Abstract
Transforming growth factor-β (TGF-β) is a pleiotropic growth factor; its overexpression has been implicated in many diseases, making it a desirable target for therapeutic neutralization. In initial safety studies, mice were chronically treated (three times per week) with high doses (50 mg/kg) of a murine, pan-neutralizing, anti-TGF-β antibody. Nine weeks after the initiation of treatment, a subset of mice exhibited weight loss that was concurrent with decreased food intake. Histopathology revealed a unique, nonneoplastic cystic epithelial hyperplasia and tongue inflammation, as well as dental dysplasia and epithelial hyperplasia and inflammation of both the gingiva and esophagus. In an effort to determine the cause of this site-specific pathology, we examined TGF-β expression in these tissues and saliva under normal conditions. By immunostaining, we found higher expression levels of active TGF-β1 and TGF-β3 in normal tongue and esophageal submucosa compared with gut mucosal tissues, as well as detectable TGF-β1 in normal saliva by Western blot analysis. Interestingly, mast cells within the tongue, esophagus, and skin co-localized predominantly with the TGF-β1 expressed in these tissues. Our findings demonstrate a novel and restricted pathology in oral and esophageal tissues of mice chronically treated with anti-TGF-β that is associated with basal TGF-β expression in saliva and by mast cells within these tissues. These studies illustrate a previously unappreciated biological role of TGF-β in maintaining homeostasis within both oral and esophageal tissues.
Transforming growth factor-β isoforms (TGF-β1, -β2, and -β3) comprise a family of growth factors possessing multiple biological functions.1 These functions include embryogenesis, regulation of immune responses, cell growth and differentiation, and the formation of extracellular matrix and bone.1,2 Overexpression of TGF-β has been implicated as a contributor to diseases such as cancer and fibrotic disorders,1,3,4,5,6 making its neutralization a desirable target for therapeutics. Because of its numerous functions, however, complications may arise as a result of the inhibition of TGF-β. Mice genetically deficient in TGF-β1 or TGF-β receptor II signaling capacity have shown profound immune dysfunction and multiorgan inflammation,7,8,9,10 increased susceptibility to epithelial cell dysregulation with cancer development,11,12,13 and diminished capacity of epithelial repair after injury.14 We addressed the possibility of immune dysregulation after chronic antibody-mediated neutralization of TGF-β in a previously published study, which demonstrated minimal effects of chronic, high-dose anti-TGF-β administration on multiple immune parameters in BALB/c mice.15 Thus, antibody-mediated neutralization of TGF-β in adult mice did not result in the immune dysregulation seen in the genetically manipulated mice. However, a subset of animals in this study showed weight loss that could not be attributed to changes in immune status or significant pathology based on a limited histological evaluation. The present studies aimed to further investigate the cause of this weight loss after chronic anti-TGF-β administration, as well as to better understand additional biological roles of TGF-β.
Materials and Methods
Animals
Female BALB/cAnTac (BALB/c), BALB/cRAG-2 knockout (RAG-2KO), C57BL/6NTac (C57BL/6), 129S6/SvEvTac (SV129), and DBA/2NTac (DBA/2) mice (Taconic Laboratories, Hudson, NY) between 6 and 8 weeks of age were used in these studies. RAG-2KO mice were housed in autoclaved cages with sterilized food and water. All mice were housed in microisolator cages on a 12-hour light cycle, with housing, handling, and procedures performed in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Office of Laboratory Animal Welfare.
Antibodies and Administration
Monoclonal anti-TGF-β (clone 1D11, mouse IgG1, neutralizes all three isoforms of TGF-β) and isotype control monoclonal antibody (13C4, mouse IgG1 antibody specific for shiga-like toxin)16 were purified from hybridoma supernatants by protein A chromatography with subsequent dialysis into physiological buffers. Endotoxin levels in the monoclonal antibody (mAb) preparations were less than 1 EU/ml. TGF-β neutralizing activity of the 1D11 mAb was confirmed with mink lung cell activity as previously described.17
Study Designs
All studies described consisted of 12 weeks of dosing with isotype control or anti-TGF-β monoclonal antibodies (mAbs). The first study treated BALB/c and RAG-2KO mice intraperitoneally three times a week for 12 weeks with 5 or 50 mg/kg of anti-TGF-β or 50 mg/kg of isotype control mAb. For studies testing the reversibility of lesions, BALB/c mice were treated with 10 mg/kg of anti-TGF-β for the 12 weeks. Tissues were collected at the end of the 12-week treatment period, as well as at 4, 8, and 12 weeks after the cessation of treatment. For the strain-specific study, BALB/c, C57BL/6, SV129, and DBA/2 mice were treated with 10 mg/kg of anti-TGF-β for the 12 weeks. Study designs are described in Table 1.
Table 1.
Study Designs
| Mouse strain | Treatment | Dose* | N | Endpoint (weeks after initiation of dosing) |
|---|---|---|---|---|
| Initial study design | ||||
| BALB/c | Control IgG | 50 mg/kg | 10 | 12 |
| BALB/c | Anti-TGF-β | 50 mg/kg | 10 | 12 |
| BALB/c | Control IgG | 5 mg/kg | 10 | 12 |
| BALB/c | Anti-TGF-β | 5 mg/kg | 10 | 12 |
| RAG-2KO | Control IgG | 50 mg/kg | 10 | 12 |
| RAG-2KO | Anti-TGF-β | 50 mg/kg | 10 | 12 |
| Reversibility study design | ||||
| BALB/c | Anti-TGF-β | 10 mg/kg | 9 | 12 + 0 weeks recovery |
| BALB/c | Anti-TGF-β | 10 mg/kg | 10 | 12 + 4 weeks recovery |
| BALB/c | Anti-TGF-β | 10 mg/kg | 10 | 12 + 8 weeks recovery |
| BALB/c | Anti-TGF-β | 10 mg/kg | 9 | 12 + 12 weeks recovery |
| Strain specificity study design | ||||
| BALB/c | Anti-TGF-β | 10 mg/kg | 5 | 12 |
| C57BL/6 | Anti-TGF-β | 10 mg/kg | 5 | 12 |
| SV129 | Anti-TGF-β | 10 mg/kg | 5 | 12 |
| DBA/2 | Anti-TGF-β | 10 mg/kg | 5 | 12 |
All mice were treated with the indicated dose intraperitoneally three times a week.
Clinical Observation, Body Weight Measurements, and Food Intake
Mice were observed weekly for signs of abnormal behavior, ill health, altered toe pinch responses, and general appearance. Mice were weighed at the initiation of the antibody treatments and weekly thereafter. Body weight change throughout the course of the study was calculated as a percentage of starting weight for each individual animal. The average amount of food consumed per mouse per week was measured by subtracting the weight of the food at the end of the week from that at the beginning of the week and dividing by the total number of mice per group.
Hematology and Serum Clinical Chemistries
Immediately before euthanasia, blood was collected from the mice by cardiac puncture into both ethylenediaminetetraacetic acid-containing tubes and serum separator tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ). One drop of blood from ethylenediaminetetraacetic acid-containing tubes was smeared onto each of two glass slides for differential cell analysis. The remaining whole blood was used for complete blood counts. Blood collected in serum separator tubes was allowed to clot, centrifuged at 10,000 × g for 15 minutes, and serum was collected for serum chemistry analyses. Blood, smears, and serum were sent to AniLytics (Gaithersburg, MD) for the hematological and serum chemistry analysis.
Measurement of Circulating Levels of Anti-TGF-β mAb
Serum samples were collected before initiation of the study (pretreatment) as well as weekly throughout the studies. The levels of the 1D11 anti-TGF-β mAb in mouse serum were measured as described previously.15
Histology and Histopathology
Tissues removed at necropsy for histological examination included heart, kidney, lungs, liver, salivary glands, spleen, lymph nodes (mesenteric), thoracic skin, ear pinna, mesentery, thymus, adrenal glands, pancreas, tongue, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, skeletal muscle, sternum with bone marrow, brain, and myocardium. In addition, the maxilla was removed from animals showing altered or broken teeth. Tissue samples were fixed in 10% neutral buffered formalin, then dehydrated in gradients of alcohol, cleared in xylene, infiltrated, and embedded in paraffin. Five-μm sections were put on positively charged slides and heated at 60°C for 15 minutes. Slides were then stained with a regressive hematoxylin and eosin stain. Slides were examined microscopically for lesions in a blinded manner by a board-certified veterinary pathologist. Scoring criteria for hyperplasia and inflammation found in tongue and esophagus of anti-TGF-β mAb-treated mice are described in Table 2.
Table 2.
Pathology Scoring System
| Score | Criteria |
|---|---|
| Hyperplasia | |
| 0 | No hyperplasia |
| 1 | Mild hyperplasia or two or fewer cysts within the tongue |
| 2 | Moderate hyperplasia or two to three large cysts within the tongue |
| 3 | Marked hyperplasia or four or more cysts within the tongue |
| 4 | Neoplastic cellular proliferation |
| Inflammation | |
| 0 | No inflammation |
| 1 | Scattered inflammatory cells |
| 2 | Moderate inflammation or a locally extensive loose lichenoid infiltrate at the junction of the mucosa and submucosa |
| 3 | Marked and multifocal inflammation or a moderately dense lichenoid infiltrate multifocally obscuring the junction of the mucosa and submucosa |
| 4 | Marked and diffuse inflammation or a dense lichenoid infiltrate diffusely obscuring the junction of the mucosa and submucosa |
Immunohistochemistry
Ear pinna, back skin, tongue, esophagus, small and large intestine, and liver of untreated BALB/c mice were collected and placed into OCT gel (Shandon, Pittsburgh, PA), followed by freezing in cold 2-methylbutane (VWR International, West Chester, PA). Immunostaining was performed on acetone-fixed cryostat sections with rabbit pan-species anti-TGF-β1 (catalog no. sc-146; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit pan-species anti-TGF-β3 (catalog no. sc-82, Santa Cruz Biotechnology), rat anti-mouse anti-F4/80 to detect macrophages (catalog no. RM2900; Caltag, Burlingame, CA), rat anti-mouse anti-CD4 to detect CD4+ T helper cells (catalog no. M074873; BD PharMingen, San Diego, CA), rat anti-mouse anti-CD8 to detect CD8+ T cells (catalog no. 553026, BD PharMingen), rat anti-mouse anti-c-Kit to detect mast cells (catalog no.14-1172-81; eBioscience, San Diego, CA), rat IgG2a isotype control (catalog no. 553926, BD PharMingen), rat IgG2b isotype control (catalog no. 553985, BD PharMingen), or control rabbit IgG (catalog no. X0903; DakoCytomation, Glostrup, Denmark) followed by detection with fluorochrome-labeled goat anti-rat antibodies (for F4/80 macrophages, CD4 T cells, and c-Kit mast cells) or goat anti-rabbit antibodies (for TGF-β1 and TGF-β3) (Molecular Probes, Eugene, OR). Confocal images of the stained sections were acquired with a Zeiss LSM510-META laser-scanning confocal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) configured with appropriate excitation laser lines and emission filters. Sequential fluorescence excitation and detection was enabled to minimize channel cross talk in multichannel images.
Image Analysis for Quantitation of TGF-β Association with Mast Cells and Macrophages
Images from fluorescently stained tissues (as described above) were analyzed using MetaMorph image analysis software (Molecular Devices, Downingtown, PA).The c-kit channel from each image was subjected to an open-close filter and intensity-based thresholding to identify mast cells. Regions were generated around identified mast cells and transferred to the TGF-β channel, which was then subjected to intensity-based thresholding as above. The total integrated intensity of TGF-β staining above background was measured for the entire image and for the regions representing mast cells and used to calculate the percentage of TGF-β present within mast cells. The percentage of mast cells positive for TGF-β staining was derived by summing the number of mast cell-derived regions, which contained some amount of TGF-β fluorescence and dividing by the total number of regions.
Saliva Collection and Western Blot Analysis
Saliva was collected from untreated BALB/c mice by repeatedly collecting fluid from underneath the tongue of the animals using pipette tips. Saliva from three to five mice was pooled and centrifuged at 500 × g. Supernatant was collected to remove any debris or cells that may have been introduced during the collection process. The saliva was diluted using 4× sample buffer (Invitrogen, Carlsbad, CA) and a 10× reducing reagent (Invitrogen). Two hundred ng of active murine TGF-β (R&D Systems, Minneapolis, MN) were treated in a similar manner and included as a positive control. Samples were loaded onto a 4 to 20% Tris-glycine gradient gel under reducing/denaturing conditions. The gel was run at constant voltage (200 V) for an hour. Proteins were transferred to a polyvinylidene difluoride membrane (1.5 hours at 250 mA, constant current). The membrane was blocked and probed with a chicken anti-human TGF-β1 (R&D Systems) (100 ng/ml) for 1 hour at room temperature followed by an anti-chicken horseradish peroxidase (Pierce, Rockford, IL) detection antibody (diluted 1:20,000) for 1 hour at room temperature. The bands were visualized using enhanced chemiluminescence substrate followed by 20 seconds of film exposure.
Results
Chronic, High Doses of Neutralizing Anti-TGF-β mAb Results in Weight Loss and Decreased Food Intake
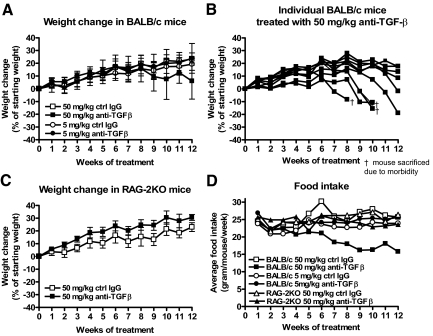
In a previous study we had observed moderate weight loss in a subset (35%) of mice treated for 12 weeks with the 1D11 pan neutralizing anti-TGF-β mAb. To more fully understand the nature of this weight loss after long-term treatment with high doses of this anti-TGF-β mAb, we repeated and expanded on the original study by treating BALB/c mice with the same dose (50 mg/kg) as well as with a 10-fold lower dose (5 mg/kg) of the anti-TGF-β mAb under the same dosing and timing regimen (three times per week for 12 weeks). To examine whether the adaptive immune system was playing a role in the weight change, we also treated RAG-2KO mice (lacking mature T and B cells) with 50 mg/kg under the same conditions as for the BALB/c mice. Other groups of BALB/c and RAG-2KO mice were concomitantly treated with corresponding doses of isotype control mAb. After 7 weeks of treatment, BALB/c mice treated with the high dose (50 mg/kg) of anti-TGF-β began to appear scruffy and displayed signs of lethargy. By the 12th week of treatment, ∼60% of these mice had exhibited significant weight loss that corresponded with decreased food intake (Figure 1, A, B, and D). BALB/c mice treated with the lower dose of anti-TGF-β (5 mg/kg) did not display a scruffy appearance, weight loss, or decreased food intake throughout the 12-week period of treatment (Figure 1, A and D). Anti-TGF-β-treated RAG-2KO mice began to appear scruffy by the 12th week of treatment, although these mice exhibited neither weight loss (Figure 1, C and D) nor a decline in food intake. Additional studies further showed that chronic treatment with anti-TGF-β at 5 mg/kg or 10 mg/kg for 20 weeks or 6 months, respectively, did not result in any evidence of weight loss in the treated animals (data not shown). Therefore, it appears that only high doses (50 mg/kg) of anti-TGF-β mAb administered for at least 8 weeks will result in weight loss that is likely attributable to decreased food intake. Even at the high doses however, this weight loss is only observed in a portion of animals (35 to 60%) and does not occur either in immunodeficient mice or in mice treated with lower doses of the antibody (10 mg/kg or less).
Figure 1.
Weight loss and decreased food intake is observed in anti-TGF-β-treated mice. Weight change and food intake after chronic treatment (three times a week for 12 weeks) with anti-TGF-β or control mAb was measured as described in the Materials and Methods. A: Weight change of BALB/c mice treated with 50 mg/kg or 5 mg/kg of anti-TGF-β or control mAb. B: Weight change of individual BALB/c mice treated with 50 mg/kg of anti-TGF-β. C: Weight change of RAG-2KO mice treated with 50 mg/kg of anti-TGF-β or control mAb. D: Changes in food intake for BALB/c mice treated with 50 mg/kg or 5 mg/kg and RAG-2KO mice treated with 50 mg/kg of anti-TGF-β as well as corresponding groups given the same doses of control mAb.
Hematological and Clinical Chemistry Parameters after Chronic Anti-TGF-β Treatment
Standard hematology (complete blood counts including differential leukocyte counts) and serum chemistry analysis was also performed in an attempt to determine whether the anti-TGF-β treatment alters hematological or clinical chemistry parameters that might indicate the cause of the weight loss observed in the mice treated with high doses of anti-TGF-β. Extensive hematological analysis only revealed a twofold elevation in average number of neutrophils in the 50 mg/kg anti-TGF-β-treated BALB/c mice and a twofold increase in average number of monocytes in the 50 mg/kg anti-TGF-β-treated RAG-2KO mice (data not shown). These findings were thought to be most consistent with a nonspecific stress response.18 Serum chemistry parameters were within the normal range in all treated animals. These findings demonstrate that high-dose TGF-β neutralization is not associated with any marked changes in hematological or serum chemistry parameters.
Chronic Anti-TGF-β Treatment Results in Unique Pathology within the Oral and Esophageal Tissues
Our earlier study had examined a limited set of thoracic and abdominal tissues histologically and found no significant changes.15 However, because the previous and present studies did not reveal any immune, hematological, or clinical chemistry parameters that would explain decreased food intake leading to weight loss in the high-dose (50 mg/kg) anti-TGF-β treated mice, we performed a more extensive histopathological analysis that, in addition to those organs examined in the previous study, included mesentery, thymus, adrenal glands, pancreas, tongue, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, skeletal muscle, sternum with bone marrow, brain, and myocardium. In addition, the maxilla was removed from animals showing altered or broken teeth. This broader pathological analysis uncovered nonneoplastic epithelial hyperplasia and/or inflammation in the oral and esophageal tissues but in no other tissue examined (Figure 2).
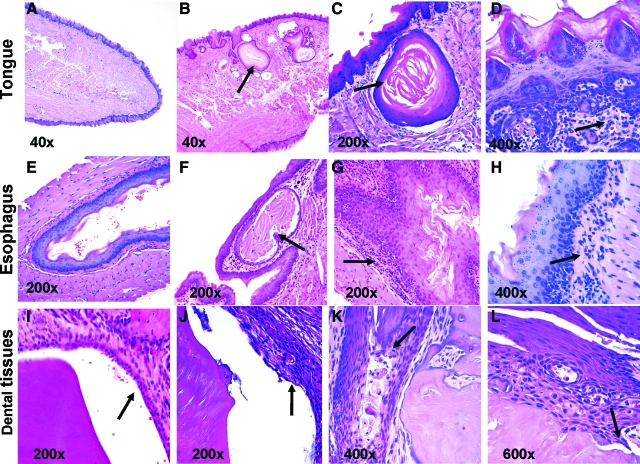
Figure 2.
Oral and esophageal pathology after chronic treatment of BALB/c mice with anti-TGF-β. Representative images are shown of H&E-stained sections of tongue (A–D), esophageal (E–H), and dental tissues (I–L) from BALB/c mice treated with 50 mg/kg of anti-TGF-β or control mAb three times a week for 12 weeks. Tissues were prepared and stained as described in the Materials and Methods. A, E, I: Tissue sections from control mAb-treated mice. B–D: Tongue section from mice treated with anti-TGF-β. B: Epithelial cysts of differing sizes were often present within the skeletal muscle of the tongue (arrow). C: Epithelial cysts in the tongue were lined by stratified squamous epithelium and contained keratin (arrow). D: Glossal inflammation consisted primarily of lymphocytes and macrophages in the submucosa (arrow). F–H: Esophageal sections from mice treated with anti-TGF-β. F: Rare cysts lined by stratified squamous epithelium and containing keratin (arrow) were present within the submucosa of the esophagus. G: Inflammatory infiltrates within the submucosa of the esophagus consisted primarily of lymphocytes and macrophages (arrow). H: Mild epithelial hyperplasia is present within the esophageal mucosa, and a dense inflammatory infiltrate is present within the superficial submucosa (arrow). J–L: Gingival/dental sections from mice treated with anti-TGF-β. J: Hyperplasia of the gingival-attaching epithelium was present (arrow). K: Rare pustules (arrow) containing necrotic cells and neutrophils were present within the hyperplastic gingival epithelium. L: The dentin (arrow) and enamel space exhibited a loss of normal smooth contour, consistent with dental dysplasia. Magnification is indicated within each image.
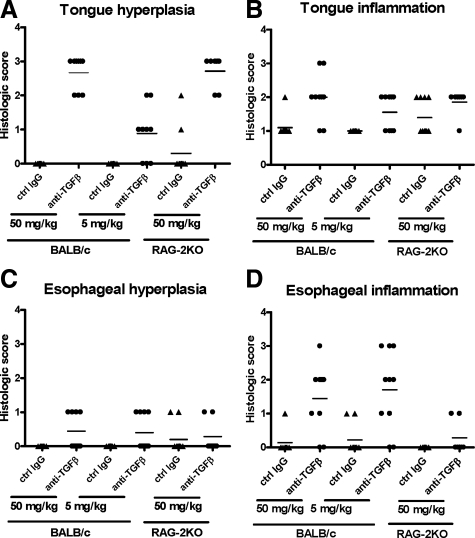
Tongue sections from affected mice contained multiple, variably sized epithelial cysts adjacent to the cranioventral glossal surface that extended into the skeletal muscle (Figure 2, A and B). These cysts were lined by well-organized stratified squamous epithelium and contained keratin (Figure 2C) and necrotic cellular debris. Glossal submucosal regions of anti-TGF-β-treated normal mice were also infiltrated by small to moderate numbers of lymphocytes, macrophages, and neutrophils (Figure 2D). We were unable to find any description in the literature of similar glossal cystic epithelial hyperplasia under any disease setting, suggesting that this is a unique and specific manifestation of TGF-β neutralization. The incidence of tongue lesions was 100% in BALB/c and RAG-2KO mice treated with the high dose (50 mg/kg) anti-TGF-β three times per week for 12 weeks and was 66% in BALB/c mice treated with the lower dose (5 mg/kg) of anti-TGF-β throughout the same time period (Figure 3, A and B). Tongue epithelial hyperplasia was slightly more severe in 50 mg/kg anti-TGF-β-treated BALB/c mice compared with 5 mg/kg anti-TGF-β-treated BALB/c mice, suggesting a dose-dependent effect of TGF-β antagonism on this pathology (Figure 3, A and B). Because the tongue epithelial hyperplasia was also present in RAG-2KO mice treated with anti-TGF-β, this indicates that this lesion is T- and B-cell-independent.
Figure 3.
Tongue and esophageal hyperplasia and inflammation in mice treated chronically with anti-TGF-β. Tongue and esophageal sections from BALB/c and RAG-2KO mice that were treated three times a week for 12 weeks with either 50 or 5 mg/kg of anti-TGF-β or control mAb were scored by a veterinary pathologist according to the criteria outlined in Table 2. Graphs represent scores of individual mice in each treatment group for tongue hyperplasia (A), tongue inflammation (B), esophageal hyperplasia (C), and esophageal inflammation (D). Means of the data are also indicated.
In esophageal lesions, the submucosa contained a moderate to marked inflammatory infiltrate consisting of lymphocytes, neutrophils, macrophages, and eosinophils (Figure 2, E–G). The esophageal mucosal epithelium was variably hyperplastic, and in rare sections, the mucosal epithelium contained small subcorneal pustules filled with neutrophils, eosinophils, and necrotic cellular debris (Figure 2H). The incidence of esophageal lesions was 77 to 80% in BALB/c mice treated with either 50 or 5 mg/kg of anti-TGF-β, but esophageal lesions were minimal or not seen in RAG-2KO mice, indicating that this change is dependent on the presence of T and B cells (Figure 3, C and D). The similar incidence and severity of esophageal lesions in both dose groups also suggests that these changes are not dose-dependent. Immunostaining for CD4+ T cells and macrophages in the esophagus also demonstrated increases in these cell types after chronic TGF-β neutralization (data not shown) suggesting a T-cell-mediated inflammatory response. Naturally occurring inflammation and circumferential epithelial hyperplasia in the mouse esophagus are very rare,19 and thus, these changes are likely attributable to the anti-TGF-β treatment. This supposition is further supported by descriptions of esophageal inflammation in TGF-β1 KO mice.7,8
During our examinations, one mouse with incisor tooth breakage was noticed at the termination of the study, and therefore, the maxilla was removed for histological evaluation. Microscopically, hyperplasia of the proximal gingiva and anchoring epithelium was associated with the broken incisor teeth (Figure 2, I–L). The periodontal ligament was infiltrated by small numbers of neutrophils admixed with fewer numbers of macrophages, plasma cells, and lymphocytes. The enamel space and dentin tissue exhibited markedly irregular contours. Unfortunately, maxillary tissues were not collected from most of the animals in these studies, but in a subsequent study, similar dental changes were seen in 60% of BALB/c mice treated with doses of anti-TGF-β in excess of 50 mg/kg for 12 weeks (data not shown). TGF-β is known to play a key role in the formation and homeostasis of dental tissues,20,21,22 and mouse incisors may be particularly susceptible to changes in TGF-β levels because these teeth grow continuously throughout the life of the animal.20 Thus, our findings support the idea that dental tissues in mice are also particularly susceptible to TGF-β neutralization.
Because the lesions we observed appeared to be restricted to keratinized, stratified squamous epithelium-lined mucosal surfaces, we also examined the vagina, another mucosal surface lined by keratinized, stratified squamous epithelium. Mild vaginal epithelial hyperplasia and mucosal and submucosal inflammation were noted at equal incidence in mice from all treatment groups (data not shown). Because of the similarity of incidence among treatment groups as well as the potential for estrus cycle stage to affect vaginal histology,23 we were unable to determine whether these changes resulted from anti-TGF-β treatment.
Oral and Esophageal Pathology Persisted after Cessation of Anti-TGF-β Treatment
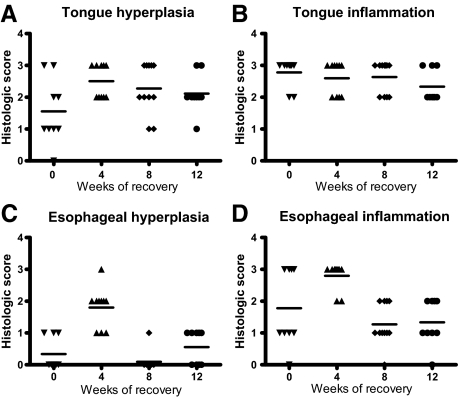
Given the high incidence and severity of the lesions in the oral cavity and esophagus as well as their likely effect on food intake, we were interested in whether cessation of anti-TGF-β treatment would result in resolution of these lesions. BALB/c mice in this study were treated with an intermediate dose of anti-TGF-β (10 mg/kg) for 12 weeks and then evaluated immediately or 4, 8, or 12 weeks after cessation of treatment. Tongue lesions were present in 90% of mice sacrificed at the end of the 12-week treatment period, and there was no evidence of diminution in severity or incidence throughout time after the cessation of treatment. In fact, higher hyperplasia scores were noted in the 4- and 12-week recovery groups compared with the group euthanized immediately after treatment cessation (Figure 4, A and B). Average esophageal hyperplasia and inflammation reached a maximum 4 weeks after treatment cessation, followed by a reduction in the 8- and 12-week recovery groups (Figure 4, C and D). However, the circulating levels of anti-TGF-β remain high for a period of time after cessation of treatment and we found that serum levels of the anti-TGF-β mAb did not decrease to background levels until 10 weeks after the last treatment (serum anti-TGF-β levels after treatment cessation were as follows: 0 weeks: 411.79 ± 101.77 μg/ml, 1 week: 381.97 ± 133.54 μg/ml, 2 weeks: 298.17 ± 93.11 μg/ml, 3 weeks: 143.50 ± 46.41 μg/ml, 4 weeks: 70.16 ± 47.74 μg/ml, 5 weeks: 51.02 ± 45.70 μg/ml, 6 weeks: 12.72 ± 5.18 μg/ml, 7 weeks: 11.71 ± 11.12 μg/ml, 8 weeks: 3.35 ± 1.87 μg/ml, 9 weeks: 2.06 ± 1.00 μg/ml; levels in several mice were below the lower limit of quantitation of 0.56 μg/ml at 10 weeks and later). Given the high doses of antibody used and extended presence of the antibody in serum, it remains possible that additional recovery time may be necessary to observe resolution of these lesions, however, our findings indicate that they are not readily reversible under the conditions of the current study.
Figure 4.
Persistence of tongue and esophageal lesions after treatment cessation. Tongue and esophageal sections from BALB/c mice that were treated three times a week for 12 weeks with 10 mg/kg of anti-TGF-β followed by recovery periods of 0, 4, 8, or 12 weeks after discontinuation of treatments were scored by a veterinary pathologist according to the criteria outlined in Table 2. Graphs represent scores of individual mice in each treatment group for tongue hyperplasia (A), tongue inflammation (B), esophageal hyperplasia (C), and esophageal inflammation (D). Means of the data are also indicated.
Strain Specificity of Tongue and Esophageal Lesions after Chronic Anti-TGF-β Treatment
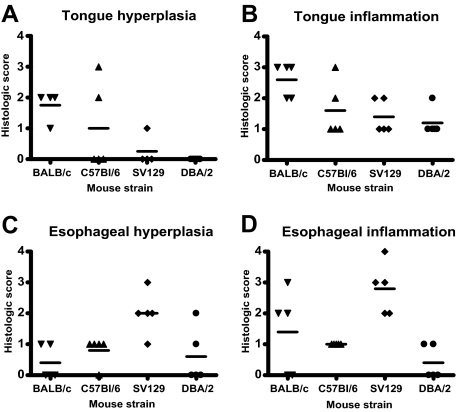
To determine whether the development of tongue and esophageal pathology after chronic anti-TGF-β treatment would also develop in other strains of mice, we treated C57BL/6, SV129, and DBA/2 mice, in addition to BALB/c mice with 10 mg/kg of anti-TGF-β for 12 weeks. Compared with the BALB/c mice, tongue hyperplasia and esophageal inflammation was milder and less common in C57BL/6 mice (Figure 5, A and B). Although SV129 mice exhibited less incidence and severity of tongue hyperplasia compared with BALB/c mice, they showed a greater incidence and severity of esophageal hyperplasia and inflammation (Figure 5, C and D). DBA/2 mice exhibited uncommon, mild lesions in the tongue and esophagus. Thus, all strains of mice examined did show the propensity to develop oral and/or esophageal lesions under the conditions tested, but were found to a lesser degree than in BALB/c mice. Similar to BALB/c mice, the other strains of mice did not exhibit significant lesions in any other organ examined.
Figure 5.
Strain differences in tongue and esophageal lesions after chronic treatment with anti-TGF-β. Tongue and esophageal sections from BALB/c, C57BL/6, SV129, and DBA/2 mice that were treated three times a week for 12 weeks with 10 mg/kg of anti-TGF-β were scored by a veterinary pathologist according to the criteria outlined in Table 2. Graphs represent scores of individual mice in each treatment group for tongue hyperplasia (A), tongue inflammation (B), esophageal hyperplasia (C), and esophageal inflammation (D). Means of the data are also indicated.
Increased Basal Expression of TGF-β in Tongue and Esophageal Tissues as Well as in Saliva
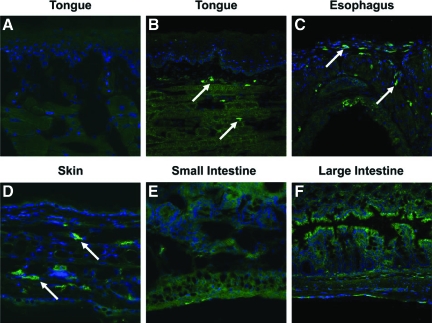
Because the appearance of lesions in mice chronically treated with anti-TGF-β appeared to be restricted to the tongue and esophagus, we were interested in whether differential TGF-β expression in these tissues was a potential cause of enhanced susceptibility to TGF-β antagonism. We performed immunofluorescent staining for active TGF-β isoforms 1 and 3 on tongue, esophagus, skin, liver, and small and large intestine, as well as Western blot analysis for TGF-β1 in the saliva of untreated BALB/c mice in an attempt to determine whether there might be increased levels of active TGF-β specifically within oral and esophageal sites. By immunofluorescent analysis, we were able to demonstrate moderate to strong TGF-β1 (Figure 6, A–C) and TGF-β3 (data not shown) staining in the submucosa of the tongue and esophagus, whereas liver (data not shown) and small and large intestine sections (Figure 6, E and F) stained similarly to control mAb stained tissues. We also found moderate positive staining in the dermis of skin sections (Figure 6D).
Figure 6.
Greater basal TGF-β1 expression in tongue, esophageal, and skin tissues in untreated BALB/c mice. Representative images are shown of isotype control-stained and TGF-β1-stained untreated BALB/c mouse tissues performed as described in the Materials and Methods. A: Isotype control-stained tongue section. B–F: TGF-β1-stained sections of tongue (B), esophagus (C), skin (D), small intestine (E), and large intestine (F). Positive staining is green and is indicated with arrows. All images were acquired with a ×20 0.7NA objective lens and are shown at the same magnification.
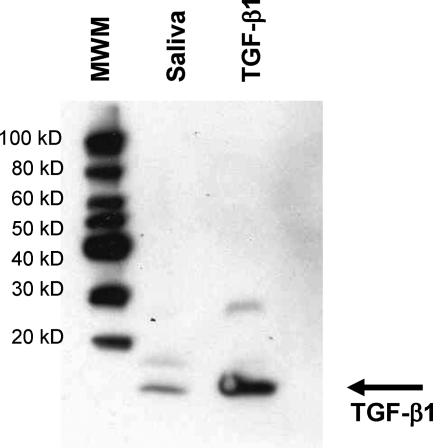
In addition to the tongue and esophagus, TGF-β1 was also detected in the saliva of untreated BALB/c mice (Figure 7). Densitometric analysis using various amounts of TGF-β determined the levels in saliva to be ∼135 ng/ml, which are considerably higher than levels reported in other biological fluids in mice (plasma, ∼4 ng/ml; amniotic fluid,: ∼1 ng/ml).24,25 Although the antibody used to detect TGF-β in the tissues is specific for active TGF-β, it is possible that TGF-β in saliva is in the latent form and is activated during the Western blot analysis. Enzyme-linked immunosorbent assays to detect active or total TGF-β in saliva were not possible because of interference of saliva on detection of TGF-β in these assays.26 Nevertheless, the presence of higher levels of active TGF-β in these oral and esophageal tissues as well as the demonstration of TGF-β1 within the saliva may contribute to the increased susceptibility of the oral and esophageal tissues to epithelial hyperplastic and inflammatory lesions after systemic TGF-β neutralization.
Figure 7.
Presence of TGF-β1 in saliva from untreated BALB/c mice. Western blot analysis was performed on 7.5 μl of normal mouse saliva for TGF-β1 as described in the Materials and Methods. Molecular weight markers (MWM) and purified active TGF-β1 as a positive control were also run on the same blot.
Co-Localization of TGF-β Expression in Tongue, Esophagus, and Skin with Mast Cells
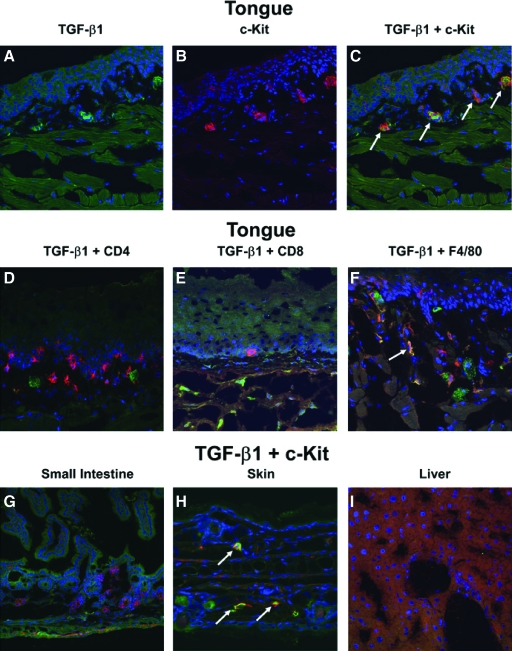
Because the TGF-β immunostaining of the tongue and esophagus from normal mice appeared to localize within discrete cells rather than staining diffusely throughout the tissues, we further explored whether this TGF-β expression was associated with a particular cell type. By confocal microscopy, we evaluated co-localization of the TGF-β1 staining with macrophages, CD4+ T cells, CD8+ T cells, and mast cells in the tongue, esophagus, skin, liver, and intestines. We found no evidence of co-localization of TGF-β1 with either CD4 or CD8 T cells and only minimal co-localization of TGF-β1 with macrophages (Figure 8, D–F). In contrast mast cells within the submucosa of the tongue, esophagus, and dermis co-localized with TGF-β1 staining in all mice examined (Figure 8, A–C, and H; and data not shown).
Figure 8.
TGF-β co-localization with mast cells in tongue, esophagus, and skin from untreated BALB/c mice. Tissues from untreated BALB/c mice were double immunostained for TGF-β1 in combination with markers for various cell types as described in the Materials and Methods. A: Single stains of TGF-β1 in tongue sections. B: Single stains of mast cells (c-Kit) in tongue sections. C: Merged image of TGF-β1 and c-Kit staining in the tongue section. D–F: Double staining for TGF-β1 and CD4 T cells (D), CD8 T cells (E), and macrophages(F4/80) (F) in tongue sections. G–I: Double staining of TGF-β1 and mast cells (c-Kit) in the small intestine (G), skin (H), and liver (I) tissue sections. In all images, green indicates TGF-β1 staining, red indicates the specific cell type staining, blue indicates DAPI or nuclear staining, and yellow indicates co-localization of TGF-β and the specific cell types stained. Co-localization is indicated with arrows. All images were acquired with a ×20 0.7NA objective lens and are shown at the same magnification.
Quantitation of the TGF-β co-localization with mast cells in the tongue determined that more than 90% of the mast cells express detectable TGF-β staining and ∼50% of the TGF-β is localized within mast cells. Because of significant autofluorescence arising from muscle fibers within the tissue, which cannot be eliminated using intensity-based thresholding, our measurements certainly underestimate the amount of TGF-β present within mast cells. Although there is some imprecision in the morphometric analysis, it should be emphasized that 50% of the TGF-β staining observed within the lamina propria was localized within a single cell type, which represents a small percentage of the tissue both in terms of mass and cell number. It was difficult to quantify the exact amount of TGF-β co-localization within macrophages because of poor signal-to-noise ratios, but the relative amount within macrophages appeared small compared with the amount of TGF-β present within mast cells. The remaining TGF-β (not associated with mast cells or macrophages) appeared diffusely dispersed throughout the tissue sections.
The expression of active TGF-β1 by mast cells in normal mice also appears to be restricted to the tongue, esophagus, and skin (Figure 8, A–I; and data not shown) because even though mast cells were detected in the intestines, they did not show any evidence of TGF-β expression (Figure 8G). Thus, the elevated TGF-β expression observed in normal tongue, esophagus, and skin predominately co-localizes with mast cells suggesting that these cells may be responsible for the TGF-β-mediated homeostasis in the oral cavity and esophagus of normal mice.
Discussion
In this series of studies, we examined the effects of chronic, antibody-mediated TGF-β neutralization in mice. Our findings demonstrate that a subset of BALB/c mice treated with high doses (>10 mg/kg) of anti-TGF-β begin to exhibit lethargy and progressive weight loss that correlates with diminished food intake starting 8 weeks after the onset of treatment. We were able to rule out the influence of hematological and serum chemistry parameters on the development of these symptoms and have previously reported that there is no evidence of systemic immune dysregulation in these animals.15 However, extensive histopathological analysis revealed that the anti-TGF-β-treated mice develop profound and persistent nonneoplastic epithelial hyperplasia and inflammation affecting the oral cavity and esophagus, but in no other tissues examined. On further investigation into the unusual and restricted location of these lesions, we found that mice under normal conditions have TGF-β in their saliva and show greater expression of active TGF-β that co-localizes predominately with mast cells in tongue and esophageal tissues compared with other mucosal tissues. Taken together, the susceptibility of these tissues to systemic TGF-β neutralization may be attributable to a TGF-β-dependent homeostatic role of mast cells within these tissues and/or dual TGF-β neutralization effects through tissue and salivary sources.
The appearance of lesions in the oral cavity and esophagus may have resulted in painful mastication, and it is likely that these lesions are the cause of the decreased food intake and subsequent weight loss. It is notable that decreased food intake and weight loss were not seen in RAG-2KO mice, which exhibited severe tongue hyperplasia but lacked significant esophageal pathology. In addition, decreased food intake and weight loss were not seen in low-dose-treated BALB/c mice, which exhibited similar esophageal pathology to the high-dose-treated BALB/c mice but less severe tongue hyperplasia. Furthermore, it is unlikely that lesions severe enough to cause decreased food intake and weight loss develop later with the lower dose because extended treatments did not result in weight loss or diminished food intake. As a result, it seems most likely that decreased food intake and weight loss are attributable to the additive severity of lesions affecting both the tongue and esophagus.
The restricted location of the lesions we observed in mice chronically treated with anti-TGF-β is surprising given the wide range of TGF-β activities and the systemic immune dysregulation seen in genetically manipulated mice with functional TGF-β inactivation.7,8,9 However, the manifestation of the lesions in our studies as epithelial hyperplasia and inflammation is consistent with the clearly established role of TGF-β in the regulation of epithelial cell growth and inflammatory responses. Epithelial cell proliferation is inhibited by TGF-β through arresting the cell cycle in the G1 phase,27,28,29 and TGF-β has been demonstrated to control the development and function of immune cell types including dendritic, Langerhans, and B cells, as well as subsets of T cells.1,30,31,32 Additionally, the increased sensitivity in BALB/c mice with regard to overall incidence and severity of lesions after anti-TGF-β treatment is also consistent with reports that BALB/c mice are the most susceptible to immune pathology in TGF-β gene knockout mice.33 Thus, although our findings in mice treated chronically with neutralizing anti-TGF-β are unique given the absence of a multiorgan inflammatory syndrome, the appearance of epithelial hyperplasia and inflammation, albeit restricted to the oral cavity and esophagus, is consistent with established biological activities of TGF-β.
Previous studies have demonstrated that the multiorgan inflammation and early mortality of the TGF-β1-null mouse is dependent on the presence of lymphocytes because crossing the TGF-β1-deficient mice onto a T- and B-cell-deficient background (scid) results in survival of the animals into adulthood.34 It is important to note that in our studies, cystic epithelia hyperplasia within the tongue of mice treated with anti-TGF-β occurs independently of mature T and B cells because RAG-2KO mice developed these lesions at the same incidence as BALB/c mice. However, and perhaps not surprisingly, esophageal inflammation was minimal in the RAG-2KO mice treated with anti-TGF-β. Furthermore, the mild esophageal hyperplasia noted in anti-TGF-β-treated BALB/c mice but not in RAG-2KO mice suggests that this change might be secondary to esophageal inflammation. As a result, it appears that the inflammatory component of the oral and esophageal pathology noted after anti-TGF-β treatment is T- and B-cell-dependent, whereas the tongue hyperplasia is T- and B-cell-independent.
It was also surprising that the profound lesions in the oral and esophageal mucosa did not extend further into the gastrointestinal tract, given the purported role of TGF-β in these tissues.14,35,36,37 However, even under condition of genetic TGF-βRII deficiency in the intestinal epithelia, effects of this deficiency were only noted after the induction of colitis,14 supporting the idea that TGF-β is not required to maintain normal homeostasis of the intestinal epithelium. One reason for this difference between gut and oral mucosa could be attributable to the higher proliferation rates of murine oral mucosa proliferation compared to skin or intestinal mucosa.38,39 Thus, it is possible that the oral and esophageal mucosa require the higher basal expression of TGF-β that we observed in normal mice to regulate this proliferation. This requirement for constant expression of TGF-β could predispose oral and esophageal tissues to the formation of hyperplastic lesions in the absence of TGF-β to a greater degree than intestine or skin. Although we also found increased expression of active TGF-β in the skin, we did not see lesions in the skin of antibody-treated mice, suggesting that TGF-β may not be as important for regulation of epithelial proliferation in this tissue, given the lower proliferation rates of the skin compared with the oral mucosa. Therefore, part of the reason for the tissue restriction of lesions in antibody-mediated neutralization of TGF-β in mice could be attributable to differences in the requirements for TGF-β expression to regulate epithelial proliferation.
The presence of TGF-β in the saliva provides additional rationale for increased susceptibility of the oral and esophageal mucosa, as well as the teeth, to anti-TGF-β-induced lesions because not only are there higher levels of active TGF-β expression within these tissues, but these tissues are also bathed in saliva containing additional TGF-β. A dual-exposure hypothesis has been suggested for gingival hyperplasia observed with other compounds, such as cyclosporine A, phenytoin, and nifedipine in which these compounds can exert effects on the gingiva through both the tissue vasculature as well as the saliva.40,41 If applied to our system, the oral cavity and esophagus are doubly exposed to TGF-β under normal conditions, and the neutralizing effects of systemically administered anti-TGF-β could be exerted through both the bloodstream as well as through secretion in the saliva42 and/or neutralization within the salivary gland. This hypothesis of both dual exposure and dual neutralization effects in the oral cavity and esophagus is further supported by the fact that although we did observe active TGF-β expression within the skin, there were no lesions found in this tissue after chronic administration of anti-TGF-β. Taken together, these data suggest that because oral and esophageal tissues are dually exposed to the effects of TGF-β neutralization through both the saliva and bloodstream, they may be more susceptible to the complications arising when TGF-β is systemically neutralized.
The appearance of lesions in the oral cavity and esophagus after chronic anti-TGF-β treatment paired with the expression of TGF-β by mast cells under normal conditions in these same tissues suggests an interesting role for mast cells in homeostasis specifically within these tissues. Although mast cells are classically thought to be involved in the initiation and potentiation of allergic and anaphylatic responses,43 recent studies have also demonstrated that mast cells can contribute to immunosuppression under a variety of conditions, including contact hypersensitivity reactions or inflammation after exposure to UV irradiation,44 immunosuppression in the skin after UV irradiation,45 glomerulonephritis,46 and the induction of tolerance in a model of skin transplantation.47 IL-10 production by mast cells was found to be a critical factor for limiting contact hypersensitivity inflammatory responses,44 but to our knowledge TGF-β production by mast cells has not yet been demonstrated to participate in these immunosuppressive activities. Although TGF-β can be produced by mast cells, this has been suggested to be detrimental in disease conditions such as scleroderma48 or porphyria cutanea tarda.49 We are also unaware of mast cell-deficient mice on strain backgrounds that show significant pathology in the tongue or esophagus after anti-TGF-β treatment making it difficult to formally test the role of mast cells in the pathology we observe. Nevertheless, the studies reported here provide evidence that mast cells, via production of TGF-β, may promote homeostasis under normal conditions in the oral cavity and esophagus.
The systemic homeostatic role of TGF-β related to control of cell proliferation, differentiation, activation, and survival (in the context of immune function, epithelial cell proliferation, and hematopoiesis) revealed in mice genetically deficient in TGF-β or TGF-β signaling was not observed in our studies with antibody-mediated neutralization. The reason for this observation is unknown but may reflect simple physical steric hindrance or lack of accessibility of the anti-TGF-β binding to the mature form of the TGF-β protein during its activation and receptor binding. Decreased accessibility of the antibody to tissues such as the skin may also explain the lack of pathology seen in this tissue after chronic TGF-β neutralization. It is also possible that very small amounts of TGF-β can maintain the natural homeostasis in antibody-treated mice, but not in genetically manipulated mice in which TGF-β1 or the functioning of the TGF-βRII are completely inactive. In addition, there is evidence that TGF-β may be active and perform important functions within cells, including stabilization of mitochondrial membrane potential that prevents T-cell apoptosis.50 Thus, neutralization of only extracellular, active TGF-β by the antibody treatment may spare these important intracellular functions of TGF-β that protect the host from development of systemic pathologies. Any or all of these possibilities could explain the differences we see between antibody-mediated TGF-β neutralization compared with genetic inactivation of TGF-β or its receptor.
In summary, we demonstrate that BALB/c mice treated chronically with high doses of neutralizing anti-TGF-β develop persistent epithelial hyperplasia and inflammation affecting the tongue and esophagus, as well as gingival hyperplasia and dental dysplasia in the region of the maxillary incisor teeth. Basal TGF-β expression by mouse mast cells in oral and esophageal tissue as well as the presence of TGF-β in saliva support a hypothesis in which mast cell-derived TGF-β and/or exposure of these tissues to TGF-β in saliva is required for regulation specifically within these tissues. Collectively, these findings demonstrate a critical role for TGF-β in the maintenance of homeostasis specifically within oral and esophageal tissues of mice.
Acknowledgments
We thank Jim Murray, Jen Tedstone, Brendan Gavin, Ken Munroe, and the Department of Comparative Medicine for their aid in performing animal studies; Cindy Rogers for her help with serum anti-TGF-β assays; the technical staff of the Department of Pathology for their expertise with processing tissues for histopathology; John McPherson and Michael O’Callaghan for their critical review of this manuscript.
Footnotes
Address reprint requests to Melanie Ruzek, Genzyme Corporation, 1 The Mountain Rd., Framingham, MA 01701-9322, E-mail: melanie.ruzek@genzyme.com.
Supported by Genzyme Corporation in collaboration with MedImmune (formerly Cambridge Antibody Technologies).
All authors are or were employees of Genzyme during the course of these studies.
Present addresses of A.V.: Pfizer Global Research and Development, San Diego, CA; R.P.: Cubist Pharmaceuticals Inc., Lexington, MA; and M.L.H.: Charter Preclinical Services, Hudson, MA.
References
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. Regulation and regulatory activities of transforming growth factor beta. Crit Rev Eukaryot Gene Expr. 1999;9:33–44. [PubMed] [Google Scholar]
- Piek E, Roberts AR. Suppressor and oncogenic roles for transforming growth factor-β and its signaling pathways in tumorigenesis. Adv Cancer Res. 2001;83:1–54. doi: 10.1016/s0065-230x(01)83001-3. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]
- Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- Lakos G, Takagawa S, Chen S, Ferriera AM, Han G, Masuda K, Wang X, DiPietro LA, Varga J. Targeted disruption of TGF-β/SMAD3 signaling modulates skin fibrosis in a mouse model of scleroderma. Am J Pathol. 2004;165:203–217. doi: 10.1016/s0002-9440(10)63289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, Huh C, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H, Geiser AG, Letterio JJ, Nakabayashi T, Kong L, Fernandes G, Talal N. SLE-like autoantibodies and Sjogren’s syndrome-like lymphoproliferation in TGF-beta knockout mice. J Immunol. 1995;155:3205–3212. [PubMed] [Google Scholar]
- Levéen P, Larsson J, Ehinger M, Cillo CM, Sundler M, Sjöstrand LJ, Holmdahl R, Karlsson S. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002;100:560–568. doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]
- Lu SL, Herrington H, Reh D, Weber S, Bornstein S, Wang D, Li AG, Tang CF, Siddiqui Y, Nord J, Andersen P, Corless CL, Wang XJ. Loss of transforming growth factor-beta type II receptor promotes metastatic head and neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Hahm KB, Lee KM, Kim YB, Hong WS, Lee WH, Han SU, Kim MW, Ahn BO, Oh TY, Lee MH, Green J, Kim SJ. Conditional loss of TGF-beta signaling leads to increased susceptibility to gastrointestinal carcinogenesis in mice. Aliment Pharmacol Ther. 2002;16(Suppl 2):115–127. doi: 10.1046/j.1365-2036.16.s2.3.x. [DOI] [PubMed] [Google Scholar]
- Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. doi: 10.1016/s0002-9440(10)63853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzek MC, Hawes MH, Pratt B, McPherson J, Ledbetter S, Richards SM, Garman RD. Minimal effects on immune parameters following chronic anti-TGF-β monoclonal antibody administration to normal mice. Immunopharmacol Immunotoxicol. 2003;25:235–257. doi: 10.1081/iph-120020473. [DOI] [PubMed] [Google Scholar]
- Strockbine NA, Marques LR, Holmes RK, O'Brien AD. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun. 1985;50:695–700. doi: 10.1128/iai.50.3.695-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielpour D, Dart LL, Flanders KC, Roberts AB, Sporn MB. Immunodetection and quantitation of two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989;138:79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Latimer KS, Prasse KW. Leukocytes. Latimer KS, Mahaffey EA, Prasse KW, editors. Ames: Iowa State Press,; Duncan and Prasse’s Veterinary Laboratory Medicine Clinical Pathology, (ed 4) 2003:pp 46–79. [Google Scholar]
- Leininger JR, Jokinen, Dangler CA, Whiteley LO. Maronpot RR, Boorman GA, Gaul BW, editors. Vienna: Cache River Press,; Oral cavity, esophagus, and stomach. Pathology of the Mouse. 1999:pp 36–37. [Google Scholar]
- Long PH, Leininger JR. Teeth. Maronpot RR, Boorman GA, Gaul BW, editors. Vienna: Cache River Press,; Pathology of the Mouse. 1999:p 16. [Google Scholar]
- Prime SS, Pring M, Davies M, Paterson IC. TGF-beta signal transduction in oro-facial health and non-malignant disease (part 1). Crit Rev Oral Biol Med. 2004;15:324–336. doi: 10.1177/154411130401500602. [DOI] [PubMed] [Google Scholar]
- D'Souza RN, Cavender A, Dickinson D, Roberts A, Letterio J. TGF-beta1 is essential for the homeostasis of the dentin-pulp complex. Eur J Oral Sci. 1998;106(Suppl 1):185–191. doi: 10.1111/j.1600-0722.1998.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Dixon D, Herbert RA. Ovary, oviduct, uterus, cervix, and vagina. Maronpot RR, Boorman GA, Gaul BW, editors. Vienna: Cache River Press,; Pathology of the Mouse. 1999:pp 440–444. [Google Scholar]
- Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor b1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan IS, Koishi K. Fetal and maternal transforming growth factor-beta 1 may combine to maintain pregnancy in mice. Biol Reprod. 2004;70:1614–1618. doi: 10.1095/biolreprod.103.026179. [DOI] [PubMed] [Google Scholar]
- Wozniak KL, Arribas A, Leigh JE, Fidel PL., Jr Inhibitory effects of whole and parotid saliva on immunomodulators. Oral Microbiol Immunol. 2002;17:100–107. doi: 10.1046/j.0902-0055.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Belizario JE, Dinarello CA. Interleukin 1, interleukin 6, tumor necrosis factor, and transforming growth factor β increase cell resistance to tumor necrosis factor cytotoxicity by growth arrest in the G1 phase of the cell cycle. Cancer Res. 1991;51:2379–2385. [PubMed] [Google Scholar]
- Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990;62:175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Tato CM, O'Shea JJ. Immunology: what does it mean to be just 17? Nature. 2006;441:166–168. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- Gorham JD, Lin JT, Sung JL, Rudner LA, French MA. Genetic regulation of autoimmune disease: BALB/c background TGF beta 1-deficient mice develop necroinflammatory IFN-gamma-dependent hepatitis. J Immunol. 2001;166:6413–6422. doi: 10.4049/jimmunol.166.10.6413. [DOI] [PubMed] [Google Scholar]
- Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci USA. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Biswas S, Chytil A, Washington K, Romero-Gallo J, Gorska AE, Wirth PS, Gautam S, Moses HL, Grady WM. Transforming growth factor beta receptor type II inactivation promotes the establishment and progression of colon cancer. Cancer Res. 2004;64:4687–4692. doi: 10.1158/0008-5472.CAN-03-3255. [DOI] [PubMed] [Google Scholar]
- Sakuraba H, Ishiguro Y, Yamagata K, Munakata A, Nakane A. Blockade of TGF-beta accelerates mucosal destruction through epithelial cell apoptosis. Biochem Biophys Res Commun. 2007;359:406–412. doi: 10.1016/j.bbrc.2007.05.117. [DOI] [PubMed] [Google Scholar]
- Kvidera A, Mackenzie IC. Rates of clearance of epithelial surfaces of mouse oral mucosa and skin. Epithelial Cell Biol. 1994;3:175–180. [PubMed] [Google Scholar]
- Kellett M, Potten CS, Rew DA. A comparison of in vivo cell proliferation measurements in the intestine of mouse and man. Epithelial Cell Biol. 1992;1:147–155. [PubMed] [Google Scholar]
- Seymour RA, Thomason JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol. 1996;23:165–175. doi: 10.1111/j.1600-051x.1996.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Das SJ, Newman HN, Olsen I. Keratinocyte growth factor receptor is up-regulated in cyclosporine A-induced gingival hyperplasia. J Dent Res. 2002;81:683–687. doi: 10.1177/154405910208101006. [DOI] [PubMed] [Google Scholar]
- Grönblad EA. Concentration of immunoglobulins in human whole saliva: effect of physiological stimulation. Acta Odontol Scand. 1982;40:87–95. doi: 10.3109/00016358209041120. [DOI] [PubMed] [Google Scholar]
- Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Limón-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180:4648–4655. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochegger K, Siebenhaar F, Vielhauer V, Heininger D, Mayadas TN, Mayer G, Maurer M, Rosenkranz AR. Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur J Immunol. 2005;35:3074–3082. doi: 10.1002/eji.200526250. [DOI] [PubMed] [Google Scholar]
- Lu L-F, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Ozbilgin MK, Inan S. The roles of transforming growth factor type beta 3 (TGF-beta3) and mast cells in the pathogenesis of scleroderma. Clin Rheumatol. 2003;22:189–195. doi: 10.1007/s10067-003-0706-5. [DOI] [PubMed] [Google Scholar]
- Lançoni G, Ravinal RC, Costa RS, Roselino AM. Mast cells and transforming growth factor-beta expression: a possible relationship in the development of porphyria cutanea tarda skin lesions. Int J Dermatol. 2008;47:575–581. doi: 10.1111/j.1365-4632.2008.03607.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Tian H, Sicurello P, Frank M, Orenstein JM, Wahl SM. Requirement for transforming growth factor beta1 in controlling T cell apoptosis. J Exp Med. 2001;194:439–453. doi: 10.1084/jem.194.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]