Abstract
Patients with respiratory failure often require supplemental oxygen therapy and mechanical ventilation. Although both supportive measures are necessary to guarantee adequate oxygen uptake, they can also cause or worsen lung inflammation and injury. Hyperoxia-induced lung injury is characterized by neutrophil infiltration into the lungs. The urokinase plasminogen activator receptor (uPAR) has been deemed important for leukocyte trafficking. To determine the expression and function of neutrophil uPAR during hyperoxia-induced lung injury, uPAR expression was determined on pulmonary neutrophils of mice exposed to hyperoxia. Hyperoxia exposure (O2>80%) for 4 days elicited a pulmonary inflammatory response as reflected by a profound rise in the number of neutrophils that were recovered from bronchoalveolar lavage fluid and lung cell suspensions, as well as increased bronchoalveolar keratinocyte-derived chemokine, interleukin-6, total protein, and alkaline phosphatase levels. In addition, hyperoxia induced the migration of uPAR-positive granulocytes into lungs from wild-type mice compared with healthy control mice (exposed to room air). uPAR deficiency was associated with diminished neutrophil influx into both lung tissues and bronchoalveolar spaces, which was accompanied by a strong reduction in lung injury. Furthermore, in uPAR−/− mice, activation of coagulation was diminished. These data suggest that uPAR plays a detrimental role in hyperoxia-induced lung injury and that uPAR deficiency is associated with diminished neutrophil influx into both lung tissues and bronchoalveolar spaces, accompanied by decreased pulmonary injury.
Patients with acute lung injury or the acute respiratory distress syndrome commonly receive supportive care consisting of low tidal volume protective ventilation and the administration of high concentrations of inspired oxygen.1,2 Prolonged exposure to high oxygen concentrations, however, can worsen or induce lung damage in already injured or previously healthy lungs.3 The mechanisms underlying hyperoxia-induced lung injury are beginning to be understood, despite difficulties in distinguishing the effects of exposure to hyperoxia from those of the inciting disease for which oxygen therapy was started. Insight into factors involved in hyperoxia-induced lung injury could help identify possible new targets for improving the management of patients who require supplemental oxygen.
The inflammatory response to hyperoxia is dominated by neutrophils and likely mediated by the generation of reactive oxygen species.1,3 The recruitment of neutrophils to the lungs on exposure to hyperoxia has been linked to the local production of CXC chemokines and the expression of CXCR2, the predominant neutrophil receptor for CXC chemokines in rodents.4,5,6 In addition, increased expression of adhesion molecules on the pulmonary endothelial cell surface has been found to contribute to neutrophil trafficking into the bronchoalveolar compartment during hyperoxia.7 We and others recently identified a pivotal role for the urokinase plasminogen activator receptor (uPAR, CD87) in neutrophil migration to the lungs during bacterial pneumonia.8,9 uPAR is a glycosylphosphatidylinositol-linked receptor expressed by a variety of cell types, including hemopoietic cells, endothelial cells and many different neoplastic cells, that has been implicated as an important player in leukocyte trafficking.10,11 At present it is unknown whether uPAR is involved in hyperoxia-induced neutrophil migration and lung injury. Therefore, in the present study we sought to investigate the expression and function of neutrophil uPAR during hyperoxia-induced lung inflammation.
Materials and Methods
Animals
Female upar gene deficient (uPAR−/−) mice on a C57Bl/6 background were from Jackson Laboratory (Bar Harbor, Maine). Female wild-type C57Bl/6 mice were from Harlan Sprague Dawley (Inc., Horst, The Netherlands). All experiments were performed with 8 to 10 weeks old mice. The Institutional Animal Care and Use Committee of the Academic Medical Center, University of Amsterdam, approved all experiments.
Induction of Hyperoxia-Induced Lung Injury
Before the experiments had started, the mice of both strains had stayed at least one week in the animal facility to become acclimatized. Animals of both genotypes were kept in the same room. Wild-type and uPAR−/− mice were exposed to either 80% oxygen or room air (6 liters/minute) under normobaric pressure in a 90 × 70 × 70 cm chamber. The mice were kept on a 12 hours light/dark cycle and received food and water ad libitum.
Tissue Handling
Before they were sacrificed at indicated time points, mice were anesthetized with ketamine (Eurovet Animal Health BV, Bladel, The Netherlands) and medetomidine (Pfizer Animal Health BV, Capelle aan de IJssel, The Netherlands). Lungs were harvested for preparation of lung cell suspensions as described.12,13
Bronchoalveolar Lavage
In separate mice, not used for pathology or preparation of lung cell suspensions, bronchoalveolar lavage fluid (BALF) was obtained and differential counts were performed as described earlier.9,14 Briefly, the trachea was exposed through a midline incision and BALF was harvested by instilling and retrieving two 0.5-ml aliquots of sterile isotonic saline. Cell counts were determined using an automated counter (Beckham Coulter, Coulter ZF, Mijdrecht, the Netherlands).
Assays
Lung cell suspensions were stained with antibodies (abs) against Gr1 (fluorescein isothiocyanate, BD Pharmingen, San Diego, CA) and uPAR (biotinylated, R&D Systems, Minneapolis, MN) and analyzed by flow cytometry using a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA). Keratinocyte-derived chemokine (KC) and interleukin (IL)-6 levels were determined by enzyme-linked immunosorbent assay (R&D Sytstems, Abingdon, United Kingdom). Protein levels were measured using the bicinchoninic acid protein kit (Pierce, Rockford, IL) using bovine serum albumin as a standard. Alkaline phosphatase levels were determined by a commercially available kit (Sigma Aldrich, St. Louis, MO) using a Hitachi analyzer (Boehringer Mannheim, Germany). Thrombin-anti-thrombin complexes (TATc) were measured by enzyme-linked immunosorbent assay (Enzygnost TAT Micro, Dade Behring, Marburg, Germany). For western blotting of the soluble receptor for advanced glycation end-products (sRAGE), 10 μl of BALF was separated by 10% polyacrylamide SDS gel electrophoreses and transferred to Immobilon P (Pharmacia, Piscataway, NJ) polyvinylidene difluoride membranes. Membranes were blocked in block buffer containing 5% nonfat dry milk proteins, and 0.1% Tween 20 in 50 mmol/L Tris, 150 mmol/L NaCl (pH 7.4; TBS), washed with TBS and incubated overnight with primary antibodies (goat anti-mouse RAGE polyclonal antibodies (Neuromics, Edina, MN) in block buffer at 4°C. After washing with TBS, membranes were probed with peroxidase-labeled secondary Ab (Cell Signaling Technology, Danvers, MA) for 1 hour at room temperature in TBS. After washing with TBS, membranes were incubated with Lumi-LightPlus Western Blotting Substrate (Roche, Mijdrecht, The Netherlands) and positive bands were detected using a GeneGnome Syngene Bio Imaging System (Syngene, Leusden, The Netherlands). Intensity of the bands was quantified using the GeneTools software supplied by Syngene.
Histological Examination
Lungs were fixed in 4% formaldehyde and embedded in paraffin. Four-micron thick sections were stained with H&E or periodic acid Schiff after diastase (PAS-D). The following parameters were scored: interstitial inflammation, edema, necrosis of the bronchial epithelium, and hyaline membrane formation. Each parameter was graded on a scale of 0 to 4 (0, absent; 1, mild; 2, moderate; severe; and 4, very severe). The total histology score was expressed as the sum of the score for all parameters. Neutrophil stainings9,14 and fibrin(ogen) stainings15,16 were performed as described. For neutrophil staining, slides were deparaffinized and rehydrated. Slides were then digested by a solution of pepsin 0.25% (Sigma, St. Louis, MO) in 0.01 M/L HCl. After being rinsed, the sections were incubated in 10% normal goat serum (Dako, Glostrup, Denmark) and then exposed to fluorescein isothiocyanate-labeled anti-mouse Ly-6-G monoclonal antibody (Pharmingen, San Diego, CA). Endogenous peroxidase activity was quenched by a solution of 0.1% NaN3/0.03% H2O2 and 3.3′-diaminobenzidine-tetrahydrochloride (Sigma, St. Louis, MO) in Tris-HCl. The sections were mounted in glycerine gelatin without counter staining and analyzed. For fibrin(ogen) staining, slides were deparaffinized and endogenous peroxidase activity was quenched with a solution of methanol/0.03% H2O2 (Merck, Darmstadt, Germany). After digestion with a solution of pepsin 0.25% (Sigma, St. Louis, MO) in 0.01 M/L HCl, the sections were incubated in 10% normal goat serum (Dako, Glostrup, Germany) and then exposed to biotin-labeled goat anti-mouse fibrin(ogen) antibody (Ixell, Accurate Chemical & Scientific Corp., Westbury, NY). After washes, slides were incubated in a streptavidin-ABC solution (Dako, Glostrup, Germany) and developed using 1% H2O2 and 3.3′-diaminobenzidine-tetra-hydrochloride (Sigma, St. Louis, MO) in Tris-HCl. The sections were mounted in glycerine gelatin without counter staining and analyzed. Surface areas immunostained with fibrin(ogen) were analyzed using a DFC500 digital camera mounted on a DM5000B microscope (both from Leica Microsystems, Wetzlar, Germany). The area for positive for fibrin(ogen) was determined with Image Pro Plus (Media Cybernetics, Silver Spring, MD) and expressed as the percentage of the total surface area.
Statistical Analysis
All data are expressed as mean ± SE. Serial data were analyzed using a Kruskal-Wallis and Dunn’s post test. Differences between wild-type and uPAR−/− mice were analyzed by Mann-Whitney U-test. Values of P < 0.05 were considered to represent a statistically significant difference.
Results
Kinetics of Lung Inflammation and Injury on Exposure to 80% Oxygen
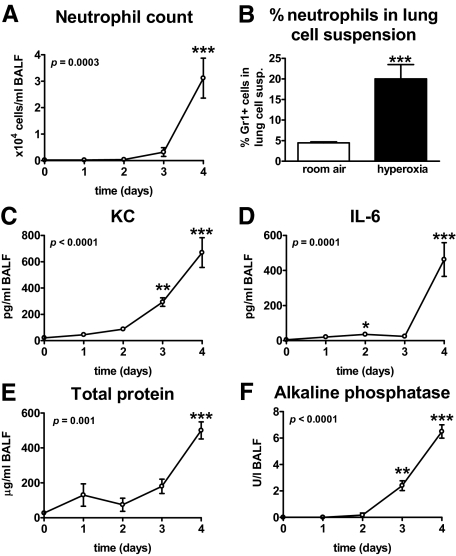
To investigate whether our hyperoxia model induced neutrophil recruitment to the lungs, mice were exposed to 80% oxygen and BALF was harvested after 0, 1, 2, 3, and 4 days for determination of neutrophil counts. Mice were not followed beyond 4 days since mortality occurred from that time point onwards. Neutrophils began to appear in BALF after 3 days and were markedly increased at 4 days after the hyperoxic challenge (P < 0.001 versus t = 0, Figure 1A). In addition, lung cell suspensions of mice exposed to hyperoxia for 4 days, contained more neutrophils than those of control mice that were exposed to room air (P < 0.001, Figure 1B). To further evaluate the pulmonary responses to oxygen exposure, KC and IL-6 were measured in BALF, as well as total protein and alkaline phosphatase (as measures of lung injury) (Figure 1, C–F, respectively). The concentrations of all parameters were clearly elevated after 4 days (all P < 0.001 versus t = 0). Further experiments were performed using the 4-day time point.
Figure 1.
Pulmonary responses to oxygen exposure. Wild-type mice were exposed to 80% oxygen for the indicated time points (A and C–F) or for 4 days (B). Neutrophil counts in BALF (A), neutrophil counts in lung cell suspensions at 4 days (B), KC (C), IL-6 (D), total protein (E), and alkaline phosphatase (AF) (F) in BALF. Data are mean ± SE of 6 to 8 mice per time point. Depicted P values are obtained from Kruskal-Wallis tests for analysis of overall differences within groups in time (A and C–F). *P < 0.05 versus t = 0; **P < 0.01 versus t = 0; ***P < 0.001 versus t = 0 (A and C–F) as analyzed by Dunn’s post tests as described in the Materials and Methods section. *P < 0.001 versus wild-type mice (B) as analyzed by Mann-Whitney test.
Hyperoxia Enhances uPAR Expression on Neutrophils
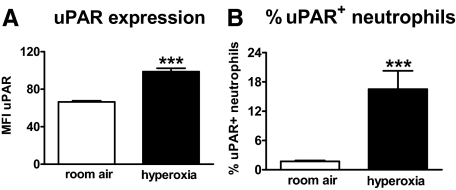
uPAR has been implicated in neutrophil recruitment to sites of inflammation, including the lungs.8,9 Having shown that oxygen exposure caused an influx of neutrophil into the lungs, we next wished to investigate whether hyperoxia-induced uPAR expression on neutrophils. Therefore, uPAR expression on neutrophils from lung cell suspensions of mice exposed to hyperoxia were compared with that of healthy mice (exposed to room air) by flow cytometry. Pulmonary neutrophils from mice exposed to hyperoxia displayed more uPAR on their cell surface than neutrophils from control mice (P < 0.001 versus room air, Figure 2A). Furthermore, pulmonary cell suspensions of hyperoxia-challenged mice showed a higher percentage of uPAR+ neutrophils (P < 0.001 vs room air, Figure 2B).
Figure 2.
Hyperoxia enhances uPAR expression on neutrophils. Wild-type mice were exposed to room air (open bars) or 80% oxygen (closed bars) for 4 days. Lung cell suspensions were collected and flow cytometry was performed as described in the Materials and Methods section. Results are represented as the mean fluorescence intensity of uPAR surface expression (A) and % uPAR+ cells (B) within the Gr1+ neutrophil population. Data are mean ± SE of 8 mice per time point. ***P < 0.001 versus wild-type mice.
uPAR−/− Mice Show a Reduced Neutrophil Recruitment during Hyperoxia
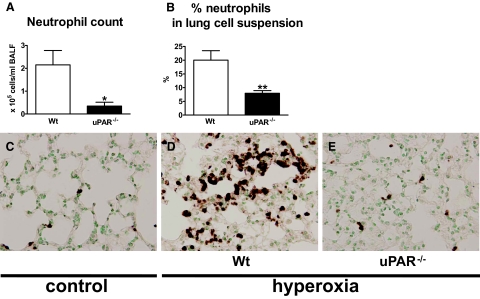
To study the contribution of endogenous uPAR to the influx of neutrophils into the lungs during hyperoxia, we exposed wild-type and uPAR−/− mice to 80% oxygen and investigated neutrophil recruitment to the lungs. In some experiments, control mice were exposed to room air. uPAR−/− mice had a reduced influx of neutrophils into the lungs, as compared with the wild-type mice, as reflected by lower neutrophil counts in BALF (P < 0.05, Figure 3A). In addition, lung cell suspensions of uPAR−/− mice consisted of fewer neutrophils compared with those of wild-type mice (P < 0.005, Figure 3B). In line, neutrophil stainings of lung tissue showed fewer neutrophils in uPAR−/− mice than in wild-type mice (Figure 3E versus 3D, Figure 3C control). Together, these data indicate that uPAR deficiency diminishes neutrophil recruitment to the lungs on exposure to hyperoxia.
Figure 3.
uPAR−/− mice display reduced neutrophil migration to the lungs. Wild-type (Wt) (open bars) and uPAR−/− mice (closed bars) were exposed to 80% oxygen and sacrificed after 4 days. Neutrophil counts in BALF (A) and neutrophil counts in lung cell suspensions (B). Neutrophil counts in BALF from healthy control wild-type and uPAR−/− mice were low (P < 0.009 for both genotypes) and did not differ (data not shown). Data are mean ± SE of 7 to 10 mice per time point. *P < 0.05 versus wild-type mice; **P < 0.01 versus wild-type mice. Representative neutrophil stainings of lung tissue of 80% oxygen exposed wild-type (D) and uPAR−/− mice (E). Control wild-type mice were exposed to room air (C).
uPAR−/− Mice Demonstrate Lower KC and IL-6 Concentrations in their Lungs
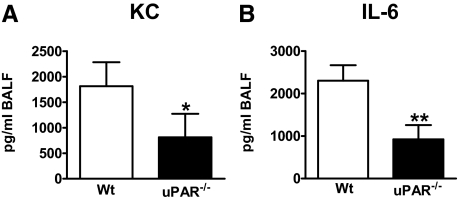
Chemokines and cytokines play an important role during hyperoxia induced lung injury.6,7 Thus, we determined the concentrations of neutrophil chemoattractant KC and IL-6 in BALF. The uPAR−/− mice showed reduced levels of both mediators after 4 days (both P < 0.05, Figure 4A and B, respectively). Chemoattractant MIP-2 levels did not increase during the 4 days of hyperoxia (data not shown).
Figure 4.
uPAR deficiency diminishes KC and IL-6 release. Wild-type (Wt) (open bars) and uPAR−/− mice (closed bars) were exposed to either 80% oxygen or room air (control mice) and sacrificed after 4 days. KC (A) and IL-6 (B) were measured in BALF. Data are mean ± SE of 7 to 10 mice per time point. Levels of healthy wild-type and uPAR−/− mice were below detection limit for both KC and IL-6 (data not shown). *P < 0.05 versus wild-type mice; **P < 0.01 versus wild-type mice.
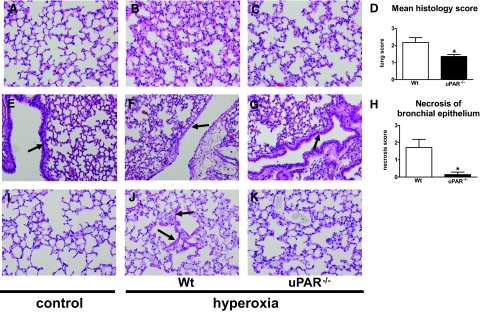
uPAR-Deficient Mice Show Less Severe Lung Injury during Hyperoxia
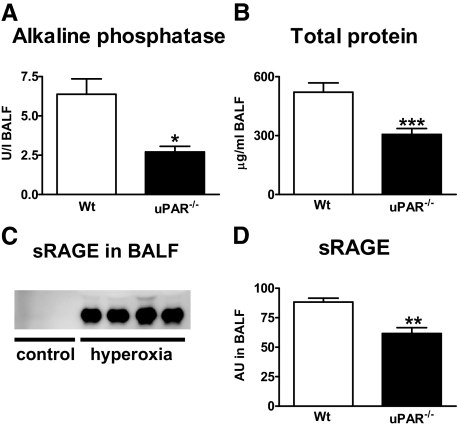
Having shown that uPAR−/− mice displayed a decreased migration of neutrophils to the lungs, we next evaluated the role of endogenous uPAR in lung inflammation and damage during hyperoxia. Therefore, we analyzed lung tissue slides from wild-type and uPAR−/− mice exposed to hyperoxia for 4 days. On histopathological examination, the lungs of hyperoxia exposed wild-type mice showed interstitial inflammation together with edema (Figure 5B versus 5A). Lung inflammation in uPAR−/− mice (Figure 5C) was less profound compared with that in wild-type mice. The mean histology score of the lungs (determined using the scoring system described in the Materials and Methods section) was significantly lower in the uPAR−/− mice (P < 0.05, Figure 5D). In addition, uPAR−/− mice demonstrated less necrosis of the bronchial epithelium (Figure 5G, arrow, versus Figure 5F, arrow, P < 0.05, Figure 5H, Figure 5E, control). The formation of hyaline membranes is an important characteristic of hyperoxia-induced lung injury.17,18 To establish whether the presence of hyaline membranes differed between the two mouse strains, we performed PAS-D stainings on lung tissue slides. Compared with control mice exposed room air, wild-type mice exposed to 80% oxygen demonstrated markedly enhanced PAS-D staining when compared with wild-type mice or hyperoxic uPAR−/− mice (Figure 5J, arrows versus 5I). Furthermore, the extent of lung injury was determined by measuring alkaline phosphatase and total protein concentrations in BALF19; both markers were reduced in uPAR−/− mice compared with wild-type mice (both P < 0.05, Figure 6A and 6B). sRAGE has been recently described as a marker of lung injury based on experimental studies in rats and in patients with acute lung injury.20 To establish whether this model is associated with increased levels of sRAGE in the bronchoalveolar space, we performed Western blot analysis on BALF samples from healthy control mice and mice exposed to 80% oxygen for 4 days and generated semiquantitative data by densitometric evaluation. Hyperoxia elicited increased sRAGE concentrations in BALF, as shown in Figure 6C. Importantly, uPAR−/− mice demonstrated significantly lower sRAGE levels in BALF than wild-type mice after exposure to hyperoxia (P < 0.005, Figure 6D). In conclusion, these data strongly suggest that uPAR−/− mice show less severe lung injury after exposure to hyperoxia.
Figure 5.
Lungs of uPAR−/− mice show less injury. Representative H&E stainings of lung tissue of wild-type (Wt) mice exposed to room air (control, A and E) and of wild-type (B and F) and uPAR−/− mice (C and G) exposed to 80% oxygen for 4 days. Original microscopic magnification A–C: ×20. Graphical representation of the mean histology score of lung inflammation (D) determined according to the scoring system described in the Materials and Methods section. Less profound necrosis of the bronchial epithelium was observed in the uPAR−/− mice (G, arrow) compared with wild-type mice (F, arrow). Original microscopic magnification E–G: ×10. Graphical representation of the degree of necrosis of bronchial epithelium (H) determined according to the scoring system described in the Materials and Methods section. Arrow in E indicates bronchial epithelium of a healthy lung from wild-type mouse exposed to room air. Representative PAS-D stainings of lung tissue of wild-type mice exposed to room air (control, I) and of hyperoxia exposed wild-type (J, arrows indicate enhanced PAS-D staining, indicative for hyaline membranes) and uPAR−/− mice (K). Original microscopic magnification I–K: ×20. *P < 0.05 versus wild-type mice.
Figure 6.
Lower total protein and alkaline phosphatase levels in uPAR−/− mice. Wild-type (Wt) (open bars) and uPAR−/− mice (closed bars) were exposed to either 80% oxygen (A–D) or room air (control mice, C) and sacrificed after 4 days. Total protein (A) and AF (B) were measured in BALF. Levels of healthy wild-type and uPAR−/− mice were 23.4 ± 1.9 and 7.6 ± 3.8, respectively, for total protein and below detection limit for alkaline phosphatase (data not shown). Western blot was performed for soluble receptor for advanced glycation end products (sRAGE) in BALF of healthy control mice (C) and of mice exposed to hyperoxia (C and D). Semiquantitative data were generated by densitometric evaluation of wild-type versus uPAR−/− mice 4 days after oxygen exposure (D). AU = arbitrary units. Data are mean ± SE of 6 to 10 mice per genotype. *P < 0.05 versus wild-type mice; **P < 0.01 versus wild-type mice; ***P < 0.001 versus wild-type mice.
uPAR−/− Mice Demonstrate Enhanced Activation of Pulmonary Coagulation
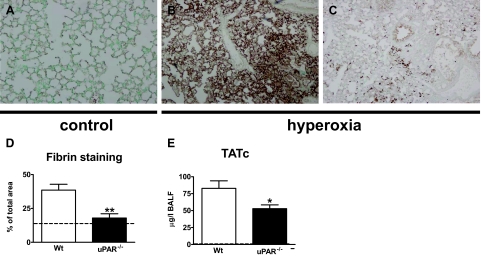
Pulmonary coagulopathy is an important feature of acute lung injury in patients21,22 and of hyperoxia-induced lung injury in experimental animal models.17 To establish whether our hyperoxia model is associated with activation of pulmonary coagulation, we performed fibrin(ogen) stainings on lung tissue slides (semiquantified as described in the Materials and Methods section) and measured TATc levels in BALF after exposure to 80% oxygen. Both wild-type and uPAR−/− mice displayed strong evidence for pulmonary activation of coagulation: relative to healthy control mice, lungs of both mouse strains revealed markedly more fibrin(ogen) depositions (Figure 7, A–D) and strongly elevated TATc concentrations in BALF (Figure 7E). Importantly, activation of coagulation was less profound in uPAR−/− mice, as reflected by diminished fibrin(ogen) deposition and lower BALF TATc levels (both P < 0.05 versus wild-type mice, Figure 7, B–E). Together, these data indicate that uPAR deficiency reduces the activation of coagulation in the lungs during hyperoxia-induced lung injury.
Figure 7.
uPAR−/− mice demonstrate decreased local activation of coagulation. Wild-type and uPAR−/− mice were exposed to 80% oxygen. Control mice were exposed to room air. Representative fibrin(ogen) immunostaining of lung tissue of control mice (A) and of wild-type and uPAR−/− mice exposed to hyperoxia for 4 days (B and C, respectively). Original magnification ×4. Graphical representation of the % of the total area with positive fibrin(ogen) staining (D) according to the scoring system described in the Materials and Methods section. Thrombin-antithrombin complex (TATc) concentrations were measured in BALF (E). Data are mean ± SE of 7 to 10 mice per time point. *P < 0.05 versus wild-type mice; **P < 0.01 versus wild-type mice. Dotted lines represent the mean values obtained from normal mice (exposed to room air).
Discussion
Although the oxygenation of hypoxic patients with acute lung injury or acute respiratory distress syndrome is an important and life-saving therapeutic measure, exposure to high oxygen concentrations via the airways can cause severe damage the lungs. In the present study we sought to obtain insight into the contribution of uPAR to hyperoxia-induced lung injury. To the best of our knowledge, we here for the first time demonstrate that hyperoxia results in the recruitment of uPAR+ neutrophils to the lungs in vivo. These results extend previous studies showing up-regulation of uPAR RNA levels during hyperoxia in vivo in premature murine pups23 and showing an increase of uPAR expression on isolated human neutrophils on activation with tumor necrosis factor-α, phorbol myristate acetate, and formyl-methionyl-leucyl-phenylalanine in vitro,24 the latter study describing enhanced uPAR expression due to translocation of intracellular uPAR reservoirs to the plasma membrane. Activation of neutrophils is an important feature of hyperoxia induced lung injury and several mediators could have attributed directly or more indirectly to uPAR up-regulation in our model, eg, several cytokines24 and reactive oxygen species.25 Further research is warranted to investigate what mechanisms underlie induction of neutrophil surface uPAR during hyperoxia.
uPAR deficiency was associated with a diminished neutrophil influx into lung tissue and the bronchoalveolar space, which was accompanied by a strong reduction in lung injury. These results show for the first time that uPAR plays a detrimental role during hyperoxia-induced lung injury.
uPAR is a multifunctional protein involved in different inflammatory responses, including cell-associated proteolysis, cell adhesion, chemotaxis, cell migration, and proliferation10,11 uPAR lacks an intracellular domain and therefore is not able to directly activate intracellular pathways; instead, uPAR can induce cellular responses by interacting with other molecules, including integrins. Neutrophil emigration from the lung circulation is unique in that it can proceed via two distinct pathways: one that requires the β2 integrin CD11b/CD18 and one that does not.26,27 uPAR has been implicated as a modulator of β2 integrin function, especially that of CD11b/CD18.8,28,29 During Gram-negative pneumonia caused by Pseudomonas aeruginosa uPAR was required for the recruitment of neutrophils to the lungs by a mechanism that involved CD11b/CD18.8 Of interest, however, our laboratory previously showed that uPAR also mediated neutrophil influx into the lungs during pneumonia caused by Streptococcus pneumonia,9 although in this Gram-positive respiratory tract infection model neutrophil migration has been established to proceed via a CD11b/CD18 independent mechanism.30,31 The influx of neutrophils into the lungs on exposure to hyperoxia occurs via mechanisms that do not rely on CD11b/CD18.32 The current study firmly establishes that uPAR expression contributes to neutrophil recruitment during hyperoxia: uPAR−/− mice displayed fewer neutrophils in BALF and whole lung cell suspensions, as well as in lung tissue. Together these data strongly suggest that uPAR mediates neutrophil trafficking during hyperoxia-induced lung inflammation by a pathway that, similar to its role in pneumococcal pneumonia,9 is independent of an interaction with CD11b/CD18, thereby further confirming that uPAR and CD11b/CD18 are not necessarily functionally linked during the process of neutrophil migration to the lungs.
uPAR−/− mice not only demonstrated a diminished neutrophil influx, but also less lung injury. Indeed, uPAR deficiency was associated a relative protection against epithelial cell injury, as demonstrated by histopathology (revealing marked reductions in hyaline membrane formation and epithelial cell necrosis), lower concentrations of total protein in BALF and a diminished release of alkaline phosphatase and sRAGE, indicative of type II and type I alveolar epithelial cell injury respectively,19,20 into the bronchoalveolar space. Several studies have indicated that neutrophils may contribute to hyperoxia-induced lung injury, but are not absolutely required. Indeed, neutrophil depletion using nitrogen mustard only partially reduced hyperoxia-induced lung edema in rabbits,33 whereas neutropenia accomplished by cyclophospamide did not alter the increased lung permeability associated with hyperoxia in rats.34 Furthermore, in the most recent study, antibody-mediated depletion of neutrophils did not impact on lung edema or protein leak on exposure of mice to hyperoxia.7 However, inhibition of neutrophil influx during hyperoxia by blocking or elimination of neutrophil CXCR2 receptors strongly attenuated lung injury.6,35 Of interest, recent evidence indicates that uPAR is expressed by respiratory epithelial cells.36,37 At present it is unknown if and how epithelial cell uPAR impacts on hyperoxia-induced lung injury.
Acute lung injury and acute respiratory distress syndrome are associated with a disturbance of the procoagulant-anticoagulant balance within the bronchoalveolar space resulting in intrapulmonary fibrin deposition.21,22 In accordance, rodents exposed to hyperoxia-demonstrated fibrin deposition in their lungs on exposure to hyperoxia.17,23 We here confirm and extend these previous findings, showing that inhalation of 80% oxygen for 4 days results in fibrin deposition in lung tissue accompanied by a rise in TATc concentrations in BALF. Remarkably, uPAR−/− mice displayed less fibrin deposition when compared with wild-type mice on exposure to hyperoxia. This finding may be counterintuitive considering that uPAR has been implicated in cell-associated fibrinolytic activity mediated by uPA.10,11 However, it should be noted that uPA−/− mice were earlier found to have an unaltered response to hyperoxia, including intra-alveolar fibrin deposition.17 Together these data suggest that uPAR/uPA do not have a major impact on fibrin clearance in the lung during hyperoxia, and that the enhanced activation of pulmonary coagulation in uPAR−/− mice likely reflects the overall increased injurious response in the airways. Moreover, the fact that uPA−/− mice responded to hyperoxia with unaltered septal thickening and epithelial cell injury,17 strongly suggests that the beneficial phenotype of uPAR−/− mice described here is related to an uPA independent mechanism. This is not unprecedented considering earlier reports on uPAR-dependent cell recruitment via uPA-independent mechanisms.9,29,38
We here show that hyperoxia results in the influx of uPAR+ neutrophils into lung tissue and the bronchoaveolar space. The contribution of uPAR in hyperoxia-induced lung injury was demonstrated using uPAR−/− mice, which displayed less neutrophil influx, less lung injury and less pulmonary coagulation. Inhibition of uPAR may be a novel strategy to reduce the lung injury that accompanies oxygen therapy.
Acknowledgments
We thank Joost Daalhuisen and Marieke S. ten Brink for expert technical assistance.
Footnotes
Address reprint requests to Marieke A.D. van Zoelen, M.D., Center for Experimental and Molecular Medicine (CEMM), G2-130, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam. E-mail: M.A.vanZoelen@amc.uva.nl.
References
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- Leaver SK, Evans TW. Acute respiratory distress syndrome. BMJ. 2007;335:389–394. doi: 10.1136/bmj.39293.624699.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CR, Paula Pinto SG, Maranhao B, Bethlem EP. Hyperoxia and lung disease. Curr Opin Pulm Med. 1998;4:300–304. doi: 10.1097/00063198-199809000-00010. [DOI] [PubMed] [Google Scholar]
- Deng H, Mason SN, Auten RL., Jr Lung inflammation in hyperoxia can be prevented by antichemokine treatment in newborn rats. Am J Respir Crit Care Med. 2000;162:2316–2323. doi: 10.1164/ajrccm.162.6.9911020. [DOI] [PubMed] [Google Scholar]
- Auten RL, Richardson RM, White JR, Mason SN, Vozzelli MA, Whorton MH. Nonpeptide CXCR2 antagonist prevents neutrophil accumulation in hyperoxia-exposed newborn rats. J Pharmacol Exp Ther. 2001;299:90–95. [PubMed] [Google Scholar]
- Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol. 2004;172:3860–3868. doi: 10.4049/jimmunol.172.6.3860. [DOI] [PubMed] [Google Scholar]
- Perkowski S, Scherpereel A, Murciano JC, Arguiri E, Solomides CC, Albelda SM, Muzykantov V, Christofidou-Solomidou M. Dissociation between alveolar transmigration of neutrophils and lung injury in hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1050–L1058. doi: 10.1152/ajplung.00067.2006. [DOI] [PubMed] [Google Scholar]
- Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J Immunol. 2000;165:1513–1519. doi: 10.4049/jimmunol.165.3.1513. [DOI] [PubMed] [Google Scholar]
- Rijneveld AW, Levi M, Florquin S, Speelman P, Carmeliet P, van der Poll T. reduced capacity to generate activated protein C have an unaltered pulmonary immune response to respiratory pathogens and lipopolysaccharide. Blood. 2004;103:1702–1709. doi: 10.1182/blood-2002-05-1380. [DOI] [PubMed] [Google Scholar]
- Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell BioI. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004;25:450–455. doi: 10.1016/j.it.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Juffermans NP, Florquin S, van Rooijen N, Vervoordeldonk MJ, Verbon A, van Deventer SJ, van der Poll T. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J Immunol. 2001;166:4604–4611. doi: 10.4049/jimmunol.166.7.4604. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Florquin S, Heikens M, Pals ST, van der Rooijen N, van der Poll T. CD44 is a macrophage binding site for Mycobacterium tuberculosis that mediates macrophage recruitment and protective immunity against tuberculosis. J Clin Invest. 2003;111:681–689. doi: 10.1172/JCI16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- Rijneveld AW, Weijer S, Florquin S, Esmon CT, Meijers JC, Speelman P, Reitsma PH, Ten Cate H, van der Poll T. Thrombomodulin mutant mice with a strongly reduced capacity to generate activated protein C have an unaltered pulmonary immune response to respiratory pathogens and lipopolysaccharide. Blood. 2004;103:1702–1709. doi: 10.1182/blood-2002-05-1380. [DOI] [PubMed] [Google Scholar]
- Weijer S, Schoenmakers SH, Florquin S, Levi M, Vlasuk GP, Rote WE, Reitsma PH, Spek CA, van der Poll T. Inhibition of the tissue factor/factor VIIa pathway does not influence the inflammatory or antibacterial response to abdominal sepsis induced by Escherichia coli in mice. J Infect Dis. 2004;189:2308–2317. doi: 10.1086/421031. [DOI] [PubMed] [Google Scholar]
- Barazzone C, Belin D, Piguet PF, Vassalli JD, Sappino AP. Plasminogen activator inhibitor-1 in acute hyperoxic mouse lung injury. J Clin Invest. 1996;98:2666–2673. doi: 10.1172/JCI119089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew E, Pun R, Simonich M, Iwamoto H, Dedman J. Cyclosporin A protects lung function from hyperoxic damage. Am J Physiol. 1999;276:L786–L795. doi: 10.1152/ajplung.1999.276.5.L786. [DOI] [PubMed] [Google Scholar]
- Henderson RF. Use of bronchoalveolar lavage to detect respiratory tract toxicity of inhaled material. Exp Toxicol Pathol. 2005;57 Suppl 1:155–159. doi: 10.1016/j.etp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, Grimminger F, Walmrath D, Temmesfeld-Wollbruck B, Seeger W. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:454–462. doi: 10.1164/ajrccm.161.2.9712038. [DOI] [PubMed] [Google Scholar]
- Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol. 2000;22:401–404. doi: 10.1165/ajrcmb.22.4.f184. [DOI] [PubMed] [Google Scholar]
- ter Horst SA, Walther FJ, Poorthuis BJ, Hiemstra PS, Wagenaar GT. Inhaled nitric oxide attenuates pulmonary inflammation and fibrin deposition and prolongs survival in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol. 2007;293:L35–L44. doi: 10.1152/ajplung.00381.2006. [DOI] [PubMed] [Google Scholar]
- Plesner T, Ploug M, Ellis V, Ronne E, Hoyer-Hansen G, Wittrup M, Pedersen TL, Tscherning T, Dano K, Hansen NE. The receptor for urokinase-type plasminogen activator and urokinase is translocated from two distinct intracellular compartments to the plasma membrane on stimulation of human neutrophils. Blood. 1994;83:808–815. [PubMed] [Google Scholar]
- Kim MH, Cho HS, Jung M, Hong MH, Lee SK, Shin BA, Ahn BW, Jung YD. Extracellular signal-regulated kinase and AP-1 pathways are involved in reactive oxygen species-induced urokinase plasminogen activator receptor expression in human gastric cancer cells. Int J Oncol. 2005;26:1669–1674. [PubMed] [Google Scholar]
- Doerschuk CM, Tasaka S, Wang Q. CD11/CD18-dependent and -independent neutrophil emigration in the lungs: how do neutrophils know which route to take? Am J Respir Cell Mol Biol. 2000;23:133–136. doi: 10.1165/ajrcmb.23.2.f193. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- Gyetko MR, Sitrin RG, Fuller JA, Todd RF, III, Petty H, Standiford TJ. Function of the urokinase receptor (CD87) in neutrophil chemotaxis. J Leukoc Biol. 1995;58:533–538. doi: 10.1002/jlb.58.5.533. [DOI] [PubMed] [Google Scholar]
- May AE, Kanse SM, Lund LR, Gisler RH, Imhof BA, Preissner KT. Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo. J Exp Med. 1998;188:1029–1037. doi: 10.1084/jem.188.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerschuk CM, Winn RK, Coxson HO, Harlan JM. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- Rijneveld AW, de Vos AF, Florquin S, Verbeek JS, van der Poll T. CD11b limits bacterial outgrowth and dissemination during murine pneumococcal pneumonia. J Infect Dis. 2005;191:1755–1760. doi: 10.1086/429633. [DOI] [PubMed] [Google Scholar]
- Keeney SE, Mathews MJ, Haque AK, Rudloff HE, Schmalstieg FC. Oxygen-induced lung injury in the guinea pig proceeds through CD18-independent mechanisms. Am J Respir Crit Care Med. 1994;149:311–319. doi: 10.1164/ajrccm.149.2.7905767. [DOI] [PubMed] [Google Scholar]
- Shasby DM, Fox RB, Harada RN, Repine JE. Reduction of the edema of acute hyperoxic lung injury by granulocyte depletion. J Appl Physiol. 1982;52:1237–1244. doi: 10.1152/jappl.1982.52.5.1237. [DOI] [PubMed] [Google Scholar]
- Boyce NW, Campbell D, Holdsworth SR. Granulocyte independence of pulmonary oxygen toxicity in the rat. Exp Lung Res. 1989;15:491–498. doi: 10.3109/01902148909087873. [DOI] [PubMed] [Google Scholar]
- Liao L, Ning Q, Li Y, Wang W, Wang A, Wei W, Liu X, Auten RL, Tanswell AK, Luo X. CXCR2 blockade reduces radical formation in hyperoxia-exposed newborn rat lung. Pediatr Res. 2006;60:299–303. doi: 10.1203/01.pdr.0000233058.08200.d6. [DOI] [PubMed] [Google Scholar]
- Chu EK, Cheng J, Foley JS, Mecham BH, Owen CA, Haley KJ, Mariani TJ, Kohane IS, Tschumperlin DJ, Drazen JM. Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2006;35:628–638. doi: 10.1165/rcmb.2006-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufort N, Leduc D, Eguchi H, Mengele K, Hellmann D, Masegi T, Kamimura T, Yasuoka S, Fend F, Chignard M, Pidard D. The human airway trypsin-like protease modulates the urokinase receptor (uPAR. CD87) structure and functions. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1263–L1272. doi: 10.1152/ajplung.00191.2006. [DOI] [PubMed] [Google Scholar]
- Gyetko MR, Sud S, Sonstein J, Polak T, Sud A, Curtis JL. Antigen-driven lymphocyte recruitment to the lung is diminished in the absence of urokinase-type plasminogen activator (uPA) receptor, but is independent of uPA. J Immunol. 2001;167:5539–5542. doi: 10.4049/jimmunol.167.10.5539. [DOI] [PubMed] [Google Scholar]