Abstract
Cell adhesion molecule 1 (CADM1), an immunoglobulin superfamily member, is expressed on superior cervical ganglion neurites and mediates cell–cell adhesion by trans-homophilic binding. In addition to the membrane-bound form, we have previously shown that a soluble form (sCADM1) generated by alternative splicing possesses a stop codon immediately downstream of the immunoglobulin-like domain. Here, we demonstrate the presence of sCADM1 in vivo and its possible role in neurite extension. sCADM1 appears to be a stromal protein because extracellular-restricted, but not intracellular-restricted, anti-CADM1 antibody stained stromal protein-rich extract from mouse brains. Murine plasmacytoma cells, P3U1, were modified to secrete sCADM1 fused with either immunoglobulin (Ig)G Fc portion (sCADM1-Fc) or its deletion form that lacks the immunoglobulin-like domain (ΔsCADM1-Fc). When P3U1 derivatives expressing sCADM1-Fc or ΔsCADM1-Fc were implanted into collagen gels, Fc-fused proteins were present more abundantly around the cells. Superior cervical ganglion neurons, parental P3U1, and either derivative were implanted into collagen gels separately, and co-cultured for 4 days. Bodian staining of the gel sections revealed that most superior cervical ganglion neurites turned toward the source of sCADM1-Fc, but not ΔsCADM1-Fc. Furthermore, immunofluorescence signals for sCADM1-Fc and membrane-bound CADM1 were co-localized on the neurite surface. These results show that sCADM1 appears to be involved in directional neurite extension by serving as an anchor to which membrane-bound CADM1 on the neurites can bind.
In the developing and regenerating nervous system during ontogeny and tissue repair, neurites are guided along a defined path toward their appropriate targets. This neurite guidance is achieved as a result of complex but highly regulated interactions among neurons, between neurons and glia, and between neurons and extracellular molecules or matrices.1 To maintain these specific interactions, growing neurites express numerous recognition molecules, such as immunoglobulin superfamily cell adhesion molecules, integrins, receptor tyrosine kinases, neuroligins, and neurexins, while neural stroma contains a variety of guidance cues, either permissive and attractive or inhibitory and repulsive, such as netrins, semaphorins, and ephrins.1,2,3,4,5,6,7 Among the best-studied subclasses of immunoglobulin superfamily cell adhesion molecules are the neural cell adhesion molecule (NCAM) and L1 families.1 Primary function of these families is to mediate cell–cell interaction, which is achieved generally via trans-homophilic binding of membrane-bound molecules, the canonical form of these families. Both families, however, are also known to serve as extracellular guidance cues, because they have soluble ectodomain (ECD) molecules that are arisen through alternative splicing or enzymatic cleavage of the membrane-bound forms.8,9,10,11
Among other members of immunoglobulin superfamily cell adhesion molecules expressed on neurons is cell adhesion molecule 1 (CADM1), which was formerly referred to as spermatogenic immunoglobulin superfamily,12 nectin-like molecule 2,13 tumor suppressor in lung cancer 1,14 or synaptic cell adhesion molecule 1.15 CADM1 is a membrane-bound glycoprotein composed of three extracellular Ig-like domains, a single transmembrane region, and a short carboxy-terminal intracellular tail with a protein 4.1 interaction sequence and a PDZ type II motif.12,13 The degree of glycosylation in the ECD varies with cell types and developmental stages,15,16,17 and enzymatic cleavage of the ECD is likely to occur in some types of cells.18,19 In neurons, CADM1 is localized primarily to synaptic plasma membranes, and is assumed to bridge the synaptic cleft via trans-homophilic binding.15 Besides neurons, other types of cells, such as mast cells18 and pancreatic islet cells,20 express CADM1 on the cell membrane. When mast or islet cells are co-cultured on a neurite network of superior cervical ganglion (SCG) neurons, trans-homophilic binding of CADM1 mediates cellular adhesion between these distinct types of cells.20,21 Interestingly, CADM1 appears to accumulate at the contact areas on both the neurites and mast or islet cells.20,21 Subcellular localization of CADM1 seems to change dynamically according to the microenvironment around cells.
As is often the case with immunoglobulin superfamily members, CADM1 has several isoforms arisen from alternative splicing, which occurs in the juxtamembranous extracellular region, ie, downstream of the exon encoding the third Ig-like loop and upstream of the exon encoding the transmembrane region.22 Previously, we isolated a soluble isoform of CADM1 (sCADM1) as an alternative splicing variant. Structurally, sCADM1 consists of the three Ig-like loops of the CADM1 ECD, with lacking the ECD juxtamembranous region.23 Functionally, sCADM1 is capable of binding to the ECD of the membrane-bound CADM1 (mCADM1), and this binding results in inhibition of cell–cell attachment mediated by trans-homophilic binding of mCADM1.23 When a cDNA expressing mouse sCADM1 fused at its C terminus to the Fc portion of human IgG1 (sCADM1-Fc) was transfected into COS-7 cells, a considerable amount of sCADM1-Fc molecules were detected in the cultured media.23 Therefore, sCADM1 molecules appear to be present mainly in extracellular spaces, ie, stroma of tissues and organs in vivo. If this is true, sCADM1 may serve as a stromal guidance molecule via trans-homophilic binding to mCADM1, as is the case with the soluble ECD molecules of NCAM or L1 families.9,24,25
In the present study, we attempt to detect sCADM1 proteins using the mouse brain, which has been shown to express a variety of CADM1 isoforms abundantly,15 and present evidence for in vivo existence of sCADM1 in brain stroma, based on the mRNA, molecular, and histological analyses. To probe possible roles for sCADM1 in the neural stroma, we devised collagen matrices containing a sCADM1 molecule gradient by implanting sCADM1-Fc-secreting cells into a small pit created on the surface of collagen gels. When SCG neurons were implanted into collagen gels together with sCADM1-Fc-secreting cells and non-secreting cells with being separated in a triangle, SCG neuritis were observed extending toward a source of sCADM1-Fc. While extending, the neurites bound to sCADM1-Fc via mCADM1 on their cell surface. As a stromal protein, sCADM1 seemed to be involved in directional neurite extension by serving as an anchorage to which mCADM1 on the neurites binds.
Materials and Methods
Mice, Cells, and Antibodies
C57BL/6 (B6) mice of indicated ages were purchased from Japan SLC (Hamamatsu, Japan). P3U1 mouse myeloma cells and NIH3T3 mouse fibroblasts were maintained as described previously.23 Cultured mast cells were established from B6 wild-type (+/+) and B6-mi/mi mice as described previously.18
Three anti-CADM1 antibodies were used, a rabbit polyclonal antibody against the C-terminal peptide (RP6), a chicken monoclonal antibody (3E1) and a goat polyclonal antibody (AF1459; R&D Systems, Minneapolis, MN) against the soluble ectodomain protein. The former two are our original as described previously.21 As a negative control for sCADM1-Fc, human IgG1 kappa was purchased from Sigma Chemical Company (St. Louis, MO). Other primary antibodies used in this study were rat anti-NCAM (12F11; BD Pharmingen, San Diego, CA), rat anti-mouse albumin (1D6; Yamasa, Tokyo, Japan), mouse anti-phosphotyrosine (PY-Plus Cocktail; Zymed, San Francisco, CA), rabbit and goat anti-human IgG Fc portion and goat anti-human IgG F(ab′)2 fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) antibodies. Peroxidase- and fluorophore-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and Jackson ImmunoResearch Laboratories, respectively.
Reverse Transcription-PCR Analyses
Total RNA was extracted from mouse brains and cultured mast cells using Trizol regent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Procedures of reverse transcription (RT)-PCR were essentially similar to our previous report,23 except that the reaction mixture of PCR contained either set of primers: for CADM1, one forward and two reverse primers (F, 5′-GTGACCAGTCAGCTGATGCTG-3′; R1, 5′-AAAATAGCGGCCCAGAATGATGAGCAA-3′; and R2, 5′-AAGCCCAGGCAGCAGCTACATGCA-3′), or for glyceraldehyde-3-phosphate dehydrogenase, one forward and one reverse primers (Toyobo, Osaka, Japan). PCR products were electrophoresed on 3% agarose gels and stained with ethidium bromide. Several bands were excised from gels, subcloned into pTA2 vector (Toyobo), and sequenced. Subclones containing the cDNA inserts identical to the reported sequence of CADM1 isoforms were amplified by PCR with the above set of primers, and a mixture of the PCR products were loaded in one lane as size markers. RT-PCR products were densitometrically analyzed according to the method described previously.26 Briefly, RT-PCR signal intensity was quantified with the BAS 3000 system (Fuji Photo Film, Tokyo, Japan) to compare mRNA levels between sCADM1 and mCADM1. Densitometric densities of single bands derived from sCADM1 mRNA and multiple bands derived from mCADM1 mRNAs were calculated for each age, and were expressed as relative values normalized to 1 for the total amount of CADM1 mRNA.
Establishment of P3U1 and NIH3T3 Subclones
The P3U1 subclone that expressed sCADM1-Fc was previously established by transfecting parent P3U1 cells with the pEFBosFc plasmid construct containing the full-length sCADM1 cDNA.23 By PCR using this construct as a DNA template with a pair of primers (forward, 5′-ACGCGTCGACGGCAGGTGCCCGACATGGCG AGTGCT-3′ and reverse, 5′-GAAGATCTTACTTACTTTGTCCTTCAATCACTGTCACGTc-3′), an N-terminal portion of the sCADM1 cDNA upstream of the first Ig-like motif was amplified. The PCR-amplified cDNA fragment was digested by SalI and BglII, and then inserted into pEFBosFc through the restriction sites of SalI and BamHI. The resultant plasmid construct was confirmed by sequencing to express a deletion form of sCADM1 lacking the three Ig-like motifs as a fusion protein with human IgG1 Fc portion (ΔsCADM1-Fc). P3U1 cells were transfected with this plasmid by electroporation, and were selected by resistance against G-418 for 1 month.
The NIH3T3 subclone that expressed mCADM1 isoform c (NCBI accession number NM_018770), a common isoform of CADM1 expressed in respiratory epithelia, spermatogonia and mast cells, was established previously.18 For establishment of sCADM1-expressing subclones, the plasmid vector containing the full-length mRNA for sCADM1 (clone #15 of a mast cell cDNA library) was used as a template in PCR together with a pair of primers (forward, 5′-CAGGAATTCGGCACGAGGGGCAGGTGCCCGACAT-3′ and reverse, 5′-CCGGAATTCCTCACGTACCGTATACATACAGCATAT-3′). The cDNA fragment amplified by PCR was digested at both ends with EcoRI, and then inserted into pMSCVpuro vector (Clontech, Mountain View, CA) through the EcoRI site. Using Fugene regents (Roche Diagnostics, GmbH, Mannheim, Germany), NIH3T3 cells were transfected with the plasmid construct that contained directionally the cDNA insert without mutations, and were selected by resistance against puromycin for a month.
For detection of molecules released from P3U1 or NIH3T3 subclones, the cells were cultured overnight at near confluency in serum-free Dulbecco’s Modified Eagle’s Medium media containing 10 mmol/L Hepes. Culture supernatants were passed through a 0.45-μm cellulose acetate filter unit (Advantec, Tokyo, Japan), and were concentrated approximately 30 times by using Microcon centrifugal filter devices (10-kDa cutoff; Millipore, Billerica, MA). Concentrated supernatants were subjected to Western blot analyses.
Preparation of Collagen Matrices and Three-Dimensional Co-Culture of SCG and P3U1 Cells
Single cell suspension of SCGs from B6 mouse neonates were prepared as described previously.20,21 As shown in supplemental Figure S1 available at http://ajp.amjpathol.org, three plastic rods with a 2.5-mm diameter were made a triplet, being separated from each other at a distance of 8 or 6.5 mm, and were fixed to a metal plate. The collagen mixture was made with 8 volumes of type I collagen stock solution (Cellmatrix type-IA, 3.0 mg/ml; Nitta Gelatin, Osaka, Japan), 1 volume of 10× concentrated F-12 medium, 1 volume of glial conditioned medium MB-X9501 (Sumitomo Bakelite, Tokyo, Japan), 100 ng/ml nerve growth factor, and 2 μmol/L Ara-C, and was then kept on ice (supplemental Figure S1B available at http://ajp.amjpathol.org). Immediately after supplementation with 1 volume of reconstruction buffer containing 0.05 N NaOH, 2.2% NaHCO3, and 200 mmol/L HEPES, the mixture containing type-I collagen at a concentration of 2.2 mg/ml was poured into 12-well tissue culture at a volume of 2.75 ml/well (supplemental Figure S1, C–E available at http://ajp.amjpathol.org), and the triplet of three plastic rods were hung down over 12-well plates, suspended (4 mm deep) into the collagen mixture (supplemental Figure S1, C–E available at http://ajp.amjpathol.org). The plates were then incubated at 37°C for about 30 minutes until the collagen solidified. The rods were pulled out so gently as to leave sharp-margined pits in the solidified collagen gels (supplemental Figure S1F available at http://ajp.amjpathol.org). For SCG neuron implantation, 2 × 104 neurons were suspended in 5 μl of the above-mentioned collagen mixture supplemented with reconstruction buffer on ice, and poured into a pit in the collagen gel. For P3U1 cell implantation, 5 × 104 cells were suspended in 5 μl of cooled type-I collagen stock solution supplemented with F-12 medium and reconstruction buffer at concentrations as above, and were poured into a pit in the collagen gels. Immediately after cell implantation, the plates were incubated at 37°C for about 30 minutes until the collagen solidified. The collagen gels implanted with cells were overlaid with 0.5 ml of 1 × F-12 medium containing 10% MB-X9501, 100 ng/ml nerve growth factor, and 2 μmol/L Ara-C, and the medium was changed every 2 days. In three-dimensional co-culture experiments, P3U1 cells were implanted 2days after SCG neuron implantation, and co-cultured for 2 (2-day-long co-culture) or 4 (4-day-long co-culture) days.
Protein Samples
B6 mouse cerebrums of indicated ages were frozen in liquid nitrogen, crushed, and vigorously vortexed in a buffer containing 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1% Triton X-100, and protease inhibitor cocktail (Sigma). Three-dimensional culture collagen gels implanted with P3U1 cells were cut into three 5-mm cubes, and were homogenized in the same buffer by sonication. Insoluble components including cellular nuclei and collagen fibers were removed by centrifugation at 10,000 × g. Resultant supernatants were referred to as whole tissue lysates of brains and gel extracts of three-dimensional cultures, respectively. To extract stromal proteins in preference to cellular proteins, mouse cerebrums of indicated ages were cut into pieces roughly with scissors, suspended in PBS, and passed gently through Pasteur pipettes. After addition of collagenase type IV-S (Sigma) at a concentration of 1 μg/ml, obtained homogenates were incubated at 37°C for 15 minutes, followed by centrifugation at 3000 × g to pellet cells and insoluble components. Resultant supernatants were gently passed through a 0.45-μm cellulose acetate filter unit (Advantec), and used as soluble protein extracts in the present study. Lysates of SCG neurons grown in three-dimensional co-cultures were prepared as follows: a 6-mm cubic gel piece that contained the implanted SCG neurons at its center was cut out from the 4-day-long co-culture, and was homogenized by sonication in a buffer containing 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1% Triton X-100, and protease inhibitor cocktail (Sigma). Insoluble components including cellular nuclei and collagen fibers were removed by centrifugation at 10,000 × g. Resultant supernatants were referred to as three-dimensional cultured SCG neuron lysates, which were expected to contain proteins derived from neurites extending within collagen matrices.
Western Blot and Immunoprecipitation Analyses
Lysates and extracts of mouse brains and three-dimensional culture gels were separated on 10% SDS-polyacrylamide gels, transferred to Immobilon (Millipore) and analyzed by Western blot, as described previously.17,18 The procedure for immunoprecipitation with RP6 was essentially similar to that described previously.27 To use 3E1 for immunoprecipitation, 100 μg of the 3E1 antibody were crosslinked with 100 μl of the affinity-purified goat anti-IgY antibody-conjugated Microbeads (2 mg IgG/ml Microbeads, 50% slurry in Tris-buffered saline; GenWay Biotech, San Diego, CA) by using dimethyl pimelimidate (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s instruction, and the beads were washed twice with 0.2 M/L glycine (pH 8) and subsequently twice with 0.1 M/L glycine (pH 2) before the usage for immunoprecipitation. 10 μl of the crosslinked beads (50% slurry in PBS) and 200 μl of brain soluble protein extracts were mixed in a single tube, and incubated overnight at 4°C with rotation. The beads were harvested by centrifugation, and incubated in 0.1 M/L glycine (pH 2) for 30 minutes at 4°C. After centrifugation, the supernatants were neutralized with 1 M/L Tris buffer (pH 9), and subjected to Western blot analyses. For negative control, the crosslinked beads were mixed with PBS instead of brain extracts, and were processed with the procedure same as above. In some experiments, SDS-polyacrylamide gels were stained with coomassie brilliant blue.
Three-Dimensional Co-Cultured Gel Stain, Immunohistochemistry, and Immunofluorescence
Three-dimensional culture collagen gels were fixed with 4% paraformaldehyde (PFA), then embedded in paraffin, and cut into thin (3 μm) or thick (12 μm) sections. To visualize neurites, thick sections were stained according to the standard Bodian’s method28 except that silver protein reaction time was shortened to 3 hours. Thin sections were immunostained according to the methods essentially similar to those for immunohistochemistry described previously.17 Briefly, deparaffinized sections were autoclaved in 0.1 M/L citrate buffer, then blocked with bovine serum albumin, and reacted with rabbit anti-human IgG Fc portion antibody in PBS containing 2% bovine serum albumin. Second antibody and signal enhancement reactions were performed using an LSAB kit, and color was developed with aminoethylcarbazole (Dako). Some sections were counterstained with hematoxylin for cell nuclear visualization. For immunofluorescence double staining, thin sections deparaffinized, autoclaved, and blocked. Then they were incubated in PBS containing 2% bovine serum albumin, firstly with a mixture of goat anti-human IgG Fc portion and rabbit anti-CADM1 C-terminal peptide antibodies, and secondly with a mixture of Cy2-conjugated anti-goat IgG and Cy3-conjugated anti-rabbit IgG antibodies. In negative controls, the anti-human IgG Fc portion antibody was replaced with the anti-human IgG F(ab′)2 fragment antibody. Immunofluorescent signals were detected under a confocal laser microscope (LSM510; Carl Zeiss, OberKochen, Germany). The procedure of immunohistochemistry was described previously.17
In Situ Detection of DNA Fragmentation
SCG neurons and P3U1 cells were 3-dimentionally co-cultured as illustrated in supplemental Figure S2 available at http://ajp.amjpathol.org. Four-day-long three-dimensional co-culture gels were fixed with 4% PFA, embedded in paraffin, and cut in a 4-μm thickness. DNA fragmentation was detected with the fluorescein-based terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) assay using In Situ Cell Death Detection Kit, POD (Roche Diagnostics). According to the manufacturer’s instruction, sections were incubated serially with the TUNEL reaction mixture and with the converter-peroxidase solution, and were then colored with aminoethylcarbazole. Nuclei were counterstained with hematoxylin. To quantify apoptosis of SCG neurons and P3U1 cells, more than 300 cells were individually judged as either TUNEL-positive or TUNEL-negative to obtain the proportion of TUNEL-positive cells to the total number of cells observed. For each set of treatment groups, the mean and SD of the TUNEL-positive cell proportion were calculated from four sections that were prepared from independent co-cultures.
Quantification of the Degree of Curve of SCG Neurites Extending in Three-Dimensional Co-Cultures
In a co-culture gel section stained with Bodian’s solution, a 650-μm wide and 250-μm high rectangular area was defined at the margin of a SCG-implanted pit nearest a co-culture triangle center, as illustrated in supplemental Figure S3A, available at http://ajp.amjpathol.org. In this rectangle, approximately 20 neurites were traceable from the bottom to the top. The bottom and top points of each neurite were connected with a straight line (red line), and the angle of this line to the midline was measured and expressed as + degrees for left side rotation from the midline and − degrees for right side rotation (black number). Schematic and real examples are shown in supplemental Figure S3, A and B–D available at http://ajp.amjpathol.org, respectively. Degrees of the angles of approximately 20 traceable neurites were averaged for each gel section, and the mean and SD were calculated from four or five sections that were prepared from independent co-cultures for each set of treatment groups.
Two-dimensional Culture of SCG Neurons
Coverslip-like-bottomed culture dishes of a 35-mm diameter (μ-Dishes; ibidi GmbH, München, Germany) coated with Matrigel (Becton Dickinson, Bedford, MA) were described previously.2,3 Poly-l-lysine-coated plastic filters (Sumilon Cell-tight, PL Cell Disk FL, for 24-well tissue culture plate) were purchased from Sumitomo Bakelite. According to the procedure described by Sakurai-Yageta et al,29 Matrigel-coated dishes and poly-l-lysine-coated filters were coated additionally with sCADM1-Fc, ΔsCADM1-Fc, and human IgG (Sigma) by dropping 50 μl of PBS containing each protein at a concentration of 50 ng/μl, then putting a Parafilm (American National Can, Neenah, WI) sheet on a drop, and leaving the dishes and filters overnight at room temperature. Single cell suspensions of SCG neurons were seeded onto the coated dishes and filters at a density of 1 × 104 neurons/dish and 3 × 104 neurons/filter, respectively, and were grown in F-12 medium (Invitrogen) containing 0.2 mmol/L l-glutamine, 10% glial conditioned medium MB-X9501; 100 ng/ml nerve growth factor (Upstate Biotechnology, Lake Placid, NY), and 2 μmol/L cytosine-β-d-arabinofuranoside (Ara-C; Sigma). The media was changed every 2 days.
Statistical Analyses
The Student’s t-test was performed for analyzing proportions of apoptotic cells and the degree of neurite extension turning by using the StatView (Abacus Concepts Inc., Cary, NC) software on a Macintosh computer. A P value <0.05 was considered to be significant.
Results
Expression of sCADM1 in the Mouse Brain at Various Ages
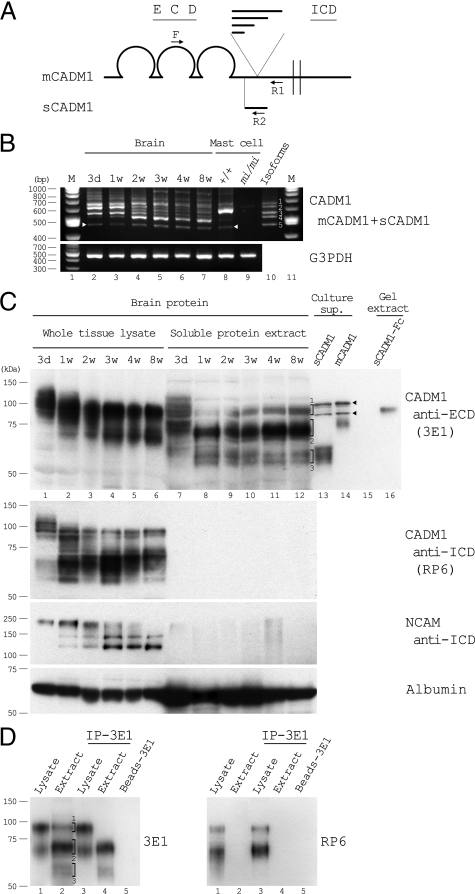
In comparison with the mCADM1 mRNA, we examined the sCADM1 mRNA expression in the mouse brain at various ages by RT-PCR analyses using one forward (F) and two reverse primers, each of which is specific to mCADM1 (R1) and sCADM1 (R2), respectively, as illustrated in Figure 1A. PCR products were detected as multiple ladders on agarose gels (Figure 1B). By making reference to the molecular sizes of known isoforms (Figure 1B, lane 10), we considered that the smallest band, indicated by white arrows in Figure 1B, corresponded to the mRNA for sCADM1, and the others corresponded to the mRNAs derived from various isoforms of mCADM1. Consistent with this judgment, the smallest bands disappeared when R2 primer was removed from the PCR mixture (data not shown). The expression level of sCADM1 mRNA was substantially lower than that of the major mCADM1 isoform at each age, but it was detectable at all ages examined, with a slight increase after 2 weeks after birth (Figure 1B and supplemental Figure S4 available at http://ajp.amjpathol.org).
Figure 1.
Expression of sCADM1 mRNA and protein in the mouse brain. A and B: RT-PCR analyses to detect CADM1 mRNAs for various isoforms. As illustrated in (A), one forward (F) and two reverse (R1 and R2) primers were designed so as to anneal the cDNA portions indicated. R1 is located downstream of the site where alternative splicing occurs multifariously, while R2 is specific to the exon unique to sCADM1. In (B), total RNAs from mouse brains of various ages were amplified by RT-PCR using either primer set for CADM1 (upper) or glyceraldehyde-3-phosphate dehydrogenase (lower), and the products were electrophoresed on 3% agarose gels. Total RNAs from cultured mast cells of +/+ and mi/mi genotypes were used as positive and negative controls for detection of sCADM1 mRNA, respectively. Equal input of total RNA per lane was verified by amplification of glyceraldehyde-3-phosphate dehydrogenase mRNA. In lane 10, cDNA fragments of reported CADM1 isoforms were amplified by PCR, and were loaded as a mixture: 1, isoform a (NCBI accession number NM_207675); 2, isoform c (NM_018770); 3, isoform b (NM_207676); 4, isoform d (NM_001025600); and 5, sCAMD1 (AB092414). White arrowheads indicate the PCR-amplified cDNA for sCADM1. Lanes 1 and 11, 100-bp ladder size marker. C: Detection of sCADM1 in soluble protein extract, but not whole tissue lysate, of the mouse brain by the anti-CADM1 ECD antibody, 3E1. Whole tissue lysate and soluble protein extract were prepared from mouse brains of indicated ages, and were blotted with the antibodies indicated. In lanes 13 and 14, concentrated supernatants (sup.) prepared from overnight culture of NIH3T3 cells expressing sCADM1 and mCADM1isoform c were loaded, respectively. The four bands indicated by arrowheads in lanes 13 and 14 are derived from cross-reactivity of the concentrated culture medium to 3E1. In lane 16, gel lysate prepared from 2-day-long three-dimensional culture of P3U1-sCADM1-Fc cells (cube A; see Figure 4) was loaded. Concerning band groups #1 to #3 indicated in lane 12, refer to the Results section. D: immunoprecipitation of the whole tissue lysate and soluble protein extract by 3E1. Brain whole tissue lysate (Lysate) and soluble protein extract (Extract) of 4-week-old mice were immunoprecipitated with 3E1, and together with the original lysate and extract (lanes 1 and 2, respectively), were blotted with 3E1 (left) or RP6 (right). Lane 5, negative control for the crosslinked Microbeads (see Materials and Methods). Band groups #1 to #3 are the same as in (C).
In an attempt to detect sCADM1 molecules in the stroma of mouse brain, we obtained soluble protein extracts by gently homogenizing brain tissues in PBS, and compared this extract with the whole tissue lysate that was prepared by vigorously homogenizing brain tissues in a detergent-containing buffer. These extract and lysate were immunoblotted with antibodies against the intracellular domain of NCAM, a protein spanning the cell membrane, and albumin, a protein abundantly present in extracellular stroma but not inside cells. NCAM molecules were detected exclusively in the whole tissue lysate, while albumin was much more abundant in the soluble protein extract, suggesting significant enrichment of stromal proteins in the extract (Figure 1C). The identical blot was subsequently probed with the anti-CADM1 intracellular domain (RP6) or ECD (3E1) antibody. RP6-specific signals were detected exclusively in the whole tissue lysate, whereas 3E1-specific signals were observed in not only the whole tissue lysate but also the soluble extract (Figure 1C). In the tissue lysate, the multiple bands detected by RP6 and those by 3E1 seemed to be mostly identical, while 3E1 appeared to recognize larger molecules preferentially, suggesting that 3E1 has higher affinity to heavily glycosylated forms of mCADM1 than RP6 does. In the soluble protein extract, 3E1-specific signals were also multiple, but apparently distinct from those in the tissue lysate. As indicated in lane 12 of Figure 1C, we categorized the multiple bands into three groups. The largest band group (#1) seemed to correspond to mCADM1 derived from contamination of cellular proteins, because the tissue lysate contained strong immunoreactivity to 3E1 at the mobility similar to #1. In contrast, the tissue lysate did not contain any signals at the mobility of the two smaller band groups (#2 and #3). To examine whether these bands corresponded to CADM1 ECD molecules, we loaded concentrated culture supernatants from NIH3T3 cells overexpressing sCADM1 or mCADM1 isoform c, and blotted with 3E1 (Figure 1C, lanes 13 and 14). sCADM1 molecules were detected as smear-like bands in lane 13 at the mobility similar to band group #3, suggesting that #3 corresponded to sCADM1. On the other hand, smear-like bands were detected around 70 kDa in lane 14. Consistent with our notion that mCADM1 should undergo enzymatic cleavage in its juxtamembranous extracellular region,18,19 these bands were regarded as ECD molecules derived from mCADM1 isoform c expressed on NIH3T3 cells. The similarity in mobility size suggested that band group #2 corresponded to ECD fragments resulting from enzymatic cleavage of mCADM1.
To further confirm the identity of immunoreactive bands detected in the soluble protein extracts, the extracts of 4-week-old mouse brains, along with the whole tissue lysates, were immunoprecipitated with 3E1, and blotted with either 3E1 or RP6. Band groups #2 and #3 were easily detected with 3E1 in the immunoprecipitates from the soluble protein extract but not the whole tissue lysate, whereas they were not recognized with RP6 (Figure 1D). As band group #1 was expected to correspond to mCADM1, it should be detected by both 3E1 and RP6. In fact, however, neither antibody detected it in the immunoprecipitates from the soluble protein extract (Figure 1D, lane 4), probably due to the small amount below the limit of detection.
Immunohistochemical staining patterns of RP6 and 3E1 were compared on adjacent sections of 4-week-old mouse brain (Figure 2). Both antibodies yielded a similar diffuse staining almost constantly throughout a para-midline sagittal plane (Figure 2A). However, in a high-power field, the staining patterns were apparently distinct between each other. Most 3E1-specific signals were punctate and distributed diffusely in interneuron soma spaces, and some signals were accumulated on nerve fibers (Figure 2B). On the other hand, RP6-specific signals were often accumulated on the neuron soma surfaces and nerve fibers (Figure 2C). Such difference in the staining patterns between the two antibodies was more clearly recognizable on the sections from 3-day-old mouse brain (supplemental Figure S5 available at http://ajp.amjpathol.org). Together with these results from mRNA, molecular and histological analyses, sCAMD1 appeared to be present physiologically in the extracellular stromal space of the mouse brain.
Figure 2.
Immunohistochemical staining of CADM1 in the mouse brain. As illustrated in the upper right, a brain section from a 4-week-old mouse was cut in a sagittal plane 0.3 mm lateral to the midline, and was incubated with 3E1 (A and B). A boxed area of the sagittal plane was photographed in (A), and enlarged in (B). The adjacent section was stained with RP6, and the area corresponding to (B) was shown in (C). Boxed areas in (B and C) are enlarged in the insets. Immunoreactive signals were colored with aminoethylcarbazole, and the nuclei were counterstained with hematoxylin. Arrowheads indicate punctate signals along the neuron soma surface and nerve fibers. Scale bars: 200 μm in (A); 50 μm in (B and C).
Formation of sCADM1 Gradient in Collagen Gels
We speculated that sCADM1 might have some activity to neurite outgrowth in SCG neuron culture. To examine this possibility in three-dimensional culture, we made a type-I collagen gel with a pit of a 3-mm diameter and 5-mm depth in a 12-well tissue culture plate (see Figure 3 and supplemental Figure S1 available at http://ajp.amjpathol.org), and implanted parental P3U1 cells and its two derivatives, ie, P3U1-sCADM1-Fc and P3U1-ΔsCADM1-Fc, into the pits of collagen gels. After 2 days of three-dimensional culture, the gel matrices around the pits implanted with P3U1 cells were carefully cut into three pieces, 5-mm cube each, as illustrated in Figure 3A. Soluble proteins were extracted from each piece of the gels, and were immunoblotted with anti-IgG Fc antibody. In both three-dimensional co-cultures implanted with P3U1-sCADM1-Fc and P3U1-ΔsCADM1-Fc cells, Fc-fused proteins were contained most abundantly in the gel piece A, the nearest one to the pits, while less and the least amounts of Fc-fused proteins were detected in gel pieces, B and C, located farther from the pits, respectively (Figure 3, A and B). Thus, both P3U1 derivatives appeared to generate a gradient of the secreted Fc-fused proteins around the pits when implanted into the pits. After 2 days of implantation, sections of the collagen gels were stained with anti-IgG Fc antibody (Figure 3C). Secreted Fc-fused proteins were detected in an area within 3.5 mm distance from the pit margin, with a maximal concentration around 2 mm distance from the margin. Although the IgG Fc immunostain finding was consistent with the Western blot result in that secreted Fc-fused proteins were present at a maximal concentration within the ‘gel piece A’ area, it did not prove that the Fc-fused protein gradient was continuous around the pit, as the Fc immunostaining showed a clear decrease in Fc-positive signals adjacent to the margin of the pits implanted with P3U1 derivatives (Figure 3C). This might be attributable to secretion of some proteolytic factors from P3U1 cells.
Figure 3.
Secretion of sCADM1-Fc proteins from P3U1 cells into collagen gels. A and B: three-dimensional culture of P3U1 cells and Western blot analyses of gel lysate. As schematically presented in (A), collagen gels with a 3-mm pit were made in 12-well plates, and P3U1-sCADM1-Fc or P3U1-ΔsCADM1-Fc cells were implanted in the pit. After 2 days of culture, the gel around the pit was cut into three pieces (A, B, and C) 5 mm cubed, as illustrated in A. The gel lysates prepared from the three gel cubes were immunoblotted with anti-IgG Fc portion antibody, together with purified sCADM1-Fc proteins of indicated amounts (B). C: Immunohistochemical detection of sCADM1-Fc in three-dimensional culture collagen gels. Two-day-long three-dimensional cultures were PFA-fixed and paraffin-embedded, and sections of the collagen gels were stained with anti-IgG Fc antibody. Right three panels are high-power view images of circled areas in the left panel. Scale bar = 20 μm.
To semiquantify the amounts of Fc-fused proteins present in the collagen matrices, purified sCADM1-Fc proteins were serially diluted, and together with the gel extracts, were blotted with anti-IgG Fc antibody. As shown in Figure 3B, the band intensity detected in the extract of gel piece A was almost equivalent to that of the purified protein of 3 μg Because the amount of the extract loaded per lane in Figure 3B was one tenth of the total volume, gel piece A, 5 mm cube, was roughly estimated to contain 10 × 3 μg of Fc-fused proteins, ie, a concentration of 30 μg/125 mm3 = 0.24 μg/mm3. This concentration seemed to be several fold as high as the physiological concentration of sCADM1, because band group #3 in brain soluble protein extracts was several fold as weak as the band in the extract from gel piece A when these extracts were blotted in an identical membrane with 3E1 (Figure 1C, uppermost panel).
Neurite Outgrowth Directing toward a Source of sCADM1-Fc
To co-culture SCG neurons with P3U1 cells within collagen gels, we made a type-I collagen gel with three pits arranged in an equilateral triangle, which base and side were 8 mm and 6.5 mm, respectively (see supplemental Figure S1 available at http://ajp.amjpathol.org). SCG neurons were implanted at the apex of the triangle, and after 2 days, when some SCG neurites were observed outgrowing over the margin of the implanted pits into the surrounding collagen gels, P3U1 cells and either P3U1-sCADM1-Fc or P3U1-ΔsCADM1-Fc cells were implanted at both ends of the base of the triangle. On the following day, most SCG neurons sprouted out neurites into the surrounding collagen gels (Figure 4A), and thereafter continued active neurite extension for 3 days. It was difficult to find out whether SCG neurite extension conformed to some particular rules by tracing the neurite alive in the three-dimensional cultures under a phase-contrast microscope, because SCG neurites were very thin and crossed with each other in a very complicated manner. At 2 and 4 days after P3U1 implantation, three-dimensional co-cultures were fixed with PFA, then embedded in paraffin, and were cut into thick (12 μm) sections in a plane parallel to the gel surface. For each co-culture, the section containing the most elaborate network of neurites was stained with Bodian’s method. Representative results of 2-day-long and 4-day-long co-cultures were shown in supplemental Figure S6 available at http://ajp.amjpathol.org and Figure 4, B and C, respectively. In 2-day-long co-cultures, there was no considerable difference in neurite extension between different sets of co-cultures. In 4-day-long co-cultures with P3U1-sCADM1-Fc cells, most SCG neurites inside the triangle were not extending radially but curving in a direction toward the pit implanted with P3U1-sCADM1-Fc cells (Figure 4B). Similar results were obtained, when SCG neurons were co-cultured with P3U1-sCADM1-Fc and P3U1-ΔsCADM1-Fc cells (supplemental Figure S3D, available at http://ajp.amjpathol.org). In contrast, such a directional preference in SCG neurite outgrowth was not recognizable in 4-day-long co-cultures with P3U1-ΔsCADM1-Fc and parent P3U1 cells (Figure 4C). These qualitative findings were supported by the quantitative analyses, in which the degree of directional neurite extension was quantified by averaging the angles at which approximately 20 traceable neurites extended from the midline of the three-dimensional co-culture triangle (supplemental Figure S3 available at http://ajp.amjpathol.org). In the three-dimensional co-cultures with P3U1-sCADM1-Fc cells, SCG neurites were found to be inclined, on the average, at approximately 10 degrees from the midline.
Figure 4.
SCG neurite extension in three-dimensional co-culture implanted with P3U1 cells secreting sCADM1-Fc or ΔsCADM1-Fc. A: A whole view of three-dimensional co-culture. SCG neurons were implanted into a 3-mm pit located at the apex of an equilateral triangle (base = 8 mm, and side = 6.5 mm). Two days later, parent P3U1 and its derivatives were implanted into 3-mm pits located both ends of the triangle base. B and C: Bodian staining of three-dimensional co-cultures implanted with P3U1-sCADM1-Fc (B) or P3U1-ΔsCADM1-Fc (C) cells. At 6 days after SCG implantation, three-dimensional co-cultures were PFA-fixed and paraffin-embedded, and thick sections of the gels were stained with the standard Bodian’s method with some modifications (see Materials and Methods). Boxed areas in upper panels are enlarged in the next lower. Scale bar = 100 μm.
Sections from 4-day-long co-cultures were subjected to TUNEL staining, and representative results were shown in supplemental Figure S2 available at http://ajp.amjpathol.org. There were few neurons that fell into apoptosis in any sets of co-cultures (supplemental Figure S2, A–C available at http://ajp.amjpathol.org). On the other hand, some P3U1 and subclone cells were apoptotic, but they were minor proportions in the two sets of co-cultures examined (less than 5%; supplemental Figure 2, D–E available at http://ajp.amjpathol.org). The amounts of Fc-fused proteins contained in the three-dimensional co-culture gels were examined with Western blot analyses by extracting proteins from a gel piece cut out from the three-dimensional co-culture triangle center (supplemental Figure S7 available at http://ajp.amjpathol.org). Both types of Fc-fused proteins, sCADM1-Fc and ΔsCADM1-Fc, appeared to be present within collagen gel matrices at similar concentrations in the three-dimensional co-cultures implanted with P3U1 subclones. These results supported the claim that directional neurite outgrowth observed in the three-dimensional co-cultures was attributable to sCDM1-Fc secreted from P3U1 cells, but neither to the other factors from P3U1 cells, nor to non-uniformity of the collagen matrices.
Binding of sCADM1 to mCADM1 on Growing SCG Neurites
Paraffin-embedded sections of three-dimensional co-cultures implanted with P3U1-sCADM1-Fc cells were stained with anti-IgG Fc antibody. Punctate signals were detected not only in the gels but also on the surface of the neurites, suggesting that sCADM1-Fc bound CADM1 expressed on the cell membrane of the neurites (Figure 5A). This possibility was clearly supported by immunofluorescence double-staining experiments, in which two antibodies against IgG Fc and CADM1 intracellular domain were used to detect Fc-fused proteins secreted in collagen matrices and mCADM1 expressed on the neurites, respectively. Both signals were dot-like and were often colocalized with each other on the surface of the neurites (Figure 5C). In contrast, when three-dimensional co-cultures implanted with P3U1-ΔsCADM1-Fc cells were stained with anti-IgG Fc antibody, punctate signals were distributed broadly in the gel area but rarely localized on the neurite surface (Figure 5B). Usage of anti-IgG (Fab′)2 antibody instead of anti-IgG Fc antibody did not yield any significant signals (supplemental Figure S8 available at http://ajp.amjpathol.org).
Figure 5.
Colocalization of secreted sCADM1-Fc with mCADM1 on the surface of SCG neurites extending within collagen gels. A and B: Immunohistochemical detection of Fc-fused molecules in three-dimensional co-cultures implanted with P3U1-sCADM1-Fc (A) or P3U1-ΔsCADM1-Fc (B) cells. At 6 days after SCG implantation, three-dimensional co-cultures were PFA-fixed and paraffin-embedded. Thin sections of the gels were incubated with anti-IgG Fc portion antibody, then colored with aminoethylcarbazole, and were counterstained with hematoxylin. Neurites extending in a direction from the SCG neurons’ pit (base) to the P3U1 cells’ pits (top) are shown. A boxed area of the left panel is enlarged in the right. C: Double-staining immunofluorescence of three-dimensional co-cultures implanted with P3U1 subclone cells secreting sCADM1-Fc. Thin sections of 4-day-long three-dimensional co-cultures were double-stained with anti-IgG Fc and RP6 antibodies, and were visualized with Cy2 (Ca) and Cy3 (Cb), respectively. Images, Ca and Cb, are merged in Cc, which is overlaid on the differential interference contrast image (Cd). Scale bar = 20 μm.
Cell lysates were prepared from the three-dimensional co-culture gel piece that contained SCG neurons and growing neurites, and were immunoprecipitated with RP6, then blotted with anti-phosphotyrosine antibodies (Figure 6). As indicated by asterisks in Figure 6, multiple immunoreactive bands were detected only in the lysates from three-dimensional co-cultures implanted with P3U1-sCADM1-Fc cells. Binding of sCADM1 to mCADM1 appeared to result in enhancement of tyrosine phosphorylation levels of the molecules associated with mCADM1 in growing neurites.
Figure 6.
Enhancement of tyrosine phosphorylation in SCG neurons co-cultured with P3U1-sCADM1-Fc cells. As illustrated in the upper panel, SCG neurons were co-cultured with various pairs of P3U1 and its subclone cells, and cell lysates were prepared from SCG neurons and growing neurites by cutting out a gel piece from 4-day-long three-dimensional co-cultures. Lysates (1 to 3) were immunoprecipitated with RP6, and together with the original lysates, were blotted with either the anti-phosphotyrosine (Tyr-P) antibody cocktail (left) or RP6 (right). Asterisks indicate specific immunoreactivity to the anti-phosphotyrosine antibody cocktail. Arrowheads and arrows indicate the bands for type-I collagen and bands for RP6, respectively.
Discussion
In the present study, we attempted to clarify whether sCADM1 molecules are actually present in the mouse brain. As we showed in Figures 1 and 2, the three analyses at mRNA, molecular, and histological levels consistently indicated the presence of sCADM1 in brain stroma. However, sCADM1 did not seem to be the major form of CADM1 ECD molecules that are present in brain stroma, because in the soluble protein extract, band group #2 was more intense than band group #3 (Figure 1C, uppermost panel). As we previously described in mast cells and mesothelial cells,18,19 mCADM1 is likely to undergo enzymatic cleavage in the juxtamembranous extracellular region and to release the N-terminal fragment composed of a nearly full-length of the mCADM1 ECD. Band group #2 seemed to correspond to this kind of ECD molecules, based on its mobility size similarity to the smear-like band detected in culture supernatant from mCADM1-overexpressing cells. To clarify this speculation, we are now under intensive investigation on the precise mechanism of enzymatic cleavage of mCADM1.
According to Figure 1C and supplemental Figure S4 (available at http://ajp.amjpathol.org), sCADM1 seemed to be present in the brain at nearly constant concentrations through life after birth, suggesting possible involvement of sCADM1 in brain development. To probe how sCADM1 influences neuronal network formation, we co-cultured SCG neurons with P3U1 cells secreting either sCADM1-Fc or ΔsCADM1-Fc within collagen matrices, and found substantial effects of sCADM1 on neurite outgrowth. When SCG neurons were induced to sprout out neurites into collagen gels by nerve growth factor, the neurites preferentially extended in a confined direction toward the side exposing to a higher concentration of sCADM1. Moreover, immunofluorescence double-staining revealed that sCADM1-Fc bound mCADM1 on the surface of neurites. As we stated in the Results section, the sCADM1 concentration in the three-dimensional co-culture gels implanted with P3U1-sCADM1-Fc cells was roughly estimated to be several-fold higher than that in the brain stroma. Although future experiments are needed to explore whether such a high concentration of sCADM1 is likely to happen in vivo in particular areas or conditions, sCADM1 appeared to be a stromal protein competent to guide attractively the direction of neurite outgrowth. In conventional 2-dimentional culture, the soluble forms of NCAM and L1 are known to promote neurite elongation through binding to their membrane-bound counterparts expressed on neurons.9,24,30 In contrast, we failed to show that sCADM1 promoted neurite extension when we added it to 2-dimentional cultures as a protein fixed on the culture dish bottoms (see supplemental Figure S9 available at http://ajp.amjpathol.org). Therefore, preferable interpretation to the present data are that sCADM1 is an attractive guidance cue by serving as not an active promoter of neurite extension, but a simple anchorage to which mCADM1 on the neurites binds during neurite extension. Neurite outgrowth turning involves asymmetric plasma membrane extension and asymmetric adhesion and detachment at the neurite tip.7 An asymmetric association of sCADM1 and mCADM1 on the neurite tip may result in selective asymmetric localization of other adhesion molecules and receptor tyrosine kinases to either the sCADM1-mCADM1 binding side or the opposite side. In fact, once sCADM1 binds to mCADM1 on growing neurites, activation of phosphorylation appears to occur on tyrosine residues of neural proteins associated with mCADM1 (Figure 6). This event may be of causative importance for directional neurite extension evoked by sCADM1.
It remains to be clarified which types of cells are the main source of sCADM1 in vivo. We previously reported that cultured mast cells expressed sCADM1 mRNA.23 However, mast cells do not seem to be the only source of cells expressing sCADM1 in vivo, because RT-PCR analyses on various tissues failed to detect any differences in sCADM1 mRNA expression between W/Wv mast cell-deficient mice and control wild-type mice (unpublished data, A. Ito and M. Hagiyama, 2007). Consistently, our preliminary RT-PCR analyses on a variety of cell lines revealed that sCADM1 mRNA was expressed by a variety of cell types, including neurons and epithelial cells. Previously and currently, we reported that CADM1 is a novel mast and pancreatic–islet cell adhesion molecule that mediates nerve–mast cell and nerve–islet cell interaction, respectively.18,19 Development of extracellular microenvironment with sCADM1-rich stroma around mast and islet cells may be important in triggering nerve extension toward mast and islet cells and establishing cellular attachment between these cells and nerves, although it remains to be addressed whether sCADM1 may exist in other organs and tissues than brains. Nerve–mast cell interaction is of clinical importance, because it is assumed to underlie neurogenic inflammation, which is involved in pathophysiology of various diseases, such as irritable bowel syndrome and contact hypersensitivity.31,32,33 Nerve–islet cell interaction is of physiological and pathological importance, because it is implicated in hormone secretion from the islet.20 Further studies on sCADM1 and its effect on mCADM1 function will lead us to discovery of new aspects of these interactions.
Supplementary Material
Footnotes
Address reprint requests to Akihiko Ito, Division of Molecular Pathology, Department of Cancer Biology, Institute of Medical Science, The University of Tokyo, 4-6-1, Shirokanedai, Minato-ku, Tokyo 108-8639, Japan. E-mail: aito@ims.u-tokyo.ac.jp.
Supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Sankyo Foundation of Life Science, and Japan Diabetes Foundation.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Ito K, Hirata T. Netrin 1 regulates ventral tangential migration of guidepost neurons in the lateral olfactory tract. Development. 2006;133:845–853. doi: 10.1242/dev.02257. [DOI] [PubMed] [Google Scholar]
- Ito K, Kawasaki T, Takashima S, Matsuda I, Aiba A, Hirata T. Semaphorin 3F confines ventral tangential migration of lateral olfactory tract neurons onto the telencephalon surface. J Neurosci. 2008;28:4414–4422. doi: 10.1523/JNEUROSCI.0372-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Giancotti FG. Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle. 2005;4:e131–e135. [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM. To move or not to move? Semaphorin signalling in cell migration. EMBO Rep. 2004;5:356–361. doi: 10.1038/sj.embor.7400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16:52–58. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Doherty P, Williams G, Williams EJ. CAMs and axonal growth: a critical evaluation of the role of calcium and the MAPK cascade. Mol Cell Neurosci. 2000;16:283–295. doi: 10.1006/mcne.2000.0907. [DOI] [PubMed] [Google Scholar]
- Secher T: Soluble NCAM. Neurochem Res 2008, in press [DOI] [PubMed] [Google Scholar]
- Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, Postina R, Fahrenholz F, Fogel M, Lemmon V, Altevogt P. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol. 2001;155:661–673. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T, Schulte M, Ludwig A, Rose-John S, Blobel C, Hartmann D, Altevogt P, Saftig P, Reiss K. L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol Cell Biol. 2005;25:9040–9053. doi: 10.1128/MCB.25.20.9040-9053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Ohashi K, Mizuno K, Iseki S. Cloning and characterization of a novel mouse immunoglobulin superfamily gene expressed in early spermatogenic cells. Mol Reprod Dev. 2001;60:158–164. doi: 10.1002/mrd.1072. [DOI] [PubMed] [Google Scholar]
- Shingai T, Ikeda W, Kakunaga S, Morimoto K, Takekuni K, Itoh S, Satoh K, Takeuchi M, Imai T, Monden M, Takai Y. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J Biol Chem. 2003;278:35421–35427. doi: 10.1074/jbc.M305387200. [DOI] [PubMed] [Google Scholar]
- Kuramochi M, Fukuhara H, Nobukuni T, Kanbe T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura T, Sekiya T, Reeves RH, Murakami Y. TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer. Nat Genet. 2001;27:427–430. doi: 10.1038/86934. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Südhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Koami H, Ariga H, Kobayashi D, Sai Y, Tsuji A, Yamamoto M, Iseki S. Expression and functional characterization of the adhesion molecule spermatogenic immunoglobulin superfamily in the mouse testis. Biol Reprod. 2003;68:1755–1763. doi: 10.1095/biolreprod.102.012344. [DOI] [PubMed] [Google Scholar]
- Ito A, Okada M, Uchino K, Wakayama T, Koma Y, Iseki S, Tsubota N, Okita Y, Kitamura Y. Expression of the TSLC1 adhesion molecule in pulmonary epithelium and its down-regulation in pulmonary adenocarcinoma other than bronchioloalveolar carcinoma. Lab Invest. 2003;83:1175–1183. doi: 10.1097/01.lab.0000081391.28136.80. [DOI] [PubMed] [Google Scholar]
- Ito A, Jippo T, Wakayama T, Morii E, Koma Y, Onda H, Nojima H, Iseki S, Kitamura Y. SgIGSF: a new mast-cell adhesion molecule used for attachment to fibroblasts and transcriptionally regulated by MITF. Blood. 2003;101:2601–2608. doi: 10.1182/blood-2002-07-2265. [DOI] [PubMed] [Google Scholar]
- Ito A, Hagiyama M, Mimura T, Matsumoto M, Wakayama T, Iseki S, Yokozaki H, Okada M. Expression of cell adhesion molecule 1 in malignant pleural mesothelioma as a cause of efficient adhesion and growth on mesothelium. Lab Invest. 2008 May;88:504–514. doi: 10.1038/labinvest.2008.15. [DOI] [PubMed] [Google Scholar]
- Koma Y, Furuno T, Hagiyama M, Hamaguchi K, Nakanishi M, Masuda M, Hirota S, Yokozaki H, Ito A. Cell adhesion molecule 1 is a novel pancreatic-islet cell adhesion molecule that mediates nerve-islet cell interactions. Gastroenterology. 2008;134:1544–1554. doi: 10.1053/j.gastro.2008.01.081. [DOI] [PubMed] [Google Scholar]
- Furuno T, Ito A, Koma Y, Watabe K, Yokozaki H, Bienenstock J, Nakanishi M, Kitamura Y. The spermatogenic Ig superfamily/synaptic cell adhesion molecule mast-cell adhesion molecule promotes interaction with nerves. J Immunol. 2005;174:6934–6942. doi: 10.4049/jimmunol.174.11.6934. [DOI] [PubMed] [Google Scholar]
- Biederer T. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics. 2006;87:139–150. doi: 10.1016/j.ygeno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Koma Y, Ito A, Wakayama T, Watabe K, Okada M, Tsubota N, Iseki S, Kitamura Y. Cloning of a soluble isoform of the SgIGSF adhesion molecule that binds the extracellular domain of the membrane-bound isoform. Oncogene. 2004;23:5687–5692. doi: 10.1038/sj.onc.1207761. [DOI] [PubMed] [Google Scholar]
- Sugawa M, Ono K, Yasui Y, Kishi T, Tsumori T. Enhancement of neurite outgrowth by the soluble form of human L1 (neural cell adhesion molecule). Neuroreport. 1997;8:3157–3162. doi: 10.1097/00001756-199709290-00030. [DOI] [PubMed] [Google Scholar]
- Naus S, Richter M, Wildeboer D, Moss M, Schachner M, Bartsch JW. Ectodomain shedding of the neural recognition molecule CHL1 by the metalloprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J Biol Chem. 2004;279:16083–16090. doi: 10.1074/jbc.M400560200. [DOI] [PubMed] [Google Scholar]
- Koma Y, Ito A, Watabe K, Kimura SH, Kitamura Y. A truncated isoform of the PP2A B56γ regulatory subunit reduces irradiation-induced Mdm2 phosphorylation and could contribute to metastatic melanoma cell radioresistance. Histol Histopathol. 2004;19:391–400. doi: 10.14670/HH-19.391. [DOI] [PubMed] [Google Scholar]
- Ito A, Kataoka TR, Watanabe M, Nishiyama K, Mazaki Y, Sabe H, Kitamura Y, Nojima H. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J. 2000;19:562–571. doi: 10.1093/emboj/19.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodian P. A new method for staining nerve fibers and nerve ending in mounted paraffin sections. Anat Rec. 1936;65:89–96. [Google Scholar]
- Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon V, Farr KL, Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. The mast cell: a neuroimmunoendocrine master player. Int J Tissue React. 1996;18:1–21. [PubMed] [Google Scholar]
- Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Ito A, Hagiyama M, Oonuma J. Nerve-mast cell and smooth muscle-mast cell interaction mediated by cell adhesion molecule-1. CADM1 J Smooth Muscle Res. 2008;44:83–93. doi: 10.1540/jsmr.44.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.