Abstract
Prominent eosinophil infiltration is a characteristic of some forms of vasculitis, such as Churg-Strauss syndrome, also known as allergic granulomatous vasculitis. In the current study, we established a mouse model of cutaneous eosinophilic vasculitis by the cutaneous reverse passive Arthus reaction using IgE injection instead of IgG. Wild-type C57BL/6 mice were injected with IgE anti-trinitrophenyl antibodies, followed immediately by intravenous administration of trinitrophenyl bovine serum albumin. IgE-mediated immune complex challenge induced substantial hemorrhage with marked infiltration of eosinophils in which neutrophils, mast cells, and macrophages were also mixed. This finding contrasted remarkably with the neutrophil-dominant infiltration pattern in IgG-mediated immune complex challenge. In the lesion, the expression level of monocyte chemotactic protein-3 was increased, and anti-monocyte chemotactic protein-3 treatment resulted in a significant but incomplete blockade of eosinophil recruitment. Furthermore, mice lacking E-selectin, P-selectin, L-selectin, or intercellular adhesion molecule-1, as well as wild-type mice that received anti-vascular cell adhesion molecule-1-blocking antibodies were assessed for the IgE-mediated Arthus reaction. After 24 hours, the loss of P-selectin resulted in a significant reduction in eosinophil accumulation compared with both wild-type mice and other mouse mutants. Collectively, the Fc class of immunoglobulins, which forms these immune complexes, critically determines the disease manifestation of vasculitis. The IgE-mediated cutaneous reverse passive Arthus reaction may serve as an experimental model for cutaneous eosinophilic infiltration in vasculitis as well as in other diseases.
Eosinophilia is associated with a variety of infectious, allergic, and inflammatory diseases, including helminth infection, asthma, allergic rhinitis, atopic skin diseases, and inflammatory bowel disease, as well as idiopathic hypereosinophilic syndrome.1,2 Eosinophils also play a major role in some forms of vasculitis.3,4 The most prominent example is Churg-Strauss syndrome (CSS; also known as allergic granulomatous vasculitis), which occurs in patients with a history of asthma and is characterized by a necrotizing vasculitis of small arteries and veins, with extravascular granulomas and a marked eosinophilia in the lesion and in the peripheral blood.3,5,6,7 Activated eosinophils induce tissue destruction and inflammation, while the mechanism that generates eosinophilic vasculitis remains unclear. Several lines of evidence have suggested a role of IgE in CSS. Serum IgE levels are elevated and often correlate with disease activity in CSS. IgE deposition in affected blood vessels is also observed occasionally.8 Therefore, the pathogenesis of CSS may involve IgE through immune complex (IC) deposition containing IgE.
IC-mediated tissue injury has been implicated in the pathogenesis of vasculitis syndrome. The classical experimental model for such IC-mediated tissue injury is the Arthus reaction, which induces edema and hemorrhage in the skin.9,10 Currently, most the frequently used is the reverse passive Arthus reaction, in which IgG antibody (Ab) is injected at the site where the investigator wishes the inflammatory response to develop, followed immediately by an intravenous application of the antigen.9,10,11,12,13 IgG-induced IC-mediated vascular tissue damage requires the accumulation of neutrophils and mast cells.11,12,14,15,16,17,18 This process is tightly regulated by chemotactic factors and adhesive interactions between leukocytes and the vascular endothelium, including selectins, integrins, and Ig superfamily members, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1).19,20,21 Nonetheless, it remains unknown which molecules are responsible for the development of eosinophilic vasculitis.
In this study, an experimental mouse model of cutaneous eosinophilic vasculitis was established using the IgE-mediated cutaneous reverse passive Arthus reaction. This model developed a marked accumulation of eosinophils surrounding the blood vessels, resulting in substantial hemorrhage. Thus, this model demonstrates that the Fc class of Ig that forms IC can critically determine the disease manifestation.
Materials and Methods
Mice
C57BL/6 mice, P-selectin−/−,22 and E-selectin−/−23 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). L-selectin−/− mice were produced as described previously.24 ICAM-1−/− mice,25 expressing residual amounts of ICAM-1 splice variants in the thymus and spleen but not in other organs including skin,26 were also obtained from The Jackson Laboratory. All mice were healthy, fertile, and did not display evidence of infection or disease. All mice were backcrossed for 10 generations into the C57BL/6 background. Mice used for experiments were 12 to 16 weeks old. All mice were housed in a pathogen-free barrier facility and screened regularly for pathogens. All studies and procedures were approved by the Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Science.
Cutaneous Reverse Passive Arthus Reaction
For the cutaneous reverse passive Arthus reaction,11,12,13 mice were anesthetized by inhalation of diethyl ether, shaved on their abdominal skin, and wiped with 70% ethanol. Mouse IgE or IgG anti-trinitrophenyl (TNP) Abs (66 μg/30 μl; BD Biosciences, San Diego, CA) were injected once intradermally into the abdominal skin of the mice using a 29-gauge needle, followed immediately by i.v. injection of 2,4,6-trinitrophenyl bovine serum albumin (TNP-BSA, 800 μg/400 μl; LSL, Japan) in PBS via the tail vein. The intradermal injection of mouse IgE or IgG anti-TNP Abs (66 μg/30 μl) followed by i.v. installation of BSA in PBS served as a control. For a blocking study using mAbs against VCAM-1 (Clone 429 MVCAM.A, rat IgG2a, 30 μg per mouse; BD Biosciences, San Diego, CA), mAb were given as an i.v. injection 30 minutes before IC challenge.13 For a blocking study using goat polyclonal Abs against monocyte chemotactic protein (MCP)-3 (50 μg per mouse; Abcam, Cambridge, UK), anti-MCP-3 Abs were give as an i.v. injection 30 minutes before IC challenge. Goat polyclonal IgG (50 μg per mouse; Sigma, St. Louis, MO) served as a control.
The amount of hemorrhage was assessed at 24 hours after IC challenge. Mice were sacrificed, and the skin containing the injection site was removed at the level of the fascia above the skeletal muscle and was reversed. The diameters of purpuric spots and bleeding vessels on the fascia side of the injection site were measured by using Adobe Photoshop 5.0 software. The diameters of the major and minor axes of the purpuric spots were averaged. Then, the ratio of purpuric spot diameter/bleeding vessel diameter was evaluated.
Histological Examination and Immunohistochemical Staining
Tissues were harvested before and at 4, 8, 12, 24, 48, and 72 hours after IC challenge using a disposable sterile 6-mm punch biopsy blade (Maruho, Osaka, Japan) and assessed for tissue damage and the numbers of infiltrating eosinophils, mast cells, and neutrophils. Tissue samples were cut into halves, fixed in 3.5% paraformaldehyde, and then embedded in paraffin. Six-micron sections were stained using H&E for inflammatory cell evaluation and toluidine blue for mast cell evaluation. Hansel staining was used for eosinophil evaluation.27 To identify neutrophils, polyclonal anti-myeloperoxidase (anti-MPO; Thermo Scientific, Fremont, CA) Abs were used on paraffin-embedded sections by the following procedure.
For immunohistochemistry, frozen tissue sections were acetone fixed and incubated with 10% normal rabbit serum in PBS to block nonspecific staining, followed by the incubation with rat monoclonal Abs; anti-mouse Siglec-F (E50–2440; BD Biosciences), anti-mouse Gr-1 (RB6–8C5; BD Biosciences), or macrophage-specific F4/80 Abs (BD Biosciences).28 Rat IgG was used as a control for non-specific staining. Sections were incubated with a biotinylated secondary Abs and then horseradish peroxidase-conjugated avidin (Vector Laboratories, Burlingame, CA).
Eosinophil, mast cell, and neutrophil infiltration was evaluated by counting extravascular cells in the entire section and averaging the numbers present in eight serial skin sections from the injection site. Each section was examined independently by three investigators in a blinded fashion, and the mean was used for analysis.
RNA Isolation and Real-Time Reverse Transcription-PCR
Skin samples were harvested before and 12 and 24 hours after IC challenge, and total RNA was isolated from frozen skin specimens using QIAGEN RNeasy spin columns (QIAGEN Ltd., Hilden, Germany) and digested using DNase I (QIAGEN Ltd.) to remove chromosomal DNA in accordance with the manufacturer’s protocol. Total RNA was reverse transcribed to cDNA using a Reverse Transcription System with random hexamers (Promega, Madison, WI). The expression levels of eotaxin-1, eotaxin-2, eotaxin-3, MCP-3, and RANTES (regulated on activation normal T cell expressed and secreted) were analyzed by using a real-time quantification method in accordance with the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Sequence-specific primers and probes were designed with Pre-Developed TaqMan Assay Reagents (Applied Biosystems). Real-time PCR (40 cycles of denaturation at 92°C for 15 seconds and annealing at 60°C for 60 seconds) was performed using an ABI Prism 7000 Sequence Detector (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize mRNA levels. Relative expression of real-time PCR products was determined using the ΔΔCT technique as described previously.29 Briefly, one of the control samples was chosen as a calibrator, and each set of samples was normalized using the difference in threshold cycle (CT) between the target gene and a house-keeping gene (GAPDH): ΔCT = (CT target gene − CT GAPDH). Relative mRNA levels were calculated using the expression 2−ΔΔCT, where ΔΔCT = ΔCT sample (n) − ΔCT calibrator (n). Each reaction was done in triplicate.
Statistical Analysis
The Mann-Whitney U-test was used for determining the level of significance of differences in sample means and Bonferroni’s test was used for multiple comparisons. A P value <0.05 was considered statistically significant. All data are shown as means ± SEM.
Results
IgE-Mediated Cutaneous Reverse Passive Arthus Reaction Results in Hemorrhage with Eosinophil Infiltration
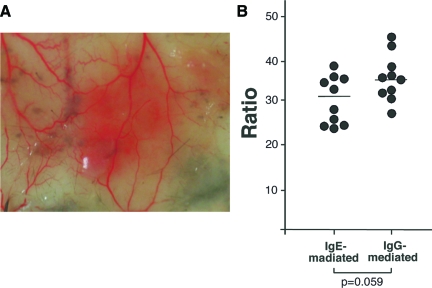
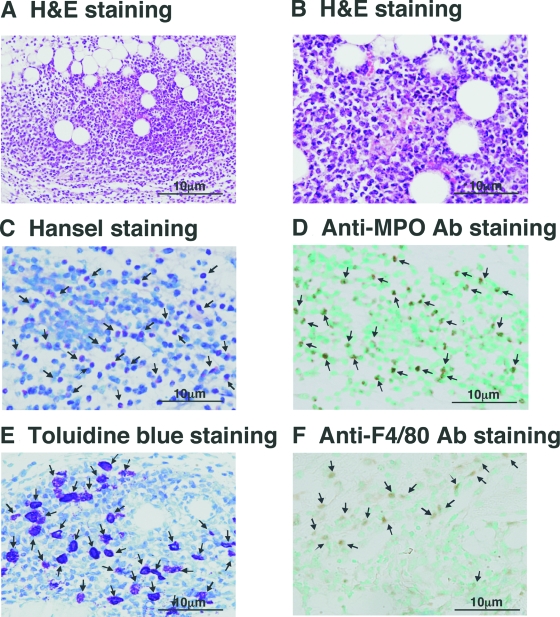
Cutaneous reverse passive Arthus reaction in mice using IgG Abs exhibits IC-mediated vascular damage with neutrophil and mast cell accumulation. To assess whether another Ig class can also induce vascular damage in the cutaneous reverse passive Arthus reaction, the injection of IgE anti-TNP Abs, followed immediately by i.v. installation of TNP-BSA, was attempted in wild-type C57BL/6 mice. IgE-mediated cutaneous reverse passive Arthus reaction demonstrated substantial hemorrhage (Figure 1A). Histopathology revealed the destruction of blood vessels with inflammatory cell infiltration and extravasation of red blood cells (Figure 2, A and B), demonstrating the presence of vasculitis. Because the size of hemorrhage may depend on the diameter of the blood vessel, the diameter ratio of the purpuric spot/bleeding vessel was evaluated for the analysis. The hemorrhage size (ie, diameter ratio) induced by IgE anti-TNP Abs was slightly smaller than that induced by IgG anti-TNP Abs, although the difference was not statistically significant (P = 0.059, Figure 1B). A remarkable finding was that the infiltrated cells were predominantly eosinophils, which was revealed on skin tissue sections with H&E staining (Figure 2, A and B) and Hansel staining (Figure 2C). Furthermore, neutrophil infiltration, which is the predominant IgG-mediated response, was also observed by anti-MPO Ab staining, although it was less intense than eosinophil infiltration (Figure 2D). Mast cells and macrophages were also present (Figure 2, E and F). After preliminary examinations of various concentrations of Ab and antigen, injections of 66 μg/30 μl IgE anti-TNP Abs and 800 μg/400 μl TNP-BSA in PBS were chosen for the following experiments because this combination was sufficient for reproducing eosinophil infiltration (data not shown).
Figure 1.
A: The macroscopic findings of IgE-mediated cutaneous reverse passive Arthus reaction in wild-type mice. B: The size of hemorrhage after 24 hours induced by intradermal IgG or IgE anti-TNP Ab injection followed by i.v. injection of TNP-BSA was assessed. The diameter ratio indicates the mean diameters of purpuric spots divided by the diameter of bleeding vessels. Horizontal bars indicate mean values for each group of mice.
Figure 2.
Histological tissue sections of the skin from wild-type mice during IgE-mediated Arthus reaction. Skin tissues were harvested at 24 hours after IC challenge. H&E staining demonstrated a damaged vessel with prominent inflammation (A) and leukocytoclastic vasculitis with clearly visible eosinophils (B). Eosinophils (arrows) were detected by Hansel staining (C); neutrophils (arrows) by anti-MPO Ab staining (D); mast cells (arrows) by metachromatic staining of granules in toluidine blue-stained sections (E); and macrophages (arrows) by anti-F4/80 Ab staining (F). Original magnification = ×100 (A) and ×200 (B–F).
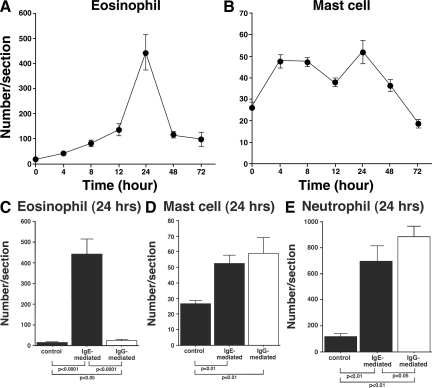
When the time course of eosinophil accumulation in skin tissue sections was assessed at 4, 8, 12, 24, 48, and 72 hours after IC challenge, eosinophil numbers reached a maximum at 24 hours (Figure 3A). In contrast, mast cell accumulation, as assessed by staining with toluidine blue, was observed at a similar density from 4 to 24 hours but also peaked at 24 hours after IC challenge (Figure 3B). Moreover, the size of hemorrhage caused by IgE-mediated IC challenge also peaked after 24 hours of IC formation (data not shown). Collectively, cutaneous inflammation induced by the Arthus reaction with IgE anti-TNP Abs peaked in intensity after 24 hours of IC formation.
Figure 3.
A, B: Time course of eosinophil (A) and mast cell (B) recruitment induced by IgE-mediated Arthus reaction in the skin tissue from wild-type mice. Mice were injected intradermally with IgE anti-TNP Ab, followed by i.v. TNP-BSA injection. The numbers of eosinophils and mast cells per skin section were determined by counting in Hansel- and toluidine blue-stained skin sections, respectively, before and at 4, 8, 12, 24, 48, and 72 hours after IC challenge. All values represent the mean ± SEM of results obtained from 5 to 10 mice. C, D, and E: Comparison of accumulated eosinophils (C), mast cells (D), and neutrophils (E) in the skin from wild-type mice between IgE- and IgG-mediated Arthus reaction at 24 hours after IC challenge. The numbers of eosinophils, mast cells, and neutrophils per skin section were determined by counting in Hansel-, toluidine blue-, and anti-MPO Ab-stained skin sections, respectively. All values represent the mean ± SEM of results obtained from 5 to 10 mice in each group.
Eosinophil Infiltration Is Specific for IgE-Mediated IC Challenge
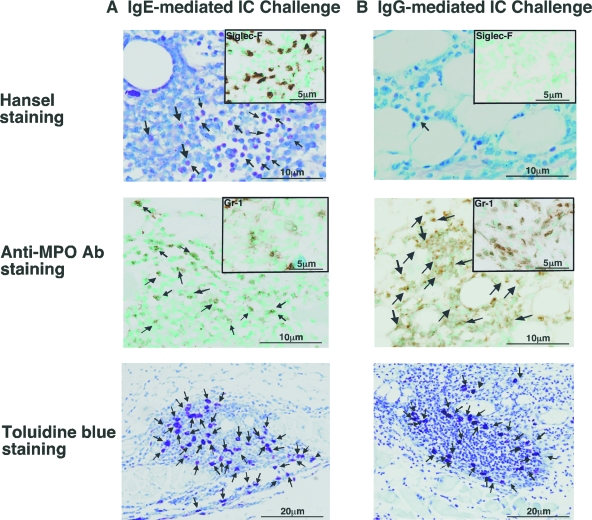
To determine whether eosinophil infiltration is specific for IgE-mediated IC challenge or is induced by IgG Ab injection as well, the numbers of infiltrated eosinophils were compared between IgG- and IgE-mediated Arthus reaction in wild-type mice (Figures 3C and 4, A and B). Control IgG or IgE Abs or IgG or IgE anti-TNP Abs were injected intradermally, followed immediately by an i.v. injection of TNP-BSA under identical condition. Injection of control IgG or IgE Abs did not induce eosinophil accumulation, while infiltrated eosinophil numbers were slightly but significantly increased by IgG anti-TNP Ab injection compared with the controls (9.2 cells/mm2, 1.8-fold, P < 0.05). However, IgE anti-TNP Ab induced strikingly increased eosinophil accumulation (122.3 cells/mm2), which was 13.4-fold higher than IC challenge mediated by IgG anti-TNP Abs after 24 hours (P < 0.0001, Figure 3C). Eosinophils were identified using Hansel staining and were also confirmed by immunostaining with anti-Siglec-F Ab (Figure 4, A and B). Thus, IgE-mediated IC challenge induced significantly more eosinophil infiltration than IgG-mediated IC challenge.
Figure 4.
Histological tissue sections showing eosinophil, neutrophil and mast cell accumulation of wild-type mice at 24 hours after IC challenge by intradermal injection of IgE (A) or (B) IgG anti-TNP. Hansel-staining (anti-Siglec-F Ab; insets) shows eosinophils (arrows; upper panels), and anti-MPO Ab staining (anti-Gr-1 Ab: insets) demonstrates neutrophils (arrows; middle panels). Mast cells were revealed by toluidine blue-staining (arrows; bottom panels). Original magnification = ×100 (toluidine blue) and ×200 (Hansel, anti-Siglec-F, anti-MPO, and anti-Gr-1).
Neutrophil infiltration was also evaluated by immunostaining using anti-MPO and anti-Gr-1 Abs (Figures 3E and 4, A and B). In contrast to IgE anti-TNP Abs, skin biopsies revealed that IgG anti-TNP Abs mainly induced the accumulation of neutrophils instead of eosinophils, as reported previously (Figure 4B).11,12 IgE anti-TNP-mediated IC challenges also the induced accumulation of neutrophils, although the numbers of neutrophils recruited by IgE-mediated IC challenges were significantly fewer than IgG-mediated IC challenges (325.3 cells/mm2 in IgG and 185.5 cells/mm2 in IgE; P < 0.05, Figure 3E). Thus, while both IgE- and IgG-mediated IC challenges induced the recruitment of neutrophils and mast cells, IgE-mediated IC challenges preferentially recruited eosinophils; IgG-mediated IC challenges induced more neutrophils than IgE-mediated IC challenges. In addition, mast cell recruitment was observed both in IgG and IgE anti-TNP-mediated IC challenges after 24 hours (Figures 3D and 4, A and B), and accumulated mast cell numbers were similar between IgE- and IgG-mediated IC challenges. When IgA Ab was used for the induction of the cutaneous reverse passive Arthus reaction, the infiltrated cell profile was almost identical with IgG-mediated IC challenges (data not shown). Therefore, the Ig class of injected Ab is an important factor for eosinophil/neutrophil accumulation in the Arthus reaction.
Chemokine Expressions in IgE-Mediated Cutaneous Reverse Passive Arthus Reaction
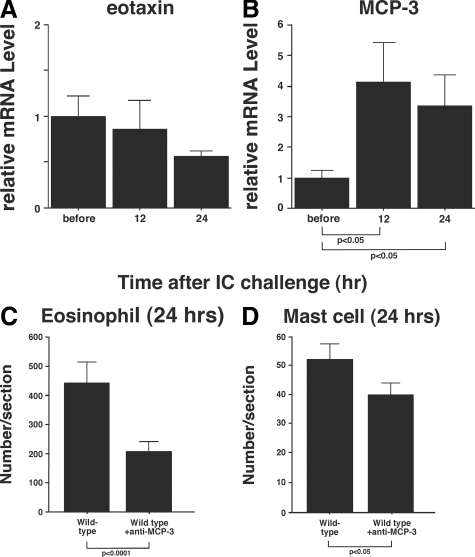
The accumulation of eosinophils is regulated by chemokines, including eotaxin and MCP-3.30 To identify the chemokine(s) responsible for eosinophil recruitment by IgE-mediated IC challenge, chemokine expression levels were measured in skin tissue sections before IC formation and after 12 and 24 hours. While there was no significant differences in eotaxin-1 mRNA levels between before and after 12 or 24 hours (Figure 5A), the MCP-3 mRNA level was increased significantly after 12 hours (4.1-fold, P < 0.05) and 24 hours (3.4-fold, P < 0.05) compared with the expression level before IC challenge (Figure 5B). In addition, there were no significant increases in the mRNA expression levels of eotaxin-2, eotaxin-3, or RANTES between before and after 12 or 24 hours (data not shown). Therefore, MCP-3 is likely to play a major role in eosinophil accumulation in IgE-mediated Arthus reaction.
Figure 5.
mRNA expression levels of (A) eotaxin-1 and (B) MCP-3 in the dermis from wild-type mice before and at 12 and 24 hours after IgE anti-TNP Ab-mediated IC challenge. mRNA expression levels were assessed by real-time reverse transcription-PCR. Relative expression of real-time PCR products was determined by using the Ct method to compare target gene and house-keeping gene (GAPDH) mRNA expression levels. One of the control samples was chosen as a calibrator sample. The accumulation of eosinophils (C) and mast cells (D) was assessed in the skin from wild-type mice treated with polyclonal anti-MCP-3 Abs at 24 hours after IC challenge by intradermal injection of IgE anti-TNP Abs. All values represent the mean ± SEM of results obtained from 5 to 10 mice in each group.
To confirm that MCP-3 is important for eosinophil accumulation, eosinophil accumulation was evaluated 24 hours after IgE-mediated IC challenge in wild-type mice treated with polyclonal Abs to MCP-3 or with control Abs. Anti-MCP-3 or control Abs were injected 30 minutes before IC challenge. Eosinophil numbers were significantly reduced in MCP-3 blockade mice compared with the control group (P < 0.0001, Figure 5C), although the blockade was incomplete. Mast cell numbers were also significantly reduced in MCP-3 blockade mice compared with the control group (P < 0.05, Figure 4D). Therefore, MCP-3 is likely to play an important role in both eosinophil and mast cell recruitment, while other chemokines may also participate in the process.
Contribution of Adhesion Molecules in IgE-Mediated Cutaneous Reverse Passive Arthus Reaction
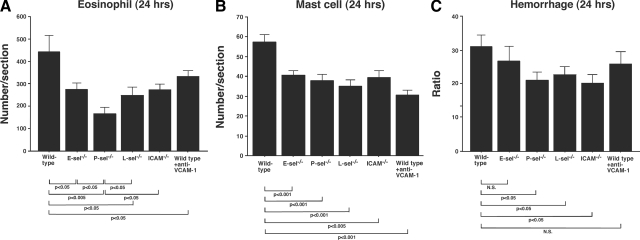
Cell adhesion molecules play critical roles in the progression of the Arthus reaction.11,12 To assess the role of adhesion molecules in IgE-mediated cutaneous reverse passive Arthus reaction, the size of hemorrhage and the numbers of eosinophils and mast cells were evaluated at 24 hours after IgE-mediated IC challenge in mice deficient in adhesion molecules. These mice included E-selectin−/−, P-selectin−/−, L-selectin−/−, and ICAM-1−/− mice. Wild-type mice treated with mAb to VCAM-1 were also assessed. There were no differences in eosinophil or mast cell numbers between mutant and wild-type mice before IC challenge (data not shown).
Eosinophil numbers were significantly reduced in E-selectin−/− (38%, P < 0.05), L-selectin−/− (44%, P < 0.05), and ICAM-1−/− mice (38%, P < 0.05), and wild-type mice treated with anti-VCAM-1 mAb (27%, P < 0.05) compared with wild-type mice (Figure 6A). P-selectin deficiency resulted in the most profound reduction in eosinophil numbers (63% reduction from wild-type mice, P < 0.005), which were even decreased significantly compared with E-selectin−/− (P < 0.05), L-selectin−/− (P < 0.05), and ICAM-1−/− mice (P < 0.05), and blockade of VCAM-1 in wild-type mice (P < 0.05). These results suggested that P-selectin plays a dominant role in eosinophil recruitment at 24 hours, although other adhesion molecules also contributed to eosinophil recruitment to a lesser degree.
Figure 6.
Recruitment of (A) eosinophils and (B) mast cells in the skin from E-selectin (E-sel−/−), P-selectin−/− (P-sel−/−), L-selectin−/− (L-sel−/−), and ICAM-1−/− (ICAM−/−) mice, wild-type mice treated with anti-VCAM-1 mAbs, and wild-type mice at 24 hours after IC challenge by intradermal injection of IgE anti-TNP Abs. The numbers of eosinophils and mast cells per section were determined by counting in Hansel- and toluidine blue-stained skin sections, respectively. C: Hemorrhages in these mice were evaluated by diameter ratio as shown in Figure 1. All values represent the mean ± SEM of results obtained from 5 to 10 mice in each group.
Mast cell numbers were also significantly reduced in E-selectin−/− (29%, P < 0.001), P-selectin−/− (38%, P < 0.001), L-selectin−/− (39%, P < 0.001), and ICAM-1−/− mice (31%, P < 0.005), and blockade of VCAM-1 in wild-type mice (42%, P < 0.001) compared with wild-type mice (Figure 6B). In contrast with eosinophil infiltration, all mutant mice showed similar decreases, with no significant differences observed among them. Thus, these selectins, ICAM-1, and VCAM-1 appear equally important for mast cell accumulation.
Hemorrhage was compared among these mice at 24 hours after IC challenges. The diameter ratio (hemorrhage/blood vessel) was reduced in E-selectin−/− mice (P < 0.05), P-selectin−/− mice (P < 0.05), L-selectin−/− mice (P < 0.05), and ICAM-1−/− mice (P < 0.05) compared with wild-type mice. Blockade of VCAM-1 in wild-type mice showed no difference between wild-type mice (Figure 6C).
Discussion
Passive systemic anaphylaxis using IgE Abs and the antigen applied intravenously is a widely used model for assessing the role of IgE in immediate hypersensitivity, although models for assessing the role of IgE in other allergic or inflammatory diseases are not yet available. This study established an experimental mouse model of eosinophilic vasculitis by the cutaneous reverse passive Arthus reaction using IgE anti-TNP Abs and TNP-BSA as an antigen. IgE-mediated IC challenge induced hemorrhage by the accumulation of inflammatory cells consisting predominantly of eosinophils as well as neutrophils, mast cells, and macrophages (Figures 1–3). The hemorrhage peaked 24 hours after IC challenge, which was similar to the IgG-mediated Arthus reaction. In the original description by Arthus, horse serum was injected repeatedly intradermally into a rabbit, resulting in edema, hemorrhage, and neutrophil infiltration in the skin.9 The cutaneous reverse passive Arthus reaction using the combination of rabbit IgG anti-chicken egg albumin Abs and chicken egg albumin also induced neutrophil accumulation.11,12 Similarly, the reverse passive Arthus reaction mediated by IgG anti-TNP Abs and TNP-BSA developed neutrophil accumulation with a very small numbers of eosinophils (Figures 3 and 4). In contrast, IgE anti-TNP Ab injection with TNP-BSA induced prominent eosinophil accumulation. IgE anti-TNP Ab injection also induced neutrophil accumulation, but the numbers were significantly fewer than in the IgG-mediated Arthus reaction. Taken together, these results indicated that eosinophil infiltration was not associated with TNP as an antigen but with the IgE class of injected Abs. This appeared to be specific for IgE because the injection of IgA Abs under the same conditions exhibited histopathology that resembled the IgG-mediated response and did not result in eosinophil recruitment (data not shown). Thus, it is likely that the interaction between IgE and FcεRI initiates the development of eosinophilic vasculitis in this model.
FcεRI is expressed in mast cells and basophils. Mast cell recruitment into the tissue is thought to occur by the release of mast cell precursors from the bone marrow into the peripheral blood, followed by migration of these precursors into tissue, and their subsequent differentiation into mature mast cells.31 The numbers of infiltrated mast cells in the IgE-mediated reverse Arthus reaction reached a maximal level 4 to 8 hours after the IC challenge, followed by eosinophil infiltration reaching a peak at 24 hours after IC challenge (Figure 3). Studies have shown that FcεRI expression can be up-regulated by as much as 32-fold after in vitro incubation of mast cells with IgE or injection of IgE in vivo.32,33 Thus, the initial mast cell accumulation induced overproduction of mediators, such as cytokines and chemokines, and contributed to eosinophil infiltration and hemorrhage. On the other hand, the standard IgG-mediated cutaneous Arthus reaction is initiated by FcγRIII on mast cells.14,18,34,35 Therefore, although both reactions appear to be initiated by mast cells, FcγRs and FcεRs may deliver distinct signal transductions in mast cells, which then trigger different cascades that lead to neutrophil or eosinophil accumulation.
Eosinophil migration is regulated by CCRs. Because CCR3 is strongly expressed by eosinophils,36,37,38,39 it is recognized that chemokines that bind to CCR3, including MCP-3 and eotaxin, are important chemotactic factors for eosinophil recruitment. MCP-3 levels were up-regulated at 12 and 24 hours after IgE-containing IC challenge (Figure 5); however, no significant increase was observed for eotaxin mRNA levels (Figure 5). Similar to these results, Chensue and colleagues demonstrated that Ab-mediated eotaxin depletion did not reduce local eosinophil accumulation significantly in type-2 lung granuloma formation, but MCP-3 was an important mediator of this reaction.40,41 The present findings indicate that MCP-3 plays a critical role in eosinophil recruitment in IgE-mediated IC challenge.
It was also shown that reduced Arthus reaction by the loss of adhesion molecules correlated with a decrease in the numbers of infiltrated eosinophils and mast cells (Figure 6). While no specific molecule appeared to have a predominant role in mast cell accumulation, the loss of P-selectin resulted in a significant reduction in eosinophil accumulation compared with E-selectin, L-selectin, and ICAM-1 deficiencies at 24 hours. This also suggested that the decrease in eosinophil infiltration in P-selectin−/− mice was not due to the defective recruitment of mast cells, but that P-selectin has a direct role in eosinophil recruitment. However, because other time points were not assessed, the apparent dominant role of P-selectin may be limited to specific time points. Furthermore, E-selectin, L-selectin, or ICAM-1 deficiency as well as VCAM-1 blockade also showed a significant decrease of eosinophil accumulation. Collectively, the results indicated that adhesion molecules cooperatively play critical roles with different relative contributions in eosinophil recruitment.
The current study established an experimental mouse model of eosinophil infiltration in skin vasculitis using IgE-mediated cutaneous reverse passive Arthus reaction. Because the reverse passive Arthus reaction is a simple model that is useful for evaluating factors contributing to the development of the disease as well as therapeutic agents, the present results suggested that this model may be useful to investigate the mechanisms of cutaneous leukocyte infiltration in eosinophilic vasculitis such as CSS. However, CSS is a complex multi-organ disease characterized by asthma, hypereosinophila, eosinophilic tissue infiltrates, necrotizing vasculitis, and extravascular granuloma formation.3,5,6 Because this model does not form extravascular granulomas, this model cannot be simply used as an experimental model for CSS. In addition, this model is limited to the skin, while vasculitis in other organs may have a different pathogenesis. Nonetheless, this model may be applicable to other non-vasculitis eosinophilic infiltrations in the skin, including bullous pemphigoid, where IgE autoantibody deposition has been proved important.42
Acknowledgments
We thank Ms. Masako Matsubara and Yuko Yamada for technical assistance.
Footnotes
Address reprint requests to Manabu Fujimoto, M.D., Department of Dermatology, Kanazawa University Graduate School of Medical Science, 13-1 Takaramachi, Kanazawa, Ishikawa 920-8641, Japan. E-mail: fujimoto-m@umin.ac.jp.
Supported in part by the Grant-in-Aid from the Ministry of Education, Science, and Culture of Japan.
References
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Silberstein DS. Eosinophil function in health and disease. Crit Rev Oncol Hematol. 1995;19:47–77. doi: 10.1016/1040-8428(94)00127-f. [DOI] [PubMed] [Google Scholar]
- Churg J. Allergic granulomatosis and granulomatous-vascular syndromes. Ann Allergy. 1963;21:619–628. [PubMed] [Google Scholar]
- Fujimoto M, Sato S, Hayashi N, Wakugawa M, Tsuchida T, Tamaki K. Juvenile temporal arteritis with eosinophilia: a distinct clinicopathological entity. Dermatology. 1996;192:32–35. doi: 10.1159/000246310. [DOI] [PubMed] [Google Scholar]
- Chumbley LC, Harrison EG, Jr, DeRemee RA. Allergic granulomatosis and angiitis (Churg-Strauss syndrome). Report and analysis of 30 cases. Mayo Clin Proc. 1977;52:477–484. [PubMed] [Google Scholar]
- Finan MC, Winkelmann RK. The cutaneous extravascular necrotizing granuloma (Churg-Strauss granuloma) and systemic disease: a review of 27 cases. Medicine (Baltimore) 1983;62:142–158. doi: 10.1097/00005792-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Abril A, Calamia KT, Cohen MD. The Churg Strauss syndrome (allergic granulomatous angiitis): review and update. Semin Arthritis Rheum. 2003;33:106–114. doi: 10.1016/s0049-0172(03)00083-0. [DOI] [PubMed] [Google Scholar]
- Manger BJ, Krapf FE, Gramatzki M, Nusslein HG, Burmester GR, Krauledat PB, Kalden JR. IgE-containing circulating immune complexes in Churg-Strauss vasculitis. Scand J Immunol. 1985;21:369–373. doi: 10.1111/j.1365-3083.1985.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Arthus M. Injections repetees de serum de cheval chez le lapin. V R Soc Biol. 1903;55:817–820. [Google Scholar]
- Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science. 1994;265:1095–1098. doi: 10.1126/science.8066448. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Kaburagi Y, Takehara K, Steeber DA, Tedder TF, Sato S. Relative contributions of selectins and intercellular adhesion molecule-1 to tissue injury induced by immune complex deposition. Am J Pathol. 2003;162:1463–1473. doi: 10.1016/S0002-9440(10)64279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaburagi Y, Hasegawa M, Nagaoka T, Shimada Y, Hamaguchi Y, Komura K, Saito E, Yanaba K, Takehara K, Kadono T, Steeber DA, Tedder TF, Sato S. The cutaneous reverse Arthus reaction requires intercellular adhesion molecule 1 and L-selectin expression. J Immunol. 2002;168:2970–2978. doi: 10.4049/jimmunol.168.6.2970. [DOI] [PubMed] [Google Scholar]
- Orito H, Fujimoto M, Ishiura N, Yanaba K, Matsushita T, Hasegawa M, Ogawa F, Takehara K, Sato S. Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 cooperatively contribute to the cutaneous Arthus reaction. J Leukoc Biol. 2007;81:1197–1204. doi: 10.1189/jlb.1006623. [DOI] [PubMed] [Google Scholar]
- Baumann U, Kohl J, Tschernig T, Schwerter-Strumpf K, Verbeek JS, Schmidt RE, Gessner JE. A codominant role of FcγRI/III and C5aR in the reverse Arthus reaction. J Immunol. 2000;164:1065–1070. doi: 10.4049/jimmunol.164.2.1065. [DOI] [PubMed] [Google Scholar]
- Hopken UE, Lu B, Gerard NP, Gerard C. Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J Exp Med. 1997;186:749–756. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhl J, Gessner JE. On the role of complement and Fc gamma-receptors in the Arthus reaction. Mol Immunol. 1999;36:893–903. doi: 10.1016/s0161-5890(99)00111-x. [DOI] [PubMed] [Google Scholar]
- Baumann U, Chouchakova N, Gewecke B, Kohl J, Carroll MC, Schmidt RE, Gessner JE. Distinct tissue site-specific requirements of mast cells and complement components C3/C5a receptor in IgG immune complex-induced injury of skin and lung. J Immunol. 2001;167:1022–1027. doi: 10.4049/jimmunol.167.2.1022. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ramos BF, Jakschik BA. Augmentation of reverse arthus reaction by mast cells in mice. J Clin Invest. 1991;88:841–846. doi: 10.1172/JCI115385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. doi: 10.1111/j.1600-065X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Bullard DC, Qin L, Lorenzo I, Quinlin WM, Doyle NA, Bosse R, Vestweber D, Doerschuk CM, Beaudet AL. P-selectin/ICAM-1 double mutant mice: acute emigration of neutrophils into the peritoneum is completely absent but is normal into pulmonary alveoli. J Clin Invest. 1995;95:1782–1788. doi: 10.1172/JCI117856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Sligh JE, Jr, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King PD, Sandberg ET, Selvakumar A, Fang P, Beaudet AL, Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J Immunol. 1995;154:6080–6093. [PubMed] [Google Scholar]
- Ma W, Bryce PJ, Humbles AA, Laouini D, Yalcindag A, Alenius H, Friend DS, Oettgen HC, Gerard C, Geha RS. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J Clin Invest. 2002;109:621–628. doi: 10.1172/JCI14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MD, Planck SR, Crespo S, Garman K, Fleischman RJ, Dullforce P, Seitz GW, Martin TM, Parker DC, Rosenbaum JT. Immunohistology of antigen-presenting cells in vivo: a novel method for serial observation of fluorescently labeled cells. Invest Ophthalmol Vis Sci. 2003;44:2004–2009. doi: 10.1167/iovs.02-0560. [DOI] [PubMed] [Google Scholar]
- Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diag. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793–805. doi: 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146:1410–1415. [PubMed] [Google Scholar]
- Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, Kinet JP, Galli SJ. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med. 1997;185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S, Matsuoka K, Taya C, Kitamura F, Takai T, Yonekawa H, Karasuyama H. Drastic up-regulation of Fcepsilonri on mast cells is induced by IgE binding through stabilization and accumulation of Fcepsilonri on the cell surface. J Immunol. 2001;167:3427–3434. doi: 10.4049/jimmunol.167.6.3427. [DOI] [PubMed] [Google Scholar]
- Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, van de Winkel JG, Verbeek JS. Impaired IgG-dependent anaphylaxis and Arthus reaction in FcγRIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- Elsner J, Kapp A. Activation of human eosinophil effector functions by CC chemokines. Allergy Asthma Proc. 1999;20:365–369. doi: 10.2500/108854199778251771. [DOI] [PubMed] [Google Scholar]
- Gao JL, Sen AI, Kitaura M, Yoshie O, Rothenberg ME, Murphy PM, Luster AD. Identification of a mouse eosinophil receptor for the CC chemokine eotaxin. Biochem Biophys Res Commun. 1996;223:679–684. doi: 10.1006/bbrc.1996.0955. [DOI] [PubMed] [Google Scholar]
- White JR, Imburgia C, Dul E, Appelbaum E, O'Donnell K, O'Shannessy DJ, Brawner M, Fornwald J, Adamou J, Elshourbagy NA, Kaiser K, Foley JJ, Schmidt DB, Johanson K, Macphee C, Moores K, McNulty D, Scott GF, Schleimer RP, Sarau HM. Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J Leukoc Biol. 1997;62:667–675. doi: 10.1002/jlb.62.5.667. [DOI] [PubMed] [Google Scholar]
- Grimaldi JC, Yu NX, Grunig G, Seymour BW, Cottrez F, Robinson DS, Hosken N, Ferlin WG, Wu X, Soto H, O'Garra A, Howard MC, Coffman RL. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3). J Leukoc Biol. 1999;65:846–853. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]
- Ruth JH, Lukacs NW, Warmington KS, Polak TJ, Burdick M, Kunkel SL, Strieter RM, Chensue SW. Expression and participation of eotaxin during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granuloma formation. J Immunol. 1998;161:4276–4282. [PubMed] [Google Scholar]
- Shang XZ, Chiu BC, Stolberg V, Lukacs NW, Kunkel SL, Murphy HS, Chensue SW. Eosinophil recruitment in type-2 hypersensitivity pulmonary granulomas: source and contribution of monocyte chemotactic protein-3 (CCL7). Am J Pathol. 2002;161:257–266. doi: 10.1016/S0002-9440(10)64177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiura N, Fujimoto M, Watanabe R, Nakashima H, Kuwano Y, Yazawa N, Echigo T, Okochi H, Tamaki K. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2008;49:153–161. doi: 10.1016/j.jdermsci.2007.08.008. [DOI] [PubMed] [Google Scholar]