Abstract
Hyaluronan (HA) occurs in the body as a large, hydrating, space-filling, carbohydrate polymer in the extracellular matrix; it has both anti-angiogenic and immunosuppressive properties. Cleavage of HA results in the generation of variably sized fragments that stimulate multiple angiogenic and inflammatory responses in a size-specific manner. In this study, we report that platelets, as well as their megakaryocyte precursors, are unusual among somatic cells in that they contain only hyaluronidase 2 (HYAL2) but not HYAL1. Platelet HYAL2 is sufficient to cleave HA into fragments that are specific for inflammatory and angiogenic signaling; this process occurs in the absence of HYAL1, which is necessary in all other tissues to perform further HA degradation. Platelets can bind to HA, some of which derives from the stressed microvessel endothelial cell surface. Platelet-derived HYAL2 cleaves HA into fragments that stimulate mononuclear leukocytes in the immediate microenvironment to produce proinflammatory cytokines, including interleukin-6 and interleukin-8. Platelets, thus, are not only involved in hemostasis, the earliest step in wound healing, but are also important in the signaling of subsequent inflammatory and angiogenic steps. We hypothesize that aberrations in these sequential steps can promote chronic inflammation, as found in inflammatory bowel disease. The platelet may thus provide an interface between acute and chronic inflammation, wound healing, and their subsequent fibrotic responses.
Hyaluronan (HA) is a ubiquitous carbohydrate polymer of the extracellular matrix. Its deposition is greatly increased during development, repair and regeneration, in malignancy, and particularly in inflamed tissues. High molecular size HA (106 to 107 Da) occurs in the body as a hydrating, space-filling polymer.1,2,3 This form of HA is found in normal, intact, healthy tissue, and contributes to local homeostasis by suppressing cell proliferation, migration, angiogenesis, inflammation, and immunogenicity.4,5,6 On the other hand, fragmentation of HA results in smaller polymers that are highly angiogenic, inflammatory, and immunogenic in a size-dependent manner.7,8,9,10,11 Such HA fragments in tissues reflect a stress response and function as endogenous danger signals.12 HA fragments promote angiogenesis by driving endothelial cell proliferation and migration, and by stimulating production of angiogenic and inflammatory factors. These fragments also impact immune responses by promoting macrophage chemotaxis and inflammatory cytokine production. This suggests that HA fragments constitute an information-rich system.13 HA levels increase during inflammation in both human and animal systems,14,15,16 as well as upon sterile damage.11 The net HA concentration and the polymer sizes deposited within the extracellular matrix are the result of a careful balance between synthesis and degradation.
HA is produced as a straight chain unmodified polymer of repeating disaccharides of d-glucuronic acid and N-acetylglucosamine. It is synthesized on the cytoplasmic surface of plasma membranes by three HA synthases, HAS1, HAS2, and HAS317,18 and extruded into the extracellular space, where it integrates into the extracellular matrix.
Catabolism of HA in somatic tissues, on the other hand, is mediated principally by two hyaluronidases, HYAL1 and HYAL2.19,20,21 The mechanisms responsible for generating and maintaining HA fragments of specific size in sufficient concentration for signaling, particularly in an inflammatory response, have not been defined. Of the two HYALs in somatic tissues, HYAL2 is a cell surface glycosylphosphatidylinositol-anchored protein, which, in cooperation with CD4422,23 catalyzes the initial reaction that cleaves the large HA polymers.24 Normally, the acid-active lysosomal enzyme, HYAL1, degrades these HA oligomers to tetrasaccharides.25,26 The completion of HA degradation to the individual sugars follows, and is assisted by two lysosomal β-exoglycosidases, β-glucuronidase, and β-N-acetyl glucosaminidase.20,21 Also contributing to degradation are the nonenzymatic reactions, most notably those involving reactive oxygen species that cause random depolymerization of HA.27 Platelets are specialized cells in circulation traditionally associated with clot formation and hemostasis. The essential role of platelets in inflammatory conditions such as in cardiovascular disease,28 inflammatory bowel disease,29 and a host of autoimmune disorders have recently become appreciated.
In this report we describe for the first time that both platelets and megakaryocytes contain exclusively HYAL2, with no evidence for HYAL1, which is found in all other tissues.30 Platelets, therefore, appear to possess a unique mechanism for promoting inflammation. Circulating platelets, and immune cells, bind initially to HA on activated microvessel endothelial cell surfaces. The platelet-derived HYAL2 then cleaves the HA produced in the inflamed local vasculature, and possibly HA provided by the platelets themselves. This results in the generation of signaling-sized fragments that are released into the local microenvironment. The cleaved HA induces the production of proinflammatory cytokines and chemokines by the local mononuclear leukocytes, as well as recruitment of additional inflammatory cells.
Materials and Methods
Isolation of Human Platelet-Rich Plasma and Platelets
Details for the isolation of platelet-rich plasma and of platelets from normal volunteers are similar to those described previously.31 Peripheral venous blood was collected with consent as approved by the Institutional Review Board of the Cleveland Clinic. Blood samples were drawn into syringes containing 10% disodium citrate in Hanks’ balanced salt solution (HBSS) (final blood concentration, 1% citrate). Platelet-rich plasma was obtained by centrifugation of 5 ml of blood in polypropylene test tubes at 180 × g for 8 minutes at room temperature, collecting the upper fraction. In vitro clot formation was achieved by the addition of calcium chloride (20 mmol/L) and thrombin (1U/ml) to the platelet-rich plasma, and incubation at 37°C for 10 minutes. The clot was gently removed and fixed in Histochoice (Sigma-Aldrich, St. Louis, MO), paraffin-embedded, and 5 mmol/L serial sections cut and stained (below). Platelets used for RNA isolation and Western blotting were collected by centrifugation (1200 × g for 2 minutes) and washed three times in HBSS containing 1% citrate before making appropriate extracts.
Isolation of Mouse Bone Marrow
Male C57/BL6 mice were conventionally housed at the Cleveland Clinic and all husbandry and euthanization were conducted according to Institutional Animal Care and Use Committee-approved protocols. Untreated mice were sacrificed and their femurs collected. After dissecting the articular surfaces, a 26-guage needle was inserted into one end of the bone, and the bone marrow was flushed out with 2 ml of HBSS fed by a syringe. The expelled bone marrow was centrifuged (400 × g for 5 minutes) and the pellet fixed in 1 ml of Histochoice (Sigma-Aldrich).
Fluorescence Histochemistry for Confocal Microscopy
Descriptions of fluorescence histochemistry and confocal microscopy were provided previously.14 Briefly, sections of in vitro clot or inflamed mouse colon were deparaffinized and incubated in a solution of HBSS containing 2% fetal bovine serum (FBS) for 30 minutes. The slides were then incubated with a solution containing biotinylated HA-binding protein (bHABP) (Seikagaku Corp., Tokyo, Japan) at 5 mg/ml, and the appropriate antibody. Rabbit polyclonal antibodies directed against HYAL1 and HYAL2 were used at 1:100 (polyclonal antibodies specific for HYAL1 and HYAL2 were prepared in rabbits using synthetic peptides corresponding to amino acids 104 to 120 and 100 to 116, respectively30; a goat polyclonal antibody against CD42b (Santa Cruz Biotechnology, Santa Cruz, CA) was used diluted at 1:100; and an affinity-purified biotinylated rabbit antibody to fibrinogen (gift of Patricia Dibello, Cleveland Clinic) was used at 4 mg/ml. All were diluted in HBSS containing 2% FBS for 16 hours at 4°C. The slides were subsequently washed three times with HBSS, and then incubated with a solution containing Alexa-488-tagged streptavidin (1:500) or Alexa-568-conjugated anti-Ig (H+L) (1:1000) directed to rabbit or Alexa-488-conjugated anti-goat antibody (1:1000) in HBSS containing 2% FBS. This secondary incubation was done for 1 hour at 25°C. The slides were washed three times in HBSS and coverslips affixed to the slides in Vectashield mounting medium containing 4,6-diamidino-2-pheny;indole (DAPI) (Vector Laboratories, Youngstown, OH) as a nuclear stain. The slides were then sealed with nail polish and stored at −20°C. Confocal images were obtained using a Leica TCS-SP laser-scanning confocal microscope (Leica, Heidelberg, Germany), which is equipped with three lasers and photodetectors that permit detection of three distinct fluorochromes.
Extraction and Expression of Platelet mRNA
RNA from washed platelets was extracted by the phenol-chloroform method using Trizol (Invitrogen, Carlsbad, CA) reagent according to the manufacturer’s protocol. Expression of mRNA was evaluated by reverse transcription and the polymerase chain reaction using oligonucleotide primers for β-actin, HYAL1, and HYAL2, as described previously.32
Identification of Hyal2 Protein by Western Blot Analysis
Washed platelets obtained from 12 ml of whole blood were collected and resuspended in 40 μl of HBSS and mixed with an equal volume of Laemmli sample buffer (Bio-Rad, Hercules, CA), and 2-mercaptoethanol (2-ME) was added to a final concentration of 5%. Equal sample volumes (2 μl) were added to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and electrophoresis was performed. After separation, the samples were electroblotted onto polyvinylidene difluoride (PVDF)-type transfer membranes (Immobilon-P; Millipore, Bedford, MA) at 4°C. Blocking was performed in phosphate-buffered saline (PBS) containing 0.1% Tween-20 (PBST) and 5% nonfat dry milk for 2 hours at 25°C. The membrane was incubated with rabbit polyclonal antiserum to Hyal2 (1:500) in PBST containing 5% nonfat dry milk for 16 hours at 4°C. After five washes with PBST (10 minutes each), horseradish peroxidase-conjugated secondary antibody (1:3000 in PBST with 5% milk) was incubated with the membrane for 1 hour at 25°C. Membranes were washed four times with PBST and twice with PBS (10 minutes each). Positive bands were reported by a chemiluminescence detection system (Amersham, Piscataway, NJ) according to the kit protocol.
Isolation and Culture of Human Intestinal Microvessel Endothelial Cells (HIMECs)
HIMECs were isolated from surgically resected human colon tissue and grown in culture as previously described.33 Mucosal strips measuring 2 to 3 cm were dissected from the surgical specimens, rinsed in sterile HBSS, and stirred for 30 to 45 minutes at room temperature in a solution of 1.5% dithiothreitol in HBSS to remove mucus. Next, three sequential 45- to 60-minute washes, using a solution of 10% ethylenediaminetetraacetic acid in HBSS, were done to remove epithelial cells. Mucosal strips were then minced into 12-mm pieces and digested in a 2 mg/ml type II collagenase solution (Worthington Biochemical Corp., Freehold, NJ) at 37°C for 15 minutes. After the enzymatic digestion, the fragments were transferred into a sterile Petri dish containing endothelial MCDB 131 growth medium (Sigma Chemical Co., St. Louis, MO), 20% heat-inactivated FBS, 2.5% penicillin-streptomycin-Fungizone solution, and 90 mg/ml heparin. Mechanical compression with the blunt edge of a no. 21 scalpel blade was applied to the center of each tissue fragment, squeezing in an outward direction to express clusters of microvascular endothelial cells. The culture medium containing expressed cells was filtered through a sterile 60-mm-pore nylon cell strainer (BD-Falcon; BD Biosciences, San Jose, CA) to remove tissue fragments. The filtered cells were suspended in 10 to 20 ml of endothelial growth medium. This cell suspension, containing clusters of endothelial cells, was then passed through a second nylon filter with a 15-mm-pore diameter (Nitex; Tetko Inc., Briarcliff Manor, NY). Trapped cells, enriched for endothelial clusters, were flushed off the filter, collected, pelleted, and suspended in endothelial growth medium supplemented with 50 mg/ml of endothelial cell growth factor (Boehringer Mannheim, Indianapolis, IN). Endothelial cell clusters were plated onto 10-cm2 tissue culture dishes precoated for 1 hour before plating with a 1-mg/ml (0.4 mg/cm2) solution of fibronectin (Boehringer Mannheim) in PBS. Plates were incubated in a 5% CO2 humidified chamber at 37°C and observed between 7 and 10 days. Clusters of endothelial cells were identified by their cobblestone appearance and positive von Willebrand factor staining (DAKO, Carpinteria, CA).
Identification of Platelet-Clipped HA Fragments
Confluent intestinal endothelial cell cultures (75 cm2 area) were metabolically labeled with 3H-glucosamine (0.1 mCi/ml), a precursor sugar of glycoseaminoglycans, during the entire 18 hours of tumor necrosis factor (TNF)-α (10 ng/ml) treatment. Labeled endothelial cell cultures were washed four times, and incubated without or with platelets (108/ml) in MCDB medium containing 2% FBS for 1 hour at 37°C. The treatment medium from each culture was collected, centrifuged (12,000 × g) to remove platelets, and the supernatant collected. The samples were treated with and equal volume of in 0.1 mol/L NH4 acetate, pH 7.0, containing 10× proteinase K (Sigma) for 3 hours at 60°C. Samples were applied to a Sephadex G50 (fine) desalting column in 0.1 mol/L NH4 acetate, pH 7.0. The samples were concentrated to 1 ml using speed-vacuum evaporator centrifuge. Samples were applied to a molecular sieve column (Superose 6) at 0.38 ml/minute in 0.5 mol/L NH4 acetate, pH 7.0, and fractions collected every minute. Fifty μl of each fraction was added to 4 ml of scintillation fluid and counted in a β-counter. Radioactive counts were plotted to give the elution profile and fraction numbers were standardized to Kav values. A method for the determination of the molecular weight and molecular weight distribution of chondroitin sulfate34 was used to convert Kav values to mass average values in Da.
Identification of Glycoseaminoglycans by Fluorophore-Assisted Carbohydrate Gel Electrophoresis
To confirm the identity and relative amounts of the 3H-labeled glycoseaminoglycans liberated by platelets co-incubated with TNF-α-activated endothelial cells, fluorophore-assisted carbohydrate gel electrophoresis was used as previously described.35,36 Consecutive fractions of samples from the molecular sieve column were pooled in groups of five and concentrated with a speed vac. Pooled samples were heat-inactivated (100°C, 5 minutes) to destroy any residual proteinase K activity, and treated with chondroitinase ABC (292 mU/ml) and hyaluronidase SD (28 mU/ml) overnight at 37°C (Both enzymes purchased from Associates of Cape Cod, MA). EtOH was then added to 90% followed by incubation overnight at −20°C to remove any remaining insoluble material. After centrifugation, the supernatants were collected and evaporated to completion. The hyaluronidase and chondroitinase digestion products were derivatized by addition of 12.5 mmol/L 2-aminoacridone (AMAC) in 85% dimethyl sulfoxide/15% acetic acid for 15 minutes at ambient temperature. An equal volume of 1.25 mol/L sodium cyanoborohydride in ultrapure water was added and the incubation continued for 18 hours at 37°C. Glycerol was added (final concentration 20%), and the samples stored at 4°C in the dark until analysis. Aliquots (5 μl) of each sample, along with derivatized disaccharide standards, were electrophoresed (500 V, 80 minutes) on MONO composition gels (Glyko, Novato, CA), in MONO gel running buffer, at 4°C. The gels were visualized with a UV light transilluminator, imaged with a Quantix CCD (Photometrics, Tucson, AZ) camera, and the results analyzed using Gel-Pro Analyzer (Media Cybernetics, Bethesda, MD) software.
Preparation of Platelet-Cleaved HA Fragments
Confluent intestinal endothelial cell cultures (75 cm2 area) were treated with or without TNF-α (10 ng/ml) in MCDB medium containing 5% FBS for 18 hours at 37°C. Endothelial cells were rinsed and platelets (108/ml) added in RPMI 1640 medium containing 2% FBS, and incubated for 1 hour at 37°C. Medium was collected and platelets were removed by centrifugation. The supernatant was treated with Proteinase K for 4 hours (250 μg added per ml initially and again after 2 hours) to degrade all proteins and the samples were heat-inactivated (90°C, 10 minutes) to destroy any residual proteinase K activity. Absolute ethanol was added to a final concentration of 75% and stored at −20°C overnight to precipitate HA. Concentration of HA was determined using the Hyaluronan ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s directions. Aliquots of 100 μl containing 100 μg of HA fragments in HBSS were treated without or with 100 mU of hyaluronidase from Streptococcus dysgalactiae (hyaluronidase-SD; Cape Cod and Associates), which specifically cleaves HA into disaccharide units. Matched samples were treated for 4 hours at 37°C, then boiled for 10 minutes to inactivate the enzyme, and applied directly to monocyte cultures.
Activation of Monocytes by Platelet-Cleaved HA Fragments
Purified monocytes were purified and provided to us by the Clinical Research Unit, Leukophoresis Core Service at the Cleveland Clinic. Briefly, blood was collected by whole body aphaeresis according to an institutional review board-approved protocol. Blood was fractionated by centrifugal elutriation, and the fraction containing monocytes was collected for these studies. The purity of the samples used in the presented studies was routinely greater than 95% monocytes.
Monocytes were resuspended to 5 × 106/ml in RPMI 1640 medium containing 2% FBS. Cells (10 × 106) were dispensed into 10-cm2 wells. Platelet-cleaved HA fragments (final concentration, 50 μg/ml), hyaluronidase SD-treated HA fragments, and medium (100 μl) were added to individual cultures. Monocytes were incubated at 37°C for 48 hours. The culture content was collected, the sample centrifuged, and the supernatant was filtered (0.2 μ).
Screening for Inflammatory Cytokines
Cytokine presence in monocyte supernatants was detected using Panomics TranSignal 36-human cytokine antibody array according to the manufacturer’s instructions Panomics, Inc., Fremont, CA). Blots were scanned on a Bio-Rad ChemiDOC XRS, and densitometry values obtained with Quantity One version 4.6.5 software. Measured densitometry units were normalized to the array internal positive control, yielding the relative densitometry unit. Relative densitometry units = (average of the experimental values)/(average of the blot internal positive control) × 100.
Results
Murine Platelets and Megakaryocytes Contain Exclusively HYAL2 With No Evidence for HYAL1
Markedly increased HA deposition is a common feature of many inflamed tissues such as the intestinal mucosa of patients with IBD.14 Similarly, a pronounced deposition of HA is observed in a mouse model of IBD16 induced by dextran sodium sulfate ingestion. HYAL1 and HYAL2, the two major somatic hyaluronidases, control HA catabolism and therefore the localization of these enzymes was examined in the dextran sodium sulfate model of IBD.
Serial sections of inflamed colon tissue from mice with colitis were stained to localize HYAL1 and HYAL2 (Figure 1, A and B), and a differential expression of the two hyaluronidases was observed. HYAL1 was observed throughout the tissue, but associated especially with nucleated infiltrating leukocytes. Figure 1C, a hematoxylin and eosin-stained section from adjacent colon, provides a tissue reference and shows areas of inflammatory infiltration. In marked contrast, HYAL2 staining was apparent in the blood vessels of the inflamed tissue, not in association with the blue-staining nucleated cells, but in association with very small, nonnucleated cells suggestive of platelets (indicated by the arrows in Figure 1B and in enlargement Figure 1B).
Figure 1.
HYAL1 and HYAL2 are differentially expressed during colitis, with HYAL2 being specifically associated within blood vessels. Serial sections of distal colon from a mouse that had developed a dextran sodium sulfate-induced inflammation were stained with antisera raised against the hyaluronidase HYAL1 and HYAL2 (green) and with DAPI stain for nuclei (blue). A: HYAL1 appears throughout the inflamed tissue and is most prominently associated with leukocytes. HYAL2-specific staining (B and higher magnification, D) is most prominent on small anuclear structures within focused areas of the mucosa, which when viewed at higher magnification (D) have the appearance of blood vessels (arrows, B). D: The red color indicates autofluorescent molecules in the tissue including the bright elastic lamina defining the blood vessels, and some red blood cells in the lumen in the magnified image. C: H&E-stained adjacent (not serial) section provided for tissue orientation. The area noted (dotted line) is characteristic of the area captured in the fluorescent images (serial sections A and B). As we have previously shown, features of colitis16 include: a highly damaged epithelial layer (E) surrounding the intestinal lumen (L); a pronounced leukocyte infiltrate (I) present above the mucosal muscle (MM) layer, and in the expanded submucosa (S); more prominent blood vessels (bv); and an expanded external muscle layer (EM).
Platelets were therefore examined to determine their identity as the HYAL2-expressing cells. Figure 2A demonstrates a magnified view of a three-dimensional reconstruction of platelet aggregates obtained from an in vivo clot present in the colitic tissue. Platelet identity was confirmed by immunohistochemistry using a platelet-specific surface marker CD42b (von Willebrand receptor GP1b), shown in green. Red staining was used for the localization of HYAL2. Perfect co-localization of the CD42b and HYAL2 patterns was observed as indicated in the yellow overlay staining, indicating that HYAL2 was associated with platelet surface membranes. Such a pattern was also consistent with the HYAL2 enzyme being a glycosylphosphatidylinositol-anchored cell surface membrane protein.22,24 No evidence of HYAL1-specific staining was obtained in serial sections of the clot within the intestinal tissue, although leukocytes in other areas of the same tissue stained intensely (Figure 1A).
Figure 2.
Murine platelets and megakaryocytes contain exclusively the HA-degrading enzyme HYAL2. A: An in vivo clot from inflamed mouse colon tissue is stained for the platelet marker CD42b (green) and HYAL2 (red) with specific antisera and fluorescently labeled secondary antibodies. Nuclei are labeled blue with DAPI and observed by confocal microscopy. The co-localization of HYAL2 and CD42b appears yellow. Lack of blue DAPI staining indicates high purity of the platelet preparation. B: Mouse bone marrow aspirate demonstrating that megakaryocytes express HYAL2 (red). Identification of megakaryocytes in this preparation is based on the characteristic large size (∼50 μm) and fragmented DAPI-stained nuclear material (blue). They contain the Hyal 2 enzyme in abundance before fragmenting into platelets.
Platelets are derived from megakaryocyte bone marrow precursors. To establish whether megakaryocytes also contained HYAL2, bone marrow aspirates from mouse femurs were stained. A stippled red-staining pattern for HYAL2 staining was observed in these cells (Figure 2B) indicating that the enzyme was present in abundance in these platelet precursor cells before becoming fragmented into platelets.
Human Platelets Also Express Exclusively HYAL2, With No Evidence for HYAL1
To determine whether human platelets also expressed hyaluronidases in a similar pattern to that of mice, sections from a clot produced in vitro from thrombin-treated human platelet-rich plasma were stained with HYAL1- and HYAL2-specific antibodies (red). We found that human platelets also contain HYAL2 that co-localizes with CD42b producing the characteristic yellow overlay in confocal micrographs (Figure 3), whereas HYAL1 was not expressed, consistent with our findings in mouse platelets.
Figure 3.
Human platelets contain exclusively the HA-degrading enzyme HYAL2. Paraffin sections of an in vitro clot generated by thrombin treatment of human platelet-rich plasma stained with antisera raised against HYAL1 and HYAL2 (red). Platelets specifically stained for HYAL2 with no evidence for HYAL1 staining. Platelet identity was confirmed with CD42b co-staining (yellow).
To ascertain whether circulating platelets also normally expressed HYAL 2 before clotting, we stained preparations of human platelet-rich plasma. We found HYAL2 was expressed on the majority of freshly isolated platelets (Figure 4A). Additionally we wanted to determine whether the substrate for HYAL2 enzyme, ie, HA, was normally present in the platelet environment and if so how it was distributed. Therefore the same preparations were co-stained for HA (green) using biotinylated HA-binding protein (bHABP). As shown in Figure 4A, platelets appeared to contain HA. Co-expression of the hyaluronidase enzyme and its HA substrate in platelets occurred with high frequency. These results demonstrate that HYAL2 enzyme and HA are in close proximity during clotting.
Figure 4.
The HA substrate for HYAL2 enzyme is also present in platelets, as well as in their megakaryocyte precursors. A, left: A human platelet-rich plasma preparation stained with antiserum to HYAL2 (red) and biotin-labeled HA-binding protein (green) demonstrates that platelets contain both HA and HYAL2. Preparations were also stained with DAPI nuclear stain, and the lack of blue staining as well as the infrequent autofluorescent, larger sized red blood cells (RBCs) indicate high purity of the platelet preparation. Right: Nonspecific staining control (NS). B: High magnification confocal image of a cytospin preparation of platelets within which membrane staining of HYAL2 (red) occurs, whereas HA granules (green) are present internally. C: Staining of mouse bone marrow aspirates with biotin-labeled HA-binding protein (green) and DAPI nuclear stain (blue) demonstrates that platelet precursor megakaryocytes are rich in HA compared with other bone marrow cells (left). Right: NS, nonspecific staining control.
A cytospin preparation of normal human platelets was obtained, to examine localization at a higher magnification. Staining for HYAL2 (red) confirmed a membranous pattern. HA (green), was observed in internal granules in the platelet (Figure 4B) and there was an obvious separation between the HYAL2 enzyme and the HA substrate. This separation physically prevents access of enzyme to substrate. Of intrinsic interest is the nature of the separation between HYAL2 and HA after platelet degranulation. The reaction must be held in abeyance for a short period after clot formation and the initiation of wound healing.
To better understand the origin of the HA in platelets, megakaryocytes from a mouse bone marrow aspirate were examined using the same bHABP. We found the cytoplasm of megakaryocytes stained abundantly for HA (Figure 4C). Little staining was observed in other bone marrow hematopoietic precursor cells. Although the function of the HA in megakaryocytes is not clear, the HA appears to fill the cytoplasm and is likely included in platelets during formation.
To confirm the presence of HYAL2 protein, immunoblot analyses were performed on platelets isolated from two normal human control donors. Figure 5A demonstrates that the major band detected in each sample corresponded with the 54K calculated molecular weight of the human HYAL2 protein. The difference in staining intensity between the two bands may reflect normal differences in HYAL2 content between individual donors. Specific HYAL1 protein bands were not detected in these samples, even when extract loading was increased 20-fold (not shown). Thus, HYAL2 is the only hyaluronidase expressed by circulating platelets, suggesting that platelets do not have the capacity to degrade HA beyond what HYAL2 digestion can achieve.
Figure 5.
Human platelets contain HYAL2 mRNA and protein. A: Western blot analysis of protein extracts derived from two different samples of human platelets isolated as described in the Materials and Methods and reacted with HYAL2-specific antiserum. Each lane contains an extract of platelets derived from 0.3 ml of whole blood. B: RT-PCR analysis of mRNA isolated from freshly isolated platelets. Analysis of RNA was performed using primers to detect HYAL1, HYAL2, and β-actin expression as described in the Materials and Methods. Lanes were loaded with reaction products from 0.1 μg of RNA per lane.
Some proteins are synthesized by platelets themselves, whereas others, such as fibrinogen, are taken up from circulating proteins. Therefore we investigated whether HYAL2 protein could be made by platelets by asking whether platelets contained HYAL2 mRNA. Using RT-PCR analysis of RNA isolated from human platelets (Figure 5B) we detected the HYAL2 mRNA, but not HYAL1 mRNA. As a positive control, however, we were able to detect HYAL1 expression in RNA samples obtained from intestinal epithelial cells using the same primers (not shown). Because the platelet has the ability to synthesize a number of proteins, even in the absence of a nucleus and without nuclear control mechanisms,37,38 the presence of HYAL2 mRNA in platelets suggests that the enzyme may be one of the proteins synthesized de novo.
Platelets Bind to HA and Mediate Fragmentation
Membrane-bound HYAL2 can degrade HA in cell culture medium at neutral pH.23 Because HYAL2 is present on platelet membranes, we asked whether platelets had the ability to cleave polymeric HA in vitro. TNF-α induces intestinal endothelial cells to produce HA structures on their surfaces, which act as leukocyte adhesion molecules.16 Because of the biological importance of platelet adhesion to the endothelial surface, we used this endothelial source of HA as substrate, to test the capacity of platelets to degrade HA.
Figure 6A shows a high-power field in which freshly isolated platelets were incubated with HA-expressing TNF-α-activated mucosal endothelial cells (indicated by their blue DAPI-stained nuclei) at 4°C. After 1 hour, the cultures were washed to remove unbound platelets. Platelets, identified by CD42b staining (red) were observed attached to HA (green). Similar additional experiments (Figure 6, B–D) tested whether bound platelets could degrade HA. Figure 6C shows that compared with control intestinal endothelial cells (Figure 6B), TNF-α activated endothelial cells produced greater amounts of HA (green). Again, platelets (positively stained red for HYAL2) bound to HA at 4°C (as magnified in Figure 6A), creating close contact of enzyme and substrate but not degradation. However, when parallel cultures were subsequently incubated at 37°C, rapid disappearance of HA from cell surfaces occurred (Figure 6D). Temperature sensitivity is consistent with the requirements for enzyme activity.
Figure 6.
Platelets bind to HA produced on TNF-α-activated intestinal endothelial cell surfaces. Intestinal endothelial cells (detected by their blue nuclei) produce HA (green) during treatment with TNF-α for 18 hours [A (magnified image) and C] compared with control (B).16 When freshly isolated platelets (CD42b, positive, red) are added to the cultures at 4°C for 1 hour, some adhere to HA [A (magnified image) and C] whereas others can be washed away. D: HA (green) is rapidly degraded on incubation with platelets at 37°C, whereas the endothelial monolayer remains intact.
Platelets Cleave HA Polymers into Signaling-Sized Fragments
HA fragments induce a variety of proinflammatory and proangiogenic responses in leukocytes and endothelial cells by signaling through CD44, RHAMM, TLR4, and TLR2.10,11,12,22,39,40 Therefore, we examined whether signaling-sized HA fragments appeared in the cell supernatant during the breakdown of endothelial cell-produced, cell-associated polymers of HA. HIMECs incubated with radiolabeled 3H-glucosamine generated labeled HA in response to TNF-α. Data presented in Figure 7A show the comparative size and quantity distribution of the radiolabeled products collected in the supernatant fluids from TNF-α-stimulated HIMECs incubated without and with platelets. Relatively little radiolabeled material, and of comparatively low (>10 kDa or fragments <50 saccharides) molecular weight, was found in the supernatant of the endothelial culture that did not receive platelets (dashed green). In platelet-containing cultures much greater quantities of larger sized products (400 down to 10 kDa, or fragments between 2000 and 50 saccharides) were detected (dotted red) in the culture supernatant fluids. Carbohydrate analyses (Figure 7B) using FACE demonstrated that the radiolabeled fractions contained HA.
Figure 7.
Platelets cleave endothelial-produced HA into signaling-sized fragments. TNF-α stimulated, 3H-glucosamine-labeled intestinal endothelial cells were incubated without or with platelets for 1 hour at 37°C. Supernatants were collected, the proteins exhaustively digested, and the glycoseaminoglycans precipitated (see Materials and Methods) and analyzed. A: HPLC analysis of radiolabeled molecules shows the relative quantity and size distribution of 3H-glucosamine-labeled glycoseaminoglycans in the endothelial cell supernatants. B: Fluorophore-assisted carbohydrate gel electrophoresis (FACE) confirms the presence of HA in the pooled fractions from endothelial cells co-incubated with platelets, but not in the control endothelial fractions.
Platelet-Cleaved HA Fragments Induce Monocyte Activation
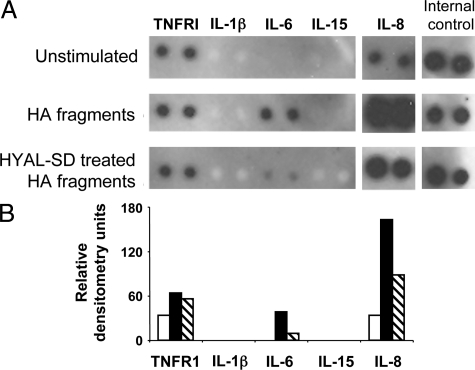
The fragments cleaved from endothelial cell surface HA by platelets fall in the range of the HA size reported to drive production of proinflammatory and proangiogenic factors.7,8,9,10,12,15,41 To determine whether the platelet-cleaved fragments have immune-activating properties, several individual preparations of platelet-cleaved HA fragments were created. HIMECs were treated with TNF-α to induce HA cable formation. Platelets were added to the cultures and the supernatants containing the platelet-liberated HA fragments were collected. Elutriation-purified populations of normal human monocytes were divided and treated with no fragments, platelet-created HA fragments, or hyaluronidase-SD (a specific bacterial enzyme that reduces HA to disaccharides)-treated platelet-cleaved HA fragments. Monocyte culture fluids collected after 48 hours of treatment were analyzed in protein screening arrays for inflammatory factor release. Figure 8 presents data from a representative experiment in which a single preparation of platelet-cleaved HA fragments was used to treat a monocyte population (99% pure). The immunoblot shows the selective up-regulation of interleukin IL-6 and IL-8 proteins in monocyte supernatant fluids after treatment with platelet cleaved HA fragments (dark bars) compared with no fragments (white bars). The stimulatory effects of the HA fragments on monocytes were substantially decreased by hyaluronidase-SD pretreatment (striped bars), indicating that the activating effects on monocytes was attributable to HA and not contaminants in the supernatant. In contrast, TNFR1 was constitutively secreted into the medium, but not regulated by HA, and IL-1β and IL-15 were neither expressed nor HA regulated in these monocyte cultures.
Figure 8.
Platelet-cleaved HA fragments activate monocytes. Fragments of HA were collected from the culture fluid of TNF-α-stimulated endothelial cells that were incubated with platelets for 1 hour at 37°C as described in the Materials and Methods. Fragments were purified by exhaustive protein digestion and ethanol precipitation, and the amount of HA determined by ELISA. Preparations of HA fragments were split and one half fully degraded with hyaluronidase-SD (HYAL-SD). Elutriation-purified monocytes (107, 99% pure) were cultured in 2 ml of RPMI 1640 medium containing 2% FBS and treated without fragments (control), with platelet-created HA fragments (50 μg/ml), or with HYAL-SD-digested platelet-created HA fragments for 48 hours. Supernatants were analyzed for cytokine content by immunoblot (A), and normalized densitometric values calculated (B). White bars, control; black bars, HA fragment treated; and striped bars, hyaluronidase-SD-degraded fragment-treated. Protein blot is representative of four experiments using different preparations of platelet-clipped HA.
Discussion
Platelets have long been acknowledged to participate in inflammation,4,6,29,42 but the mechanism by which this is accomplished is incompletely understood. Here we provide novel evidence that platelets mediate a previously undescribed mechanism to degrade HA and generate fragments that promote inflammation and angiogenesis. This hitherto unknown pathway provides a molecular basis for platelet-associated inflammation, as shown diagrammatically (Figure 9). The discovery of this new platelet-associated HA-HYAL2 axis necessitates reevaluation of pathological disorders in which platelets are abundant and in which microthrombi play important roles.
Figure 9.
Diagram of platelet HYAL2 generating signaling fragments of HA, and their interactions in the catabolic cascades associated with wound healing and inflammation. A diagram of interactions that occur between the signaling fragments of HA generated by the HYAL2 of platelets and their involvement in the earliest stages of wound healing and the subsequent inflammatory phase. Aberrations in this pathway can lead to chronicity of inflammation and possibly to autoimmune disorders.
This report is the first to identify platelets as a prominent source of the enzyme HYAL2, even though hyaluronidase activity associated with platelet fractions has been documented previously using biochemical techniques.43 Platelets have no trace of the HYAL1 found in nearly all other tissues, as determined by Northern blot analysis with a cDNA probe using an expression library of vertebrate tissues.30 Although it is unknown whether platelets are even capable of internalizing HA, the lack of HYAL1 suggests that platelets, uniquely, do not have the machinery to further degrade HA fragments. Histochemical studies reveal that HYAL2 is primarily associated with the platelet surface membrane, consistent with the enzyme being predominantly a glycosylphosphatidylinositol-anchored protein.44 The enzyme is also expressed in megakaryocytes, the platelet precursors. mRNA expression in platelets suggests that HYAL2 protein synthesis may be occurring in mature, anucleated platelets, similar to the platelet protein synthesis process described by Weyrich and colleagues.37,38 HYAL2 is also expressed by megakaryocytes before platelet formation, however we do yet know what role it may serve for these precursors either in the bone marrow niche or during platelet formation.
We recently reported that TNF-α-activated human intestinal microvascular endothelial cells produce large HA structures on their surfaces.16 Platelets bind to and degrade these HA matrix structures, and do so at neutral pH. The optimal pH for HYAL2 enzyme activity is controversial, although Harada and Takashi23 recently showed in a cell transfection model that membrane-bound HYAL2, in concert with CD44, mediates HA degradation at neutral pH. CD44 is the predominant receptor for HA.45 Cleavage of HA by HYAL2 in breast cancer cells also requires the presence of CD44, together with Na+ H+ exchanger1 (NHE1).22 However, we were unable to detect CD44 in platelets by immunofluorescent staining in several human platelet preparations tested (data not shown). Others, however, have successfully demonstrated CD44 on murine platelets,46 and this discrepancy with our data may reflect a difference in reagents low abundance of CD44 protein. Whether CD44 is required only at low levels, or whether some other HA receptor or binding protein is present in platelets that facilitates HA cleavage by HYAL2 is not yet known.
Importantly, we have demonstrated in this report that HA cleaved by platelet HYAL2 can activate monocytes. The fragmented HA preparation resulting from platelet-mediated degradation contains a polydisperse size range, and includes fragments of sizes known to induce proinflammatory, proangiogenic effects. We confirmed that the platelet-generated fragments created in our system were signaling sized by demonstrating that naive human peripheral blood monocytes, exposed to protein-free, platelet-cleaved HA preparations, were induced to secrete specific inflammatory cytokines. Reproducibly throughout our experiments, IL-6 and IL-8 were regulated by platelet-cleaved HA fragments. Increased expression of the potent immune-regulating cytokines IL-6 and IL-8 by HA fragments has been previously been reported in other cell systems including cumulus cells47 and endothelial cells.10 Additional cytokines, including RANTES and MIP1β were also induced by platelet-cleaved HA in some experiments (de la Motte, unpublished). Whether these additional cytokines resulted from shifts in the HA fragment spectrum or different responsiveness of the monocytes from individual donors is unknown. In line with our theory that HA fragments contribute to certain inflammatory diseases, in particular IBD, patient disease activity may correlate with the levels as well as the spectrum of cytokines induced by HA.
Based on this work and our recent observation that HA is present within intestinal blood vessels during colitis,16 we propose the following model for the perpetuation of inflammation, and for the chronicity of the inflammatory process (Figure 9). Circulating HYAL2-positive platelets bind to the HA on the surface of activated inflamed endothelial cells. Thrombi form at the areas of tissue damage, which provides close proximity of the HA and platelets. Fragments of HA are generated, which induce chemokine and cytokine production in local leukocytes. The HA fragments also activate endothelial cells and smooth muscle cells to proliferate, migrate, and also to produce angiogenic factors through TLR-4 receptors. This becomes a chronic self-perpetuating inflammatory process. In some pathological situations, fibrosis may result as the end product of this process, such as found in Crohn’s disease, scleroderma, and systemic lupus erythematosus (SLE). The severe scarring and fibrosis found in some patients as a result of failed attempts at wound healing may be a variation of this same process. TLR regulation of IL-6, likely through a HA-mediated pathway, was shown recently to contribute to experimental lung fibrosis.48
After tissue damage, HA is rapidly deposited in high molecular size form. The initial edema associated with early wound healing is a result of HA accumulation together with its large volume of water of hydration. The HA apparently is contributed not only by local tissue synthesis but, as we now realize, may also arise from platelet degranulation (Figure 2a). High molecular size HA opens up tissue spaces that facilitate neutrophil invasion, and clearance of debris, bacteria, and necrotic tissue. This implies that HYAL2 activity, initially, is held in abeyance, presumably through the action of an unidentified inhibitor. Inhibitors of the hyaluronidases are virtually unexplored, although they were first detected more than half a century ago.49,50 We propose that fibrinogen, because of its large size and ability to bind to HA51,52 functions as such an inhibitor by preventing association between enzyme and substrate. Alternatively, another plasma protein, inter-α-inhibitor (IαI), whose protein side chains associate with HA also has hyaluronidase inhibitory activity,53 and may participate in the temporal control of this important reaction.
HA fragments, formed by endothelial-platelet interactions in the microvasculature during the course of tissue injury or infection, induce a strong initial call for action with highly specific inflammatory monocyte-derived cytokines being produced. Platelets may also contribute HA fragments, likely in lower amounts, after platelet degranulation. The quantity of HA fragments generated is likely proportional to the extent of tissue damage and microvasculature activation, and hence is a way to signal the first responders to call for appropriate-magnitude immune response backup. Using our model, one can easily envision multiple steps in which an initial dysregulated response may result in inappropriate downstream immune activation. IL-6 and TNF-α are both initiators and end products in this experimental cascade. This could drive a self-perpetuating loop that contributes to chronic inflammatory disease conditions, including IBD.
Acknowledgments
We thank Dr. Vincent Hascall for his advice and for many useful discussions.
Footnotes
Address reprint requests to Carol de la Motte, Ph.D., Lerner Research Institute, NC2, Cleveland Clinic Foundation, 9500 Euclid Ave., Cleveland, OH 44195. E-mail: delamoc@ccf.org.
See related Commentary on page 1993
Supported by the National Institutes of Health (grant DK069854 to C.F. and C.dlM. and National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, OH) and the Department of Pathobiology, Cleveland Clinic (start-up funds to C.dlM.).
References
- Toole BP. Hyaluronan is not just a goo! J Clin Invest. 2000;106:335–336. doi: 10.1172/JCI10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- Day AJ, Sheehan JK. Hyaluronan: polysaccharide chaos to protein organization. Curr Opin Struct Biol. 2001;11:617–622. doi: 10.1016/s0959-440x(00)00256-6. [DOI] [PubMed] [Google Scholar]
- McBride WH, Bard JB. Hyaluronidase-sensitive halos around adherent cells. Their role in blocking lymphocyte-mediated cytolysis. J Exp Med. 1979;149:507–515. doi: 10.1084/jem.149.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg RN, Beebe DC. Hyaluronate in vasculogenesis. Science. 1983;220:1177–1179. doi: 10.1126/science.6857242. [DOI] [PubMed] [Google Scholar]
- Delmage JM, Powars DR, Jaynes PK, Allerton SE. The selective suppression of immunogenicity by hyaluronic acid. Ann Clin Lab Sci. 1986;16:303–310. [PubMed] [Google Scholar]
- West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- West DC, Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res. 1989;183:179–196. doi: 10.1016/0014-4827(89)90428-x. [DOI] [PubMed] [Google Scholar]
- McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- Stern R, Asari AR, Sugahara KN. Size-specific fragments of hyaluronan: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alpha-trypsin inhibitor is crucial to structure and function. Am J Pathol. 2003;163:121–133. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- Kessler S, Rho H, West G, Fiocchi C, Drazba J, de la Motte C. Hyaluronan (HA) deposition precedes and promotes leukocyte recruitment in intestinal inflammation. Clin Transl Sci. 2008;1:57–61. doi: 10.1111/j.1752-8062.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317–325. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LYW, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J Biol Chem. 2007;282:5597–5607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- Lepperdinger G, Strobl B, Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem. 1998;273:22466–22470. doi: 10.1074/jbc.273.35.22466. [DOI] [PubMed] [Google Scholar]
- Frost GI, Csoka TB, Wong T. Stern: Purification, cloning and expression of human plasma hyaluronidase. Biochem Biophys Res Commun. 1997;236:10–15. doi: 10.1006/bbrc.1997.6773. [DOI] [PubMed] [Google Scholar]
- Frost GI, Stern R. A microtiter-based assay for hyaluronidase activity not requiring specialized reagents. Anal Biochem. 1997;251:263–269. doi: 10.1006/abio.1997.2262. [DOI] [PubMed] [Google Scholar]
- Stern R, Kogan G, Jedrzejas MJ, Soltes L. The many ways to cleave HA. Biotechnol Adv. 2007;25:537–557. doi: 10.1016/j.biotechadv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Lindemann S, Krämer B, Seizer P, Gawaz M. Platelets, inflammation and atherosclerosis. J Thromb Haemost. 2007;5(Suppl 1):203–211. doi: 10.1111/j.1538-7836.2007.02517.x. [DOI] [PubMed] [Google Scholar]
- Danese S, de la Motte C, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004;99:938–945. doi: 10.1111/j.1572-0241.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- Csoka AB, Scherer SW, Stern R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics. 1999;60:356–361. doi: 10.1006/geno.1999.5876. [DOI] [PubMed] [Google Scholar]
- Danese S, de la Motte C, Sturm A, Vogel JD, West GA, Strong SA, Katz JA, Fiocchi C. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124:1249–1264. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- Selbi W, de la Motte C, Hascall V, Phillips A. BMP-7 modulates hyaluronan-mediated proximal tubular cell-monocyte interaction. J Am Soc Nephrol. 2004;15:1199–1211. doi: 10.1097/01.asn.0000125619.27422.8e. [DOI] [PubMed] [Google Scholar]
- Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895–1907. doi: 10.1053/gast.1997.v112.pm9178682. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971;59:87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Calabro A, Benavides M, Tammi M, Hascall VC, Midura RJ. Microanalysis of enzyme digests of hyaluronan and chondroitin/dermatan sulfate by fluorophore-assisted carbohydrate electrophoresis (FACE). Glycobiology. 2000;10:273–281. doi: 10.1093/glycob/10.3.273. [DOI] [PubMed] [Google Scholar]
- Calabro A, Hascall VC, Midura RJ. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology. 2000;10:283–293. doi: 10.1093/glycob/10.3.283. [DOI] [PubMed] [Google Scholar]
- Weyrich AS, Lindemann S, Tolley ND, Kraiss LW, Dixon DA, Mahoney TM, Prescott SP, McIntyre TM, Zimmerman GA. Change in protein phenotype without a nucleus: translational control in platelets. Semin Thromb Hemost. 2004;30:491–498. doi: 10.1055/s-2004-833484. [DOI] [PubMed] [Google Scholar]
- Weyrich AS, Prescott SM, Zimmerman GA. Platelets, endothelial cells, inflammatory chemokines, and restenosis: complex signaling in the vascular play-book. Circulation. 2002;106:1433–1435. doi: 10.1161/01.cir.0000033634.60453.22. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, Stein CS, Nieswandt B, Wang Y, Davidson BL, Ratliff TL. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- Fiszer-Szafarz B, Litynska A, Zou L. Human hyaluronidases: electrophoretic multiple forms in somatic tissues and body fluids. Evidence for conserved hyaluronidase potential N-glycosylation sites in different mammalian species. J Biochem Biophys Methods. 2000;45:103–116. doi: 10.1016/s0165-022x(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Liu SL, Miller AD. Transformation of Madin-Darby canine kidney epithelial cells by sheep retrovirus envelope proteins. J Virol. 2005;79:927–933. doi: 10.1128/JVI.79.2.927-933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Koshiishi I, Shizari M, Underhill CB. CD44 can mediate the adhesion of platelets to hyaluronan. Blood. 1994;84:390–396. [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, Richards JS. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- Yoshizaki A, Iwata Y, Komura K, Ogawa F, Hara T, Muroi E, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, Tedder TF, Sato S. CD19 regulates skin and lung fibrosis via Toll-like receptor signaling in a model of bleomycin-induced scleroderma. Am J Pathol. 2008;172:1650–1663. doi: 10.2353/ajpath.2008.071049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E. On the mechanism of invasion. I. Invasin I, an enzyme in plasma. J Biol Chem. 1946;163:63–88. [PubMed] [Google Scholar]
- Dorfman A, Ott ML, Whitney R. The hyaluronidase inhibitor of human blood. J Biol Chem. 1948;174:621–629. [PubMed] [Google Scholar]
- LeBoeuf RD, Raja RH, Fuller GM, Weigel PH. Human fibrinogen specifically binds hyaluronic acid. J Biol Chem. 1986;261:12586–12592. [PubMed] [Google Scholar]
- Frost SJ, Weigel PH. Binding of hyaluronic acid to mammalian fibrinogens. Biochim Biophys Acta. 1990;1034:39–45. doi: 10.1016/0304-4165(90)90150-u. [DOI] [PubMed] [Google Scholar]
- Mio K, Carrette O, Maibach HI, Stern R. Evidence that the serum inhibitor of hyaluronidase may be a member of the inter-alpha-inhibitor family. J Biol Chem. 2000;275:32413–32421. doi: 10.1074/jbc.M005428200. [DOI] [PubMed] [Google Scholar]