Abstract
Experimental autoimmune glomerulonephritis (EAG), an animal model of Goodpasture’s disease, can be induced in Wistar Kyoto (WKY) rats by immunization with the noncollagenous domain of the α 3 chain of type IV collagen, α3(IV)NC1. Recent studies have identified an immunodominant peptide, pCol (24-38), from the N-terminus of rat α3(IV)NC1; this peptide contains the major B- and T-cell epitopes in EAG and can induce crescentic nephritis. In this study, we investigated the mechanisms of mucosal tolerance in EAG by examining the effects of the nasal administration of this peptide after the onset of disease. A dose-dependent effect was observed: a dose of 300 μg had no effect, a dose of 1000 μg resulted in a moderate reduction in EAG severity, and a dose of 3000 μg produced a marked reduction in EAG severity accompanied by diminished antigen-specific, T-cell proliferative responses. These results demonstrate that mucosal tolerance in EAG can be induced by nasal administration of an immunodominant peptide from the N-terminus of α3(IV)NC1 and should be of value in designing new therapeutic strategies for patients with Goodpasture’s disease and other autoimmune disorders.
Goodpasture’s, or anti-glomerular basement membrane (GBM), disease is an autoimmune disorder characterized by rapidly progressive glomerulonephritis and lung hemorrhage.1,2 The disease is caused by autoimmunity to a component of the GBM, the non–collagenous domain of the α3 chain of type IV collagen, α3(IV)NC1.3,4 Epitope mapping studies have localized the immunodominant region for antibody binding to the amino terminal of the α3(IV)NC1 molecule.5,6 Experimental autoimmune glomerulonephritis (EAG), an animal model of Goodpasture’s disease, can be induced in susceptible strains of rats and mice by immunization with GBM7,8,9 or with recombinant α3(IV)NC1.10,11,12 This results in the development of circulating and deposited anti-GBM antibodies, with focal necrotizing crescentic glomerulonephritis and lung hemorrhage. EAG shares many features with the human disease, in that the renal and lung pathology are very similar,13 and the anti-GBM antibodies show the same specificity for the main target antigen, α3(IV)NC1.10,11,12
There is now compelling evidence for the role of both humoral and cell-mediated immunity in the pathogenesis of EAG. The pathogenic role of anti-GBM antibodies has been demonstrated in a variety of passive transfer studies.9,14,15,16 Transfer of disease has been demonstrated using antibodies pooled from the serum of nephritic mice,9 antibodies purified from the urine of nephritic rats,14 monoclonal antibodies derived from rats with EAG,15 and antibodies eluted from the kidney of nephritic rats.16 In the latter study, it was shown that deposited anti-GBM antibody has a higher functional affinity for GBM than circulating antibody.
The pathogenic role of T cells in EAG has also been demonstrated in several studies. T cells have been shown to be present in the glomeruli of animals with EAG,11,13 to proliferate in response to α3(IV)NC1,12,17 and to transfer disease to naive recipients.9,18 Glomerular T cells from rats with EAG show restricted T-cell receptor CDR3 spectratypes, demonstrating that they are an oligoclonal antigen-driven population.19 Anti-T-cell immunotherapy has been shown to be effective in preventing or ameliorating disease.20,21,22,23 Anti-CD4 mAb therapy is effective in the prevention of EAG,20 anti-CD8 mAb therapy is effective in both prevention and treatment of established disease,21 and inhibition of T-cell co-stimulation by blockade of either the CD28-B7 pathway22 or the CD154-CD40 pathway23 has been shown to reduce the severity of glomerulonephritis.
Further evidence supporting the role of T-cell-mediated cellular immunity in the pathogenesis of EAG is documented in recent studies demonstrating that synthetic peptides derived from α3(IV)NC1 can induce glomerulonephritis in WKY rats.24,25,26,27 Recent studies from our group have identified a 15-mer immunodominant peptide, pCol, (24-38) from the N-terminus of rat α3(IV)NC1, which contains the major B- and T-cell epitopes in EAG, and which can induce crescentic nephritis.24 Previous studies by Luo and colleagues25 showed that a 24-mer synthetic peptide, pCol, (28-51) from the N-terminus of α3(IV)NC1 was capable of inducing glomerulonephritis, although this was mild and inconsistent, whereas Wu and colleagues26 showed that a 13-mer peptide, pCol, (28-40) containing a T-cell epitope from α3(IV)NC1, induced severe crescentic glomerulonephritis. In further characterization of this T-cell epitope, it was shown that only three residues were critical for disease induction.27 In addition, it has been reported that peptides containing the T-cell epitope pCol (28-40) not only induced severe glomerulonephritis, but also triggered a diversified anti-GBM antibody response through B-cell epitope spreading, suggesting that the autoantibody response to GBM antigens could be induced by a single nephritogenic T-cell epitope.28,29,30
Mucosal tolerance is a phenomenon whereby peripheral immunological tolerance may be induced by the mucosal administration of autoantigens.31,32,33,34,35 The inhibitory effect of orally or nasally administered autoantigens, or immunodominant peptides, has been widely reported in experimental models of autoimmune disease in rodents, including encephalomyelitis,36,37,38 arthritis,39,40,41 myasthenia gravis,42,43 interstitial nephritis,44 and glomerulonephritis.9,45,46 In several of these studies, it has been shown that nasal administration of lower doses of antigen than those given orally has been effective in inducing mucosal tolerance,36,40,42 and in treating established disease.37,38,43 Our previous work in EAG has shown that both oral administration of GBM antigen45 and nasal administration of recombinant α3(IV)NC146 are effective in preventing the development of crescentic nephritis. However, neither of these studies demonstrated successful treatment of established EAG, which would clearly be more applicable to patients with glomerulonephritis.
In the present study, we have examined the effect of nasal administration of an immunodominant peptide, pCol, (24-38) from α3(IV)NC1 after the onset of disease in EAG. We show for the first time that mucosal tolerance and reduction in glomerular injury in established EAG can be induced by nasal administration of an immunodominant peptide. This work may lead to new antigen-specific treatment strategies for patients with anti-GBM disease and other autoimmune disorders.
Materials and Methods
Experimental Animals
Male WKY rats, age 8 to 10 weeks and weighing 120 to 150 g, were purchased from Charles River (Margate, UK). All animals were housed in standard conditions and had free access to normal laboratory diet and water. All experimental procedures were conducted in accordance with the UK Animals (Scientific Procedures) Act.
Production of Recombinant Rat α3(IV)NC1
Recombinant rat α3(IV)NC1 was produced from a stably transfected HEK293 cell line, as previously described.46 Purification of recombinant rat α3(IV)NC1 from the supernatant was performed by affinity chromatography using an anti-FLAG M2 affinity column (Sigma-Aldrich Company Ltd., Poole, UK). Recombinant rat α3(IV)NC1 was then characterized by Western blotting, using serum from an animal with EAG and control serum, as previously described.46
Production of Synthetic Peptides
Two 15-mer peptides from rat α3(IV)NC1, immunodominant peptide pCol (24-38) (FTRHSQTTANPSCPE) and control peptide pCol (38-52) (EGTQPLYSGFSLLFV) were synthesized by the Advanced Biotechnology Centre, Charing Cross Campus, Imperial College London, UK.
Immunization with Recombinant Rat α3(IV)NC1
Groups of WKY rats (n = 5 to 6) were given a single intramuscular injection of recombinant rat α3(IV)NC1 at a dose of 100 μg/rat in an equal volume of Freund’s complete adjuvant (FCA, Sigma-Aldrich Company Ltd.).11 Blood samples were taken by tail artery puncture under light anesthesia (isofluorane), and 24-hour urine specimens were obtained at different time points by placing animals in metabolic cages. All animals were sacrificed at day 28 after immunization.
Experimental Protocol for Nasal Administration of Peptides
Groups of WKY rats with EAG were given pCol (24-38) nasally at total cumulative doses of 300 μg (n = 6), 1000 μg (n = 6), or 3000 μg (n = 6), over 3 consecutive days after the onset of proteinuria (day 18 after immunization). In addition, positive control groups (POS) (n = 5) (immunized with α3(IV)NC1 in FCA) and negative control groups (NEG) (n = 5) (injected with FCA alone) were both given control peptide pCol (38-52) nasally at a total cumulative dose of 3000 μg, or saline alone.
Assessment of Disease
Serum Creatinine and Urea
Levels of creatinine and urea were measured in sera of experimental animals at day 28 after immunization on an Olympus AU2700 analyzer (Olympus Diagnostics, London, UK), as previously described.22,23 Creatinine was measured by a kinetic Jaffe method (alkaline picrate), and urea was measured using an enzymatic method (urease and glutamate dehydrogenase). All samples were analyzed in a single batch, to enable comparison between different groups.
Albuminuria
Urinary albumin concentrations were measured at different time points in 24-hour collections by rocket immunoelectrophoresis (Amersham Bioscience UK Limited, Little Chalfont, UK), as previously described.8,11,13 Briefly, urine samples from experimental animals were subjected to immunoelectrophoresis at 60 v in an electrophoresis tank containing Barbitone buffer (BDH Laboratory Supplies, Poole, UK), pH 9.5, for 6 hours, using a 1% agarose gel (BDH Laboratory Supplies) containing rabbit anti–sera to rat albumin raised in our laboratory. Results were calculated using rat serum albumin standards (which were run at the same time) and expressed in mg per 24 hours.
Glomerular Abnormalities
Kidney tissue was fixed in 10% neutral buffered formalin, processed, and embedded in paraffin wax for light microscopy by standard techniques (Histopathology Department, South Kensington Campus, Imperial College London). Briefly, 3-μm sections were stained with hematoxylin and eosin, and periodic acid-Schiff. Fifty glomeruli per section were assessed and graded by a blinded observer as: severe (extensive segmental necrosis/crescent formation), abnormal (segmental necrosis and/or extracapillary proliferation), or normal, and expressed as a percentage of glomeruli examined.13
Glomerular T Cells and Macrophages
Kidney sections were stained for T cells and macrophages using a standard avidin-biotin complex immunoperoxidase staining technique, as previously described.13 Briefly, formalin-fixed, paraffin-embedded kidney sections were stained with monoclonal antibodies W3/13 (T cells) and ED1 (macrophages) (Serotec Ltd., Kidlington, UK). Numbers of glomerular T cells and macrophages were detected using a biotinylated secondary antibody and avidin-biotin complex (DAKO Ltd., Cambridge, UK). The cellular infiltrate was assessed by a blinded observer, by counting the number of positively stained cells per 50 consecutive glomeruli in cross section.
Splenocyte Proliferation Assay
Splenocyte proliferative responses in the experimental animals were measured by standard tritiated thymidine incorporation assays, as previously described.45,46 Briefly, spleens were dissociated into a single cell suspension, and plated out in round-bottom 96-well plates (Invitrogen, Paisley, UK) at a concentration of 5 × 105 cells/well. Cells were then cultured with α3(IV)NC1 at a concentration of 10 μg/ml, in a humidified environment with 5% CO2 at 37°C for 72 hours. Tritiated thymidine (Amersham Bioscience UK Ltd.) was added at a concentration of 1 μCi/well at 16 hours before harvesting, and thymidine incorporation was measured using an automated β counter (Amersham Bioscience UK Ltd.). Results were expressed as a stimulation index, which was calculated by dividing the cpm in wells cultured with antigen by the cpm in wells with no antigen.
Circulating Anti-α3(IV)NC1 Antibody Concentrations
Circulating levels of IgG antibody were measured in sera of experimental animals at day 28 after immunization, by a solid-phase enzyme-linked immunosorbent assay (ELISA), as previously described.8,11,13 Briefly, recombinant rat α3(IV)NC1 was coated onto ELISA plates (Life Technologies, Paisley, UK) at a concentration of 5 μg/ml by overnight incubation at 4°C. An optimum dilution of test or control sera, as determined in preliminary experiments, was then applied for 1 hour at 37°C. Bound anti-α3(IV)NC1 antibody was detected by alkaline phosphatase-conjugated sheep anti-rat IgG (Sigma-Aldrich Company Ltd.), and developed using the substrate p-nitrophenyl phosphate (NPP, Sigma-Aldrich Company Ltd.). The absorbencies for each well were read at 405 nm using an Anthos Multiskan ELISA plate reader (Lab Tech International, Uckfield, UK), and the results calculated as mean optical density for each triplicate sample.
Deposition of IgG on the GBM
Deposits of IgG in the glomeruli were detected by direct immunofluorescence, as previously described.8,11,13 Tissue was embedded in OCT II embedding medium (Miles Inc., Elkhart, IN) on cork disks, snap-frozen in isopentane (BDH Laboratory Supplies), pre–cooled in liquid nitrogen, and stored at −70°C. Cryostat sections were cut at 5–μm in thickness and were incubated with fluorescein isothiocyanate-labeled rabbit anti-rat IgG (Serotec Ltd.). The degree of IgG deposition was assessed by a blinded observer, by grading the intensity of immunostaining from 0 to 3+ per 50 consecutive glomeruli in cross section.
Circulating Anti-α3(IV)NC1 Antibody Isotypes
Circulating levels of IgG1, IgG2a, IgG2b, and IgG2c antibodies were measured in sera of experimental animals at day 28 after immunization, by an indirect ELISA, as previously described.22,23 Briefly, recombinant rat α3(IV)NC1 was coated onto ELISA plates (Life Technologies), and sera applied, as described above. The isotypes of circulating anti-α3(IV)NC1 antibodies were detected by mouse monoclonal antibodies specific for rat IgG1 and IgG2a IgG2b and IgG2c (Serotec Ltd.), followed by goat anti-mouse, IgG (Serotec Ltd.). The levels of bound antibodies were detected by alkaline phosphatase-conjugated rabbit anti-goat IgG (Sigma-Aldrich Company Ltd.), and developed and analyzed as described above.
Statistical Analysis
Differences between data were determined by nonparametric Kruskal-Wallis test followed by a Dunn post–hoc test.
Results
Serum Creatinine and Urea
All positive control rats showed an increase in the level of serum creatinine and urea by day 28 after immunization. Animals treated with immunodominant peptide pCol (24-38) nasally at a total dose of 300 μg or 1000 μg also showed an increase in their creatinine and urea levels, whereas those given pCol (24-38) at 3000 μg showed no significant increase in the level of serum creatinine or urea, when compared with positive controls. Negative control animals showed no increase in the level of serum creatinine or urea. Results are shown in Figure 1, A and B.
Figure 1.
Effect of nasal administration of pCol (24-38) in groups of WKY rats (n = 5 to 6) after the onset of disease on serum creatinine (A) and serum urea (B). Results shown represent the mean ± SD of each group at day 28 after immunization. *P < 0.05, positive control (POS) versus pCol (24-38) 3000 μg nasally.
Albuminuria
All positive control rats immunized with recombinant rat α3(IV)NC1 in FCA, and given control peptide pCol (38-52) nasally at 3000 μg or saline alone, produced detectable levels of albuminuria by day 18 after immunization, which increased to high levels by day 28. Nasal administration of pCol (24-38) after the onset of albuminuria had a dose-dependent effect. Animals treated with pCol (24-38) nasally at 300 μg showed no reduction in albuminuria, whereas those given 1000 μg showed a moderate reduction, and those given 3000 μg showed a marked reduction, when compared with positive controls. Negative control animals given FCA alone, and given control peptide pCol (38-52) nasally at 3000 μg or saline alone, did not develop albuminuria. Results are shown in Figure 2A.
Figure 2.
Effect of nasal administration of pCol (24-38) in groups of WKY rats (n = 5 to 6) after the onset of disease on albuminuria at different time points after immunization: positive control (POS) (filled circles), negative control (NEG) (open circles), pCol (24-38) 300 μg nasally (filled squares), pCol (24-38) 1000 μg nasally (open squares), and pCol (24-38) 3000 μg nasally (filled triangles) (A) and glomerular abnormalities at day 28 after immunization: severe (extensive segmental necrosis/crescent formation), abnormal (segmental necrosis and/or extracapillary proliferation), or normal (B). Results shown represent the mean ± SD of each group. *P < 0.01, positive control (POS) versus pCol (24-38) 1000 μg nasally; **P < 0.001, positive control (POS) versus pCol (24-38) 3000 μg nasally.
Glomerular Abnormalities
Light microscopy of kidney tissue at day 28 revealed that all positive control rats developed extensive segmental necrosis of the glomerular tuft with crescent formation. Nasal administration of pCol (24-38) had a dose-dependent effect on the severity of the glomerular abnormalities. Animals treated with pCol (24-38) nasally at 300 μg showed no significant reduction in the level of segmental necrosis and/or crescent formation. By contrast, those given pCol(24-38) nasally at 1000 μg showed a moderate reduction in severity of the glomerular abnormalities, whereas those given pCol(24-38) at 3000 μg showed a marked reduction in the number and severity of the glomerular abnormalities, when compared with positive controls. Negative control animals showed normal renal histology. Results are shown in Figure 2B and illustrated in Figure 3, A and B.
Figure 3.
Kidney tissue at day 28 from WKY rats with EAG showing marked segmental necrosis of the glomerular tuft with crescent formation in a positive control animal by H&E (A); marked reduction in the severity of glomerular abnormalities in an animal given pCol (24-38) at 3000 μg nasally after the onset of disease (H&E) (B); large numbers of macrophages in a cellular crescent in a positive control animal by immunoperoxidase (IP) (C); marked reduction in the number of glomerular macrophages in an animal given pCol (24-38) at 3000 μg nasally after the onset of disease (IP) (D); strong linear deposits of IgG on the GBM in a positive control animal by direct immunofluorescence (DIF) (E); and no reduction in the deposition of IgG on the GBM in an animal given pCol (24-38) at 3000 μg nasally after the onset of disease (DIF) (F). Original magnifications, ×300.
Glomerular T Cells and Macrophages
Immunohistochemistry of kidney tissue at day 28 showed that positive control rats had a large number of glomerular T cells and macrophages within the glomerular tuft and crescents. Animals treated with pCol (24-38) nasally at 300 μg or 1000 μg showed no significant reduction in the numbers of T cells and macrophages infiltrating the glomeruli, whereas those given pCol (24-38) at 3000 μg showed a significant reduction in the number of glomerular T cells and macrophages, when compared with positive controls. Negative control animals showed no cellular infiltrate. Results are shown in Figure 4, A and B, and illustrated in Figure 3, C and D.
Figure 4.
Effect of nasal administration of pCol(24-38) in groups of WKY rats (n = 5 to 6) after the onset of disease on the number of glomerular T cells (A) and glomerular macrophages (B). Results shown represent the mean ± SD of each group at day 28 after immunization. *P < 0.015; **P < 0.02, positive control (POS) versus pCol (24-38) 3000 μg nasally.
Splenocyte Proliferative Responses
Spleen cells from positive control rats showed an increased proliferative response when cultured with α3(IV)NC1 in vitro at a concentration of 10 μg/ml. Splenocytes from animals treated with pCol (24-38) nasally at 3000 μg showed a marked reduction in proliferation, when compared with positive controls. Negative control animals showed only background proliferative responses. Results are shown in Table 1.
Table 1.
Effect of Nasal Administration of Immunodominant Peptide pCol(24-38) on the in Vitro Splenocyte Proliferative Responses from Groups of Experimental Rats Cultured with α3(IV)NC1
| Experimental groups | Stimulation index |
|---|---|
| Positive control (n = 5) | 2.7 ± 0.4 |
| Negative control (n = 5) | 0.85 ± 0.3 |
| pCol(24-38) (3000 μg) (n = 6) | 0.76 ± 0.2* |
Results shown represent the mean ± SD of each group at day 28 after immunization.
P < 0.004, positive control versus pCol(24-38) 3000 μg nasally.
Circulating Anti-α3(IV)NC1 Antibody Concentrations
All positive control rats produced high levels of circulating anti-α3(IV)NC1 IgG antibody by day 28 after immunization. Animals treated with immunodominant peptide pCol (24-38) nasally at a total dose of 300 μg, 1000 μg, or 3000 μg showed no significant reduction in the level of circulating IgG antibodies, when compared with positive controls. Negative control animals showed no increase in the level of circulating IgG antibody. Results are shown in Figure 5A.
Figure 5.
Effect of nasal administration of pCol (24-38) in groups of WKY rats (n = 5 to 6) after the onset of disease on circulating levels of IgG antibodies directed toward α3(IV)NC1 (A) and glomerular deposits of IgG on the GBM (B). Results shown represent the mean ± SD of each group at day 28 after immunization.
Deposits of IgG on the GBM
Direct IF for IgG on kidney tissue day at 28 after immunization revealed that positive control rats showed strong linear deposits of IgG along the GBM. Animals treated with pCol (24-38) nasally at a total dose of 300 μg, 1000 μg, or 3000 μg showed no significant reduction in the deposits of IgG on the GBM, when compared with positive controls. Negative control animals showed no IgG deposition. Results are shown in Figure 5B and illustrated in Figure 3, E and F.
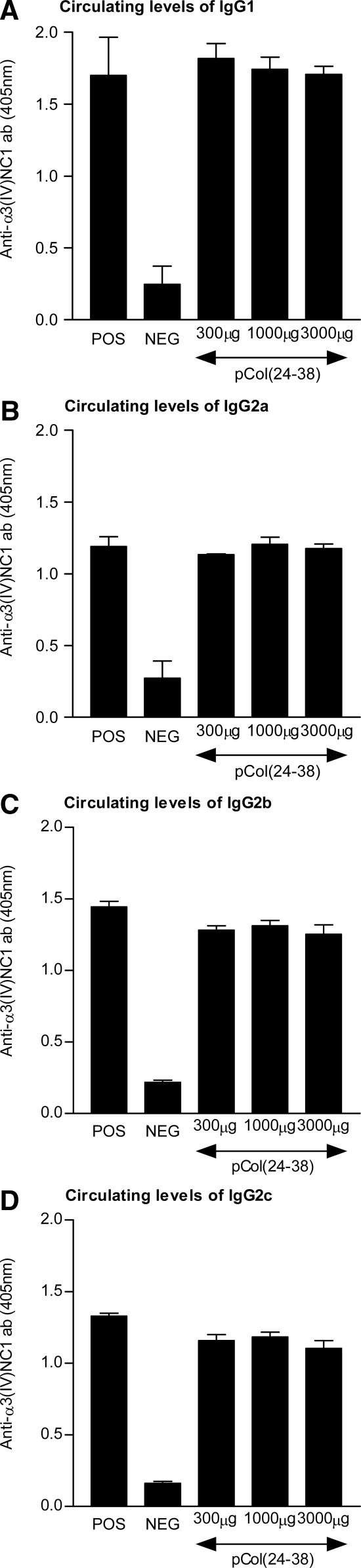
Circulating Anti-α3(IV)NC1 Antibody Isotypes
All positive control rats produced high levels of circulating anti-α3(IV)NC1 IgG1, IgG2a, IgG2b, and IgG2c antibody by day 28 after immunization. Animals treated with immunodominant peptide pCol (24-38) nasally at a total dose of 300 μg, 1000 μg, or 3000 μg showed no significant reduction in the levels of IgG1, IgG2a, IgG2b, or IgG2c antibody, when compared with positive controls. Negative control animals showed no increase in the level of circulating isotypes. Results are shown in Figure 6.
Figure 6.
Effect of nasal administration of pCol (24-38) in groups of WKY rats (n = 5 to 6) after the onset of disease on levels of circulating IgG1 (A), IgG2a (B), IgG2b (C), and IgG2c (D) anti-α3(IV)NC1 antibody concentrations. Results shown represent the mean ± SD of each group at day 28 after immunization.
Discussion
Although our previous studies in EAG demonstrated that oral administration of GBM antigen45 or nasal administration of recombinant α3(IV)NC146 were effective in preventing the development of crescentic nephritis, neither approach was successful therapeutically. Successful treatment of established EAG would clearly be more relevant to the management of patients with anti-GBM disease. To investigate further the potential of mucosal tolerance induced by immunodominant peptides in the treatment of established disease, we examined the effect of nasal administration of pCol (24-38)24 after the onset of disease in EAG induced by recombinant α3(IV)NC1 in FCA.
A clear-cut dose-dependent effect of nasal administration of pCol (24-38) was observed. A dose of 300 μg had no effect on the development of disease, 1000 μg resulted in a moderate reduction in the severity of nephritis, and 3000 μg led to a marked reduction in albuminuria, serum creatinine, and urea, severity of glomerular abnormalities, numbers of glomerular T cells and macrophages, and in vitro T-cell proliferative responses to α3(IV)NC1. In other models of autoimmunity, nasal administration of synthetic immunodominant peptides has been shown to be more effective than crude preparations of antigen in the treatment of established disease.37,38,43 This may be because peptides containing defined T-cell epitopes induce a more specific regulatory immune response than complex antigens. Previous studies have also shown that higher doses of peptides are necessary to treat established disease than to induce disease.37,43 In the present study, six times more peptide was necessary to treat established disease (3000 μg) than to induce disease (500 μg),24 confirming previous results.
Nasal administration of pCol (24-38) seemed to have a preferential inhibitory effect on cell-mediated immunity. There was a significant reduction in the number of infiltrating glomerular T cells and macrophages, despite the presence of deposited antibody, suggesting that nasal administration of pCol (24-38) had a direct effect on cell-mediated immunity within the glomerulus. In relation to this, a marked reduction in the number and severity of glomerular abnormalities was observed, especially in crescent formation, along with the preservation of renal function. Furthermore, we demonstrated a reduced proliferative response of splenocytes from the animals treated with pCol (24-38) nasally to recombinant α3(IV)NC1 in vitro, suggesting that tolerance had been induced at the level of autoreactive T cells directed toward the autoantigen. By contrast, nasal administration of pCol (24-38) had no discernable effect on humoral immunity. This may be because both circulating and deposited antibodies are well established by day 18 in our model, and there may have been insufficient time to see any inhibitory effect of pCol(24-38) on humoral immunity by day 28.
The mechanisms by which mucosal tolerance to autoantigens is mediated remain unclear, but a major factor seems to be the dose of antigen administered.30,31,32,33,34,35 High doses of antigen may induce clonal deletion or anergy, whereas a regimen of multiple feeding with low doses of antigen favors the induction of regulatory cells, whose suppressive activities are mediated through the production of anti-inflammatory cytokines. In low-dose tolerance studies, several types of regulatory cells have been reported to be involved in the induction of mucosal tolerance. The balance between Th1 and Th2 cells has been shown to be important in the induction of mucosal tolerance.47,48 The role of transforming growth factor-β-producing Th3 cells found within gut-associated lymphoid tissue, which are triggered in an antigen-specific manner, has also been well studied.49,50 However, more recent evidence suggests a role for interleukin-10-producing CD4+CD25+ regulatory T cells (Tr1) in mucosal tolerance.51,52 Finally, it is important to consider the pivotal role of different populations of mucosal dendritic cells in mucosal tolerance.53,54 Dendritic cells from the nasal-associated lymphoid tissue have been shown to induce Tr1 cells to produce interleukin-10, whereas dendritic cells from the gut-associated lymphoid tissue induced Th3 cells to produce transforming growth factor-β. Thus, there may be functional links between different regulatory T-cell subsets involved in mucosal tolerance, despite the fact that they originate from different compartments and display different cytokine profiles. The involvement of regulatory T cells in mucosal tolerance in our model is under investigation.
Mucosal tolerance is an attractive approach for the treatment of autoimmune disease because of lack of toxicity, ease of administration throughout time, and antigen-specific mechanisms of action. However there is a theoretical risk of exacerbating disease. Antigenic form, for example, whole protein or synthetic peptide, dose of antigen, and route of administration are critical factors in the efficacy of mucosally induced tolerance.31,32 Clinical trials involving the treatment of multiple sclerosis by myelin,31 and rheumatoid arthritis by type II collagen,32 have never progressed beyond phase II. Although such trials have not been associated with significant toxicity or exacerbation of disease, the results have not demonstrated clinical efficacy. However, these trials involved the administration of crude preparations of antigenic protein via the oral route, requiring high doses of antigen. The focus has now turned to the use of soluble peptides containing defined T-cell epitopes that can be administered nasally at much lower doses than antigenic proteins. Development of antigen-specific treatment strategies for patients with autoimmune disease is of great clinical relevance because current pharmaceutical treatments are nonspecific and have many undesirable side effects.
In conclusion, we have demonstrated for the first time that therapeutic nasal administration of an immunodominant peptide from the N-terminus of rat α3(IV)NC1, pCol, (24-38) produces mucosal tolerance in EAG induced by the whole antigen. The most likely explanation for these findings is that nasal administration of pCol (24-38) has resulted in the induction of regulatory T cells. These findings should be of value in designing new antigen-specific therapeutic strategies for patients with anti-GBM disease and other autoimmune disorders.
Acknowledgments
We thank John Meek, Department of Clinical Chemistry, Hammersmith Hospital, London, UK, for analyzing the serum creatinine and urea.
Footnotes
Address reprint requests to Dr. John Reynolds, Renal Section, Division of Medicine, Imperial College London, Hammersmith Campus, Du Cane Rd., London W12 ONN, UK. E-mail: john.reynolds@imperial.ac.uk.
Supported by The Sir Jules Thorn Charitable Trust (grant 02/03A) and the Hammersmith Hospital Charity Trustees.
This work was presented in part at the 38th Annual Meeting of the American Society of Nephrology, Philadelphia, November 13, 2005.
References
- Goodpasture EW. The significance of certain pulmonary lesions to the etiology of influenza. Am J Med Sci. 1919;158:863–870. [Google Scholar]
- Wilson CB, Dixon FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973;3:74–89. doi: 10.1038/ki.1973.14. [DOI] [PubMed] [Google Scholar]
- Saus J, Wieslander J, Langeveld JPM, Quinones S, Hudson BG. Identification of the Goodpasture antigen as the α3 chain of collagen IV. J Biol Chem. 1988;263:13374–13380. [PubMed] [Google Scholar]
- Turner N, Mason PJ, Brown R, Fox M, Povey S, Rees AJ, Pusey CD. Molecular cloning of the human Goodpasture antigen demonstrates it to be the α3 chain of type IV collagen. J Clin Invest. 1992;89:592–601. doi: 10.1172/JCI115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JJ, Mason PJ, Pusey CD, Turner N. Recombinant α-chains of type IV collagen demonstrate that the amino terminal of the Goodpasture antigen is critical for antibody binding. Clin Exp Immunol. 1998;113:17–27. doi: 10.1046/j.1365-2249.1998.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer KO, Leinonen A, Boutaud A, Borza DB, Todd P, Gunwar S, Langeveld JP, Hudson BG. The Goodpasture autoantigen: mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17-31 and 127-141 of the NC1 domain. J Biol Chem. 1999;274:11267–11274. doi: 10.1074/jbc.274.16.11267. [DOI] [PubMed] [Google Scholar]
- Sado Y, Okigaki T, Takamiya H, Seno S. Experimental autoimmune glomerulonephritis with pulmonary haemorrhage in rats. The dose-effect relationship of the nephritogenic antigen from bovine glomerular basement membrane. J Clin Lab Immunol. 1984;15:199–204. [PubMed] [Google Scholar]
- Reynolds J, Mavromatidis K, Cashman SJ, Evans DJ, Pusey CD. Experimental autoimmune glomerulonephritis (EAG) induced by homologous and heterologous glomerular basement membrane in two sub-strains of Wistar Kyoto rat. Nephrol Dial Transplant. 1998;13:44–52. doi: 10.1093/ndt/13.1.44. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Danoff TM, Okada H, Neilson EG. Susceptibility to anti-glomerular basement membrane disease and Goodpasture’s syndrome is linked to MHC class II genes and the emergence of T cell-mediated immunity in mice. J Clin Invest. 1997;100:2263–2275. doi: 10.1172/JCI119764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sado Y, Boutaud AA, Kagawa M, Naito I, Ninomiya Y, Hudson BG. Induction of anti-GBM nephritis in rats by recombinant α3(IV) NC1 and α4(IV) NC1 of type IV collagen. Kidney Int. 1998;53:664–671. doi: 10.1046/j.1523-1755.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Reynolds J, Norgan VA, Pusey CD. Expression and characterisation of recombinant rat α3(IV)NC1 and its use in the induction of experimental autoimmune glomerulonephritis. Nephrol Dial Transplant. 2001;16:253–261. doi: 10.1093/ndt/16.2.253. [DOI] [PubMed] [Google Scholar]
- Hopfer H, Maron R, Butzmann U, Helmchen U, Weiner HL, Kalluri R. The importance of cell-mediated immunity in the course and severity of autoimmune anti-glomerular basement membrane disease in mice. FASEB J. 2003;17:860–868. doi: 10.1096/fj.02-0746com. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Moss J, Duda MA, Smith J, Karkar AM, Macherla V, Shore I, Evans DJ, Woodrow DF, Pusey CD. The evolution of crescentic nephritis and alveolar haemorrhage following induction of autoimmunity to glomerular basement membrane in an experimental model of Goodpasture’s disease. J Pathol. 2003;200:118–129. doi: 10.1002/path.1336. [DOI] [PubMed] [Google Scholar]
- Sado Y, Naito I, Okigaki T. Transfer of anti-glomerular basement membrane antibody-induced glomerulonephritis in inbred rats with isologous antibodies from urine of nephritic rats. J Pathol. 1989;158:325–332. doi: 10.1002/path.1711580410. [DOI] [PubMed] [Google Scholar]
- Kohda T, Okada S, Hayashi A, Kanzaki S, Ninomiya Y, Taki M, Sado Y. High nephritogenicity of monoclonal antibodies belonging to IgG2a and IgG2b subclasses in rat anti-GBM nephritis. Kidney Int. 2004;66:177–186. doi: 10.1111/j.1523-1755.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Albouainain A, Duda MA, Evans DJ, Pusey CD. Strain susceptibility to active induction and passive transfer of experimental autoimmune glomerulonephritis in the rat. Nephrol Dial Transplant. 2006;21:3398–3408. doi: 10.1093/ndt/gfl523. [DOI] [PubMed] [Google Scholar]
- Wu J, Hicks J, Ou C, Singleton D, Borillo J, Lou Y-H. Glomerulonephritis induced by recombinant collagen IV alpha 3 chain noncollagen domain 1 is not associated with glomerular basement membrane antibody: a potential T cell-mediated mechanism. J Immunol. 2001;167:2388–2395. doi: 10.4049/jimmunol.167.4.2388. [DOI] [PubMed] [Google Scholar]
- Wu J, Hicks J, Borillo J, Glass WF, Lou Y-H. CD4+ T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest. 2002;109:517–525. doi: 10.1172/JCI13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters G, Habib A-M, Reynolds J, Wu H, Knight JF, Pusey CD. Glomerular T cells are of restricted clonality and express multiple CDR3 motifs across different Vβ T cell receptor families in experimental autoimmune glomerulonephritis. Nephron Exp Nephrol. 2004;98:71–81. doi: 10.1159/000080682. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Pusey CD. In vivo treatment with a monoclonal antibody to T helper cells in experimental autoimmune glomerulonephritis. Clin Exp Immunol. 1994;95:122–127. doi: 10.1111/j.1365-2249.1994.tb06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J, Norgan VA, Bhambra U, Smith J, Cook HT, Pusey CD. Anti-CD8 monoclonal antibody therapy is effective in the prevention and treatment of experimental autoimmune glomerulonephritis. J Am Soc Nephrol. 2002;13:359–369. doi: 10.1681/ASN.V132359. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Tam FWK, Chandraker A, Smith J, Karkar AM, Cross J, Peach R, Sayegh MH, Pusey CD. CD28-B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J Clin Invest. 2000;105:643–651. doi: 10.1172/JCI6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J, Khan SB, Allen AR, Benjamin CD, Pusey CD. Blockade of the CD154-CD40 T cell costimulatory pathway prevents the development of experimental autoimmune glomerulonephritis. Kidney Int. 2004;66:1444–1452. doi: 10.1111/j.1523-1755.2004.00907.x. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Haxby J, Juggapah JK, Evans DJ, Pusey CD. Identification of a nephritogenic immunodominant B and T cell epitope in experimental autoimmune glomerulonephritis. Clin Exp Immunol. 2008;155:311–319. doi: 10.1111/j.1365-2249.2008.03833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AM, Fox JW, Chen L, Bolton WK. Synthetic peptides of Goodpasture’s antigen in anti-glomerular basement membrane nephritis in rats. J Lab Clin Med. 2002;139:303–310. doi: 10.1067/mlc.2002.123623. [DOI] [PubMed] [Google Scholar]
- Wu J, Borillo J, Glass WF, Hicks J, Ou C-N, Lou Y-H. T-cell epitope of α3 chain of type IV collagen induces severe glomerulonephritis. Kidney Int. 2003;64:1292–1301. doi: 10.1046/j.1523-1755.2003.00227.x. [DOI] [PubMed] [Google Scholar]
- Robertson J, Wu J, Arends J, Glass W, Southwood S, Sette A, Lou Y-H. Characterization of the T-cell epitope that causes anti-GBM glomerulonephritis. Kidney Int. 2005;68:1061–1070. doi: 10.1111/j.1523-1755.2005.00498.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Ardens J, Borillo J, Zhou C, Merszei J, McMahon J, Lou Y-H. A self T cell epitope induces autoantibody response: mechanism for production of antibodies to diverse glomerular basement membrane antigens. J Immunol. 2004;172:4567–4574. doi: 10.4049/jimmunol.172.7.4567. [DOI] [PubMed] [Google Scholar]
- Bolton WK, Chen L, Hellmark T, Wieslander J, Fox J. Epitope spreading and autoimmune glomerulonephritis in rats induced by a T cell epitope of Goodpasture’s antigen. J Am Soc Nephrol. 2005;16:2657–2666. doi: 10.1681/ASN.2004100823. [DOI] [PubMed] [Google Scholar]
- Chen L, Hellmark T, Pedchenko V, Hudson BJ, Pusey CD, Fox JW, Bolton WK. A nephritogenic peptide induces intermolecular epitope spreading on collagen IV in experimental autoimmune glomerulonephritis. J Am Soc Nephrol. 2006;17:3076–3081. doi: 10.1681/ASN.2006070688. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Current issues in the treatment of human diseases by mucosal tolerance. Ann NY Acad Sci. 2004;1029:211–224. doi: 10.1196/annals.1309.053. [DOI] [PubMed] [Google Scholar]
- Staines NA, Derry CJ, Marinova-Mutafchieva L, Ali N, Davis DH, Murphy JJ. Constraints on the efficacy of mucosal tolerance in the treatment of human and animal arthritis diseases. Ann NY Acad Sci. 2004;1029:250–259. doi: 10.1196/annals.1309.056. [DOI] [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- Nicolson KS, Wraith DC. Natural and induced regulatory T cells: targets for immunotherapy in autoimmune disease and allergy. Inflamm Allergy Drug Targets. 2006;5:141–148. doi: 10.2174/187152806778256098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-L, Liu J-Q, Bai X-F, Van der Meide PH, Link H. Dose-dependent mechanisms relate to nasal tolerance induction and protection against experimental autoimmune encephalomyelitis in Lewis rats. Immunology. 1998;94:431–437. doi: 10.1046/j.1365-2567.1998.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X-F, Li H-L, Shi F-D, Liu J-Q, Xiao B-G, Van der Meide PH, Link H. Complexities of applying nasal tolerance induction as a therapy for ongoing relapsing experimental autoimmune encephalomyelitis (EAE) in DA rats. Clin Exp Immunol. 1998;111:205–210. doi: 10.1046/j.1365-2249.1998.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler B, Wraith DC. Inhibition of T-cell responsiveness by nasal peptide administration: influence of the thymus and differential recovery of T-cell-dependent functions. Immunology. 1999;97:257–263. doi: 10.1046/j.1365-2567.1999.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines NA, Harper N, Ward FJ, Malmstrom V, Holmdahl R, Bensal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal inhalation of synthetic peptide 184-198 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin Exp Immunol. 1996;103:368–375. doi: 10.1111/j.1365-2249.1996.tb08289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G, Komagata Y, Slavin AJ, Maron R, Weiner HL. Suppression of collagen-induced arthritis by oral or nasal administration of type II collagen. J Autoimmun. 1999;13:315–324. doi: 10.1006/jaut.1999.0320. [DOI] [PubMed] [Google Scholar]
- Derry CJ, Harper N, Davies DH, Murphy JJ, Staines NA. Importance of dose of type II collagen in suppression of collagen-induced arthritis by nasal tolerance. Arthritis Rheum. 2001;44:1917–1927. doi: 10.1002/1529-0131(200108)44:8<1917::AID-ART330>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Barchan D, Souroujon MC, Im SH, Antozzi C, Fuchs S. Antigen-specific modulation of experimental myasthenia gravis: nasal tolerisation with recombinant fragments of the human acetylcholine receptor alpha-subunit. Proc Natl Acad Sci. 1999;96:8086–8091. doi: 10.1073/pnas.96.14.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S-H, Barchan D, Fuchs S, Souroujon MC. Mechanism of nasal tolerance induced by recombinant fragment of acetylcholine receptor for treatment of experimental myasthenia gravis. J Neuroimmunol. 2000;111:161–168. doi: 10.1016/s0165-5728(00)00395-7. [DOI] [PubMed] [Google Scholar]
- Pham K, Smoyer WE, Archer DC, Gabbai F, Kelly CJ. Oral feeding of renal tubular antigen abrogates interstitial nephritis and renal failure in Brown Norway rats. Kidney Int. 1997;52:725–732. doi: 10.1038/ki.1997.388. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Pusey CD. Oral administration of glomerular basement membrane prevents the development of experimental autoimmune glomerulonephritis. J Am Soc Nephrol. 2001;12:61–70. doi: 10.1681/ASN.V12161. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Prodromidi EI, Juggapah JK, Abbott DS, Holthaus KA, Kalluri R, Pusey CD. Nasal administration of recombinant rat α3(IV)NC1 prevents the development of experimental autoimmune glomerulonephritis. J Am Soc Nephrol. 2005;16:1350–1359. doi: 10.1681/ASN.2004121026. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–573. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- Van Eden W, Van Der Zee R, Van Kooten P, Berlo SE, Cobelens PM, Kavelaars A, Heijnen CJ, Prakken B, Roord S, Albani S. Balancing the immune system: Th1 and Th2. Ann Rheum Dis. 2002;61:ii25-ii28. doi: 10.1136/ard.61.suppl_2.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- Faria AM, Maron R, Ficker SM, Slavin AJ, Spahn T, Weiner HL. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor-beta/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20:135–145. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- Unger WW, Jansen W, Wolvers DA, Van Halteren AG, Kraal G, Samsom JN. Nasal tolerance induces antigen-specific CD4+CD25+ regulatory T cells that can transfer their regulatory capacity to naïve CD4+ T cells. Int Immunol. 2003;15:731–739. doi: 10.1093/intimm/dxg069. [DOI] [PubMed] [Google Scholar]
- Mowat AM, Donachie AM, Parker LA, Robson NC, Beacock-Sharp H, McIntyre LJ, Millington O, Chirdo F. The role of dendritic cells in regulating mucosal immunity and tolerance. Novartis Found Symp. 2003;252:291–302. doi: 10.1002/0470871628.ch22. [DOI] [PubMed] [Google Scholar]
- Weiner HL. The mucosal milieu creates tolerogenic dendritic cells and T(R)1 and T(H)3 regulatory cells. Nat Immunol. 2001;2:671–672. doi: 10.1038/90604. [DOI] [PubMed] [Google Scholar]