Abstract
Matrix metalloproteinase 9 (MMP-9) is a critical mediator of leukocyte migration in hepatic ischemia/reperfusion (I/R) injury. To test the relevance of inducible nitric oxide synthase (iNOS) expression on the regulation of MMP-9 activity in liver I/R injury, our experiments included both iNOS-deficient mice and mice treated with ONO-1714, a specific iNOS inhibitor. The inability of iNOS-deficient mice to generate iNOS-derived nitric oxide (NO) profoundly inhibited MMP-9 activity and depressed leukocyte migration in livers after I/R injury. While macrophages expressed both iNOS and MMP-9 in damaged wild-type livers, neutrophils expressed MMP-9 and were virtually negative for iNOS; however, exposure of isolated murine neutrophils and macrophages to exogenous NO increased MMP-9 activity in both cell types, suggesting that NO may activate MMP-9 in leukocytes by either autocrine or paracrine mechanisms. Furthermore, macrophage NO production through the induction of iNOS was capable of promoting neutrophil transmigration across fibronectin in a MMP-9-dependent manner. iNOS expression in liver I/R injury was also linked to liver apoptosis, which was reduced in the absence of MMP-9. These results suggest that MMP-9 activity induced by iNOS-derived NO may also lead to detachment of hepatocytes from the extracellular matrix and cell death, in addition to regulating leukocyte migration across extracellular matrix barriers. These data provide evidence for a novel mechanism by which MMP-9 can mediate iNOS-induced liver I/R injury.
Ischemia/reperfusion (I/R) injury is the pathophysiological process in which the hypoxic insult is further accentuated by restoration of blood flow to the compromised organ. This process causes up to 10% of early transplant failures and can lead to a significantly higher incidence of acute and chronic rejection.1 Hepatic I/R injury is observed in many clinical situations other than transplantation, such as hepatectomy, shock, and cardiac arrest. Liver damage caused by I/R is the result of complex interactions between various inflammatory mediators, which include infiltrating leukocytes, reactive nitrogen species, reactive oxygen species, and cytokines.2,3,4,5 A better understanding of the molecular pathophysiology of I/R injury may eventually lead to advanced therapeutic strategies that could improve the success rate of organ transplantation.
Intracellular nitric oxide synthase (NOS) converts l-arginine to l-citrulline and to a free radical nitric oxide (NO).6 NO is a short-lived signaling molecule capable of regulating many physiological and pathological processes. There are at least three different isoforms of NOS able to generate NO; the neuronal NOS (nNOS or NOS1), the inducible NOS (iNOS or NOS2), and the endothelial NOS (eNOS or NOS3).6 While nNOS and eNOS are constitutively expressed, iNOS is triggered in many cell types by cytokines such as tumor necrosis factor-α or interferon (IFN-γ).7 Under normal conditions, only eNOS is present in the liver and low levels of NO regulate the hepatic perfusion.8 Alternatively, the excess production of nitric oxide, generated primarily by iNOS,9 has been implicated as a mediator of cellular injury at sites of inflammation, including liver I/R injury.10,11,12 Under these circumstances, nitric oxide reacts with molecular oxygen or superoxide and generates reactive nitrogen species, which are capable of modifying bioorganic molecules13 and mediating many biological processes, including extracellular matrix (ECM) degradation.14
Leukocyte migration across ECM proteins is dependent on matrix degradation, not only for facilitating “matrix permeability” but also for generating ECM-derived fragments, which are biologically active, and can be highly chemotactic for leukocytes.15,16 Matrix metalloproteinase (MMP)-9 is one of two major gelatinases in the MMP family responsible for the turnover and degradation of several ECM proteins, including fibronectin,17 a key ECM protein expressed very early by liver endothelial cells in response to injury,18 including to I/R injury.19 The expression of MMP-9 has been linked to numerous pathological conditions, such as tumor invasion,20 inflammation,17 arthritis,21 cerebral I/R injury22 liver I/R injury,15,23 and liver transplantation.24
In general, MMPs have a large propeptide containing cysteine, a catalytic domain with zinc at the active center, and a hemopexin-like domain.25 MMP activation typically requires dissociation of cystein from the zinc ion, which is recognized as the switch that leads to enzymatic activation.26 However, it has been recently shown that NO can interact with zinc ions and cysteine residues and activates MMP-9 in neuronal cells22 and in a macrophage cell line27 in vitro. Similarly to iNOS, MMP-9 is virtually absent in naive livers, and it is highly up-regulated in damaged livers after I/R injury.15,19,23
In this study, we use iNOS deficient mice and mice treated with a specific iNOS inhibitor to test the hypothesis that iNOS expression has a regulatory function on MMP-9 activation in liver I/R injury. We demonstrate that specific iNOS inhibition markedly down-regulates MMP-9 activity, disrupts leukocyte migration, and reduces apoptosis in liver I/R injury. We present evidence that NO, possibly acting by paracrine mechanisms, regulates MMP-9 activity in neutrophils, which are critical mediators of acute inflammatory liver injury.28 Moreover, we also show that macrophage-derived NO production through the induction of iNOS is capable of regulating neutrophil transmigration across fibronectin in a MMP-9 dependent manner.
Materials and Methods
Mice and Model of Hepatic I/R Injury
C57BL/6-NOS2−/− (B6;129P2-Nos2tm1Lau) and matched iNOS+/+ wild-type littermates (B6;129PF2/J), MMP-9−/− (FVB.Cg-Mmp9tm1tvu), and matched MMP-9+/+ wild-type littermates (FVB/NJ), and C57BL6 male mice 8 to 10 weeks old were purchased from the Jackson Laboratory. Mice were housed in the University of California at Los Angeles animal facility under specific pathogen-free conditions. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health. A warm hepatic I/R model was performed as previously described.15 Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg intraperitoneally) and injected with heparin (100 U/kg). Arterial and portal venous blood supplies were interrupted to the cephalad lobes of the liver for 90 minutes using an atraumatic clip. Mice were sacrificed at 6 hours and 24 hours after reperfusion and liver and blood samples were collected.
ONO-1714 Administration
ONO-1714 (0.05 mg/kg), a novel selective iNOS inhibitor, kindly provided by Drs. Naka and Maruyama from ONO Pharmaceutical Co. Ltd. (Osaka, Japan), was administrated subcutaneously to C57BL6 mice 5 minutes before ischemia. Control mice were treated with vehicle in a similar fashion to ONO-1714 administration. ONO-1714 or vehicle administration had no effect in naïve animals.
Assessment of Liver Damage
Serum alanine transaminase (sALT), serum glutamate pyruvate transaminase, serum aspartate transaminase, and serum glutamic oxaloacetic transaminase, levels were measured with an autoanalyzer by ANTECH Diagnostics (Los Angeles, CA). Liver specimens were fixed with a 10% buffered formalin solution, embedded in paraffin, and processed for H&E staining.
Measurement of Nitrate and Nitrite Contents
Nitrite/nitrate levels in serum, liver homogenates, and cell supernatants were measured using Griess Reagent System (Promega, Madison, WI) according to manufacturer’s instructions.
Myeloperoxidase Assay
Myeloperoxidase activity was evaluated as previously described.15 Frozen tissue was homogenized in an iced solution of 0.5% hexadecyltrimethyl-ammonium (Sigma, St. Louis, MO) and 50 mmol/L of potassium phosphate buffer solution (Sigma) with pH adjusted to 5. Samples were centrifuged at 15,000 rpm for 15 minutes at 4°C. Supernatants (100 μl) were mixed in a solution of hydrogen peroxide-sodium acetate and tetramethyl benzidine (Sigma). The absorbance change at 655 nm in 1 minute was measured with PowerWave XS spectrophotometer (Bio-Tek, Winooski, VT). The quantity of enzyme degrading 1 μmol/L of peroxide per minute at 25°C per g of tissue was defined as 1U of myeloperoxidase activity.
Immunohistochemistry
Liver specimens embedded in Tissue Tec OCT compound (Miles, Elkhart, IN) and snap frozen in liquid nitrogen were used for immunostaining, as previously described.19 Appropriate primary antibodies against mouse CD3 (17A2; BioLegend San Diego, CA), CD4 (L3T4; BD Biosciences, San Jose, CA), macrophage antigen-1 (Mac-1, M1/70; BD Biosciences), Ly-6G (1A8; BD Biosciences), MMP-9 (AF909; R&D Systems, Minneapolis, MN), and vascular cell adhesion molecule1 (VCAM-1, MVCAM A 429; Serotec Inc., Raleigh, NC) were used at optimal dilutions. Bound primary antibody was detected using biotinylated anti-rat or anti-goat IgG, and then streptavidin peroxidase-conjugated complexes (Vector Laboratories, Burlingame, CA). Negative controls included sections in which the primary antibody was replaced with dilution buffer. Control sections from inflammatory tissues known to be positive for each stain were included as positive controls. The peroxidase reaction was developed with DAB Substrate Kit (Vector Laboratories). The sections were evaluated blindly by counting the labeled cells in triplicates within 40 high-power fields per section. Triple staining was detected by immunoflorescence with Alexa Fluor 488-green anti-rat IgG (H+L), Alexa Fluor 594-red anti-goat IgG (H+L), Alexa Fluor 647 anti-rabbit IgG (H+L) antibodies (Molecular Probes, Carlsbad, CA), and slides were analyzed using a Leica Confocal Microscope (University of California at Los Angeles Brain Research Institute, Confocal Microscope Core Facility).
RNA Extraction and Reverse Transcription-PCR
For evaluation of cytokine gene expression, livers were harvested and RNA was extracted with Trizol (Life Technologies Inc., Grand Island, New York) using a Polytron RT-3000 (Kinematica AG, Littau-Luzem, Switzerland), as previously described.29 Reverse transcription was performed using 5 μg of total RNA in a first-strand cDNA synthesis reaction with SuperScript II RNaseH Reverse Transcriptase (Life Technologies Inc), as recommended by the manufacturer. The cDNA product was amplified by PCR using primers specific for mouse cytokines and b-actin.
Western Blot and Zymography Analyses
Snap-frozen liver tissue was immediately homogenized as previously described.19 Protein content was determined using a BCA Protein Assay Kit (Pierce Chemical, Rockford, IL). For Western blots 40 μg of protein in SDS-loading buffer were electrophoresed through 12% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The gels were then stained with Coomassie blue to document equal protein loading. The membranes were blocked with 5% dry milk and 0.05% Tween 20 (USB, Cleveland, OH) in Tris-buffered saline and incubated with specific primary antibodies against iNOS (Chemicon, Temecula, CA), and Bcl-xl (Cell Signaling Technology, Danvers, MA). The filters were washed and then incubated with horseradish peroxidase conjugated secondary antibodies, followed by detection with SuperSignal West Pico Chemiluminescent Substrate (Pierce). After development, membranes were striped and re-blotted with an antibody against β-actin (Abcam). Relative quantities of protein were determined using a densitometer (Kodak Digital Science 1D Analysis Software, Rochester, NY).
Gelatinolytic activity was detected in liver extracts (100 μg) or 200 μl of cell supernatant by 10% SDS-polyacrylamide gel electrophoresis contained 1 mg/ml of gelatin (Invitrogen, Carlsbad, CA), under non-reducing conditions.23 After SDS-polyacrylamide gel electrophoresis, the gels were soaked twice with Novex Zymogram Renaturating Buffer (Invitrogen) for 30 minutes each time, rinsed in water, and incubated overnight at 37°C in Novex Zymogram Developing Buffer (Invitrogen). The gels were then stained with Coomassie brilliant blue R-250 (Bio-rad, Hercules, CA), and destained with methanol/acetic acid/water (20:10:70). A clear zone indicates the presence of enzymatic activity. Positive controls for MMP-9 (BIOMOL International, Plymouth, PA), and prestained molecular weight markers (Kaleidoscope Prestained Standards; Bio-Rad) served as standards. Relative quantities of protein were determined using a densitometer (Kodak Digital Science 1D Analysis Software, Rochester, NY).
MMP-9 Protein Levels
Total MMP-9 protein levels were detected in cell supernatants using a Quantikine Mouse MMP-9 (total) Immunoassay Kit (RGD, Minneapolis, MN) according to the manufacturer’s instructions.
MMP-9 Activity
MMP-9 activity was detected in liver homogenates (100 μg of protein) and in cell supernatants using an Amersham Matrix Metalloproteinase-9 Biotrak Activity Assay System (GE Health care Bio-Sciences, Piscataway, NJ) according to the manufacturer’s instructions.
Leukocyte Isolation
Isolation of adult murine neutrophils from bone marrow was performed as previously published.29 Briefly, femurs and tibias were harvested and stripped of all muscle and sinew, and bone marrow was flushed with 2.5 ml of RPMI-1640 containing 5% fetal calf serum on ice. Cells were pelleted, and erythrocytes were removed by hypotonic lysis. The entire bone marrow preparation was resuspended at 5 × 107 cells/ml in Hanks’ balanced saline solution. Cells were layered on a Percoll (Sigma–Aldrich) gradient (3 ml of 55%, top; 3 ml of 65%, middle; 4 ml of 80% Percoll) and centrifuged at 2000 rpm for 30 minutes at 10°C. Mature neutrophils were recovered at the interface of the 65% and 80% fractions and were >90% pure and >95% viable in the neutrophil-rich fraction as determined by Ly-6G immunostaining/morphology and trypan blue exclusion, respectively.
Murine macrophages were prepared using published methods. Briefly, 1 ml of 3% thioglycollate medium was injected into the peritoneal cavity 72 hours before collecting macrophages. The peritoneal cavities were lavaged with 5 ml of PBS, and the aspirate was placed on ice and centrifuged at 1200 rpm for 5 minutes at 4°C. The pellets are cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum. Cell viability was determined by trypan blue exclusion.
iNOS Inhibition/NO Donor in Vitro Assays
Isolated leukocytes were cultured in medium without fetal bovine serum for 24 hours before being stimulated by lipopolysaccharide 1 to 100 ng/ml (LPS, Sigma) or by Formyl-Met-Leu-Phe-OH 5–50 nmol/L (fMLP, Calbiochem, San Diego, CA) for 24 hours in the presence or absence of ONO-1714. LPS and fMLP are commonly used to activate macrophages and neutrophils, respectively. After incubation, cell supernatants were collected for NO measurements. In addition, isolated leukocytes were also cultured for 6 hours with a NO donor with a long half-life of 27 hours, 2,2′-(hydroxynitrosohydrazono)-bis-ethanamine 5 to 500 μmol/L (DETA NONOate; Sigma). Cell supernatants were collected for MMP-9 activity measurements by zymography.
Neutrophil Migration Assay
Macrophages previously stimulated with LPS (1 μg/ml, Sigma) for 1 hour, and washed three times in Hanks’ balanced saline solution (GIBCO BRL, Gaithersburg, MD) to remove LPS, were seeded (0.5 × 106 cells/250 μl) in fresh LPS-free DMEM in 24-well tissue culture plates and incubated for 3 hours at 37°C and 5% CO2 before neutrophil transmigration. Wells not seeded with macrophages had an equal volume of DMEM added to them. NO release by the adherent macrophages was significantly detected at 3 hours to 6 hours after LPS stimulation (not shown). Transmigration through fibronectin of isolated neutrophils, resuspended in DMEM without fetal bovine serum at a final concentration of 2.0 × 106 cells/ml, was performed using a commercially available in vitro cell migration assay kit (BD Bioscience, Bedford, MA), as previously described.15 Transwell inserts with 3-μm pore size either coated with fibronectin or uncoated (control invasion chambers) were placed in the 24-well plates, and then neutrophils (4 × 105 cells/well) were added to the upper chambers. Where indicated, 10 nmol/L of MMP-9 inhibitor-I (C27H33N3O5S; Calbiochem, La Jolla, CA) or 20 nmol/L of iNOS inhibitor (ONO-1714) were included in the DMEM medium of the lower chambers. Cells were incubated at 37°C and 5% CO2 for 4 hours, and the neutrophils that had migrated into the lower chambers were collected, stained and counted. NO contents and MMP-9 activity were also evaluated as previously described.
Cytokine-Mediated Neutrophil Stimulation
Isolated neutrophils were cultured in serum free medium for 24 hours before being treated with interleukin (IL)-6, 25 to 100 ng/ml, or IFN-g, 25 to 100U/ml (eBioscience, San Diego, CA) for 24 hours. After incubation, cell supernatants were collected for MMP-9 activity measurements by gelatin zymography. Gels were visualized using a Foto/Analyst FX (Fotodyne, Hartland, WI), and the bands were quantified by densitometry using Image J software (NIH, Bethesda, MA). Data are presented as fold increase over the unstimulated controls.
Caspase-3 Activity
Caspase-3 activity was determined in liver samples using a commercially available ApoAlert Caspase 3 Colorimetric Assay Kit (Clonetech, Mountain View, CA) according to the manufacturer’s instructions. Optical density measurements at 405 nm were performed using a microplate reader (Bio-TeK). Caspase activity was expressed in units with 1 unit being the amount of enzyme activity liberating 1 pmol of pNA per minute.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay
The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was performed on 5-μm cryostat sections using the In Situ Cell Death Detection kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s protocol. TUNEL-positive(+) cells were detected under light microscopy. Terminal transferase was omitted as a negative control. Positive controls were generated by treatment with DNase 1 (30 U/ml in 40 mmol/L of Tris-Cl, pH 7.6, 6 mmol/L MgCl2, and 2 mmol/L CaCl2 for 30 minutes).
Data Analysis
Data in the text and figures are expressed as means ± SEM. Two-group comparisons were analyzed by the two-tailed Student’s t-test for independent samples. Probability values of less than 0.05 were considered statistically significant.
Results
iNOS Expression in Hepatic I/R Injury
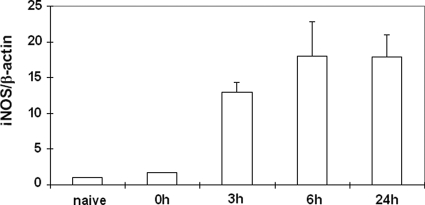
iNOS expression, as detected by Western blotting, was virtually undetectable and mildly detectable in naive wild-type livers, and in livers after 90 minutes of warm ischemia (before reperfusion), respectively. However, iNOS expression was readily up-regulated at protein level at 3 hours, 6 hours, and 24 hours of I/R injury (Figure 1). These results were consistent with our previous observations in a rat model of liver transplantation, in which iNOS was highly expressed in damaged livers after I/R injury.19 The expression of iNOS in iNOS −/− deficient mice was undetectable in naïve livers and in livers after 3 hours, 6 hours, and 24 hours of I/R injury.
Figure 1.
Western blot detection of iNOS in wild-type livers. iNOS expression was virtually absent or mildly expressed in naïve livers and in livers after 90 minutes of warm ischemia. In contrast, iNOS expression was highly detected at protein level in wild-type livers at 3 hours, 6 hours, and 24 hours of I/R injury.
Reduced I/R Injury Response in Livers from iNOS-Deficient Mice
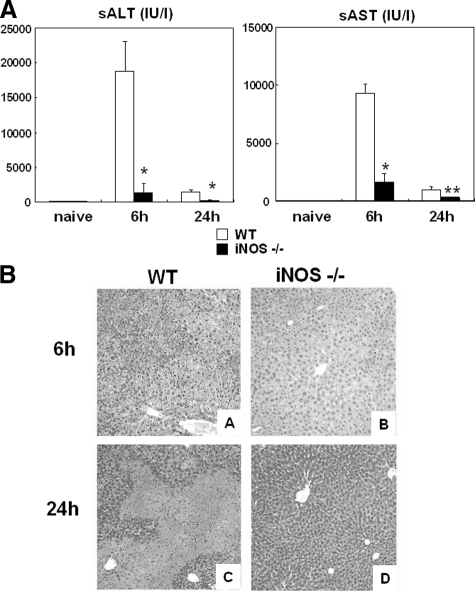
There were no apparent differences either in transaminase levels or in liver histology between naïve iNOS−/− and naïve wild-type mice. We then evaluated the liver injury produced by I/R in iNOS−/− deficient mice; mice were sacrificed at 6 hours and 24 hours after liver I/R injury. iNOS−/− mice showed significantly less liver damage, as evidenced by the reduced serum ALT levels (sALT, U/L: 1368 ± 1240 vs. 18,810 ± 4317; P < 0.001; and serum AST (sAST), U/L: 1587 ± 828 vs. 9293 ± 1166; P < 0.001, n = 6/g) at 6 hours after I/R injury (Figure 2A). A sustained protection was observed in iNOS−/− mice, with sALT (U/L: 242 ± 98 vs. 1488 ± 306; P < 0.001, n = 6/g), and sAST (U/L: 329 ± 193 vs. 974 ± 193; P < 0.003, n = 6/g) levels depressed at 24 hours after I/R injury (Figure 2A). Moreover, improvement in liver function in iNOS−/− mice was associated with significantly better histological preservation (Figure 2B). Elevated sinusoidal congestion and extensive areas of necrosis characterized livers from wild-type mice at 6 and 24 hours post-I/R injury, respectively. In contrast, iNOS knockout mice showed only mild signs of vascular changes and necrosis after liver I/R injury.
Figure 2.
A: Liver transaminases and histological preservation in iNOS−/− and wild-type (WT) mice. sALT and sAST levels (IU/L) were measured in the blood samples taken at 6 hours and 24 hours after I/R injury. sALT and sAST levels in the iNOS−/− mice were significantly lower than those in the respective wild-type control littermates at both 6 hours, and 24 hours. Representative H&E staining of livers at 6 hours and 24 hours post-I/R injury. B: Control wild-type livers were mostly characterized by elevated sinusoidal congestion at 6 hours (A), and by large necrotic areas at 24 hours (C). In contrast, iNOS−/− livers showed reduced sinusoidal congestion and rather good histological preservation at both 6 hours (B) and 24 hours (D) after liver I/R injury. H&E staining magnification = original ×100; *P < 0.001, and **P < 0.003.
iNOS Deficiency Profoundly Disrupted Leukocyte Recruitment in Liver I/R Injury
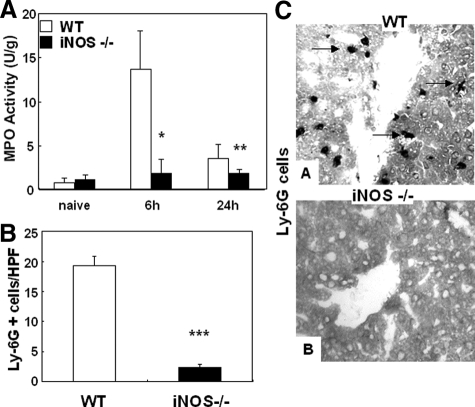
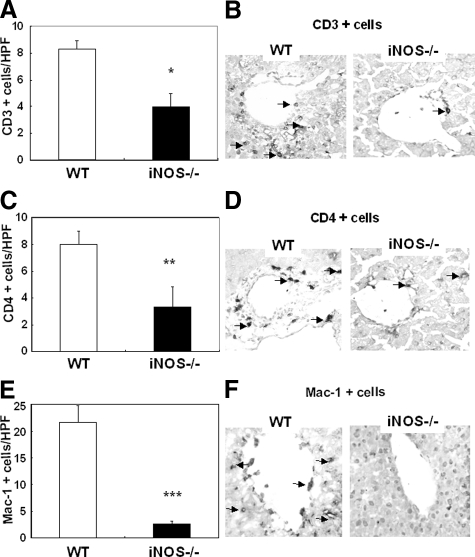
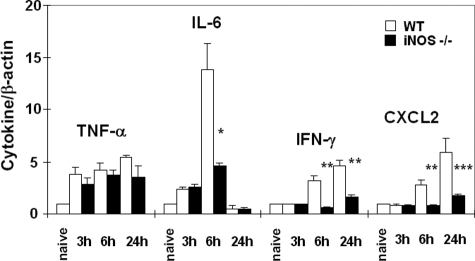
We evaluated the contribution of iNOS expression on leukocyte infiltration in liver I/R injury. Myeloperoxidase activity (U/g), an index of neutrophil infiltration, was profoundly depressed in iNOS deficient livers at 6 hours (1.9 ± 1.5 vs.13.7 ± 4.3, P < 0.004; n = 6/g) and 24 hours (1.9 ± 0.4 vs. 3.6 ± 1.6, P < 0.04; n = 6/g) of I/R injury, as compared with respective controls, (Figure 3A). Moreover, the myeloperoxidase activity results were correlated with the number of Ly-6G positive cells, a marker expressed primarily on granulocytes.30 iNOS−/− livers showed significantly lower numbers of Ly-6G neutrophils (2.3 ± 0.6 vs. 19.3 ± 1.5, P < 0.001; n = 6/g), particularly at 6 hours after I/R, a time point that coincides with the highest serum transaminase levels, (Figure 3, B and C). Moreover, the numbers of CD3 lymphocytes (6 hours: 4.0 ± 1.0 vs. 8.3 ± 0.6, P < 0.003; n = 6/g), CD4 T cells (6 hours: 3.3 ± 1.5 vs. 8.0 ± 1.0, P < 0.01; n = 6/g), and Mac-1 leukocytes (6 hours: 2.7 ± 0.6 vs. 21.7 ± 3.1, P < 0.001; n = 6/g), a mouse macrophage antigen that is abundantly expressed on stimulated macrophages and, in lower amounts, on granulocytes,31 were profoundly depressed in iNOS−/− livers as compared with respective wild-type controls after 6 hours of livers I/R injury (Figure 4, A–F). The extent of leukocyte infiltration was highly correlated with the degree of liver function and with the histological preservation observed in the different groups. Moreover, it was also correlated with the expression of pro-inflammatory cytokines (Figure 5). IL-6 expression, which is iNOS dependent in damaged livers and lungs after hemorrhagic shock,32 was profoundly depressed in iNOS−/− livers (P < 0.005; n = 4/g) at 6 hours after I/R insult. IFN-γ expression, an initiator of liver reperfusion injury,33 was also depressed in iNOS−/− livers at 6 hours (P < 0.01; n = 4/g), and 24 hours (P < 0.01; n = 4/g) of reperfusion. However, the expression of tumor necrosis factor-α, a pro-inflammatory cytokine associated to iNOS-derived NO,34 was up-regulated early (3 hours post-I/R) in both iNOS−/− and wild-type livers after I/R, and it was virtually unchanged in both groups, suggesting that iNOS−/− mice are capable of expressing tumor necrosis factor-α by an iNOS independent pathway. In addition, CXCL-2, a neutrophil chemoattractant,35 was down-regulated in the iNOS−/− livers at both 6 hours (P < 0.01; n = 4/g), and 24 hours (P < 0.005; n = 4/g) after I/R injury (Figure 5).
Figure 3.
Intrahepatic myeloperoxidase enzyme activity and Ly-6G neutrophil infiltration in iNOS−/− and wild-type (WT) mice. Myeloperoxidase enzymatic activity (A), an index of neutrophil infiltration, was markedly reduced in the iNOS−/− mice at 6 hours and 24 hours of reperfusion following 90 minutes of warm ischemia. In addition, Ly-6G neutrophil infiltration (B) was predominantly detected in wild-type livers at 6 hours after I/R injury, contrasting with very little Ly-6G cell infiltration detected in iNOS−/− livers. Representative immunostaining of Ly-6G neutrophils (C) in wild-type livers (A), and in iNOS−/− livers (B) at 6 hours of I/R injury. Arrows indicate Ly-6G cell labeling in liver specimens. Immunostaining magnification = original ×200; *P < 0.004, **P < 0.04, and ***P < 0.001.
Figure 4.
T and Mac-1 leukocyte infiltration in iNOS−/− and wild-type (WT) mice. CD3 (A), CD4 (C), and Mac-1 (E) leukocyte infiltration was significantly reduced in both iNOS−/− livers, as compared with respective controls at 6 hours post-I/R injury. Representative staining for CD3, CD4, and Mac-1 cells is illustrated in panels B, D, and F, respectively. Arrows indicate leukocyte labeling in liver specimens. Immunostaining magnification = original ×200: *P < 0.03, **P < 0.01, and ***P < 0.001.
Figure 5.
Cytokine and chemokine gene expression in iNOS−/− and wild-type (WT) livers. Cytokine induction ratios were determined at 3 hours, 6 hours, and 24 hours of reperfusion following 90 minutes of warm ischemia. Pro-inflammatory IL-6, IFN-γ, and CXCL-2 expression was profoundly depressed in iNOS deficient livers as compared with respective controls. In contrast, tumor necrosis factor-α was comparably expressed in iNOS−/− and wild-type livers after I/R injury, *P < 0.006, **P < 0.01, and ***P < 0.005.
MMP-9+ Leukocytes Were Detected in iNOS-Rich Areas of Damaged Livers
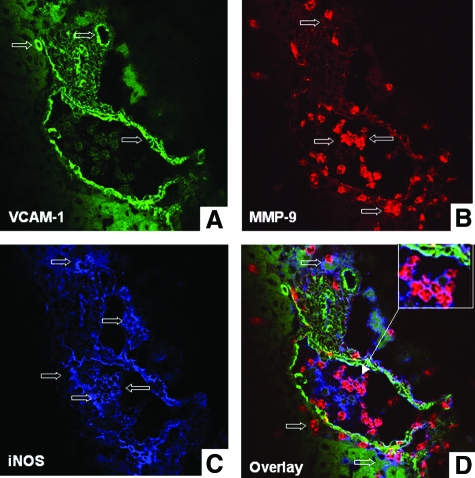
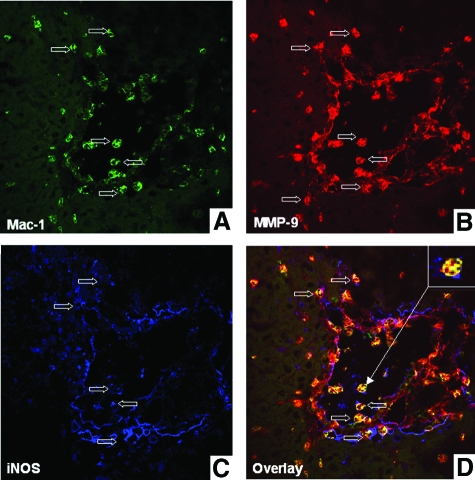
Leukocyte transmigration across endothelial and ECM barriers is a complex process, which is dependent on cell activating chemokines, and matrix degradation mechanisms. We have recently shown that MMP-9 is an important mediator in liver I/R injury.15 Others have shown that NO is capable of regulating MMP-9 activity in macrophages and neuronal cells22,27 in vitro. To evaluate whether iNOS and MMP-9 colocalize in damaged livers, we performed series of triple immunofluorescent assays in wild-type livers at 6 hours after I/R, a time point that coincides with high levels of iNOS expression, serum transaminases, and leukocyte infiltration in this experimental model. As shown in Figure 6, A–D, MMP-9+ leukocytes were detected in wild-type livers in the proximity of the vascular endothelium (stained for VCAM-1), either in the lumen of the vessels, before transmigration, or in the damaged liver tissues. Interestingly, MMP-9+ leukocytes were either positive for iNOS or were localized adjacent to iNOS-positive cells (Figure 6D). We have previously identified Mac-1+ macrophages and Ly-6G+ neutrophils as major sources of MMP-9 in this model of liver injury.15 To evaluate whether these cells were able to express iNOS, we stained wild-type-livers after 6 hours of I/R insult for simultaneous detection of leukocyte markers, iNOS, and MMP-9. While MMP-9+ Ly-6G+ neutrophils were virtually negative for iNOS (not shown), Mac-1 cells readily stained for both iNOS and MMP-9 (Figure 7, A–D). Therefore, these data show that Mac-1 macrophages co-expressed MMP-9 and iNOS, while other MMP-9+ leukocytes were localized adjacent to iNOS+ cells in damaged wild-type livers after I/R injury.
Figure 6.
Confocal imaging of VCAM-1, MMP-9, and iNOS in wild-type livers. Triple immunofluorescence labeling of VCAM-1 (A, brilliant green), MMP-9 (B, brilliant red), iNOS (C, blue), and overlay image (D) of A, B, and C in wild-type livers at 6 hours post-I/R injury. MMP-9+ leukocytes were detected in damaged livers neighboring the vascular endothelium (VCAM-1 staining), before and after transmigration. MMP-9+ leukocytes either co-expressed iNOS (magenta) or were detected adjacent to iNOS+ cells (blue) in damaged livers. Open arrows indicate positive labeling; inset in D shows colocalization of MMP-9 and iNOS in leukocytes nearby the endothelium at higher magnification.
Figure 7.
Confocal imaging of Mac-1, MMP-9, and iNOS in wild-type livers. Triple immunofluorescence labeling of Mac-1 (A, brilliant green), MMP-9 (B, brilliant red), iNOS (C, blue), and overlay image (D) of A, B, and C in wild-type livers at 6 hours post-I/R injury. Colocalization of Mac-1, MMP-9, and iNOS was detected in damaged livers. Open arrows indicate positive labeling; inset in D shows iNOS positive staining in MMP-9+ Mac-1 leukocytes at higher magnification.
iNOS Deficiency Down-Regulated MMP-9 Activity after Liver I/R Injury
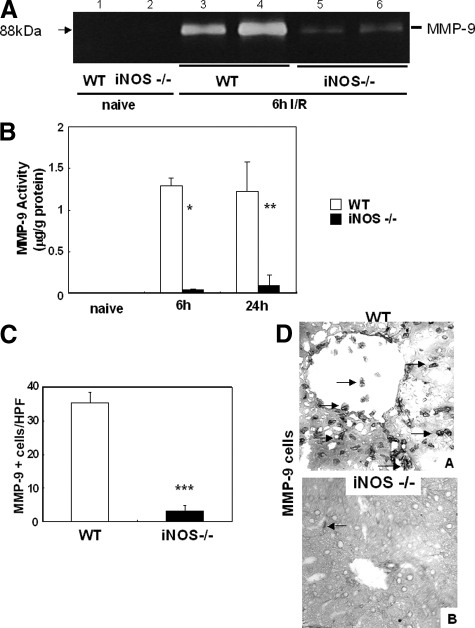
Gelatin zymography and a specific MMP-9 enzymatic activity kit were used to assess whether iNOS deficiency affected MMP-9 activity in liver I/R injury. MMP-9 activity, assessed by zymography, was markedly depressed in iNOS−/− deficient livers (∼sixfold decrease) at 6 hours after I/R injury (Figure 8A). In addition, iNOS−/− deficient livers showed a significant decrease in the amount of active MMP-9 (μg/g) at both 6 hours (0.042 ± 0.009 vs. 1.289 ± 0.091, P < 0.0008; n = 6/g) and 24 hours (0.098 ± 0.128 vs. 1.225 ± 0.352, P < 0.006; n = 6/g), as compared with wild-type control livers after I/R injury (Figure 8B). The numbers of MMP-9+ leukocytes (6 hours: 3.3 ± 1.5 vs. 35.3 ± 5.1, P < 0.001; n = 6/g) were also profoundly depressed in iNOS−/− livers (Figure 8, C and D). Thus, these results show that MMP-9 activity was strongly reduced in the absence of iNOS in liver I/R injury.
Figure 8.
MMP-9 activity in iNOS−/− and wild-type (WT) livers. MMP-9 activity detected by zymography (A) was virtually negative in wild-type (lane 1), and in iNOS−/− (lane 2) naïve livers. It was mildly detected in iNOS−/− deficient livers at 6 hours of I/R (lanes 5, and 6) and highly up-regulated in the respective wild-type controls (lanes 3, and 4). Indeed, the amount of active MMP-9 (B) was several-fold decreased in iNOS−/− livers as compared with controls at both 6 hours and 24 hours after I/R injury. In addition, MMP-9+ leukocyte infiltration was profoundly reduced in iNOS−/− livers as compared with respective wild-type controls at 6 hours post-I/R injury (C). Representative staining for MMP-9 in wild-type livers (A) and in iNOS−/− livers (B) is shown in D. Arrows indicate positive labeling in liver specimens. Immunostaining magnification = original ×200; *P < 0.0008, **P < 006, and ***P < 0.001.
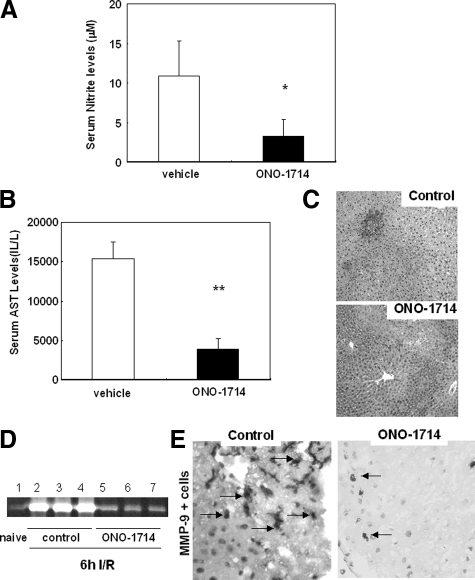
ONO-1714-Mediated iNOS Inhibition Down-Regulated MMP-9 Activity and Ameliorated Liver I/R Injury
Knockout mice represent an important research tool; however, they often possess redundant mechanisms. Therefore, we performed additional experiments with ONO-1714, a powerful specific iNOS inhibitor.36 The administration of the iNOS inhibitor to wild-type C56BL6 mice significantly decreased serum NO levels, transaminase levels (sAST: 3907 ± 1371 vs. 15,400 ± 2107 U/L, P < 0.005; n = 5/g), reduced liver vascular congestion, and improved liver preservation after 6 hours of I/R insult, (Figure 9, A–C). Moreover, ONO-1714 mediated iNOS inhibition significantly down-regulated MMP-9 activation (∼threefold decrease), and profoundly decreased the number of infiltrating MMP-9+ leukocytes (3.2 ± 1.0 vs. 22.4 ± 2.5, P < 0.001; n = 4/g), (Figure 9, D and E). Therefore, these results support our observations in iNOS-deficient mice, and are in agreement with previous studies in both pigs10 and rats,37 which show that iNOS specific inhibition ameliorates liver I/R injury. The results also support the concept that MMP-9 is an important mediator of the effects of iNOS-derived NO in liver I/R injury.
Figure 9.
Liver function and MMP-9 activity in ONO-1714 treated livers at 6 hours post-I/R injury. ONO-1714 mediated iNOS selective inhibition in liver I/R injury profoundly depressed serum nitrite (A) and AST levels (B), and reduced sinusoidal congestion (C). Furthermore, amelioration of liver I/R injury by ONO-1714 was accompanied by a profound inhibition of MMP-9 activity (D). MMP-9 activity was highly detected in vehicle treated controls (lanes 2–4), and little expressed in ONO-1714 treated livers (lanes 5–7). In addition, MMP-9+ leukocyte infiltration (E) was depressed in ONO-1714 treated liver at 6 hours post-I/R injury. Arrows indicate positive labeling in liver specimens. H&E staining magnification = original ×100; Immunostaining magnification = original ×200; *P < 0.05; and **P < 0.005.
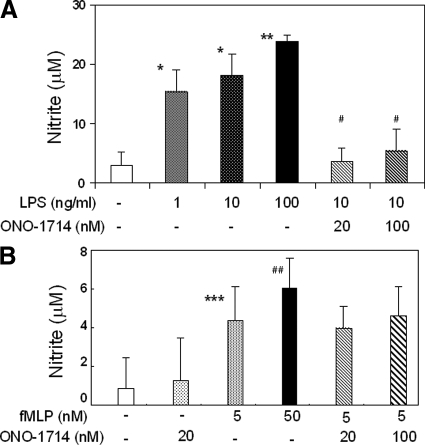
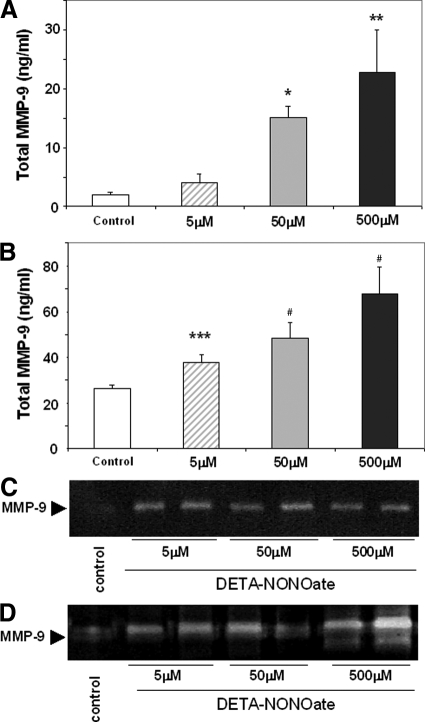
NO Regulated MMP-9 Activity in Isolated Murine Neutrophils
Cultured isolated murine macrophages, in the absence of LPS stimulation, released low NO levels (<5 μmol/L). LPS mediated activation of macrophages significantly increased NO release levels (∼15 to 25 μmol/L); however, addition of ONO-1714 to LPS-activated macrophages returned NO release to almost unstimulated values (∼4 to 6 μmol/L), (Figure 10A). Alternatively, fMLP-activated neutrophils showed only a relatively modest increase in NO release levels (5 to 7 μmol/L), which was not considerably affected by ONO-1714 mediated inhibition (Figure 10B). These results were somewhat correlated with our in vivo observations, in which iNOS expression was readily detectable in Mac-1 macrophages and virtually undetectable in Ly-6G neutrophils in the damaged wild-type livers after I/R injury. To test whether NO is capable of regulating MMP-9 expression and activity in isolated murine macrophages and neutrophils, we cultured these cells in the presence of a NO-generating agent, DETA-NONOate. We found that DETA-NONOate significantly up-regulated the expression of total MMP-9 protein levels in both macrophages and neutrophils, the latter being the cells that expressed higher levels of this gelatinase (Figure 11, A and B). For example, MMP-9 protein levels in macrophages and neutrophils were approximately 15 ng/ml and 50 ng/ml, respectively, at a DETA-NONOate concentration of 50 μmol/L. Moreover, it also up-regulated MMP-9 activity in both cell types (Figure 11, C and D). In macrophages, the higher levels of MMP-9 activity were predominantly detected in cells treated with DETA-NONOate at concentrations of 5 μmol/L and 50 μmol/L (Figure 11C). DETA-NONOate at a concentration of 500 μmol/L seemed less effective in MMP-9 activation by macrophages. These results are supported by data obtained with a macrophage cell line in which NO up-regulates MMP-9 activity in this cell line; however, very high concentrations of the NO donor are less effective in increasing MMP activity by these cells.27 On the other hand, MMP-9 enzymatic activity in neutrophils was increased at all studied concentrations of DETA-NONOate, with a more substantial increase observed at high concentrations of the NO donor (500 μmol/L), (Figure 11D). Therefore, these data provide evidence that NO is capable of regulating MMP-9 activity in neutrophils in addition to macrophages, and support our in vivo results of a regulatory function for iNOS-derived NO on activation of MMP-9 in liver I/R injury.
Figure 10.
Nitrite levels in isolated murine macrophages and neutrophils. Nitrite levels, expressed as mean ± SD of three experiments, in macrophages (A) and in neutrophils (B). Nitrite levels in macrophages were significantly increased on LPS stimulation, and addition of ONO-1714 to LPS-activated macrophages returned nitrite release to almost unstimulated values. In contrast, compared with LPS-activated macrophages, release of nitrite by neutrophils was mildly detected on fMLP stimulation, and virtually unchanged on iNOS specific inhibition *P < 0.001, **P < 0.0001, ***P < 0.03, and #P < 0.01, relative to unstimulated cells-white bars; ##P < 0.001, relative to stimulated cells-black dotted bars.
Figure 11.
Regulation of MMP-9 expression and activity in isolated murine macrophages and neutrophils. Exposure of macrophages (A and C), and neutrophils (B and D), to exogenous NO increased MMP-9 expression/activity by both cell types with higher expression and activation levels detected in neutrophils. Total MMP-9 protein levels expressed as mean ± SD of three experiments *P < 0.003, **P < 0.02, ***P < 0.007, and #P < 0.01, relative to controls.
Macrophage-Derived NO Up-Regulated MMP-9 Activation and Promoted Neutrophil Migration across Fibronectin
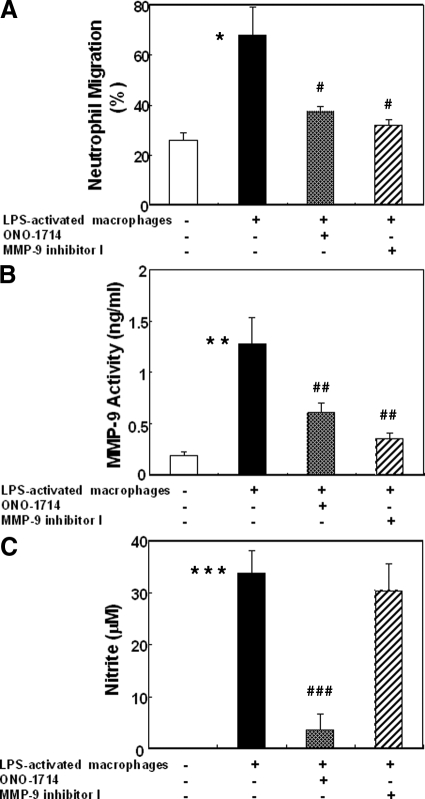
Neutrophils are considered to be critical mediators in acute liver injury.28 Having in consideration that neutrophils were nearly negative for iNOS in damaged livers and, that fMLP-activated neutrophils were only capable of releasing very low levels of NO, MMP-9 activity induced by iNOS-derived NO in neutrophils is likely mediated by NO produced by neighboring cells. Transwell experiments were performed to test whether macrophage-derived NO is capable of regulating neutrophil migration across fibronectin, (Figure 12). MMP-9 activity and neutrophil migration across fibronectin-coated transwell membranes were modestly detected in the absence of activated macrophages plated in the lower chambers. In contrast, MMP-9 activity and neutrophil migration were increased by approximately sixfold and threefold, respectively, in the presence of activated macrophages; however, specific iNOS inhibition significantly depressed MMP-9 activity (0.61 ± 0.09 vs. 1.27 ± 0.26, ng/ml, P < 0.01; n = 4/g) and neutrophil migration (37.22 ± 2.36 vs. 67.98 ± 11.04, P < 0.02; n = 4/g) in a similar fashion to MMP-9 inhibition, (Figure 12, A and B). In fact, MMP-9 activity (0.35 ± 0.05 vs. 1.27 ± 0.26, ng/ml, P < 0.01; n = 4/g) and neutrophil migration (32.09 ± 2.09 vs. 67.98 ± 11.04, P < 0.02; n = 4/g) were markedly depressed in the MMP-9 inhibitor treated group as compared with controls, (Figure 12, A and B). While iNOS inhibition was highly effective in depressing NO release (3.5 ± 3.1 vs. 33.8 ± 4.4, μM, P < 0.003; n = 4/g) and MMP-9 activity/neutrophil migration, MMP-9 inhibition depressed MMP-9 activity and neutrophil migration without disturbing the release of NO (30.4 ± 5.1 vs. 33.8 ± 4.4, μM; n = 4/g), (Figure 12, B and C), evidencing that MMP-9 is required for NO mediated neutrophil migration. Therefore, these results support our in vivo observations in which macrophage NO production, through the induction of iNOS, increases MMP-9 activity and promotes neutrophil migration. Overall, they support the concept that iNOS mediates leukocyte migration in a MMP-9-dependent manner.
Figure 12.
Regulation of neutrophil migration by macrophage NO produced through the induction of iNOS. Migration of neutrophils across fibronectin (A) was markedly increased in the presence of macrophages previously activated with LPS; however, selective iNOS inhibition as well as MMP-9 inhibition significantly reduced neutrophil migration to levels comparable with those observed in the absence of LPS-activated macrophages. MMP-9 activity (B) was profoundly depressed by iNOS and by MMP-9 inhibition. In contrast, nitrite release (C) was clearly reduced on iNOS inhibition, and remained unchanged on selective MMP-9 inhibition, suggesting that NO promoted neutrophil migration through MMP-9 activation, *P < 0.02, **P < 0.006, and ***P < 0.003, relative to unstimulated controls-white bars; #P < 0.02, ##P < 0.01, and ###P < 0.003, relatively to stimulated controls-back bars.
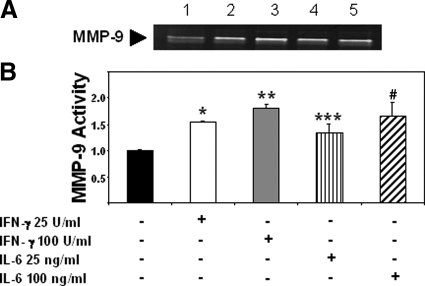
Effects of IFN-γ and IL-6 on MMP-9 Activity in Isolated Murine Neutrophils
The regulation of MMP activity is a complex process and it can be done at transcriptional, post-transcriptional, and at protein levels.38 It is important to consider that in addition to a possible NO-mediated metalloproteinase S-nitrosylation,22 NO may also contribute to MMP-9 activity via induction of cytokine or growth factor expression.38 Indeed, IFN-γ and IL-6 were both found significantly depressed by iNOS deficiency in livers after I/R injury. In an attempt to evaluate whether these pro-inflammatory cytokines are capable of regulating MMP-9 activity, we performed additional experiments in isolated neutrophils. As shown in Figure 13, A–B, IFN-γ (∼1.5- to 1.8-fold increase; n = 3/g) and IL-6 (∼1.3- to 1.7-fold increase; n = 3/g) were capable of significantly up-regulating the levels of MMP-9 activity in cultured neutrophils, suggesting that these pro-inflammatory cytokines may contribute to NO-mediated MMP-9 activity in liver I/R injury.
Figure 13.
Regulation of MMP-9 activity by IFN-γ and IL-6. Conditioned media obtained from neutrophils stimulated with IFN-γ or IL-6 was subjected to a gelatin zymography assay (A); IFN-γ 25 and 100U/ml (lanes 2 and 3, respectively), and IL-6 25 and 100 ng/ml (lanes 4 and 5, respectively) were capable of increasing MMP-9 activity in cultured neutrophils relative to unstimulated cells (lane 1). Graph (B) represents fold increases in enzymatic activity over unstimulated neutrophils, *P < 0.0003, **P < 0.0002, ***P < 0.05, and #P < 0.03, relative to unstimulated controls.
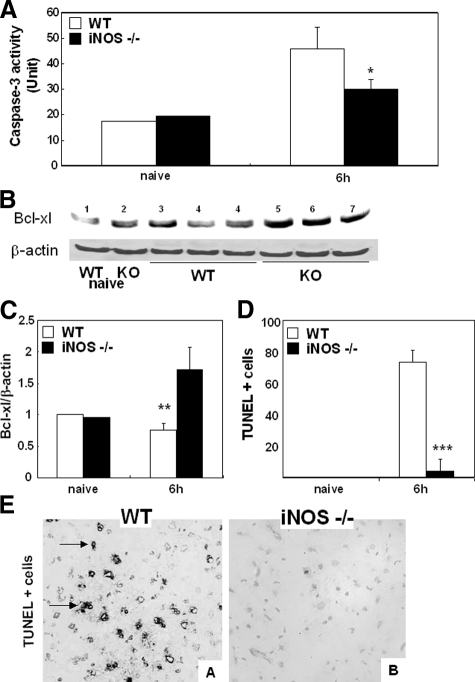
iNOS−/− Deficiency Decreased Caspase-3 Activity and TUNEL Staining in Liver I/R Injury
NO has been associated to adhesion-related apoptosis.39 MMPs may not only facilitate leukocyte migration, but they may also lead to detachment of liver cells resulting in apoptosis. Activation of caspase-3 causes DNA fragmentation,46 and caspase-3 activity is linked to I/R-induced liver apoptosis and damage.29,41 NO appears to have the dual capability of increasing42 and inhibiting caspase-3 activation.43 Here, we show that caspase-3 activity was decreased in iNOS−/− deficient livers at 6 hours (29.9 ± 3.7 vs. 45.8 ± 8.4 U/g, P < 0.03; n = 6/g) of reperfusion as compared with the respective wild-type controls, (Figure 14A). Moreover, a decrease in caspase-3 activity in the iNOS−/− livers was accompanied by an approximately twofold increase in Bcl-XL, an important anti-apoptotic factor, and by a significant reduction in TUNEL + cells, with hepatocyte morphology, at 6 hours (4.3 ± 2.1 vs. 73.4 ± 7.7, P < 0.001; n = 6/g), as compared with respective controls after I/R injury, (Figure 14, B–E). Indeed, liver TUNEL+ cells were negative for the pan-leukocyte marker CD45 (not shown). We also used MMP-9−/− deficient mice to evaluate a possible contribution of MMP-9 on apoptosis. Indeed, TUNEL+ cells in the MMP-9−/− deficient livers (32.8 ± 2.3 vs. 76.4 ± 6.5, P < 0.004; n = 3/g) were detected in significantly fewer numbers as compared with respective controls at 6 hours after I/R injury. These results support the concept that iNOS deficiency is associated with decreased liver apoptosis after I/R injury, and that iNOS-derived NO-induced liver apoptosis may, in part, be mediated by MMP-9.
Figure 14.
Apoptotic markers and TUNEL staining in iNOS−/− and wild-type (WT) mice. Caspase-3 activity (A) was significantly depressed in iNOS−/− livers at 6 hours post-I/R injury, when compared with respective controls. Alternatively, Bcl-XL expression (B) was up-regulated in iNOS−/− livers at 6 hours after I/R injury (lanes 5–7), as compared with wild-type controls (lanes 3–4), to wild-type naïve (lane 1), and to knockout naïve (lane 2) livers. The densitometric ratios of Bcl-XL/β-actin are shown in (C). TUNEL+ cells (D) were readily detected in wild-type livers, and significantly depressed in iNOS−/− livers at 6 hours of hepatic I/R injury. Representative TUNEL staining (E) in wild-type livers (A) and iNOS−/− livers (B) at 6 hours post-I/R injury. Arrows denote TUNEL+ cells. TUNEL staining magnification = original ×200; *P < 0.03, **P < 0.02, and ***P < 0.001.
Discussion
In the present study, we investigated the functional significance of iNOS expression on MMP-9 activation in a well-established 90 minutes mouse model of partial liver warm I/R injury.4,15,29,41,44 We show here that MMP-9+ leukocytes either co-expressed iNOS or were detected adjacent to iNOS+ cells in damaged wild-type livers after the I/R insult. iNOS deficient mice showed (a) profound improvement in liver transaminases and in histological outcomes, (b) markedly inhibition of MMP-9 activity, (c) reduced leukocyte infiltration, (d) inhibition of cytokine and chemokine expression, and (e) decreased caspase-3 activity and apoptotic cell labeling after liver I/R injury. Moreover, specific iNOS inhibition with ONO-1714 down-regulated MMP-9 activity and significantly ameliorated liver I/R injury. We also show that activated neutrophils produced relatively negligible levels of iNOS and NO in contrast to activated macrophages, which expressed/released high levels of iNOS and NO; however, exogenous NO up-regulated MMP-9 activity in both leukocyte types. Furthermore, macrophage NO production through the induction of iNOS was capable of regulating neutrophil transmigration across fibronectin in a MMP-9-dependent manner.
While it is generally accepted that eNOS is beneficial to liver I/R injury, iNOS has generated more controversy. Our data shows that iNOS is highly expressed in wild-type livers after the I/R insult, and that iNOS-deficient mice, as compared with their wild-type counterparts, were significantly less susceptible to liver I/R reperfusion injury. iNOS-deficient mice showed reduced sALT and sAST levels and significantly improved histological preservation after the I/R insult, which indicates that liver damage was reduced in these mice, as compared with wild-type controls. However, studies performed by others in iNOS-deficient mice using a model of 45-minute partial warm liver ischemia followed by reperfusion, have indicated that iNOS has neither detrimental nor beneficial effects in liver during the acute phase of I/R injury,45 or that iNOS deficiency renders these mice more sensitive to liver damage.46 This apparent contradiction may in part be explained by substantial differences between experimental models of liver I/R injury. Indeed, wild-type livers submitted to the 45-minute partial liver ischemia have undetectable iNOS expression after reperfusion, and absence of infiltrating neutrophils,45,46 which are critical mediators in inflammatory liver injury.28 Therefore, as previously suggested, results obtained with the of 45-minute partial liver ischemia model may be explained by factors independent of liver iNOS.47 Other reports of a protective role for NO in liver I/R injury have been mostly based in studies using non-selective NOS inhibitors, such as N ω-nitro-l-arginine methyl ester hydrochloride, which inhibit both iNOS and eNOS.48,49 There is a growing body of evidence that the toxic effects of NO vary according to the source of NO, concentration of NO, redox conditions, and the tissue environment.11,32,50 Reactive oxygen species/reactive nitrogen species are important mediators of I/R injury, and for example, peroxynitrite, which is a superoxide derivative of NO, has been shown to destroy proteins, lipids, and DNA.13 Our observations that lack of iNOS confers a protective role in our model of liver I/R injury are in line with several other studies in models of 60-minute partial liver I/R injury, ConA-induced liver injury, and hemorrhagic shock, in which liver damage is significantly ameliorated in iNOS−/− mice.7,32,34,51 Furthermore, they are also supported by our own ONO-1714 studies, in which selective iNOS inhibition ameliorated mouse liver I/R injury, and by other publications showing that iNOS-specific inhibition is beneficial in pig and in rat liver I/R injury.10,37
Infiltrating leukocytes have been implicated as major mediators of I/R injury in several organs, including liver.2,4 Infiltration of CD3, CD4, Mac-1, and Ly-6G leukocytes was markedly reduced in the iNOS-deficient livers after I/R injury. CXCL-2, a cytokine-induced neutrophil chemoattractant, was down-regulated in the iNOS−/− livers after I/R, providing an indication that this chemokine may participate in neutrophil activation and recruitment in this model.29 We have previously shown that MMP-9 facilitates leukocyte migration in liver I/R injury.15 We report here that iNOS deficiency, and ONO-1714-mediated iNOS selective inhibition, profoundly depressed MMP-9 activity and significantly reduced leukocyte recruitment to livers after I/R injury. In contrast to control livers, in which MMP-9+ leukocytes were detected in elevated numbers after I/R injury, iNOS-deficient livers, and ONO-1714 treated livers showed very little MMP-9+ leukocyte infiltration. MMP-9+ leukocytes either co-expressed iNOS or were detected adjacent to iNOS+ cells in damaged wild-type control livers after the I/R insult. Moreover, in addition to mediating MMP-9 activation in isolated macrophages in vitro, which is in line with a previous publication using a macrophage cell line,27 we show here that NO is also capable of regulating MMP-9 expression and activity in neutrophils. In our experimental settings, cultured LPS-activated murine macrophages released relatively high levels of NO, which were profoundly depressed on selective iNOS inhibition. In contrast, fMLP-activated neutrophils released almost negligible NO, which was unchanged by iNOS inhibition. Furthermore, Mac-1 macrophages expressed both iNOS and MMP-9, while Ly-6G neutrophils expressed MMP-9, but were virtually negative for iNOS in damaged wild-type livers; thus, suggesting that NO-dependent MMP-9 activity in neutrophils may primarily be mediated by NO produced by adjacent cells. Fibronectin is a key ECM protein, which is expressed very early by liver endothelial cells in response to injury,18 including I/R injury.19 Interestingly, macrophage NO production through the induction of iNOS was capable of markedly up-regulating MMP-9 activity and significantly promoting neutrophil transmigration across fibronectin. Moreover, the observations that MMP-9 selective inhibition disrupted neutrophil migration, in the presence of high levels of iNOS-derived NO, provide evidence that MMP-9 is required for NO mediated neutrophil migration. Therefore, iNOS-derived NO regulates MMP-9 activity in neutrophils, likely by paracrine mechanisms, and promotes MMP-9-dependent neutrophil migration.
The extracellular matrix proteolysis mediated by metalloproteinases may not only facilitate leukocyte migration, but it may also lead to detachment of liver cells resulting in apoptosis, by a phenomenon called “anoikis.”52 The molecular mechanisms initiating anoikis are still incompletely understood. In our experimental settings, Bcl-xL, which inhibits apoptosis in response to many cytotoxic insults,53 was up-regulated in the iNOS-deficient livers after I/R injury. Moreover, activation of caspase-3, which triggers apoptosis,33 and it is linked to liver damage,29,41 was significantly reduced in iNOS−/− livers as compared with wild-type controls after I/R injury. Inhibition of caspase-3 activation was accompanied by a markedly reduced number of TUNEL-positive parenchyma cells in iNOS deficient livers after the I/R insult. Moreover, specific iNOS inhibition with ONO-1714 was also associated with a significant decrease in TUNEL-positive cells in the livers after the I/R insult (not shown). There is a growing evidence that NO induces adhesion-related apoptosis/anoikis,39 possibly by NO-mediated MMP activity via metalloproteinase S-nitrosylation,22 and/or via induction of cytokines, or growth factors.38 The regulation of MMP activity is a complex process, and the mechanisms by which NO may regulate MMP-9 activity in liver I/R injury are perhaps multifaceted. For example, we show that IL-6 and IFN-γ, which were found down-regulated by iNOS deficiency in livers after I/R injury, were capable of up-regulating MMP-9 activity in isolated neutrophils. Others have reported that S-nitrosylation mediates activation of MMP-9 causing neuronal cell dead/anoikis.22 Thus, it is reasonable to postulate that MMP-9+ leukocytes infiltrating livers after I/R injury can cause parenchyma cell detachment from ECM and, consequently to promote apoptosis/anoikis of these cells, perhaps by a similar mechanism involved in neuronal cell death. Indeed, we have observed that MMP-9-deficient livers showed considerably fewer cells undergoing apoptosis after I/R injury, and others have shown that MMP inhibition with BB-94 leads to significant protection against apoptosis and necrosis of hepatocytes.54
In conclusion, our data support the novel view that the pathological functions of iNOS-derived NO are, at least in part, mediated by MMP-9 in liver I/R injury. As compared with wild-type mice, iNOS deficient mice and mice treated with a selective iNOS inhibitor, showed significantly greater protection against liver I/R injury. This study shows, for the fist time, that specifically targeting iNOS-disrupted MMP-9+ leukocyte infiltration in livers after the I/R insult. Furthermore, it also shows that NO was capable of up-regulating MMP-9 expression/activation in neutrophils in vitro and that iNOS-derived NO regulated neutrophil transmigration across fibronectin in a MMP-9-dependent manner.
Footnotes
Address reprint requests to Dr. Ana J. Coito, The Dumont-UCLA Transplant Center, 77-120 CHS, Box: 957054, Los Angeles, CA 90095-7054, E-mail: acoito@mednet.ucla.edu.
Supported in part by the National Institutes of Health RO1 AI57832 grant to A.J.C.
References
- Howard TK, Klintmalm GB, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103–107. doi: 10.1097/00007890-199001000-00023. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology. 1991;13:83–95. [PubMed] [Google Scholar]
- Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup JM, Battle P, Waller H, Edmiston KH, Stolz DB, Watkins SC, Locker J, Skena K. Reactive nitrogen and oxygen radicals formed during hepatic ischemia-reperfusion kill weakly metastatic colorectal cancer cells. Cancer Res. 1999;59:1825–1829. [PubMed] [Google Scholar]
- Marletta MA, Hurshman AR, Rusche KM. Catalysis by nitric oxide synthase. Curr Opin Chem Biol. 1998;2:656–663. doi: 10.1016/s1367-5931(98)80098-7. [DOI] [PubMed] [Google Scholar]
- Koerber K, Sass G, Kiemer AK, Vollmar AM, Tiegs G. In vivo regulation of inducible no synthase in immune-mediated liver injury in mice. Hepatology. 2002;36:1061–1069. doi: 10.1053/jhep.2002.36155. [DOI] [PubMed] [Google Scholar]
- Li J, Billiar TR. Nitric oxide. IV. Determinants of nitric oxide protection and toxicity in liver. Am J Physiol. 1999;276:G1069–G1073. doi: 10.1152/ajpgi.1999.276.5.G1069. [DOI] [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M, Katsuramaki T, Hirata K, Kimura H, Nagayama M, Matsuno T. Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation. 1999;68:803–813. doi: 10.1097/00007890-199909270-00013. [DOI] [PubMed] [Google Scholar]
- Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata M, Suzuki S, Miyazawa N, Miyashita A, Nagashima Y, Inoue S, Kaneko T, Okubo T. Inhibition of inducible nitric oxide synthase prevents LPS-induced acute lung injury in dogs. J Immunol. 1998;160:3031–3037. [PubMed] [Google Scholar]
- Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, Wink DA. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem. 2004;385:1–10. doi: 10.1515/BC.2004.001. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Akuta T, Tamura F, van Der Vliet A, Akaike T. Molecular mechanism for activation and regulation of matrix metalloproteinases during bacterial infections and respiratory inflammation. Biol Chem. 2004;385:997–1006. doi: 10.1515/BC.2004.130. [DOI] [PubMed] [Google Scholar]
- Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47:186–198. doi: 10.1002/hep.21922. [DOI] [PubMed] [Google Scholar]
- Lohr KM, Kurth CA, Xie DL, Seyer JM, Homandberg GA. The amino-terminal 29- and 72-Kd fragments of fibronectin mediate selective monocyte recruitment. Blood. 1990;76:2117–2124. [PubMed] [Google Scholar]
- Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amersi F, Shen XD, Moore C, Melinek J, Busuttil RW, Kupiec-Weglinski JW, Coito AJ. Fibronectin-alpha 4 beta 1 integrin-mediated blockade protects genetically fat Zucker rat livers from ischemia/reperfusion injury. Am J Pathol. 2003;162:1229–1239. doi: 10.1016/s0002-9440(10)63919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Tchetverikov I, Lard LR, DeGroot J, Verzijl N, TeKoppele JM, Breedveld FC, Huizinga TW, Hanemaaijer R. Matrix metalloproteinases-3, -8, -9 as markers of disease activity and joint damage progression in early rheumatoid arthritis. Ann Rheum Dis. 2003;62:1094–1099. doi: 10.1136/ard.62.11.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Moore C, Shen XD, Gao F, Busuttil RW, Coito AJ. Fibronectin-{alpha}4{beta}1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. Am J Pathol. 2007;170:567–577. doi: 10.2353/ajpath.2007.060456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyvenhoven JP, Verspaget HW, Gao Q, Ringers J, Smit VT, Lamers CB, van Hoek B. Assessment of serum matrix metalloproteinases MMP-2 and MMP-9 after human liver transplantation: increased serum MMP-9 level in acute rejection. Transplantation. 2004;77:1646–1652. doi: 10.1097/01.tp.0000131170.67671.75. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci USA. 2007;104:16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- Hamada T, Tsuchihashi S, Avanesyan A, Duarte S, Moore C, Busuttil RW, Coito AJ. Cyclooxygenase-2 deficiency enhances th2 immune responses and impairs neutrophil recruitment in hepatic ischemia/reperfusion injury. J Immunol. 2008;180:1843–1853. doi: 10.4049/jimmunol.180.3.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TN, Beaman BL. Murine polymorphonuclear neutrophils produce interferon-gamma in response to pulmonary infection with Nocardia asteroides. J Leukoc Biol. 2002;72:373–381. [PubMed] [Google Scholar]
- Ho MK, Springer TA. Mac-1 antigen: quantitative expression in macrophage populations and tissues, and immunofluorescent localization in spleen. J Immunol. 1982;128:2281–2286. [PubMed] [Google Scholar]
- Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- Sass G, Koerber K, Bang R, Guehring H, Tiegs G. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest. 2001;107:439–447. doi: 10.1172/JCI10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Naito Y, Takagi T, Ichikawa H, Tomatsuri N, Kuroda M, Isozaki Y, Katada K, Uchiyama K, Kokura S, Yoshida N, Okanoue T, Yoshikawa T. A novel potent inhibitor of inducible nitric oxide inhibitor. ONO-1714, reduces intestinal ischemia-reperfusion injury in rats. Nitric Oxide. 2004;10:170–177. doi: 10.1016/j.niox.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi S, Kaldas F, Chida N, Sudo Y, Tamura K, Zhai Y, Qiao B, Busuttil RW, Kupiec-Weglinski JW. FK330, a novel inducible nitric oxide synthase inhibitor, prevents ischemia and reperfusion injury in rat liver transplantation. Am J Transplant. 2006;6:2013–2022. doi: 10.1111/j.1600-6143.2006.01435.x. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro HP, Silva EF, Stern A. Nitric oxide: a potential inducer of adhesion-related apoptosis–anoikis. Nitric Oxide. 2004;10:1–10. doi: 10.1016/j.niox.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Llacuna L, Mari M, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44:561–572. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- Tamatani M, Ogawa S, Niitsu Y, Tohyama M. Involvement of Bcl-2 family and caspase-3-like protease in NO-mediated neuronal apoptosis. J Neurochem. 1998;71:1588–1596. doi: 10.1046/j.1471-4159.1998.71041588.x. [DOI] [PubMed] [Google Scholar]
- Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IkappaB degradation. Hepatology. 1998;28:1022–1030. doi: 10.1002/hep.510280417. [DOI] [PubMed] [Google Scholar]
- Kawachi S, Hines IN, Laroux FS, Hoffman J, Bharwani S, Gray L, Leffer D, Grisham MB. Nitric oxide synthase and postischemic liver injury. Biochem Biophys Res Commun. 2000;276:851–854. doi: 10.1006/bbrc.2000.3559. [DOI] [PubMed] [Google Scholar]
- Hines IN, Harada H, Bharwani S, Pavlick KP, Hoffman JM, Grisham MB. Enhanced post-ischemic liver injury in iNOS-deficient mice: a cautionary note. Biochem Biophys Res Commun. 2001;284:972–976. doi: 10.1006/bbrc.2001.5069. [DOI] [PubMed] [Google Scholar]
- Hines IN, Kawachi S, Harada H, Pavlick KP, Hoffman JM, Bharwani S, Wolf RE, Grisham MB. Role of nitric oxide in liver ischemia and reperfusion injury. Mol Cell Biochem. 2002;234–235:229–237. [PubMed] [Google Scholar]
- Wang Y, Mathews WR, Guido DM, Farhood A, Jaeschke H. Inhibition of nitric oxide synthesis aggravates reperfusion injury after hepatic ischemia and endotoxemia. Shock. 1995;4:282–288. doi: 10.1097/00024382-199510000-00009. [DOI] [PubMed] [Google Scholar]
- Cottart CH, Do L, Blanc MC, Vaubourdolle M, Descamps G, Durand D, Galen FX, Clot JP. Hepatoprotective effect of endogenous nitric oxide during ischemia-reperfusion in the rat. Hepatology. 1999;29:809–813. doi: 10.1002/hep.510290317. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Lee VG, Johnson ML, Baust J, Laubach VE, Watkins SC, Billiar TR. The roles of iNOS in liver ischemia-reperfusion injury. Shock. 2001;16:355–360. doi: 10.1097/00024382-200116050-00006. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Wielockx B, Lannoy K, Shapiro SD, Itoh T, Itohara S, Vandekerckhove J, Libert C. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]