, and this in turn leads to down-regulation of E-cadherin through transactivation of the Slug gene, and consequent changes in morphology toward a mesenchymal phenotype. Cooperation with the NF-κB pathway may be a minor event during the process.
Figure 5.
Schematic representation of possible roles of the Akt/β-catenin pathway in determination of phenotypic characteristics of UCS.
In conclusion, the present study provided evidence that the Akt/β-catenin pathway, as well as alterations in Rb expression, may be essential for establishment and maintenance of phenotypic characteristics in UCSs, playing key roles in regulation of E-cadherin through transactivation of the Slug gene.
Supplementary Material
Acknowledgments
We thank Dr. Roderick H. Dashwood for the generous gift of the pGL-HBCP reporter plasmid used in this study.
Footnotes
Address reprint requests to Makoto Saegusa, M.D., Department of Pathology, Kitasato University School of Medicine, 1-15-1 Kitasato, Sagamihara, Kanagawa 228-8555, Japan. E-mail: msaegusa@med.kitasato-u.ac.jp.
Supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant 20590352).
Supplementary material for this article can be found on http://ajp.amjpathol.org.
References
- McCluggage WG. Malignant biphasic uterine tumors: carcinosarcomas or metaplastic carcinomas? J Clin Pathol. 2002;55:321–325. doi: 10.1136/jcp.55.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Yoshida M, Gong ZX, Matsumoto T, Hamano Y, Fukunaga M, Hruban RH, Gabrielson E, Shirai T. Frequent genetic heterogeneity in the clonal evolution of gynecological carcinosarcoma and its influence on phenotypic diversity. Cancer Res. 2000;60:114–120. [PubMed] [Google Scholar]
- Matsumoto T, Fujii H, Arakawa A, Yamasaki S, Sonoue H, Hattori K, Kajiyama Y, Hirose S, Tsurumaru M. Loss of heterozygosity analysis shows monoclonal evolution with frequent genetic progression and divergence in esophageal carcinosarcoma. Hum Pathol. 2004;35:322–327. doi: 10.1016/j.humpath.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Christiansen JJ, Rajasekara AK. Reassessing epithelia to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Tarin D. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. [DOI] [PubMed] [Google Scholar]
- Sarrió D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2006;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Hajra KM, Chen DY, Fearon ER. The Slug zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh C-M, Maemura K, Layne MD, Yet S-F, Lee K-H, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee M-E. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Ruiz S, Santos M, Lara MF, Segrelles C, Ballestin C, Paramio JM. Unexpected roles for pRb in mouse skin carcinogenesis. Cancer Res. 2005;65:9678–9686. doi: 10.1158/0008-5472.CAN-05-1853. [DOI] [PubMed] [Google Scholar]
- Menges CW, Baglia LA, Lapoint R, McCance DJ. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res. 2006;66:5555–5559. doi: 10.1158/0008-5472.CAN-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, Garcia de Herreros A, Bellacosa A, Larue L. Activation of NF-κB by Akt upregulates Snail expression and induces epithelial mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. β-Catenin simultaneously induces activation of the p53–p21WAF1 pathway and overexpression of cyclin D1 during squamous differentiation of endometrial carcinoma cells. Am J Pathol. 2004;164:1739–1749. doi: 10.1016/s0002-9440(10)63732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Upregulation of TCF4 expression as a transcriptional target of β-catenin/p300 complexes during trans-differentiation of endometrial carcinoma cells. Lab Invest. 2005;85:768–779. doi: 10.1038/labinvest.3700273. [DOI] [PubMed] [Google Scholar]
- Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Crosstalk between NF-κB/p65 and β-catenin/TCF4/p300 signaling pathway through alterations in GSK-3β expression during trans-differentiation of endometrial carcinoma cells. J Pathol. 2007;213:35–45. doi: 10.1002/path.2198. [DOI] [PubMed] [Google Scholar]
- Nishida M. Ishikawa cells: opening of in vitro hormone research on endometrial carcinoma. Kuramoto H, Nishida M, editors. Tokyo: Springer-Verlag,; Cell and Molecular Biology of Endometrial Carcinoma. 2003:pp 35–60. [Google Scholar]
- Kuramoto H, Hamano M, Imai M, Fujisawa T, Kamata Y, Arai T, Kawaguchi M. Hec-1 cells: establishment of an in vitro experimental system in endometrial carcinoma. Kuramoto H, Nishida M, editors. Springer-Verlag,; Cell and Molecular Biology of Endometrial Carcinoma. 2003:pp 3–34. [Google Scholar]
- Arima Y, Inoue Y, Shibata T, Hayashi H, Nagano O, Saya H, Taya Y. Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-mesenchymal transition. Cancer Res. 2008;68:5104–5112. doi: 10.1158/0008-5472.CAN-07-5680. [DOI] [PubMed] [Google Scholar]
- Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Induction of p16INK4A mediated by β-catenin in a TCF4-independent manner: implication for alterations in p16INK4A and pRb expression during trans-differentiation of endometrial carcinoma cells. Int J Cancer. 2006;119:2294–2303. doi: 10.1002/ijc.22112. [DOI] [PubMed] [Google Scholar]
- Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A, Testa JR, Moore R, Larue L. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol Ther. 2004;3:268–275. doi: 10.4161/cbt.3.3.703. [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. Signal transduction: signaling specificity—a complex affair. Science. 2001;292:2439–2440. doi: 10.1126/science.1063279. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumor necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Criswell TL, Arteaga CL. Modulation of NF-κB activity and E-cadherin by the type III transforming growth factor Β receptor regulates cell growth and motility. J Biol Chem. 2007;282:32491–32500. doi: 10.1074/jbc.M704434200. [DOI] [PubMed] [Google Scholar]
- Storci G, Sansone P, Trere D, Tavolari S, Taffurelli M, Ceccarelli C, Guarnieri T, Paterini P, Pariali M, Montanaro L, Santini D, Chieco P, Bonafe M. The basal-like breast carcinoma phenotype is regulated by Slug gene expression. J Pathol. 2007;214:25–37. doi: 10.1002/path.2254. [DOI] [PubMed] [Google Scholar]
- Roose J, Clevers H. TCF transcription factors: molecular switches in carcinogenesis. Biochem Biophys Acta. 1999;1424:M23–M37. doi: 10.1016/s0304-419x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Damals A, Kahan S, Shtutman M, Ben-Ze'ev A, Oren M. Deregulated β-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 2001;20:4912–4922. doi: 10.1093/emboj/20.17.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F. Cloning and characterization of three Xenopus Slug promoters reveal direct regulation by Lef/β-catenin signaling. J Biol Chem. 2001;276:30350–30358. doi: 10.1074/jbc.M103167200. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of β-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutation in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.