Abstract
Recent evidence suggests that interleukin-17-producing CD4+ T cells (Th17 cells) are the dominant pathogenic cellular component in autoimmune inflammatory diseases, including multiple sclerosis. It has recently been demonstrated that all-trans retinoic acid can suppress Th17 differentiation and promote the generation of Foxp3+ regulatory T cells via retinoic acid receptor signals. Here, we investigated the effects of AM80, a synthetic retinoid with enhanced biological properties to all-trans retinoic acid, on Th17 differentiation and function and evaluated its therapeutic potential in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. AM80 treatment was more effective than all-trans retinoic acid in inhibiting Th17 differentiation in vitro. Oral administration of AM80 was protective for the early development of EAE and the down-modulation of Th17 differentiation and effector functions in vivo. Moreover, AM80 inhibited interleukin-17 production by splenic memory T cells, in vitro-differentiated Th17 cells, and central nervous system-infiltrating effector T cells. Accordingly, AM80 was effective when administered therapeutically after the onset of EAE. Continuous AM80 treatment, however, was ineffective at inhibiting late EAE symptoms despite the maintained suppression of RORγt and interleukin-17 expression levels by central nervous system-infiltrating T cells. We reveal that continuous AM80 treatment also led to the suppression of interleukin-10 production by a distinct T cell subset that expressed both Foxp3 and RORγt. These findings suggest that retinoid signaling regulates both inflammatory Th17 cells and Th17-like regulatory cells.
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease affecting the central nervous system (CNS).1 Previous studies of experimental autoimmune encephalomyelitis (EAE), a murine model of MS,2,3 indicated that autoimmune responses were initiated by a subset of myelin-specific CD4+ T cells secreting the inflammatory cytokine interferon (IFN)-γ, termed Th1 cells.4,5 More recently, the identification of an additional subset of differentiated inflammatory helper T cells secreting large amount of the cytokine interleukin (IL)-17 (Th17 cells) have allowed new insights into the pathology of a range of inflammatory autoimmune diseases.6,7 Indeed, the presence of such Th17 cells among CNS-infiltrating leukocytes has been demonstrated in EAE animals.7 Furthermore, induction of EAE in IL-17-deficient mice leads to less severe disease8 and mice that lack IL-23, a cytokine required for Th17 cell survival,9 are resistant to EAE.6 Critically, increased levels of IL-17 are detected in MS patients10 and the presence of IL-17-secreting T cells has been shown to link with acute CNS lesions in MS.11
Differentiation of naïve T cells into Th17 cells in vitro requires culture with a combination of IL-6, an inflammatory cytokine elaborated by innate immune cells subsequent to ligation of pathogen-associated molecular pattern receptors, and transforming growth factor (TGF)-β,12,13,14 a cytokine classically regarded as anti-inflammatory and also associated with the differentiation of regulatory T cells.15 The requirement for IL-6 and TGF-β in Th17 differentiation has also been demonstrated in vivo12,16 Phenotypically, Th17 cells express the retinoic acid (RA)-related orphan receptor γt (RORγt) in a Stat3-dependent manner, and produce high levels of many inflammatory cytokines, including IL-17, IL-6, tumor necrosis factor (TNF)-α, IL-21, and IL-22.7,17,18,19 Interestingly, naïve T cells receiving TGF-β signaling alone are induced to become a CD4+CD25+ regulatory T cell population (Treg cells).13 Treg cells are capable of suppressing inflammation, mediating self-tolerance, and produce suppressive cytokines such as TGF-β and/or IL-1020 The generation of Treg cells requires the forkhead/winged-helix transcription factor Foxp3 and its functional impairment leads to autoimmunity.21,22 Foxp3+ Treg cells in the CNS have been shown to ameliorate EAE via the production of IL-1023, which has recently been associated with restraining Th17-mediated pathology in EAE.24 Furthermore, IL-10-producing RORγt+Foxp3+ T cells have been identified in vivo, suggesting the existence of a regulatory Th17-like cell type.25
RA, the active metabolite of vitamin A, has multiple effects on cell differentiation and survival through ligation to the two families receptors: retinoic acid receptors (RAR) and retinoid X receptor (RXR), each of which has multiple isoforms.26 Recently, all-trans retinoic acid (ATRA) has been reported to suppress the differentiation of Th17 cells through ligation to the RAR-α,27,28 accompanied by a down-regulation of RORγt and reciprocal induction of Treg cells expressing Foxp3.27,29 Possible mechanisms of action of ATRA for the suppression of Th17 cell function have been reported to be a result of reduced expression of IL-6 receptor and IL-23 receptor as well as enhanced TGF-β signaling in a Smad3-dependent manner.30
RA has been shown to ameliorate EAE.31,32 But as these studies were carried through before the discovery of Th17 cells, the amelioration was attributed to suppression of Th1 cells. In addition, the therapeutic application of RA to date has been limited by instability and poor bioavailability of this compound as well as by its nonselective binding to a broad range of retinoid receptors, which conceivably leads to unexpected side effects.26,33,34 To circumvent these potential problems in the clinical use of RAR agonists, a variety of synthetic RAR agonists with improved biological properties in vivo have been developed. One of these synthetic retinoids, AM80, is already available as medication under the trade name Tamibarotene for human diseases such as acute promyelocytic leukemia (APL) and psoriasis.35,36,37 AM80 is specific for the RARα/β and characterized by a higher stability, fewer potential side effects, and superior bioavailability compared with ATRA.35,36,37,38 Therefore, we may open up new therapeutic avenues for treating Th17-mediated autoimmune diseases by testing AM80 in an immunoregulatory context.
In this study, we demonstrate for the first time that AM80 inhibits Th17 differentiation in vitro with a higher potency than ATRA. Treatment with AM80 ameliorates EAE and inhibits both the differentiation of Th17 cells and the effector function of Th17 cells in vivo without generating general immunosuppression. In addition, AM80 proved to be effective in rescue from acute EAE when administered after the onset of the disease. Interestingly, continuous AM80 treatment failed to improve chronic disease despite of apparent suppression of T cell expression of IL-17 and RORγt. We demonstrate that continuous AM80 treatment results in the suppression of IL-10 production by a unique subset of T cells, which is identified as T cells that co-expresses RORγt and Foxp3. We conclude that treatment with the synthetic retinoid AM80 is a considerable intervention strategy for the acute phase of Th17-mediated autoimmune diseases such as MS.
Materials and Methods
Animals and EAE Induction
C57BL/6J (B6) mice (CLEA Laboratory Animal Corp., Tokyo, Japan) were maintained in specific pathogen-free conditions in accordance with institutional guidelines (National Institute of Neuroscience, NCNP, Tokyo, Japan). This study used female mice at 8 to 10 weeks of age. For EAE induction mice were injected subcutaneously with 100 μg of myelin oligodendrocyte glycoprotein (MOG) amino acids 35–55 (MOG35–55 peptide MEVGWYRSPFSRVVHLYRNGK)39 and 1 mg of heat-killed Mycobacterium tuberculosis H37RA emulsified in complete Freund’s adjuvant (Difco, Lawrence, KS). 200 ng of pertussis toxin (List Biological Laboratories) was injected intraperitoneally on days 0 and 2 after immunization. Some groups of mice also received 3 mg/kg AM80 in 0.5% carboxymethylcellulose (CMC) solution (WAKO Chemicals, Osaka, Japan) by oral gavage.
EAE was clinically scored daily (0, no clinical signs; 1, partial tail paralysis; 2, flaccid tail; 3, partial hindlimb paralysis; 4, total hindlimb paralysis; 5, Hind- and foreleg paralysis).39 Disease was also assessed using histological examination of CNS tissue as previously described.40 Briefly, animals were perfused with 20 ml of cold phosphate-buffered saline, and CNS tissue was excised and fixed in formal saline. Paraffin-embedded sections were prepared and stained with either Luxol fast blue or H&E and photomicrographs acquired with a light microscope (Eclipse E800M, Nikon, Japan).
Cell Isolation and Purification
CNS-infiltrating lymphocytes were isolated from spinal cords and brains as previously described.39 Briefly, tissue was cut into small pieces and digested for 40 minutes at 37°C in media (GIBCO, Auckland, New Zealand) supplemented with 1.4 mg/ml collagenase H (Roche, Mannheim, Germany) and 100 μg/ml DNase I (Roche). Resulting tissue homogenates were forced through a 70-μm cell strainer and leukocytes were enriched using a discontinuous 40%/80% Percoll density gradient centrifugation. Leukocytes were collected from the interface and where indicated cell numbers for leukocytes and/or T cell subsets per mouse were counted with an improved Neubauer counting chamber and via flow cytometry with reference to a cell number curve as previously described.41 T cells were purified from splenocytes, draining lymph nodes and CNS infiltrates using a pan T cell MACS isolation kit with an AutoMACS separator according to manufacturer’s instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). Where required, naïve CD4+CD44−CD25−CD62Lhigh T cells or memory CD4+CD44+CD25−CD62Llow T cells were further sorted using a fluorescence-activated cell sorter ARIA (BD Cytometry System, Franklin Lakes, NJ).
Cell Culture
RPMI 1640 medium (GIBCO) supplemented with 10% fetal calf serum, 2 mmol/L l-glutamine, 100 U/ml penicillin-streptomycin, and 50 μmol/L 2-mercaptoethanol (Invitrogen, Carlsbad, CA) was used for all cultures. Cells were activated with immobilized anti-CD3 monoclonal antibody (mAb) (2C-11; 2 μg/ml) and soluble anti-CD28 mAb (BD PharMingen) or, when measuring recall responses of secondary lymphoid tissue 10 days after immunization, with MOG35–55 peptide (0–100 μmol/L). Where indicated, cells were cultured under Th17 polarizing conditions: 2 ng/ml TGF-β, 20 ng/ml IL-6 (PeproTech, London, UK), anti-IFN-γ mAb (HB170; 10 μg/ml), and anti-IL-4 mAb (HB188; 10 μg/ml). Into some cultures ATRA (Sigma-Aldrich, Steinheim, Germany) or AM80 dissolved in dimethyl sulfoxide (Sigma) was added at the required concentrations. Supernatants were harvested at 72 or 96 hours for cytokine measurement and proliferation was determined by incubating with [3H]thymidine (1 μCi/well) for the final 12 hours of culture assessing incorporation of radioactivity with a beta 1205 counter (Pharmacia Biotech, Freiburg, Germany). In some experiments, to measure differentiation of T cells, after 96 hours of activation cells were rested for 48 hours before restimulation with anti-CD3 for a further 96 hours.
Cytokine Measurement
IL-17 was assessed via a mouse IL-17 DuoSet (R&D Systems). All other cytokines were assessed by cytometric bead array using a mouse inflammation kit or a mouse Th1/Th2 cytokine kit (BD Biosciences).
Intracellular Staining for Cytokines and Transcription Factors
To stain cells for production of cytokines, cells were restimulated with 50 ng/ml phorbol 12-myristate 13-acetate and 500 ng/ml ionomycin (Sigma-Aldrich) for 4 to 6 hours before surface staining in the presence of monensin-containing GolgiPlug (BD Biosciences). A LIVE/DEAD fixable dead cell stain kit (Invitrogen) was used to exclude dead cells. Intracellular staining was then performed using a Cytofix/cytoperm kit (BD PharMingen), according to manufacturer’s instructions. To stain intracellular transcription factors, we used an anti-mouse/rat Foxp3 staining set (eBioscience, San Diego, CA) with anti-RORγt antibody (BioLegend, San Diego, CA) and visualized with a secondary PE-conjugated goat anti-rabbit antibody (Invitrogen).
RNA Extraction and Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from cell populations using an RNeasy Mini Kit (Qiagen, Maryland) according to the manufacturer’s instructions. cDNA was prepared using a first-strand cDNA Kit (Takara, Otsu, Shiga, Japan). Quantitative real-time PCR was performed using a LightCycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics) with a LightCycler real-time PCR machine (Roche). Gene expression values were normalized to the expression of the GAPDH housekeeping gene. Primers used in this study were GAPDH fw: 5′-AACGACCCCTTCATTGAC-3′ rv: 5′-TCCACATACTCAGCAC-3′, RORc fw: 5′-TGTCCTGGGCTACCCTACTG-3′ rv: 5′-GTGCAGGAGTAGGCCACATT-3′, mFOXP3 fw: 5′-TTCTCACAACAAGGCCACTTG-3′ rv: 5′-CCCAGGAAAGACAGCAACCCT-3′, mT-bet fw: 5′-GCCAGGGAACCGCTTATATG-3′ rv: 5′-GACGATCATCTGGGTCACATTGT-3′, mIL-10 fw: 5′-CATGGGTCTTGGGAAGAGAA-3′ rv: 5′-CATTCCCAGAGGAATTGCAT-3′.
Statistics
EAE clinical scores for groups of mice are presented as the mean group clinical score ± SEM, and statistical differences were analyzed by two-way analysis of variance (analysis of variance) for repeated measures and significance calculated with a Bonferroni post-test. Cytokine secretion data were analyzed with a two-tailed Student’s t-test or with one-way analysis of variance with Bonferroni’s multiple comparison test. Unless otherwise stated, P < 0.05 was considered significant and indicated on plots by asterisks.
Results
RAR Agonists AM80 Inhibit Th17 Cell Differentiation in Vitro
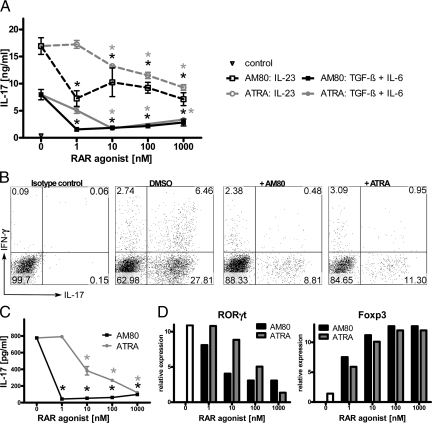
The synthetic retinoid AM80 is already available as medication for human diseases such as APL and psoriasis and characterized by superior pharmacological properties compared with ATRA. As previous studies demonstrated that Th17 cells are the dominant pathogenic cellular component in autoimmune inflammatory diseases, such as MS, and ATRA modulates Th17 differentiation,27,28,29 we examined whether AM80 could be used to treat autoimmune diseases. To this end, whole splenocytes or purified naive T cells were stimulated under a range of Th17-inducing conditions (ie, with either TGF-β plus IL-6, or IL-23) with or without either AM80 or ATRA and were assessed for their IL-17 production. Addition of retinoids to the splenocyte culture significantly reduced the amount of IL-17 in supernatants (Figure 1A). Accumulation of IL-17-producing T cells after restimulation of the culture was also suppressed by AM80 or ATRA with minimal effect on the development of IFN-γ-producing T cells (Figure 1B). Interestingly, AM80 appeared to be more effective than ATRA at inhibiting IL-17 production especially at low doses. Furthermore, both AM80 and ATRA inhibited Th17 cell differentiation of naive T cells, as revealed by the reductions in IL-17 secretion (Figure 1C). Importantly, the effect of retinoid treatment on naïve T cell differentiation is not merely due to a suppression of T cell activation or an increase in cell death, as such treatment did not reduce proliferation, or total live cell number in the cultures (data not shown). Also, treatment with AM80 or ATRA led to reduced expression of RORγt, a key Th17 cell-specific transcription factor, as compared with untreated controls (Figure 1D). Such reductions in Th17 phenotype were accompanied by the reciprocal increase of Foxp3 expression, indicative of a regulatory T cell phenotype (Figure 1D). AM80 was also more effective at modulating the transcription factors specific for Th17 cells as compared with ATRA (Figure 1D). In addition, no up-regulation in T-bet and GATA-3 was observed, indicating that the inhibition of Th17 differentiation by retinoid treatment was not a result of an altered Th1/Th2 phenotype. These results suggest that AM80 is a superior modulator of in vitro Th17 differentiation as compared with ATRA.
Figure 1.
AM80 is a potent inhibitor for Th17 cell differentiation in vitro. Whole splenocytes were stimulated with soluble anti-CD3 with retinoids added at a range of concentrations. A: Cells were cultured in the presence of IL-23 (broken line), TGF-β and IL-6 (solid line), or without the addition of cytokines (closed triangle) for 3 days and IL-17 production assessed by enzyme-linked immunosorbent assay (ELISA). B: Intracellular cytokine staining of IL-17 and IFN-γ among TcR+CD4+ cells with or without 10 nmol/L retinoid treatment assessed after 3 days of culture. Data depicted in A and B are representative of three independent experiments. In C, CD4+CD44−CD25−CD62Lhigh naïve T cells were stimulated under Th17-priming conditions with retinoids at a range of concentrations for 96 hours. Cells were rested for 48 hours before restimulation and IL-17 production was measured in culture supernatants after further 96 hours of stimulation. *P < 0.001. RNA from these cells was analyzed by quantitative RT-PCR for the transcription factors RORγt and Foxp3 (D). Data depicted in C and D are representative of four similar experiments.
AM80 Treatment Ameliorates Acute Autoimmune Inflammation
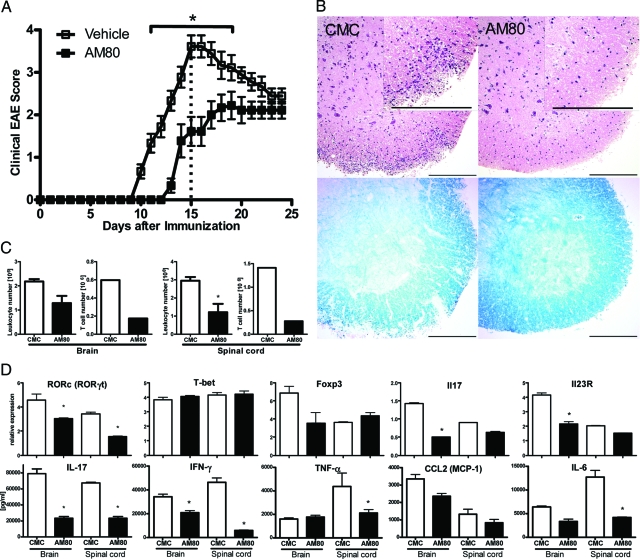
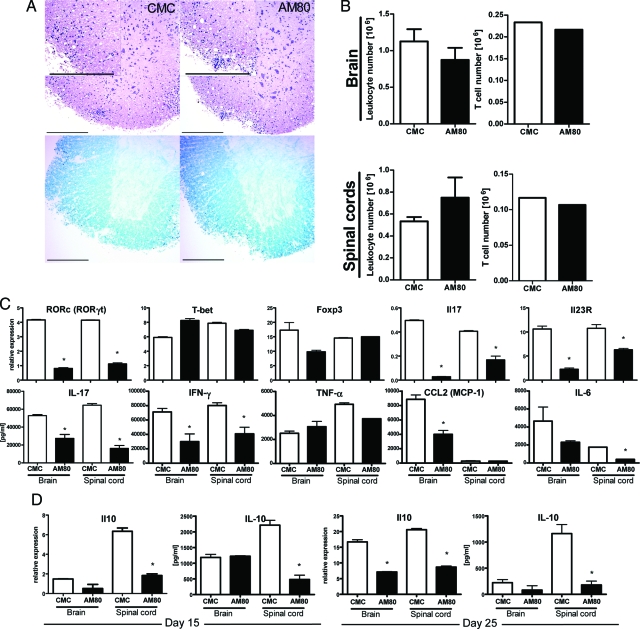
EAE, the murine model of MS,3 is an autoimmune disease in which Th17 cells play an important pathogenic role.42 At high doses ATRA can delay onset of this disease putatively via mechanisms that affect the development of Th17 cell function.30 As AM80 is more effective in inhibiting Th17 development in vitro, we tested whether or not administration of this compound could modulate EAE. Thus, EAE was induced in B6 mice, and some groups of mice received AM80 orally every other day from the day of immunization. The onset of clinical disease was delayed and maximal clinical score was significantly reduced in animals treated with AM80 as compared with control mice treated with vehicle alone (Figure 2A). Histological examination of spinal cords at the peak of clinical disease (day 15) showed that AM80 treatment led to a marked reduction in cellular infiltrates into spinal cord and maintained normal myelin structure (Figure 2B). Flow cytometric analysis confirmed that AM80 treatment led to reduced numbers of infiltrating cells in the brains and spinal cords and this difference was particularly apparent when T cell numbers were compared (Figure 2C). As some T cell infiltration was still observed in the CNS of AM80-treated mice, we examined the functional properties of such cells to ascertain whether or not AM80 modulated T cell differentiation in vivo. The expression of Th17-specific genes including RORγt, IL-17 and IL-23 receptor in T cells isolated from CNS was reduced in the group treated with AM80 (Figure 2D). In contrast, the expression of Foxp3 and T-bet (specific for Treg cells and Th1 cells respectively) were not elevated by AM80 treatment. Flow cytometric analysis confirmed that fewer IL-17-producing cells but similar numbers of Foxp3-positive cells were present among the CNS infiltrating T cells in AM80-treated mice as compared with vehicle treated controls (data not shown). Also, we note that AM80 treatment did not affect the expression of activation markers (including CD62L, CD44 and CD25) by CNS-infiltrating T cells (data not shown). Furthermore, on anti-CD3 mAb stimulation, CNS-infiltrating T cells isolated from animals treated with AM80 produced significantly reduced levels of pro-inflammatory cytokines and chemokines (Figure 2D). Myeloid cell and T cell phenotyping via flow cytometric analysis revealed no significant differences between treatment groups (data not shown). Taken together, these results indicate that AM80 treatment decreases the number of T cells infiltrating the CNS during EAE and also lowers their IL-17 producing capacity.
Figure 2.
AM80 treatment ameliorates EAE with reduction of IL-17 production in vivo. EAE was induced in B6 mice by immunization with MOG35–55. Groups of mice received either CMC or 3 mg/kg AM80 in CMC orally every other day from day 0. Mice were scored daily for clinical disease (A) analysis of variance for repeated measures shows significant differences from day 11 to 18 (*P < 0.001). On day 15 after immunization, groups of mice were sacrificed and spinal cords were examined histologically. Representative sections are shown in B. H&E staining (upper panels), Luxol fast blue staining (lower panels). Scale bar = 200 μm. Leukocytes and T cells were purified from the CNS at day 15 after immunization and cell numbers evaluated by hemocytometer (C). Quantitative RT-PCR was used to measure an array of transcription factors and cytokines (D, upper row) in which error bars represent duplicated PCR of the same samples. In the lower row, CNS-infiltrating T cells were restimulated with immobilized anti-CD3 antibody and supernatants were measured after 72 hours for the presence of a range of cytokines and chemokines using ELISA or CBA. Error bars represent measurements from duplicate wells. Data are representative of at least two independent experiments.
We have performed adoptive transfer experiments to determine whether AM80 ameliorates EAE through a direct effect on T cells or not. Draining lymph node cells isolated from immunized mice with oral administration of AM80 or vehicle were restimulated with MOG peptide and cultured for one week without retinoid treatment. After transfer of MOG-reactive T cell blasts into naive mice, AM80-treated T cells caused only minimal disease compared with a significant disease development after transfer of vehicle-treated T cells (see Supplemental Figure S1 at http://ajp.amjpathol.org). In addition, when encephalitogenic T cells isolated from vehicle-treated animals were incubated ex vivo with AM80 for 2 hours before adoptive transfer into naïve mice, no disease developed (see Supplemental Figure S1 at http://ajp.amjpathol.org). Taken together, the suppressive capacity of AM80 is attributed, at least in part, to a direct inhibitory effect on encephalitogenic T cells in vivo.
AM80 Suppresses Antigen-Specific IL-17 Production of T Cells
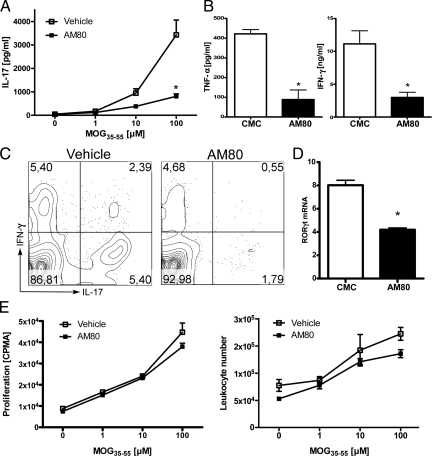
Since AM80 treatment suppressed the onset of clinical EAE and also inhibited inflammatory cellular responses in the target organ, we set out to elucidate the cellular and molecular mechanism by which AM80 suppresses EAE development by examining antigen-specific effector T cells responses with or without AM80 treatment. MOG-specific production of pro-inflammatory IL-17 by draining lymph node cells was dramatically reduced after in vivo treatment with AM80 (Figure 3A). In addition, production of other proinflammatory cytokines such as IFN-γ and TNF-α was also significantly reduced (Figure 3B). The reduction in IL-17 secretion following retinoid treatment could result from decreased frequency of IL-17 producing T cells, or decreased production of cytokines by each T cell. Therefore, we examined cytokine production among draining lymph node T cells on a per cell basis using flow cytometric intracellular cytokine staining. Also, Th17 differentiation was estimated by quantifying RORγt expression among draining lymph node, using quantitative RT-PCR. As shown in Figure 3C, the IL-17-positive population in TCR+CD4+ subset was reduced after treatment with AM80, indicating that the treatment resulted in a lower frequency of IL-17 producing T cells developing in draining lymph node. In line with these findings, isolated T cells from animals that had received AM80 treatment expressed lower levels of RORγt (Figure 3D). Interestingly, unlike the effect of AM80 in vitro, such reductions in RORγt expression were not associated with an increase in Foxp3 expression (data not shown).
Figure 3.
RAR agonist AM80 suppresses Th17 differentiation without inhibiting typical proliferative responses in vivo. B6 mice were immunized with MOG35–55 in CFA and vehicle (CMC) or AM80 (3 mg/kg in CMC) were administered orally every other day starting from day 0 until day 8. Draining lymph node cells were isolated at day 10 and restimulated with MOG peptide at various concentrations. After 72 hours, antigen-specific IL-17 production was assessed by ELISA (A). In B, TNF- α and IFN-γ production after restimulation with 100 μmol/L MOG were assessed by CBA. C: Intracellular cytokine staining of draining lymph node cells in the presence of 10 μmol/L MOG35–55 after 96 hours of culture shows reduced percentages of IL-17+ and IL-17+/IFN-γ+ double producing cells. Plots are gated on TCR+CD4+ lymphocytes. D: Expression of RORγt in T cells obtained from C was assessed by quantitative RT-PCR. E: Cell proliferation was assessed either by [3H]thymidine incorporation or cell number evaluation by fluorescence-activated cell sorting. Data in A, B, and E are representative of four independent experiments with three to five mice per group and C and D depict results from two experiments with three mice per group.
It is conceivable that AM80 may protect from EAE by generating systemic immunosuppression. Indeed, a previous study using relatively high doses of the broad spectrum RAR agonist ATRA to treat EAE was unable to rule out this mechanism of action as administration of ATRA resulted in a suppression of peripheral proliferative T cell responses.30 With our treatment approach, however, cellular proliferation as measured by [3H]thymidine incorporation and increase in cell numbers determined by fluorescence-activated cell sorting following re-stimulation with antigen did not differ between treated and control groups (Figure 3E). Collectively, these data suggest that rather acting as a systemic immunosuppressive agent, AM80 acts as an immunomodulary agent for antigen-specific T cell responses, primarily affecting Th17 cell function in vivo.
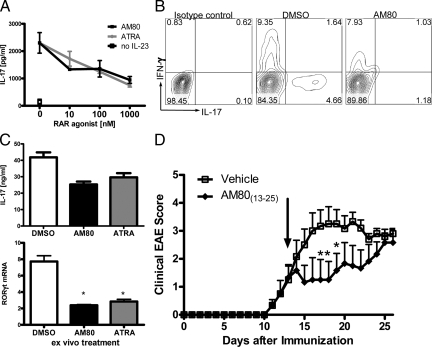
AM80 Treatment Ameliorates Ongoing Inflammatory Responses by Suppressing IL-17 Production from Differentiated Th17 Effector Cells
As we wished to evaluate the therapeutic potential of AM80 for MS treatment, we tested whether or not AM80 had an inhibitory effect on Th17 effector function in addition to its effect on Th17 differentiation. To this end, we stimulated memory cells in the presence of IL-23, a cytokine that promotes Th17 memory cell function and survival,9 and in the presence of increasing doses of AM80 or ATRA. AM80 and ATRA suppressed IL-17 production by memory T cells responding to anti-CD3 mAb activation (Figure 4A). Intracellular cytokine staining also demonstrated that those retinoids inhibit IL-17 production by differentiated Th17 cells (AM80, Figure 4B; ATRA, data not shown). Furthermore, we also confirmed that IL-17 production by T cells differentiated in vitro by a combination of TGF-β and IL-6, was effectively inhibited in the presence of AM80 after restimulation with immobilized anti-CD3 mAb (data not shown). As Th17 cells are shown to have an unstable phenotype when differentiated ex vivo,43 we examined how retinoids affect the function of Th17 cells that have stably differentiated in vivo. We stimulated CNS-infiltrating T cells isolated from mice at peak EAE, which consist of a high proportion of Th17 cells,42 in the presence of AM80 or ATRA. Both RAR agonists successfully suppressed IL-17 production by those CNS infiltrating T cells concomitant with significant reduction of RORγt expression (Figure 4C). We further tested whether or not this effect was sufficient to modulate established EAE. Rescue treatment with AM80 starting after the onset of disease significantly suppressed the increase of disease scores that was observed in control vehicle-treated EAE mice (Figure 4D). The maximal disease score was reduced from 3.4 ± 0.39 in untreated EAE mice to 2.58 ± 0.47 in AM80-treated animals. Taken together, these data indicate the retinoid treatment is effective at inhibiting the function of activated Th17 cells.
Figure 4.
AM80 suppresses IL-17 production by differentiated Th17 cells and ameliorates EAE in therapeutic intervention. A: CD4+CD44+CD25−CD62Llow memory T cells were stimulated by plate-bound anti-CD3 antibody for 4 days in the presence of IL-23. Dose-dependent decrease of IL-17 production by RAR agonists was shown in left panel. Intracellular cytokine staining of TCR+CD4+ cells shows decreased percentage of IL-17+ memory T cells after treatment with 100 nmol/L AM80 (B). Graphs are representative of two independent experiments. C: CNS-infiltrating T cells from mice with severe EAE (score 3–4) were isolated and restimulated with immobilized anti-CD3 in the presence of RAR agonists (100 nmol/L) for 3 days. Supernatants were analyzed for IL-17 production by ELISA and cells were subjected to quantitative RT-PCR for RORγt expression. Data are a representative of four similar experiments. D: Clinical EAE scores of animals treated daily from day 13 on (arrow) either with vehicle (CMC) or AM80. Displayed scores are representative of two experiments with n = 6.
Continuous AM80 Treatment Alters Cytokine Profile in the Chronic Phase of Disease
Our data have demonstrated that treatment with AM80 can both protect and rescue from EAE, associated with a suppression of pathogenic Th17 cell differentiation and function. However, when observed at later time points after EAE induction, the disease scores of both groups became alike (Figures 2A and 4D). Accordingly at such time points, there is no clear differences between treatment groups in terms of cellular infiltrates in CNS tissue as observed by histology (Figure 5A) or flow cytometry (Figure 5B). We then investigated the effector properties of CNS-infiltrating T cells derived from either vehicle-treated or AM80-treated animals with equivalent disease scores at day 25 after EAE induction. CNS-infiltrating T cells derived from AM80-treated animals contained strongly decreased levels of mRNA transcripts for RORγt, IL-23 receptor and IL-17 (Figure 5C). In addition, CNS-infiltrating T cells of AM80-treated animals produce significantly lower amounts of proinflammatory cytokines (IL-17, IFN-γ, and IL-6) and chemokines (CCL2) on restimulation in vitro (Figure 5C). Furthermore, although we have observed disease development until day 45, there were no further differences between treatment groups over this time period (data not shown). Collectively, these data suggest that inhibition of Th17 cell function alone is not sufficient to protect mice from later onset of the disease. Foxp3 expression in CNS infiltrating T by quantitative RT-PCR (Figure 5C) or intracellular Foxp3 staining (data not shown) showed no apparent differences between treatment groups, albeit a trend toward lower levels of Foxp3 expression in brain infiltrating T cells of AM80 treated animals could be observed. Next, we investigated several regulatory cytokines and found that levels of IL-10 were reduced in CNS-infiltrating T cell of animals that had received AM80 (Figure 5D). Interestingly, we observed a more profound inhibitory effect of AM80 on IL-10 production by CNS-infiltrating T cells at the later time point after EAE induction (day 25) compared with those in earlier phases of the disease (day 15). Despite simultaneous suppression of IL-17 production, continuous AM80 treatment may deplete the host immune system of its intrinsic T cell production of IL-10, leading to a possible loss of the protective function of retinoids.
Figure 5.
Continuous AM80 treatment is less effective on EAE suppression with a differentially modulated cytokine profile. A: The H&E-stained sections and the Luxol fast blue-stained sections in EAE mice treated with or without AM80 are shown. The control animal (score 2) and AM80-treated animal (score 2) at day 25 were subjected to histological examination. Scale bar = 200 μm. B: Total leukocyte numbers and isolated T cell numbers from spinal cords were evaluated in animals that had received CMC or AM80 at day 25 after immunization. The upper row of C depicts purified T cells from B that were subjected to quantitative RT-PCR. Error bars represent duplicated PCR of the same samples. In the lower row of C, CNS-infiltrating T cells were restimulated with immobilized anti-CD3 mAb and cytokine or chemokine production in culture supernatants were assessed by ELISA or CBA after 72 hours. Error bars represent measurements from duplicate wells (*P < 0.001). D: IL-10 production by stimulated CNS-infiltrating T cells derived from CMC-treated or AM80-treated animals were assessed by either quantitative RT-PCR or CBA. CNS-infiltrating T cells were isolated at day 15 or day 25 after EAE induction. B is a representative of four independent experiments; A, C, and D of two with six animals per group.
Continuous Treatment with RAR Agonists Abrogate IL-10 Production by T Cells, Which Are Identified To Be RORγtFoxp3 Double Positive
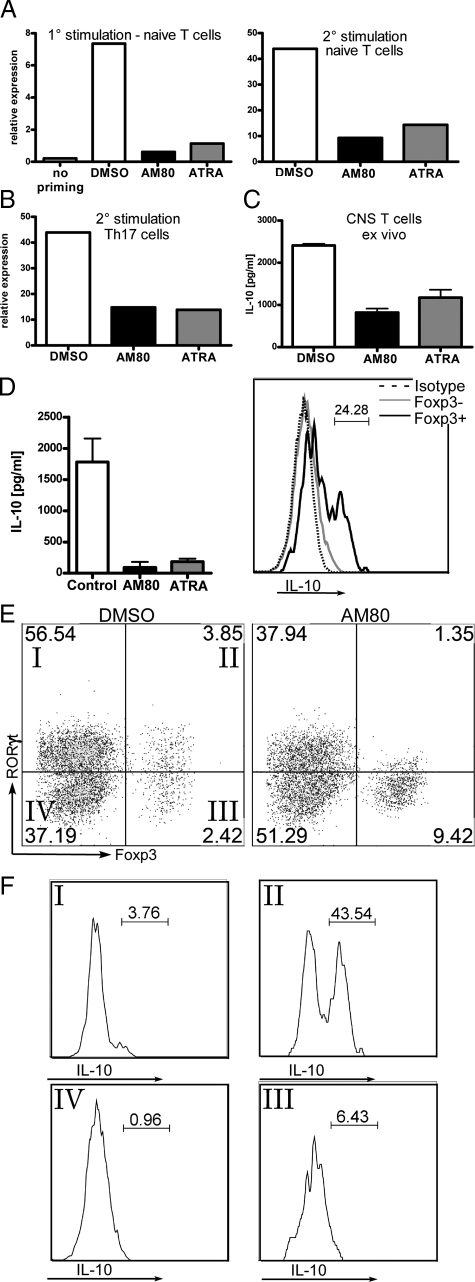
As continuous AM80 treatment suppressed the production of IL-10 by CNS-infiltrating T cells, we further investigated the effect of AM80 on the production of IL-10 in vitro. As shown in Figure 6A, we detected higher levels of IL-10 transcripts among effector T cells activated under Th17 priming conditions as compared with those primed under neutral conditions, supporting a previous study in which demonstrate increase IL-10 secretion in Th17 cultures on TGF-β and IL-6 signaling.24 Addition of RAR agonists during the primary culture confers reduced expression of IL-10 transcripts, even when cells underwent a secondary stimulation in the absence of retinoids, pointing toward a stable phenotype. To assess the effect of retinoids on differentiated Th17 cells, T cells were primed under Th17 priming conditions without retinoids, but restimulation in the presence of retinoids decreased the expression of IL-10 (Figure 6B). In addition, ex vivo restimulation of CNS-infiltrating T cells in the presence of retinoids also reduced IL-10 expression (Figure 6C). Taken together, treatment with either AM80 or ATRA inhibits the production of IL-10 by Th17 cells, which have differentiated in vitro or in vivo.
Figure 6.
Continuous AM80 treatment impairs IL-10 production by T cells, which are identified as RORγt+/FOXP3+ population. A: Naïve T cells were cultured under neutral (no priming) or Th17-priming conditions in the presence of retinoids (100 nmol/L). After 96 hours, expression of IL-10 mRNA was assessed by quantitative RT-PCR. For further analysis, cells were rested for 48 and restimulated with anti-CD3 antibody for another 96 hours and examined for expression of IL-10 transcript. B: Naive T cells stimulated under Th17 priming conditions without RAR agonists were then restimulated as described above. RAR agonists (100 nmol/L) were added during the secondary stimulation and assessed for their expression of IL-10 mRNA by quantitative RT-PCR. Results are representative of two independent experiments. C: CNS-infiltrating T cells were isolated from EAE animals (score 3) and restimulated with immobilized anti-CD3 mAb in the presence of AM80 or ATRA (100 nmol/L) for 72 hours. The amount of IL-10 in culture supernatants was examined by ELISA. D: Whole splenocytes were cultured for 96 hours with TGF-β and IL-6 and RAR agonists (10 nmol/L) and CBA analysis were performed for analyzing the levels of IL-10 production in the culture supernatants. Cells were then subjected to intracellular cytokine staining by fluorescence-activated cell sorting. The histogram gated on CD45highTCR+CD4+ lymphocytes displays the comparative population of IL-10+ cells by Foxp3+ (black line) and Foxp3− (gray line) cell populations. Broken line represents the data stained with isotype control antibody. E: CD45highTCR+CD4+ lymphocytes derived from D were analyzed for their expression of RORγt and Foxp3. Comparative dot plots were shown with or without AM80 treatment (10 nmol/L). Addition of AM80 decreased the number of RORγt+ cells, including the RORγt+FOXP3+ cells. F: The levels of IL-10 producing cells from the quadrants labeled in E are shown.
Regulatory T cells have previously been indicated as main source of IL-10-producing T cell subsets.44 In Figure 6D, we confirm that Foxp3+ cells are the source of IL-10 in whole splenocyte stimulated in the presence of TGF-β and IL-6. Interestingly, addition of retinoids expanded the number of Foxp3+ cells (data not shown), but abolished their production of IL-10 (Figure 6D). Recently, a further subset of IL-10 producing regulatory T cells that express of RORγt and Foxp3 simultaneously have been highlighted.25 Therefore, we hypothesized that such cells represent the major population of IL-10-secreting T cells in Th17 differentiation cultures and thus-it is these cells on which retinoids act to abolish IL-10 production. In fact, Foxp3+ cells could be successfully divided into RORγt+ and RORγt − populations (Figure 6E). Addition of AM80 to cultures reduced the proportion of RORγt+ and RORγt+Foxp3+ double positive cells, but increased the proportion of Foxp3+RORγt− cells (Figure 6E), at the same time as abolishing IL-10 production (Figure 6D). Intracellular IL-10 staining revealed that the RORγt+Foxp3+ double positive population was the predominant source of IL-10 (Figure 6F). Taken together, these data suggests that retinoid treatment reduces the production of IL-10 by inhibiting the effector function of a distinct RORγt+Foxp3+ population, which might serve as a regulatory T cell subset.
Discussion
ATRA, the physiologically active metabolite of vitamin A, inhibits the differentiation of Th17 cells and reciprocally promotes the generation of Treg cells in vitro.27,29 In this study, we demonstrate for the first time that the synthetic RAR agonist AM80 inhibits the differentiation and effector function of Th17 cells more effectively than ATRA. Oral administration of AM80 attenuates antigen-specific Th17 cell differentiation, thus such treatment is able to reduce disease in the acute phase of EAE.
ATRA suppresses Th17 differentiation and effector function via RARα signaling,27,28 but ATRA can also bind to RARβ and RARγ, which can form a variety of homo- and heterodimers with three RXR receptors.26,34 Non-selective receptor binding is thought to be a major cause of the side effects associated with the administration of ATRA and other pan-RAR agonists in the clinic. AM80 is a synthetic RAR agonist that has high affinity to the RARα/β and is currently available as medication for human diseases such as APL and psoriasis.35,36,37,45 In addition to greater specificity for RARα, AM80 offers several other advantages over ATRA as a therapeutic agent: it has a lower toxicity, higher stability, fewer potential side effects, and a superior bioavailability. Also, we demonstrate that a lower dose of AM80 is required to inhibit IL-17 production by T cells than similar treatment with ATRA. Interestingly, at very low doses, AM80 treatment reduced IL-17 production dramatically, while RORγt expression was only slightly reduced. Although RORγt expression is a pre-requisite for Th17 development,17 recent studies have demonstrated that Foxp3 can physically interact with RORγt inhibiting IL-17 production when both are co-expressed.25,46,47 It is conceivable that the disconnection between RORγt expression and IL-17 secretion following low dose AM80 treatment we observed may result from concomitant Foxp3 up-regulation. However, at higher doses of AM80, the inhibition of IL-17 production was associated with a reduction in RORγt expression; thus there may be multiple mechanisms of action in operation.
A recent study demonstrated that intraperitoneal injection of high doses of ATRA protected animals from EAE induction and that this protection was associated with reduced IL-17 and IFN-γ production.30 However, such treatment was also found to reduce proliferative T cells responses following antigen restimulation ex vivo,30 suggesting that, in this study, ATRA may ameliorate EAE by generating systemic immune-suppression. We have observed similar immunosuppression by ATRA and other retinoids at a high dose (data not shown). In our study, we treated with AM80 at a dose 5–10 times lower than the dose that ATRA has been previously tested at and we were able to administer the retinoid orally. Our treatment regimen also suppressed Th17 cell differentiation and IL-17 production, but antigen-specific T cell proliferation was not altered. Thus, we were able to target pathogenic Th17 cells specifically, without inducing general immunosuppression.
We and others have found that there is no induction of regulatory T cells when treating inflammatory diseases with RAR agonists,29,30 and it has been speculated that this may be due to a lack of TGF-β in vivo.29 An alternative hypothesis is that Treg generation is inhibited by the strong induction of inflammatory cytokines, including IL-6, TNF- α, and IL-1.30 Thus, under both of these suggestions, it is likely that AM80 suppresses EAE by inhibiting the generation and activity of Th17 cells.
AM80 treatment inhibited acute EAE in mice, but continuous administration of AM80 did not suppress chronic inflammation. Interestingly, T cells isolated from the CNS tissue of AM80-treated mice during the chronic phase of the disease continued to express only low levels of RORγt, IL-23 receptor, and IL-17. This was in marked contrast to vehicle-treated control mice, which, despite analogous clinical disease scores, had CNS-infiltrating T cells that expressed high levels of Th17-related factors. Thus, we suggest that inhibition of Th17 cell function alone is not sufficient to protect mice from chronic CNS inflammation.
In fact, we found that treatment with RAR-agonists suppressed T cell production of IL-10 at late stage disease. Recently, a unique T cell subset that co-expresses RORγt and Foxp3, and predominantly secretes regulatory IL-10 has been identified in vivo.23 Intriguingly, we show that RAR agonists not only suppress pathogenic Th17 cells, but also suppress IL-10 production by these RORγt+Foxp3+ T cells. Korn et al propose a model of sequential infiltration by different subsets of differentiated CD4+ T cells during organ-specific autoimmunities such as EAE. In this model, Th17 cells mediate the acute phase of disease, while Th1 cells are more prominent in the chronic phase, and at much later phase of disease, there is a moderate up-regulation of IL-10 production.48,49 In addition, previous studies indicate that IL-10 is a key cytokine for the suppression of T cell-mediated autoimmune inflammation in the CNS.23,49 Therefore, the residual expression of IL-17 or IFN-γ at later time points with continuous AM80 treatment may cause moderate progression of EAE development under the condition with reduced IL-10 production in vivo. We have little information about how those two subsets of IL-17-producing inflammatory RORγt+ T cells and IL-10-producing immunoregulatory RORγt+Foxp3+ T cells developed during immunological responses. Although we demonstrate here that the suppressive effect of AM80 on EAE is most likely through modulation of T cell production of both IL-17 and IL-10 in vivo, we don’t exclude the possible involvement other factors, as EAE/MS are complex diseases that do not depend only on a IL-17 versus IL-10 dichotomy. For example, ongoing neurodegeneration50 may also contribute significantly to the observed phenotype especially at the later phase of disease. As Th1 cells also play a significant role in the induction or EAE/MS, we should also point out that retinoic acid has been previously shown to exert direct effects on T cells, suppressing Th1 development and enhancing Th2 development via retinoic acid receptors.51 This indicates that AM80 has multiple beneficial effects for disease protection through oral administration. The immunomodulatory effect of RAR agonists on those Th17-like regulatory T cells in inflammatory autoimmune diseases is to be considered if conducting in vivo attenuation of Th17 cells for treatment of autoimmune diseases with retinoids. At this point in time, we advocate that the use of AM80 on inflammatory autoimmune diseases should target the acute phases of Th17-mediated pathogenesis.
In addition, we found that T cells that had infiltrated the spinal cord produced much lower amounts of CCL2 (MCP-1) as compared with brain-infiltrating T cells. CCL2 plays a crucial role in the progression of EAE52,53 and previously we have shown that human Th17 cells express the corresponding receptor, CCR2.54 Our data showing reductions in CCL2 in the spinal cord may represent previously reported differences in inflammatory cell populations at different CNS sites.55
In summary, we demonstrate that oral treatment with AM80 effectively inhibits IL-17 production without generating systemic immunosuppression. In addition, AM80 treatment protected mice from the development of acute EAE and rescued mice with established EAE from acute autoimmune inflammation. Collectively, these data advocate AM80 as a potent therapeutic agent against acute Th17-mediated autoimmune diseases including MS.
Supplementary Material
Footnotes
Address reprint requests to Takashi Yamamura, M.D., Ph.D., Director, Department of Immunology, or Shinji Oki, Ph.D., Section Chief, Department of Immunology, National Institute of Neuroscience, NCNP, 4-1-1 Ogawahigashi, Kodaira, Tokyo, 187-8502, Japan. E-mail: yamamura@ncnp.go.jp and soki@ncnp.go.jp.
Supported in part by Health and Labor Sciences Research Grants on Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Shevach EM, Davidson TS, Huter EN, Dipaolo RA, Andersson J. Role of TGF-beta in the induction of Foxp3 expression and T regulatory cell function. J Clin Immunol. 2008;28:640–646. doi: 10.1007/s10875-008-9240-1. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, Housseau F, Yu H, Pardoll DM, Drake CG. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–256. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O'Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massacesi L, Abbamondi AL, Giorgi C, Sarlo F, Lolli F, Amaducci L. Suppression of experimental allergic encephalomyelitis by retinoic acid. J Neurol Sci. 1987;80:55–64. doi: 10.1016/0022-510x(87)90220-6. [DOI] [PubMed] [Google Scholar]
- Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, McFarlin DE, Scott DE. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995;154:450–458. [PubMed] [Google Scholar]
- Patatanian E, Thompson DF. Retinoic acid syndrome: a review. J Clin Pharm Ther. 2008;33:331–338. doi: 10.1111/j.1365-2710.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Martin PJ, Flajollet S, Dedieu S, Billaut X, Lefebvre B. Transcriptional activities of retinoic acid receptors. Vitam Horm. 2005;70:199–264. doi: 10.1016/S0083-6729(05)70007-8. [DOI] [PubMed] [Google Scholar]
- Tobita T, Takeshita A, Kitamura K, Ohnishi K, Yanagi M, Hiraoka A, Karasuno T, Takeuchi M, Miyawaki S, Ueda R, Naoe T, Ohno R. Treatment with a new synthetic retinoid. Am80, of acute promyelocytic leukemia relapsed from complete remission induced by all-trans retinoic acid. Blood. 1997;90:967–973. [PubMed] [Google Scholar]
- Miwako I, Kagechika H. Tamibarotene. Drugs Today (Barc) 2007;43:563–568. doi: 10.1358/dot.2007.43.8.1072615. [DOI] [PubMed] [Google Scholar]
- Ohnishi K. PML-RARalpha inhibitors (ATRA, tamibaroten, arsenic troxide) for acute promyelocytic leukemia. Int J Clin Oncol. 2007;12:313–317. doi: 10.1007/s10147-007-0694-6. [DOI] [PubMed] [Google Scholar]
- Kagechika H. Novel synthetic retinoids and separation of the pleiotropic retinoidal activities. Curr Med Chem. 2002;9:591–608. doi: 10.2174/0929867024606975. [DOI] [PubMed] [Google Scholar]
- Doi Y, Oki S, Ozawa T, Hohjoh H, Miyake S, Yamamura T. Orphan nuclear receptor NR4A2 expressed in T cells from multiple sclerosis mediates production of inflammatory cytokines. Proc Natl Acad Sci USA. 2008;105:8381–8386. doi: 10.1073/pnas.0803454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol. 2006;7:987–994. doi: 10.1038/ni1370. [DOI] [PubMed] [Google Scholar]
- Raveney BJ, Richards C, Aknin ML, Copland DA, Burton BR, Kerr E, Nicholson LB, Williams NA, Dick AD. The B subunit of Escherichia coli heat-labile enterotoxin inhibits Th1 but not Th17 cell responses in established experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2008;49:4008–4017. doi: 10.1167/iovs.08-1848. [DOI] [PubMed] [Google Scholar]
- Aranami T, Yamamura T. Th17 Cells and autoimmune encephalomyelitis (EAE/MS). Allergol Int. 2008;57:115–120. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- Lexberg MH, Taubner A, Forster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- Zhou X, Schmidtke P, Zepp F, Meyer CU. Boosting interleukin-10 production: therapeutic effects and mechanisms. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:465–475. doi: 10.2174/156800805774912926. [DOI] [PubMed] [Google Scholar]
- Takeuchi M. Clinical experience with a new synthetic retinoid, tamibarotene (Am-80) for relapsed or refractory acute promyelocytic leukemia. Gan To Kagaku Ryoho. 2006;33:397–401. [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Herranz EPL, Gold R, Linker RA. Pattern of axonal injury in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neurobiol Dis. 2008;30:162–173. doi: 10.1016/j.nbd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan RN, Elhofy A, Karpus WJ. Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J Immunol. 2008;180:7376–7384. doi: 10.4049/jimmunol.180.11.7376. [DOI] [PubMed] [Google Scholar]
- Sato W, Aranami T, Yamamura T. Cutting edge: human Th17 cells are identified as bearing CCR2+CCR5- phenotype. J Immunol. 2007;178:7525–7529. doi: 10.4049/jimmunol.178.12.7525. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.