Abstract
In this study, we investigated the role of interleukin (IL)-1 signaling in wound healing. IL-1 receptor type I (IL-1R) knockout (KO) mice showed reduced fibrosis in both cutaneous and deep tissue wounds, which was accompanied by a reduction in inflammatory cellular infiltration in cutaneous but not in deep tissue wounds. There were no differences in either total collagenolytic activity or in the expression of selected matrix metalloproteinases or tissue inhibitors of metalloproteinases between the wound fluids from wild-type or IL-1R KO mice. However, wound fluids from IL-1R KO mice contained lower levels of IL-6 compared with wild-type controls. In addition, the infusion of IL-6 into wounds in IL-1R KO mice did not increase fibrosis. Skin wounds in IL-1R KO animals had lower levels of collagen and improved restoration of normal skin architecture compared with skin wounds in wild-type mice. However, neither the tensile strength of incisional skin wounds nor the rate of closure of excisional wounds differed between IL-1R KO and wild-type animals. The reduced fibrotic response in wounds from IL-1R KO mice could be reproduced by the administration of an IL-1R antagonist. These findings suggest that pharmacological interference with IL-1 signaling could have therapeutic value in the prevention of hypertrophic scarring and in the treatment of fibrotic diseases.
Progress in the therapeutic management of abnormal wound healing has fallen short of expectations. The promise of molecular medicine to normalize impaired healing, as seen in diabetes, vascular insufficiency, or other chronic diseases, through the use of exogenous cytokines or growth factors has not been realized. At the other end of the abnormal wound healing spectrum, no reliable prophylactic or therapeutic measures exist to address the pathologies of excessive repair, exemplified by hypertrophic burn scars, keloids, and stenosing gastrointestinal or vascular anastomoses. The availability of effective therapies that allow for the modulation of the wound healing response would be of substantial clinical relevance. Recent reports demonstrate a markedly reduced cellular inflammatory response in models of sterile inflammation1,2,3,4 and decreased scarring after experimental myocardial infarction in mice deficient in the interleukin (IL)-1 receptor type I (IL-1R).4
The present studies tested the hypothesis that genetic or pharmacological interference with IL-1 signaling would modulate the inflammatory response in skin and deep tissue wounds and reduce scar formation. Results using IL-1R knockout (KO) mice demonstrated that signaling through the IL-1R is required for the constitution of a normal cellular inflammatory response in cutaneous but not in deep tissue wounds. Most importantly, the quality of wound healing was different in IL-1R KOs, with cutaneous wounds in these animals attaining better restoration of normal skin architecture and a marked reduction in fibrosis without compromise in tensile strength. Additionally, deep tissue wounds in IL-1R KO mice showed a substantial reduction in collagen content, an observation that was reproduced by the administration of a human recombinant IL-1 R antagonist.
Findings demonstrate a role for the IL-1/IL-1R axis in the regulation of wound healing. They suggest that interference with IL-1 signaling through the use of an IL-1R antagonist may find a clinical application in the prevention of excessive or hypertrophic scar formation.
Materials and Methods
Animals
Male interleukin-1 receptor type I KO (B6.129S7-Il1r1tm1Imx/J) and C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6D2F1 male mice were purchased from Taconic (Germantown, NY) and were used in Anakinra infusion experiments. All animals were housed in the Central Research Facilities at Rhode Island Hospital, and fed mouse chow and water ad libitum. Mice were certified free of common pathogens by the suppliers and were monitored by Brown University/Rhode Island Hospital veterinary personnel. Animal protocols were approved by the Rhode Island Hospital Institutional Animal Care and Use Committee. At the time of experimentation, mice were 8 to 12 weeks of age, weighing ∼27 to 30 grams.
Incisional Skin Wounds
Mice were anesthetized with pentobarbital (50 mg/kg i.p.; Abbott Laboratories, North Chicago, IL) and their backs shaved. Under aseptic surgical conditions, a dorsal midline incision extending from the scapulae to the base of the tail was created with a scalpel and closed with clips, which were removed on day 7 after wounding. Animals were euthanized by CO2 asphyxiation 14 days later. Their pelts, including the wound, were affixed to a tissue holder retaining original size and shape, fixed in 10% buffered formalin, and processed for histology.
Excisional Skin Wounds
Mice were anesthetized with pentobarbital and their backs shaved. Under aseptic conditions, the dorsal skin was elevated and a 6-mm-diameter punch was used to create bilateral wounds that extended through the panniculus carnosus, 1 cm on either side of the lumbar midline. Digital photographs were taken at 3-day intervals on a standardized image stage. Wound surface area was measured using ImageJ software (National Institutes of Health, Bethesda, MD).5 At 14 days, the animals were euthanized, and their pelts fixed in buffered formalin. After 2 days of fixation, a 1-cm-diameter punch was used to remove each healed wound from the pelt and the tissue was processed for histology.
Deep Tissue Wounds
Sterile polyvinyl alcohol sponges (PVAs) were implanted subcutaneously as described previously.6 Briefly, six sponges (PVA Unlimited, Warsaw, IN) were aseptically implanted into individual subcutaneous pockets created through a dorsal midline incision in pentobarbital-anesthetized animals. Wound cells and cell-free wound fluids were recovered from the sponges as published from this laboratory.7 Differential wound cell counts were performed on cytocentrifuge preparations stained with Hema-3 (Fisher Scientific, Kalamazoo, MI). Additional sponges were retrieved and processed for histological examination 5, 10, or 14 days after implantation.
Sterile osmotic pumps (0.2 ml, Alzet model 2002; Durect Corp., Cupertino, CA) were fitted with a polypropylene mesh collar containing a PVA sponge and inserted subcutaneously into B6D2F1 mice (see Supplemental Figure S1 at http://ajp.amjpathol.org). The pumps contained either hrIL-1R antagonist (Anakinra, Kineret, 200 mg/ml; Amgen, Thousand Oaks, CA) or carrier solution [10 mmol/L sodium citrate, 140 mmol/L sodium chloride, 0.5 mmol/L EDTA, and 0.1% (w/w) Tween 80, pH 6.5]. Anakinra was delivered into the sponges at 5 mg/kg/hour for 14 days. Animals were euthanized at that time, and the sponges harvested and processed for histology.
Murine rIL-6 was either delivered to PVA sponges at 0.1 or 1 μg/sponge before implantation into IL-1R KO animals, or continuously infused into wounds in these animals using osmotic pumps (0.1 ml, Alzet model 1003D; Durect Corp.). The pumps were fitted with a 2-cm polypropylene catheter attached to a PVA sponge under sterile conditions and primed overnight in phosphate-buffered saline (PBS) at 37°C before subcutaneous implantation under anesthesia. The pumps contained either mrIL-6 (100 μg/ml; R&D Systems, Minneapolis, MN) or sterile PBS (Gibco, Invitrogen, Carlsbad, CA). IL-6-treated animals received the cytokine at 100 ng/hour for 3 days. All animals were euthanized 14 days later, and the sponges removed, fixed, and processed for histology.
Tissue Processing and Histomorphometric Analysis
After 24 to 48 hours of fixation in 10% buffered formalin, tissue and sponges were dehydrated, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E), trichrome, or sirius red. Samples were obtained from two separate regions of each wound, and care was taken to ensure uniformity of cross-sectional orientation for both incisional and excisional wounds. Multiple sections of each wound were examined by a pathologist blinded to the experimental groups and analyzed using quantitative histomorphometric techniques on digital images taken with a MicroPublisher 3.3 RTV camera (Q Imaging, Surrey, Canada). Analytical software included Image Pro-Plus version 5.1 (Media Cybernetics, Bethesda, MD) and ImageJ.
Mechanical Testing of Skin Wounds
Incisional wounds were inflicted as described above. Animals were euthanized 14 days after wounding and their pelts removed. A dog-bone shaped punch measuring 5.2 cm in length and 0.5 cm wide at the narrowest point was used to cut samples of normal and wounded skin for mechanical testing.8 Tensile strength measurements were conducted on fresh, unfixed tissue, using a Minimat 2000 miniature tensile device (Rheometric Scientific Inc., Piscataway, NJ) by stretching at 5 mm/minute until rupture.
Collagen Zymography and Matrix Metalloproteinases (MMP)/Tissue Inhibitors of Metalloproteinase (TIMP) Detection by Immunoblotting
Wound fluids were recovered from wild-type or IL-1R KO mice at the times indicated. Fluids from three animals at each time point were pooled and used for collagen zymography and immunoblotting. Collagen zymography was performed as described in Gogly and colleagues.9 Briefly, wound fluids were dispensed onto 7.5% sodium dodecyl sulfate acrylamide gels containing rat tail collagen type I (BD Biosciences, San Jose, CA) and electrophoresed using Tris-glycine running buffer. The gels were then exposed to renaturing buffer (Triton X-100, 25%, v:v in water) for 30 minutes at room temperature. After decanting of the renaturing buffer, gels were incubated overnight in developing buffer (Tris, 50 mmol/L; NaCl, 0.2 mol/L, CaCl2, 5 mmol/L, Brij 35, 0.02%) and then stained with 0.25% Coomassie blue in methanol (40%)/acetic acid (10%) for 30 minutes. Collagenolytic activity was evidenced by clear (unstained) bands.
For immunodetection of MMPs and TIMPS, wound fluids were size-fractionated in 15% sodium dodecyl sulfate-polyacrylamide gel by electrophoresis, with equal amounts of protein loaded in each lane. Proteins were transferred to a nitrocellulose membrane, incubated in blocking buffer, and probed with the antibody of interest. Appropriate peroxidase-conjugated secondary antibody was added for detection. Antibodies and antibody dilutions used for Western blotting included: MMP-2, rabbit polyclonal, 1:500 (Santa Cruz Biotechnology, Santa Cruz, CA); MMP-3, rabbit monoclonal, 1:1000 (Abcam, Cambridge, MA); MMP-8, rabbit polyclonal, 1:500 (Santa Cruz); MMP-13, rabbit polyclonal, 1:500 (Santa Cruz); TIMP-1 and TIMP-3, mouse monoclonals, 1:500 (Chemicon, Billerica, MA); TIMP-3, rabbit polyclonal, 1:2000 (Abcam); and TIMP-4, rabbit polyclonal, 1:750 (Abcam).
Cytokine Measurement
Wound fluid cytokine concentrations were measured by enzyme-linked immunosorbent assay. IL-1α, IL-1β, IL-6, IL-10, IL-12p70, interferon-β, and transforming growth factor (TGF)-β assay kits were purchased from BD Biosciences. CCL5, CCL17, and vascular endothelial growth factor assay kits were from R&D Systems. A monoclonal hamster anti-mouse tumor necrosis factor (TNF)-α antibody (BD Biosciences) and a polyclonal rabbit anti-mouse TNF-α antibody (Pierce Biotechnologies, Rockford, IL) were used for TNF-α enzyme-linked immunosorbent assay.
Wound cells taken from animals 1 day after injury were incubated (106 cells/ml) in RPMI (BD Biosciences) with 1% fetal bovine serum and 100 U/ml penicillin-streptomycin for 6 hours in the presence of Brefeldin A (1 μl/106 cells, Golgiplug; BD Biosciences). Cells were stained for intracellular TNF-α, using an anti-CD68 antibody for the identification of wound neutrophils and macrophages, as described previously.7,10 Cells were analyzed using a Becton-Dickinson FACSort with CellQuest software (BD Biosciences).
Statistical Analysis
Data shown are means ± SD from a representative of replicate experiments, unless otherwise indicated, with the number (n) of subjects noted. Data were analyzed by Mann Whitney’s U-test or analysis of variance-Holm (sequential Bonferroni), as specified. Wound fluid cytokine results were log-transformed before statistical analysis to account for unequal variance.
Results
IL-1R Deficiency Does Not Alter Cellular Infiltration in Deep Tissue Wounds but Reduces Fibrosis
Previous reports demonstrated reductions in inflammatory cell infiltration in sterile peritonitis and in myocardial infarction in IL-1R KO mice.1,2,4 This was not the case in deep tissue wounds. Results presented in Table 1 demonstrate that the number of inflammatory cells in deep wounds from IL-1R KOs harvested at 1, 5, 10, and 14 days after wounding was the same as that in wild-type animals. Moreover, differential wound cell counts did not differ between groups at any time (not shown), and confirmed the normal temporal progression of the cellular infiltrate of the wound from a neutrophil-predominant phase to one containing mostly macrophages.7
Table 1.
Inflammatory Cellular Infiltration of Deep Tissue Wounds in Wild-Type and IL-1R KO Mice
| Group | Day 1 | Day 5 | Day 10 | Day 14 |
|---|---|---|---|---|
| Wild type | 1.3 ± 0.4 | 3.2 ± 0.7 | 5.9 ± 0.9 | 6.2 ± 1.7 |
| IL-1R KO | 1.3 ± 0.3 | 2.8 ± 0.8 | 5.2 ± 1.1 | 6.2 ± 1.1 |
PVA sponges were implanted into wild-type or IL-1R KO animals. Wound cells were retrieved and counted at days 1, 5, 10, and 14 after injury. Data are mean number of wound cells per animal (×106) ± SD. n = 3 animals per group. The total number of inflammatory cells was not different between groups at any time point tested (analysis if variance).
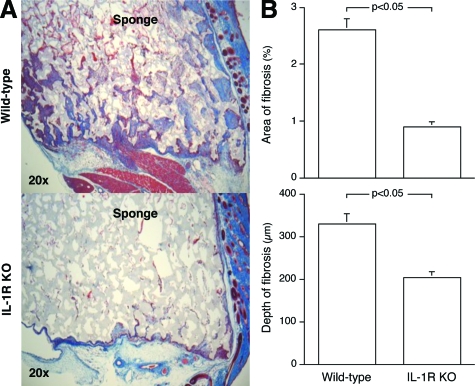
Wild-type and IL-1R KO mice differed markedly in their fibrotic response to the deep tissue wounds (Figure 1A). Wounds in IL-1R-deficient animals had threefold less fibrosis than those in wild-type mice, and the maximal depth of fibrotic invasion into the sponge was reduced by 45% in the IL-1R KOs, 14 days after wounding (Figure 1B). Similar reductions in fibrosis were seen 5 and 10 days after sponge implantation (not shown).
Figure 1.
IL-1 signaling is essential for the fibrotic response seen in deep tissue sterile wounds. PVA sponges were implanted into IL-1R KO or wild-type mice and harvested 14 days later. A: Low-power photomicrographs of trichrome-stained sponge wounds. Sponge material stains lightly, whereas fibrotic tissue appears dark blue. Sirius red staining confirmed that the areas of fibrosis stained by trichrome reflect collagen deposition (not shown). Overlying skin is oriented to the right. B: Quantitative histomorphometric analysis of the sponge wounds, expressed as total area of fibrosis per microscopic field and depth of fibrotic buds. n = 6 animals per group, Mann Whitney’s U-test (P < 0.05).
Collagenolytic Activity and Accumulation of MMPs and TIMPs in Wound Fluids from Wild-Type and IL-1R KO Mice
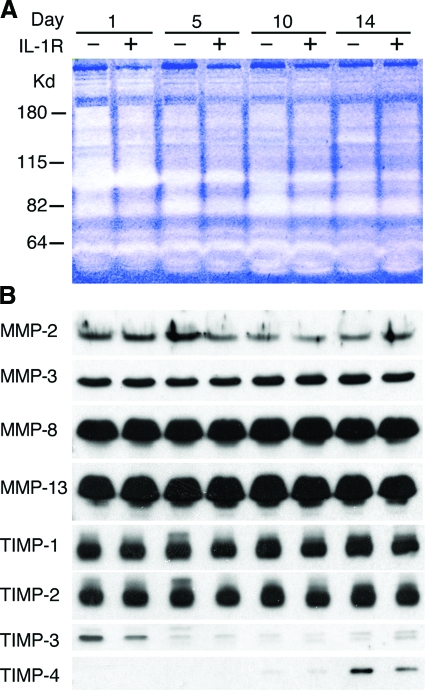
The collagenolytic activity of wound fluids obtained 1 to 14 days after wounding was examined using zymography on rat collagen 1 gels. Figure 2A shows more collagenolytic activity in wound fluids harvested 1 day after wounding than in those retrieved at later times, and no differences between fluids from wild-type and IL-1R KO mice at any time point. The accumulation of selected MMPs and TIMPs 1 to 4 were determined by Western blotting in the same wound fluids used for zymography (Figure 2B). There were no detectable differences between the accumulation of any of the examined proteins in wound fluids from wild-type mice and those from IL-1R KOs. TIMP3 and 4 were found to be segregated in time, with most TIMP-3 detected in fluids harvested in the first postwound day, and TIMP-4 in samples harvested in postwound days 10 and 14.
Figure 2.
Zymographic analysis and immunodetection of MMPs and TIMPs in wound fluids from wild-type and IL-1R KO mice. PVA sponges were implanted into IL-1R KO or wild-type mice and harvested 1 to 14 days later. A: Pooled wound fluids from three animals at each time point were used for zymography using rat collagen type 1 as substrate. B: Immunoblots for selected MMPs and TIMPs were performed using the same wound fluids used in the zymographic analysis.
Cytokine/Chemokine Concentrations in Wound Fluids from Wild-Type and IL-1R KO Animals
The cytokine profile of wound fluids obtained from IL-1R KO and wild-type animals was examined to further define the impact of IL-1R deficiency on the wound environment. As first reported from this laboratory,11 IL-6 was present at its highest concentration in day 1 wound fluids, and rapidly declined thereafter (Table 2). Wound fluids from IL-1R KO animals contained less IL-6 than those from wild-type at all examined time points, and less TGF-β and vascular endothelial growth factor 1 day after wounding. The concentrations of TNF-α, IL-1α, IL-10, IL-12p70, interferon-β, and CCL5 showed no temporal variation and were not different in wild-type and KO mice (not shown).
Table 2.
Cytokine Concentrations in Wound Fluids from Deep Tissue Wounds in Wild-Type and IL-1R KO Animals
| Day 1 | Day 3 | Day 5 | Day 10 | Day 14 | |
|---|---|---|---|---|---|
| IL-6 (ng/ml) | |||||
| wt | 73 ± 9 | 0.7 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 3.8 ± 3.0 |
| KO | 18 ± 6* | 0.2 ± 0.1* | 0.2 ± 0.1* | 0.1 ± 0.1* | 0.1 ± 0.1* |
| TGF-β (pg/ml) | |||||
| wt | 8 ± 0.5 | 9 ± 0.7 | 14 ± 0.5 | 29 ± 2.5 | 33 ± 5.6 |
| KO | 5 ± 0.5* | 9 ± 1.1 | 16 ± 2.2 | 24 ± 2.8 | 33 ± 4.1 |
| VEGF (pg/ml) | |||||
| wt | 137 ± 17 | ND | 237 ± 6 | 444 ± 87 | 289 ± 67 |
| KO | 68 ± 17* | ND | 397 ± 121 | 540 ± 31 | 489 ± 192 |
Wound fluids were recovered from PVA sponges implanted in wild-type (wt) or IL-1R KO mice (n = 3 animals per group per time point). Cytokine concentrations were determined by ELISA. ND = not determined. Results were analyzed by analysis of variance-Holm.
P < 0.05 versus wild-type animals.
IL-6 Replacement Does Not Increase Wound Fibrosis in IL-1R KO Mice
IL-6 has been shown to exert profibrotic effects in wounds (see Discussion). Because of its marked and persistent reduction in wounds in IL-1R KO mice, experiments examined whether the administration of mrIL-6 into the wounds would revert the anti-fibrotic phenotype detected in these animals. Neither the implantation of sponges containing 0.1 or 1 μg of mrIL-6 per sponge, nor the continued infusion of the cytokine for 3 days at 0.1 μg/hour increased wound fibrosis in IL-1R KO animals (control IL-1R KO: area of fibrosis 2.5 ± 0.9%, depth of fibrosis 19.3 ± 8.6% of sponge material; mrIL-6-infused IL-1R KO: area of fibrosis 2.5 ± 0.9%, depth of fibrosis 14.8 ± 3.1% of sponge material, P > 0.05 for both measurements, n = 3 to 4 animals per group).
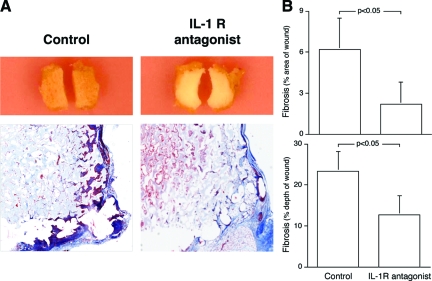
The Delivery of the IL-1R Antagonist Anakinra into Deep Wounds Reproduces the Reduction in Wound Fibrosis Found in IL-1R KO Mice
Sponge wounds were fitted with osmotic pumps delivering Anakinra (5 mg/kg/hour) or carrier solution and implanted into wild-type animals. Gross and microscopic examination of the sponges 14 days after wounding revealed less fibrotic invasion into the sponge material in Anakinra-treated wounds (Figure 3A). Histomorphometric analysis of the wounds demonstrated that the IL-1R antagonist reduced the area and depth of fibrosis by more than twofold over controls (Figure 3B).
Figure 3.
Administration of the IL-1R antagonist Anakinra recapitulates the diminished fibrotic response seen in IL-1R KOs. PVA sponges of equal size and weight were implanted in wild-type animals fitted with osmotic pumps delivering either the IL-1R antagonist (IL-1-Ra) Anakinra (5 mg/kg/hour) or carrier solution. Sponges were harvested at 14 days and processed as described in the Materials and Methods. A: Macro- and trichrome-stained microphotographs of sponges from control or IL-1R antagonist-treated mice. B: Quantitative histomorphometric analysis of the area and depth of fibrosis, expressed as a percentage of total sponge area and depth. n = 5 animals per group. Mann Whitney’s U-test (P < 0.05).
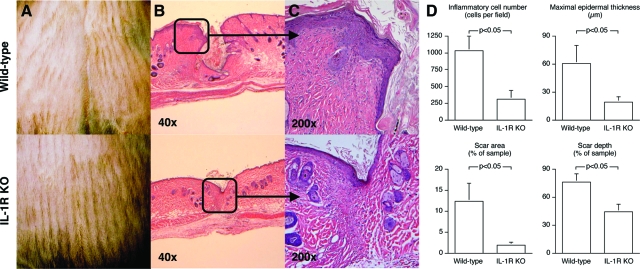
Improved Restoration of Skin Architecture in Wounds from IL-R KO Mice
Figure 4 shows healing of incisional skin wounds in wild-type and IL-1R KO mice. Macroscopic examination of the wounds showed improved wound healing in the IL-1R KOs, with closer approximation and decreased elevation of the wound edges (Figure 4A). Microscopically, wounds from wild-type animals were not fully epithelialized by 14 days after injury, whereas those in IL-1R KO were covered by normal epidermis (Figure 4, B and C). Quantitative histomorphometric analysis of the wounds demonstrated a 70% reduction in inflammatory cell infiltration, diminished epidermal thickening, and decreased area and depth of scarring in samples harvested from IL-1R KO versus wild-type mice (Figure 4D). Overall, wounds in IL-1R KOs showed a more complete and consistent return toward normal skin architecture than those in wild-type animals.
Figure 4.
Influence of IL-1 signaling on the repair of incisional wounds. Cutaneous dorsal midline linear incisional wounds were created in IL-1R KO and wild-type animals. Pelts containing the incision were harvested at 14 days, processed for histology, and stained with H&E. A: Photographs of representative skin wounds. B: Photomicrographs of cross sections through the wound. C: Photomicrographs of the epidermal/dermal junction at the repair site. D: Histomorphometric analysis of healing incisional wounds. n = 6 animals per group. Mann Whitney’s U-test (P < 0.05).
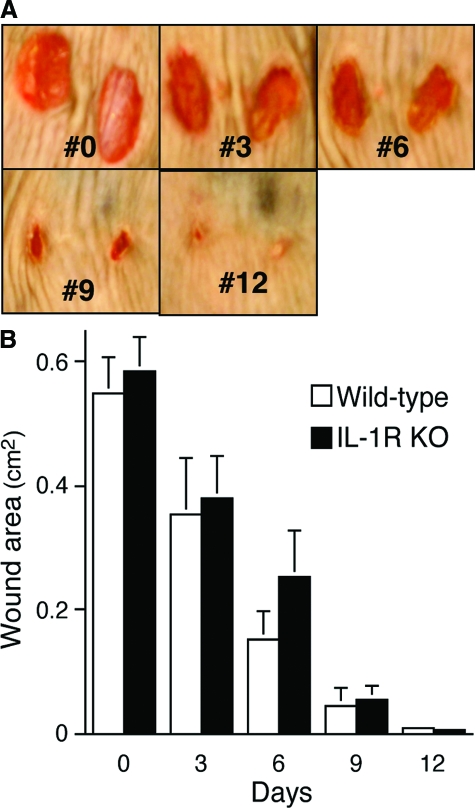
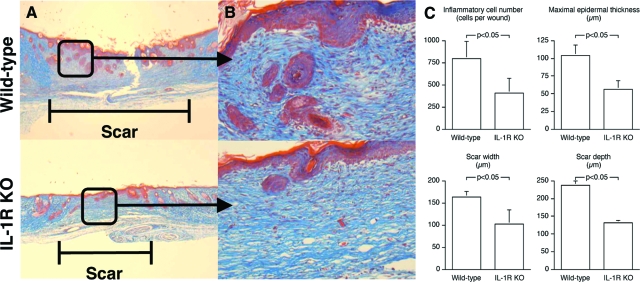
As previously reported by Graves and colleagues,12 excisional skin wounds in wild-type and IL-1R KOs closed at equal rates (Figure 5, A and B). By 14 days, the wounds were completely closed in all animals in both groups. Just as in the incisional wounds, however, there were differences in the quality of wound repair, as healed wounds from IL-1R KOs contained fewer total inflammatory cells, had decreased maximal epidermal thickness, and exhibited reduced width and depth of scar formation (Figure 6, A–C).
Figure 5.
The rate of cutaneous excisional wound closure is independent of the IL-1 signaling pathway. Bilateral dorsal 6-mm cutaneous excisional wounds through the panniculus carnosus were created in IL-1R KO and wild-type animals. A: Digital photographs from a standardized stage were taken at 3-day intervals and total wound area calculated. Shown here are the wounds of one representative wild-type mouse throughout time. B: Rate of wound closure in wild-type and IL-1R KO mice. n = 6 animals per group. There were no differences in the rate of wound closure at any time point (analysis of variance).
Figure 6.
IL-1 signaling affects the quality of repair in excisional wounds. Cutaneous excisional wounds were created in IL-1R KO and wild-type animals. Wounds were harvested at 14 days, after complete closure. A: Photomicrographs of cross sections through the scar. B: Photomicrographs of the epidermal/dermal junction at the healed excisional wound site. C: Histomorphometric analysis of healed excisional wounds. n = 6 animals per group. Mann Whitney’s U-test (P < 0.05).
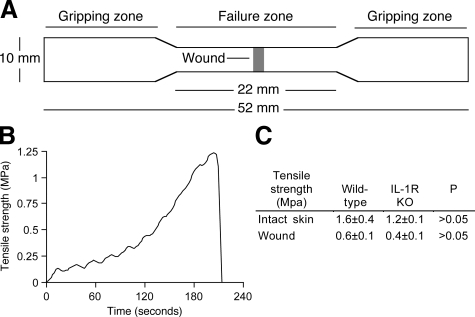
IL-1R Deficiency Does Not Impair the Tensile Strength of Skin Wounds
The development of tensile strength was investigated in incisional wounds in wild-type and IL-1R KO mice by mechanical testing. Microscopic examination revealed identical structure and thickness in normal, uninjured skin from wild-type and IL-1R KO mice (not shown). Results in Figure 7, A–C, show that the tensile strength of wounded and normal skin from IL-1R KOs did not differ from wild-type controls.
Figure 7.
IL-1 signaling does not regulate the development of tensile strength in linear incisional wounds. Dorsal midline linear cutaneous incisional wounds were created in IL-1R KO and wild-type mice. Pelts containing the incision and uninjured skin were harvested at 14 days. A: Diagram of the skin samples including the healing wounds that were used for mechanical testing. B: Representative stress-time tracing of a mechanical test of wounded skin. C: Table summarizing the tensile strength of uninjured and wounded samples from IL-1R KO and wild-type skin. n = 6 mice per group, 3 skin samples per mouse. Mann Whitney’s U-test.
Discussion
Experiments reported here were undertaken to investigate the role of IL-1 signaling in the constitution of the inflammatory cellular infiltrate and the healing response that follows cutaneous and deep tissue wounds in mice. Incisional and excisional skin wounds, and an implanted PVA sponge wound model were used in these studies. The subcutaneous implantation of PVA sponges initiates a response that chronologically duplicates mammalian wound healing, with an initial vigorous sponge infiltration by neutrophils and macrophages, followed by an angiogenic/fibrotic phase that culminates in the encasement of the sponge by a collagenous capsule.6,7,13 It models for deep tissue wounds and avoids potential epithelial-mesenchymal signal involvement in the healing response.
The most salient findings of the current studies were the reductions in inflammatory cell infiltration, epidermal hyperplasia, and fibrosis found in incisional wounds in IL-1R KOs. In addition, IL-1R KOs had a marked decrease in fibrosis in deep tissue wounds. Most importantly, the administration of the IL-1R antagonist Anakinra reproduced the antifibrotic phenotype of IL-1R KOs. Although a decrease in fibrotic responses through the use of IL-1R antagonists would be of substantial clinical relevance in the prevention or treatment of hypertrophic scars, keloids, and other fibrotic diseases, a reduction in the capacity to heal wounds would clearly be undesirable. In this regard, results demonstrate that neither the healing rate of excisional skin wounds nor the development of tensile strength in incisional skin wounds was affected in IL-1R KO mice.
The lack of IL-1 signaling in IL-1R KOs and in those receiving Anakinra may provide, in itself, a direct mechanism for the observed changes in repair. In this regard, an increase in the production of IL-1 in injured murine and human skin has been reported.14,15 Moreover, IL-1 has been shown to induce the migration of inflammatory cells and keratinocytes in vivo.16,17,18 In addition, IL-1 stimulates fibroblast proliferation19 and the synthesis of fibronectin, collagen, metalloproteases (MMPs), and tissue inhibitors of metalloproteases (TIMP).20,21,22 IL-1 also promotes the production of profibrotic cytokines, including TGF-β and IL-6.23
Present findings do not appear to have resulted from a reduction in inflammatory cell migration into wounds because the number of such cells was not altered by IL-1R deficiency or blockade by its antagonist in deep tissue wounds, and yet these showed marked reductions in fibrosis. Results are also unrelated to enhanced collagenolytic activity in the wound environment in the KO animals because zymographic analysis on collagen and Western blotting for MMPs and TIMPs in wound fluids 1 to 14 days after wounding did not reveal differences between wild-type and IL-1R KOs.
Failure to detect differences in collagenolytic activity suggests that the reduced fibrosis found in IL-1R KOs may have resulted from a reduction in collagen synthesis. In this regard, IL-6 is endowed with profibrotic activities.24,25 Its marked and persistent reduction in wounds in IL-1R KOs was considered as a potential mechanism for the reduced collagen accumulation found in these animals. However, the direct infusion of murine rIL-6 into sponge wounds failed to increase collagen deposition in IL-1R KOs. It is conceivable that both IL-1 and IL-6 are required simultaneously for fibrotic repair, or alternatively, that the scarring effects of IL-6 are mediated through IL-1. It is also possible that the dose and/or administration schedule for IL-6 delivery into the wounds failed to reproduce a physiological response.
Deep tissue wounds in IL-1R KO mice contained less TGF-β and vascular endothelial growth factor than those in controls 1 day after wounding. This early reduction in these profibrotic/angiogenic mediators found in the KO animals could have resulted in decreased fibrotic response to deep wounds seen 5 or more days after injury. Contrasting findings in deep tissue wounds, the reduced fibrosis and improved outcome in skin wounds in IL-1R KOs were associated with substantial reduction in inflammatory cell infiltration. In this, results mirror those in sterile peritonitis and in myocardial infarction in which IL-1R deficiency markedly reduced inflammatory cell accumulation at the site of injury.1,2,4 Together with those in deep wounds, these observations suggest that the effects of IL-1 on the cellular response to tissue injury differ depending on site-specific characteristics of the wound environment. As mentioned earlier, the deep tissue wound resulting from the implantation of PVA sponges does not allow for contact between wounded tissue and the skin. This characteristic of the model may have prevented potential IL-1-mediated epithelial-to-mesenchymal signaling from regulating the inflammatory response.26
Although no direct evidence was found on the ultimate mechanisms responsible for the observations reported here, the biological relevance of present findings is enhanced by the demonstration that the local delivery of a Food and Drug Administration-approved IL-1R antagonist can markedly reduce the fibrotic response to tissue injury. It is easily conceivable that a therapeutic application could emerge from these observations, in which the IL-1R antagonist would be delivered locally to wounds with high potential for severe adverse cosmetic or functional outcomes (such as those after burns or inflicted in the course of the surgical removal of facial keloids), minimizing the potential immunosuppressive risks that can be associated with its systemic administration.
Supplementary Material
Acknowledgments
We thank William L. Henry, Jr., Balduino Mastrofrancesco, and James Rotenberg for technical assistance; Paul Monfils for tissue processing; Patricia J. Young for support in manuscript preparation; and Amgen for the generous gift of Anakinra.
Footnotes
Address reprint requests to Jorge E. Albina, M.D., Division of Surgical Research, Rhode Island Hospital, NAB 216, 593 Eddy St., Providence, RI 02903. E-mail: albina@brown.edu.
Supported by the National Institutes of Health (grants GM-042859 to J.E.A., GM-066194 to J.S.R., GM-079227 to J.M.D., and P20-RR17695 to the Molecular Pathology Core of the COBRE Center for Cancer Research Development, awarded by the National Center for Research Resources, Institutional Development Award Program to E.S.) and the Carter Family Trust (to the Department of Surgery at Rhode Island Hospital, a Lifespan partner to A.A.T., an Armand D. Versaci Research Scholar in Surgical Sciences).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, Akira S, Rock KL. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Nance SC, Yi AK, Re FC, Fitzpatrick EA. MyD88 is necessary for neutrophil recruitment in hypersensitivity pneumonitis. J Leukoc Biol. 2008;83:1207–1217. doi: 10.1189/jlb.0607391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W. Bethesda: National Institutes of Health,; ImageJ. 1997–2008 [Google Scholar]
- Albina JE, Mills CD, Barbul A, Thirkill CE, Henry WL, Jr, Mastrofrancesco B, Caldwell MD. Arginine metabolism in wounds. Am J Physiol. 1988;254:E459–4-E67. doi: 10.1152/ajpendo.1988.254.4.E459. [DOI] [PubMed] [Google Scholar]
- Daley JM, Reichner JS, Mahoney EJ, Manfield L, Henry WL, Jr, Mastrofrancesco B, Albina JE. Modulation of macrophage phenotype by soluble product (s) released from neutrophils. J Immunol. 2005;174:2265–2272. doi: 10.4049/jimmunol.174.4.2265. [DOI] [PubMed] [Google Scholar]
- Strong A. Plastics: Materials and Processing. Upper Saddle River: Prentice Hall,; 1999:pp 138–139. [Google Scholar]
- Gogly B, Groult N, Hornebeck W, Godeau G, Pellat B. Collagen zymography as a sensitive and specific technique for the determination of subpicogram levels of interstitial collagenase. Anal Biochem. 1998;255:211–216. doi: 10.1006/abio.1997.2318. [DOI] [PubMed] [Google Scholar]
- Daley J, Thomay A, Connolly M, Reichner J, Albina J. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Mateo RB, Reichner JS, Albina JE. Interleukin-6 activity in wounds. Am J Physiol. 1994;266:R1840–R1844. doi: 10.1152/ajpregu.1994.266.6.R1840. [DOI] [PubMed] [Google Scholar]
- Graves DT, Nooh N, Gillen T, Davey M, Patel S, Cottrell D, Amar S. IL-1 plays a critical role in oral, but not dermal, wound healing. J Immunol. 2001;167:5316–5320. doi: 10.4049/jimmunol.167.9.5316. [DOI] [PubMed] [Google Scholar]
- Berger AC, Feldman AL, Gnant MF, Kruger EA, Sim BK, Hewitt S, Figg WD, Alexander HR, Libutti SK. The angiogenesis inhibitor, endostatin, does not affect murine cutaneous wound healing. J Surg Res. 2000;91:26–31. doi: 10.1006/jsre.2000.5890. [DOI] [PubMed] [Google Scholar]
- Ansel JC, Luger TA, Green I. The effect of in vitro and in vivo UV irradiation on the production of ETAF activity by human and murine keratinocytes. J Invest Dermatol. 1983;81:519–523. doi: 10.1111/1523-1747.ep12522862. [DOI] [PubMed] [Google Scholar]
- Sauder DN, Semple J, Truscott D, George B, Clowes GH. Stimulation of muscle protein degradation by murine and human epidermal cytokines: relationship to thermal injury. J Invest Dermatol. 1986;87:711–714. doi: 10.1111/1523-1747.ep12456681. [DOI] [PubMed] [Google Scholar]
- Granstein RD, Margolis R, Mizel SB, Sauder DN. In vivo inflammatory activity of epidermal cell-derived thymocyte activating factor and recombinant interleukin 1 in the mouse. J Clin Invest. 1986;77:1020–1027. doi: 10.1172/JCI112354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck G, Habicht GS, Benach JL, Miller F. Interleukin 1: a common endogenous mediator of inflammation and the local Shwartzman reaction. J Immunol. 1986;136:3025–3031. [PubMed] [Google Scholar]
- Sauder DN, Orr FW, Matic S, Stetsko D, Parker KP, Chizzonite R, Kilian PL. Human interleukin-1 alpha is chemotactic for normal human keratinocytes. Immunol Lett. 1989;22:123–127. doi: 10.1016/0165-2478(89)90178-8. [DOI] [PubMed] [Google Scholar]
- Schmidt JA, Mizel SB, Cohen D, Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982;128:2177–2182. [PubMed] [Google Scholar]
- Havemose-Poulsen A, Holmstrup P. Factors affecting IL-1-mediated collagen metabolism by fibroblasts and the pathogenesis of periodontal disease: a review of the literature. Crit Rev Oral Biol Med. 1997;8:217–236. doi: 10.1177/10454411970080020801. [DOI] [PubMed] [Google Scholar]
- Postlethwaite AE, Lachman LB, Kang AH. Induction of fibroblast proliferation by interleukin-1 derived from human monocytic leukemia cells. Arthritis Rheum. 1984;27:995–1001. doi: 10.1002/art.1780270905. [DOI] [PubMed] [Google Scholar]
- Matsushima K, Bano M, Kidwell WR, Oppenheim JJ. Interleukin 1 increases collagen type IV production by murine mammary epithelial cells. J Immunol. 1985;134:904–909. [PubMed] [Google Scholar]
- Aoki H, Ohnishi H, Hama K, Ishijima T, Satoh Y, Hanatsuka K, Ohashi A, Wada S, Miyata T, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Yasuda H, Mashima H, Sugano K. Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am J Physiol. 2006;290:C1100–C1108. doi: 10.1152/ajpcell.00465.2005. [DOI] [PubMed] [Google Scholar]
- Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- Gallucci RM, Sugawara T, Yucesoy B, Berryann K, Simeonova PP, Matheson JM, Luster MI. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001;21:603–609. doi: 10.1089/10799900152547867. [DOI] [PubMed] [Google Scholar]
- Boxman I, Lowik C, Aarden L, Ponec M. Modulation of IL-6 production and IL-1 activity by keratinocyte-fibroblast interaction. J Invest Dermatol. 1993;101:316–324. doi: 10.1111/1523-1747.ep12365474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.