Abstract
Prostaglandin E2 is one of several eicosanoid products of the cyclooxygenase isozymes and is a key regulator of innate immune responses; it also possesses paracrine effects on mature neurons. The prostaglandin E2 receptor family consists of four subtypes of which EP1 and EP2 are known to be expressed by microglia. Lipopolysaccharide (LPS)-induced innate immune activation leads to the degeneration of intermediate progenitor cells (IPCs) that are destined for neuronal maturation in the hippocampal subgranular zone (SGZ); these cells can be identified by the expression of the transcription factor T-box brain gene 2 (Tbr2). Importantly, depletion of LPS-induced IPCs from the SGZ is suppressed by cyclooxygenase inhibitors. We therefore tested the hypothesis that either EP1 or EP2 is critical to LPS-induced depletion of Tbr2+ IPCs from the SGZ. Expression of either EP1 or EP2 was necessary for Toll-like receptor 4-dependent innate immune-mediated depletion of these Tbr2+ IPCs in mice. Moreover, EP1 activation was directly toxic to murine adult hippocampal progenitor cells; EP2 was not expressed by these cells. Finally, EP1 modulated the response of murine primary microglia cultures to LPS but in a manner distinct from EP2. These results indicate that prostaglandin E2 signaling via either EP1 or EP2 is largely to completely necessary for Toll-like receptor 4-dependent depletion of IPCs from the SGZ and suggest further pharmacological strategies to protect this important neurogenic niche.
Activation of innate immune response in diseased regions of the central nervous system has been associated with aging as well as several degenerative diseases including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, traumatic brain injury, and HIV encephalitis.1 Central to this response are microglia that can have both beneficial and deleterious effects on mature neurons2: beneficial actions include elaboration of neurotrophic factors and phagocytosis of neurotoxic peptides; deleterious actions, such as secretion of free radicals, culminate in paracrine damage to neurons. An important regulator of the microglial innate immune response is prostaglandin (PG) E2, which derives from the combined actions of cyclooxygenases (COXs) and specific PG synthases; for PGE2 these are the constitutive cytosolic (cPGES) and the inducible membranous (mPGES-1) forms. PGE2 is different from other eicosanoids in having a family of four G-protein-coupled receptors that are linked to functionally antagonistic second messenger systems; these receptors are called EP1-4.3 All four EP receptors are expressed in rodent brain where their cellular and regional distribution varies widely.4 We and others have investigated EP2 receptor subtypes in mechanisms of microglia-mediated paracrine injury to mature primary culture neurons from innate immune activation by lipopolysaccharide (LPS) or amyloid (A) β42,5,6,7 injury to mature neurons in vivo following intracerebroventricular (ICV) injection of low dose LPS,8,9 and injury to mature neurons in transgenic mouse models of cerebral Aβ deposition or amyotrophic lateral sclerosis.10,11 EP receptor subtypes also have been investigated in the context of rodent models of cerebral ischemia. For example, EP1−/− mice or pharmacological inhibition of EP1 signaling rescues cerebrum in a model of transient focal ischemia.12,13,14 A broad interpretation of these many studies is that microglial EP2 is necessary for microglial activation and paracrine damage to neurons following innate immune activation from a variety of stimuli, while neuronal EP1 renders mature neurons more vulnerable to ischemic injury.
An emerging concept in brain pathophysiology is that intermediate-stage progenitor cells (IPCs) may be especially vulnerable to pathological stressors, and that depletion of this critical reservoir of cells may contribute significantly to dysfunction and degeneration.15 Indeed, we have shown that IPCs in the oligodendroglial lineage are especially vulnerable to hypoxic/ischemic injury of newborns.16 A similar situation may exist for IPCs in the neuronal lineage that reside within specialized brain regions, known as neurogenic niches, that include the subgranular zone (SGZ) and the subventricular zone.17,18,19 Expression of transcription factors is now being used to identify the different proliferating cell populations in adult mouse SGZ neurogenesis; indeed the sequential expression of these transcription factors precisely recapitulates that observed in embryonic neocortical neurogenesis.20,21,22,23,24 Among this cascade of transcription factors, Tbr2+ IPCs have been shown to play a major role in adult hippocampal neurogenesis22,23,24 Unlike the data reviewed above for mature neurons, we are unaware of any data investigating contributions of EP receptor subtypes to cellular functioning in the neurogenic niche.
The cellular and extracellular elements that comprise neurogenic niches not only support progenitor cells structurally but also regulate their activity and development.25,26,27,28,29 Until recently, microglial cells have been ignored as part of the environment that could affect the proliferation and differentiation of neuron progenitor cells.30 However, recent studies using LPS to specifically activate microglial innate immune response, presumably via CD14 and Toll-like receptor 4 (TLR4) coreceptors, have shown up to 85% reduction in newly born adult neurons without suppression of their proliferation or alteration of their maturation, but with increased deoxynucleotidyl transferase-mediated dUTP nick end-labeling staining; these findings have been interpreted as degeneration of SGZ IPCs following innate immune activation with LPS, and some of these studies identified specific cytokines that have pro- or anti-neurogenic effects.30,31,32,33,34,35 Interestingly, SGZ IPCs that survive the inflammatory stress induced by LPS can differentiate and integrate as granule neurons, although with a heightened degree of synaptic plasticity.36 Importantly, LPS-induced microglial activation and subsequent depletion of SGZ IPCs can be blocked by indomethacin,33 an inhibitor of COX-1 and COX-2, as well as NS398,35 a relatively COX-2-selective inhibitor, strongly suggesting involvement of the PG pathway, although other pharmacological activities have been ascribed to these drugs in addition to COX isozyme inhibition.37 The studies presented here tested the hypothesis that paracrine depletion of SGZ IPCs by innate immune activation is critically regulated by EP1 or EP2 expression.
Materials and Methods
Mice
All experiments were done with approval of the University of Washington Institutional Review Board. Mice homozygous-deficient (−/−) for EP1 or EP2 were produced by us and have been maintained for over 15 generations on the C57Bl/6 strain. TLR4−/− mice were a generous gift from Dr. Francis Kim (University of Washington) and also were maintained on the C57Bl/6 background. Polymerase chain reaction determination of mouse genotype followed published protocols.9 Mice were housed in a vivarium with 12 hours:12 hours light:dark cycle and ad libitum access to food and water. All experiments were initiated with 12-week-old mice except the experiment with TLR4−/− mice and their controls, which was performed on 6-week-old mice.
Cell Cultures
Primary microglia were derived from the cerebral cortex of postnatal (P1-3) C57Bl/6 mice as previously described.5,6 Briefly, blood vessels and meninges were removed while in ice-cold Dulbecco’s modified Eagle’s medium, the cortical tissue incubated for 30 minutes at 37°C in Dulbecco’s modified Eagle’s medium, 0.2 mg/ml l-cysteine, 0.5 mmol/L EDTA, 15U/ml papain (Worthington Biochemical, Lakewood, NJ), and 200 μg/ml DNase I (Worthington Biochemical), washed with warm Dulbecco’s modified Eagle’s medium, triturated with culture medium (Dulbecco’s modified Eagle’s medium-F12, 10% fetal bovine serum, 100 U/ml penicillin, and 100 g/ml streptomycin), and plated on a polyornithine (Sigma-Aldrich, St. Louis, MO) bottom-coated T-175 flask (Sarstedt, Newton, NC). Cell cultures were kept in a 37°C incubator with 5% CO2 and 95% air. The culture medium was changed the day following harvest and again 6 to 7 days later. At 14 to 17 days in vitro, microglia were separated from the underlying astrocytic monolayer by gentle agitation and dispersed as needed. Purity of microglia was checked by cytochemistry for CD11b and was greater than 98%.
Primary hippocampal neuronal progenitor cells were isolated according to established protocols.38 Briefly, 8- to 12-week-old C57Bl6 wild-type, EP1−/−, and EP2−/− mice were anesthetized with Avertin and transcardially perfused with ice cold saline. The brains were removed and the hippocampus dissected away from overlying cortex. The tissue was enzymatically digested with collagenase and DNase, dissociated by gentle trituration, and the cells separated using a Percoll gradient. Progenitors were grown in Dulbecco’s modified Eagle’s medium-F12 (1:1) media supplemented with 2 mmol/L l-glutamine, N-2 supplement (Invitrogen, Carlsbad, CA), 50 μg/ml heparin (Invitrogen), and 20 ng/ml epidermal growth factor (Peprotech, Rocky Hill, NJ) and basic fibroblast growth factor (Peprotech). Neuronal differentiation was achieved by plating cells on laminin-coated glass chamber slides (LabTek, Rochester, NY) in neurobasal media with B27 and 50 ng/ml brain-derived neurotrophic factor (Peprotech) for 1 week. Neuronal differentiation was confirmed by morphology and immunofluorescence (monoclonal anti-Tuj-1, 1:400; Sigma).
Cell Survival and Proliferation Assays
Wild-type, EP1−/−, and EP2−/− neuronal progenitor cells (NPCs) between passage 8 and 12 were used for proliferation and survival assays. Fifty thousand NPCs were plated per well in 96-well plates in the presence of variable concentrations of LPS, 16-phenyl-tetranor-PGE2 (16-PT-PGE2; Caymen, Ann Arbor, MI), interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (Peprotech). Exposure to vehicle only or cytosine arabinoside (Ara-C; Sigma) was used as a negative or positive control. Cells were incubated at 37°C for 24 hours and then analyzed for cell survival and proliferation using an in vitro toxicology lactate dehydrogenase-based assay kit (Sigma) and Cell Titer96 aqueous non-radioactive cell proliferation assay protocol (Promega, Madison, WI) according to supplied protocols and absorbance measured with a SpectraMax 96-well plate reader.
Innate Immune Activation
LPS from Escherichia coli O55:B5 (Calbiochem, San Diego, CA) was prepared in phosphate-buffered saline (PBS), pH 7.4, at a final concentration of 1.0 mg/ml as previously described.5,6 Anesthesia was induced and maintained with isoflurane. Mice were placed in a stereotactic frame and a sterile 1-cm scalp incision was made. A burr hole was drilled at the following coordinates in relation to bregma (caudal 0.6 mm, right 1.5 mm, ventral 2.0 mm), with the tooth bar set to −3 mm. After cannula placement, 1 μl of LPS solution was injected per minute. The cannula was slowly withdrawn at a rate of 1 mm per minute. A total of 2 μg of LPS was injected into the ventricle according to our well-established methods.9,39
Protein Determinations
Protein concentrations in cell lysates were determined by Bio-Rad protein assay (Bio-Rad, Hercules, CA) using a cellular lysis buffer (50 mmol/L Tris, 150 mmol/L NaCl, 0.02% NaN3, 0.5% Nonidet P-40, 0.5% Triton X-100, and protease inhibitor mixture). Standardized amounts of protein from cell lysates were separated by Tris-HCl sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to membrane, blocked, and probed with antibodies. Enhanced chemiluminescence was used for detection. Corresponding bands were quantified with Image J (National Institutes of Health imaging software). Primary antibodies included goat polyclonal antibodies against COX-1, COX-2, and iNOS (Santa Cruz Biotechnology, Santa Cruz, CA), and monoclonal antibody against mPGES-1 as well as rabbit polyclonal antibodies against cPGES (Cayman).
Immunofluorescence Microscopy
BrdU injections (5 μg/g body weight) following LPS delivery were given IP 2 hours and every additional 12 hours before sacrifice for a total of two or six injections for mice sacrificed at 24 or 72 hours, respectively. Mice were anesthetized with Avertin (0.02 ml/g body weight intraperitoneally) and perfused with cold buffered 4% paraformaldehyde, as described.22,40,41 Brains were removed, postfixed in the same fixative overnight at 4°C, cryoprotected with sucrose (10%, 20%, 30%), and embedded in OCT. Brains were cryosectioned in the coronal plane on a freezing sliding microtome at 40 μm thickness, and free-floating sections were processed for immunofluorescence microscopy. Primary antibodies were mouse monoclonal anti-BrdU (1:400; Chemicon, Bellerica, MA), rabbit-anti Iba-1 (1:400; Wako, Richmond, VA), and anti-Tbr2 (1:2000; Hevner Laboratory22). Secondary antibodies (fluorescent conjugates of Alexa 488 or Alexa 594) were purchased from Invitrogen and used at 1:200 dilution. Double-label immunofluorescence followed methods from previous work in our laboratory.22,40,41 Cells were counted using the modified optical dissector method as previously described by us.24 Briefly, counts were obtained using an AxioImager epifluorescence microscope and Axiovision 4.6 software (Zeiss, Thornwood, NY) with a modified stereological procedure, where cells were excluded from counts if they intersected the top focal plane of the section, as described previously.42,43,44 Cells were counted on every sixth 40-μm coronal section through the dentate gyrus, and the sum of these counts was multiplied by 6 to generate estimates of absolute numbers.
Real-Time Polymerase Chain Reaction
Total RNA was collected using the Qiagen RNeasy Protect Cell Mini Kit and quality checked by gel electrophoresis. cDNA was generated in an Eppendorf Mastercycler using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). FAM dye-labeled TaqMan gene expression assays (Applied Biosystems) for murine transcripts were used for quadruplicate runs of triplicate cultures with an ABI 7500 real-time polymerase chain reaction instrument (SDS v1.3.1). The comparative threshold cycle method was used to determine relative message abundance.
Results
Real-time polymerase chain reaction was performed to assess relative expression of transcripts important to PGE2 signaling, LPS signaling, and Tbr2 in mouse hippocampus following ICV injection (Table 1). As expected, all of the assayed transcripts were detected at varying levels in mouse hippocampus 2 hours after ICV vehicle (PBS) injection. Further, response to ICV LPS produced the anticipated outcomes of increased expression of COX-2, mPGES-1, and CD14, but not cPGES or COX-1. Interestingly, TLR4 expression was not changed by ICV LPS. Importantly, ICV LPS also did not induce a change in expression of EP1, EP2, or Tbr2.
Table 1.
Real-Time Polymerase Chain Reaction Results for Mouse Hippocampus and aHPCs
| Wild-type mouse hippocampus 2 hours after injection
|
Wild-type mouse aHPCs (ΔCt) | ||
|---|---|---|---|
| ΔCt for ICV PBS | ΔCt(ICV LPS)/ΔCt(ICV PBS) | ||
| mPges-1 | 10.5 ± 0.1 | 3.0 ± 0.6* | 16.1 ± 0.2 |
| cPges | 7.7 ± 0.1 | 1.1 ± 0.1 | 6.1 ± 0.1 |
| ptger1 (EP1) | 13.8 ± 0.1 | 1.1 ± 0.3 | 16.5 ± 0.6 |
| ptger2 (EP2) | 14.9 ± 0.3 | 0.9 ± 0.3 | Not detected |
| ptgs1 (COX-1) | 7.9 ± 0.1 | 0.9 ± 0.2 | 19.4 ± 0.1 |
| ptgs2 (COX-2) | 10.2 ± 0.2 | 4.4 ± 0.9* | 17.7 ± 0.2 |
| CD14 | 10.6 ± 0.2 | 4.9 ± 1.1* | 14.4 ± 0.1 |
| TLR4 | 12.4 ± 0.1 | 1.0 ± 0.2 | 10.5 ± 0.1 |
| Eomes (Tbr2) | 13.0 ± 0.4 | 0.9 ± 0.3 | 12.4 ± 0.1 |
Data are means ± SEM for quadruplicate experiments measured in triplicate; ΔCt, Ct(gene) − Ct(GAPDH) in the same sample.
P < 0.05 compared with unity.
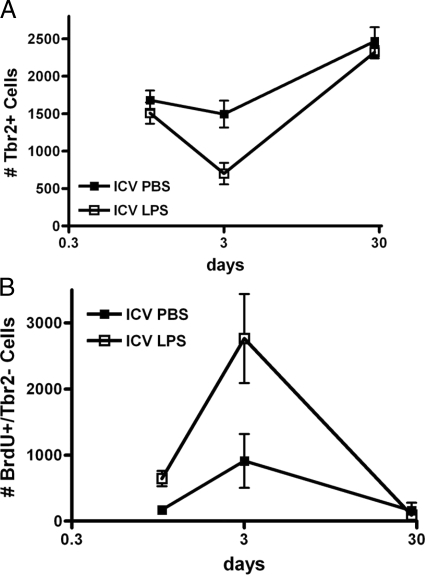
Our next series of experiments investigated directly the outcomes of LPS-activated innate immune activation on SGZ Tbr2+ IPCs. Untreated wild-type mice that did not receive any ICV injection had 1540 ± 118 (n = 4) subventricular zone Tbr2+ cells at 12 weeks of age. This was not different from ICV PBS mice of the same age either 1 or 3 days after injection (Figure 1A). An increase in Tbr2+ cells was observed in vehicle-treated mice after 4 weeks, presumably due to stressors caused by ICV injection. Indeed, others have shown an even larger delayed rebound in the number of SGZ Tbr2+ cells following exposure to antimitotic agents.24 In contrast, Tbr2+ cells showed a significant reduction in number 3 days after ICV LPS that followed a time course similar to spinodendritic degeneration of mature hippocampal pyramidal neurons following ICV LPS as previously described8 (Figure 1A). This Tbr2+ IPC depletion was not present 1 day after ICV LPS and the difference from vehicle-treated was transient, since the number of SGZ Tbr2+ cells was not significantly different between the two groups 4 weeks after ICV injection. With the BrdU labeling protocol used here, 95% labeling of Tbr2+ cells was achieved; therefore, data for Tbr2+/BrdU+ double-labeled cells (not shown) were not significantly different from Tbr2+ counts shown. A merged immunofluorescence photomicrograph of SGZ Tbr2+ and BrdU+ cells from a wild-type mouse 72 hours after ICV PBS is presented in Figure 2A.
Figure 1.
Mice received ICV LPS or vehicle (PBS) followed by BrdU injections (5 μg/g body weight intraperitoneally) at 2 hours and every additional 12 hours before sacrifice. Brains were fixed, stained, and counted as described in Materials and Methods. Data are number of SGZ cells at 24 hours, 72 hours, and 28 days after ICV injection of wild-type mice with PBS or LPS. A: Two-way analysis of variance for number of SGZ Tbr2+ cells had P < 0.01 for ICV PBS versus ICV LPS, P < 0.0001 for time, and P < 0.05 for interaction. Bonferroni-corrected post-tests comparing ICV PBS versus ICV LPS had P < 0.01 for 72 hours but P > 0.5 for 24 hours and 28 days after injection. B: Two-way analysis of variance for number of SGZ BrdU+/Tbr2− cells had P < 0.01 for ICV PBS versus ICV LPS, P < 0.0001 for time, and P < 0.05 for interaction. Bonferroni-corrected post-tests comparing ICV PBS versus ICV LPS had P < 0.01 for 72 hours but P > 0.5 for 24 hours and 28 days after injection.
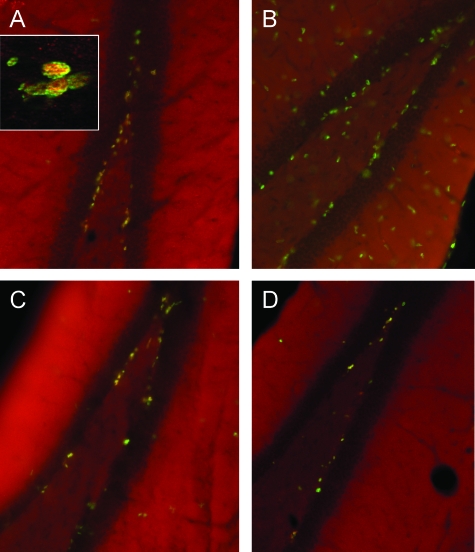
Figure 2.
Photomicrographs of mouse hippocampus 72 hours after ICV injection with vehicle (PBS) or LPS. Images are merged from immunofluorescence detection of Tbr2 (red) and BrdU (green). Yellow (colocalization, see inset) indicates a proliferative Tbr2+ cell while green indicates a proliferative Trb2− cell. The dark band is the dentata fascia. A: Wild-type mouse following ICV PBS. Tbr2 and BrdU are primarily colocalized as yellow SGZ nuclei with few Tbr2− proliferative cells. B: Wild-type mouse following ICV LPS. In contrast to A, note depletion of Tbr2+ cells and increase in Tbr2− BrdU+ (green) cells in SGZ and elsewhere, indicating a proliferative response among Tbr2− cells. C: EP1−/− mouse following ICV LPS. Tbr2+ cells were protected and appeared similar to A, accompanied by only partial increase in proliferative response among Tbr2− cells. D: EP2−/− mouse following ICV LPS. Tbr2+ cells were in between A and B, suggesting partial protection, with no apparent increase in Tbr2− cell proliferative response. Magnifications, ×20; inset, ×400.
While >95% of SGZ Tbr2+ IPCs were also BrdU+, there was a population of SGZ BrdU+ cells that were not immunoreactive for Tbr2 (Tbr2−) (Figure 1B). In contrast to depletion of SGZ Tbr2+ cells following ICV LPS, a proliferative response in SGZ BrdU+/Tbr2− cells was observed 1 day after ICV LPS that increased dramatically by 3 days and that then returned to baseline by 4 weeks after injection. Figure 2B shows a merged Tbr2+ and BrdU+ immunofluorescence photomicrograph 72 hours after ICV LPS in a wild-type mouse. The majority (83 ± 6%) of these BrdU+/Tbr2− cells were Iba1-immunoreactive. Others already have shown microglial proliferation in the SGZ accompanies LPS exposure.32,33,35 Finally, the data from ICV PBS mice support the proposal that this control experiment did induce a low level response to injection. In summary, these results showed that, in contrast to mature hippocampal pyramidal neurons, the number of SGZ Tbr2+ IPCs was reduced following innate immune activation with ICV LPS. Moreover, SGZ Tbr2+ IPC depletion is roughly coincident with proliferation of other cell types in the SGZ, which are mostly microglia.
LPS is natural product purified from bacteria with varying degrees of purity among commercial preparations. This is significant because the impurities can be inflammogens that activate pathways other than those described for LPS. Moreover, LPS can activate cells via TLR4-dependent and -independent pathways.45 We are unaware of any previous investigation of in vivo neurogenesis using the LPS model that has controlled for this potentially important variable. Therefore, the number of SGZ Tbr2+ cells in 6-week-old TLR4−/− mice or wild-type controls 72 hours after ICV injection was determined. As expected, untreated 6-week-old C57Bl/6 mice had more SGZ Tbr2+ cells (2045 ± 175) than the 12-week-old mice described above. Relative to ICV PBS wild-type mice, the percentages of SGZ Tbr2+ cells were: 86 ± 9% for TLR4−/− ICV PBS (P > 0.05), 105 ± 8% for TLR4−/− ICV LPS (P > 0.05), and 26 ± 4% for wild-type ICV LPS (P < 0.001). These results show that expression of TLR4 was necessary for ICV LPS-induced reduction in SGZ Tbr2+ cells.
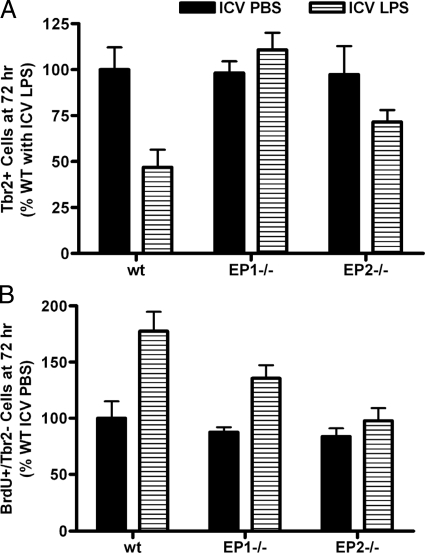
Our next series of experiments determined the contribution of EP receptor subtypes to the ICV LPS-induced changes in the SGZ described above in 12-week-old mice. It was observed that lack of EP1 expression completely protected SGZ Tbr2+ IPCs 3 days postactivation of innate immunity with ICV LPS (Figures 2C and 3A). Mice lacking EP2 also had SGZ Tbr2+ cell numbers following ICV LPS that were not significantly different from ICV PBS; however the number of Tbr2+ cells in EP2−/− 72 hours after ICV LPS was in between control values and wild-type mice exposed to ICV LPS, suggesting only partial protection of Tbr2+ cells in EP2−/− mice (Figure 2D). The sensitive but nonspecific assessment of glial reaction to ICV injection showed that EP1−/− mice had partially reduced SGZ Tbr2− cell proliferative reaction to ICV LPS, while EP2−/− mice had no Tbr2− cell proliferative reaction to ICV LPS (Figures 2C, 2D, and 3B). We interpret these data as suggesting complete suppression of response to LPS by microglia, and perhaps other cell types as well, in EP2−/− mice and partial suppression in EP1−/− mice.
Figure 3.
Data are SGZ cell counts from wild-type (wt), EP1−/−, and EP2−/− mice 72 hours after ICV injection presented as percentage of wild-type ICV PBS values (corresponding absolute numbers are in Figure 1). A: Two-way analysis of variance for SGZ Tbr2+ cells had P < 0.05 for ICV PBS versus ICV LPS, genotype, and interaction between these two terms. Bonferroni-corrected post-tests comparing ICV PBS versus ICV LPS had P < 0.01 for wild type but P > 0.05 for EP1−/− and EP2−/−. B: Two-way analysis of variance for SGZ BrdU+/Tbr2− cells at 72 hours after ICV injection had P < 0.001 for LPS versus PBS, P < 0.01 for genotype, and P > 0.05 for interaction. Bonferroni-corrected post-tests comparing ICV PBS versus ICV LPS had P < 0.001 for wild type, P < 0.05 for EP1−/−, and P > 0.05 for EP2−/− mice.
Since EP1 and EP2 are both expressed on mature neurons and microglia, the next step was to determine which cell type might be responsible for the IPC protective effects observed in vivo. To achieve this goal, primary murine cultures of adult hippocampal progenitor cells (aHPCs) were prepared. It was determined by real-time polymerase chain reaction that wild-type aHPCs expressed comparable relative levels of the assayed transcripts as murine adult hippocampus, except for EP2 that was not detected in wild-type aHPCs (Table 1). Although Tbr2 was expressed by aHPCs, it is important to stress that these cultures are not composed exclusively of Tbr2+ progenitor cells and so cannot be compared directly to the in vivo results shown above. Others already have shown that TLR4 is expressed by murine neural stem/progenitor cells in culture.46
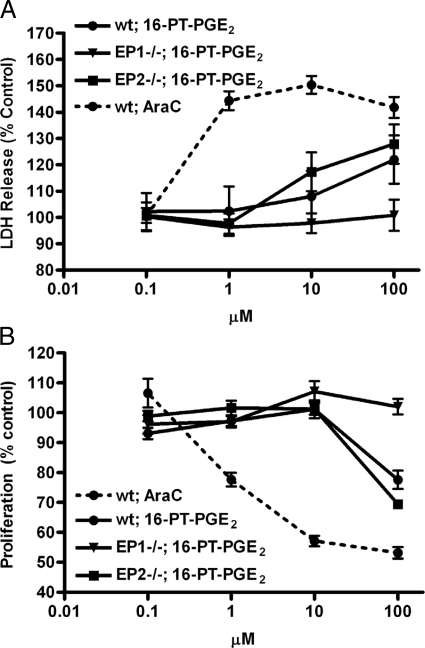
aHPCs from wild-type, EP1−/−, or EP2−/− mice were exposed to varying concentrations of a metabolically stable PGE2 analog, 16-phenyl-tetranor-PGE2 (16-PT-PGE2), or LPS. Cell death was estimated by LDH release (Figure 4A) and proliferation by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay (Figure 4B). Cultures of all three genotypes were exposed to AraC as a positive control; all three types of cultures gave similar results with AraC, so only the data for wild-type mice are shown. EP2−/− aHPCs behaved as wild-type aHPCs in response to 16-PT-PGE2 in both the cell death and proliferation assays; this is not surprising since EP2 expression was not detected in wild-type aHPCs. In contrast, EP1−/− aHPCs were unresponsive to 16-PT-PGE2 in both assays. We interpret these data as showing that expression of EP1 on aHPCs is responsible for direct cytotoxic effects of increasing concentrations of PGE2 in cell culture. Despite expression of CD14 and TLR4, LPS (0.001 to 1 μg/ml) exposure for 24 hours did not significantly alter LDH release or MTT conversion relative to PBS-treated controls in aHPCs from wild-type, EP1−/−, or EP2−/− mice (not shown).
Figure 4.
Adult hippocampal progenitor cells (aHPCs) were prepared from wild-type (wt), EP1−/−, and EP2−/− mice and then exposed to a metabolically stable analog of PGE2, 16-PT-PGE2, or cytosine arabinoside (AraC) for 24 hours before estimating cell death by LDH release (A) or proliferation by MTT assay (B). Data are presented as percentage of vehicle-exposed controls. A: AraC caused a significant increase in LDH release at 1 μmol/L (P < 0.0001) that was not further changed (P < 0.05 compared with 1 μmol/L) at 10 and 100 μmol/L. Exposure to 16-PT-PGE2 yielded concentration-dependent increase in LDH release from 1 to 100 μmol/L that was not significantly different between wild-type and EP2−/− aHPCs. Exposure of EP1−/− aHPCs to 16-PT-PGE2 up to 100 μmol/L did not significantly change LDH release at any concentration compared with 0.1 μmol/L (P > 0.05). B: AraC caused a concentration-dependent decrease in MTT conversion from 0.1 to 10 μmol/L that was not further significantly changed at 100 μmol/L. Exposure to 100 μmol/L 16-PT-PGE2 (but not lower concentrations) decreased MTT conversion in wild-type and EP2−/− aHPCs (P < 0.001) but not EP1−/− aHPCs.
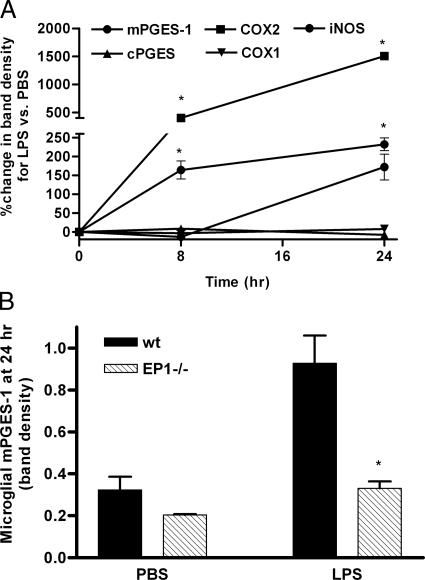
While aHPCs were not responsive to LPS, the in vivo data for BrdU+/Tbr2− cells confirmed that microglia responded to ICV LPS. We already have published that primary mouse microglia that lack EP2 have completely suppressed paracrine neurotoxicity following activation by LPS, and that primary microglia from EP2−/− mice do not induce COX-2 or iNOS in response to LPS exposure.6 The in vivo results with BrdU+/Tbr2− cells presented in Figure 2 are consistent with these previous findings. Others have published limited data suggesting that primary mouse microglia that lack EP1 are activated by LPS in culture similar to wild-type microglia.12 However, the in vivo data from BrdU+/Tbr2− cells in EP1−/− mice suggested partial suppression of microglial response to LPS in these mice. Therefore, the role of EP1 in microglial activation by LPS was explored more extensively by determining cellular levels of enzymes involved in PGE2 synthesis and iNOS at 8 and 24 hours after incubation with LPS. As expected, COX-1 and cPGES protein levels were unchanged, while levels of COX-2, mPGE-1, and iNOS were induced by LPS exposure of primary wild-type mouse microglia (Figure 5A). Of these five enzymes, only mPGES-1 was altered in EP1−/− microglia where its induction was significantly suppressed compared with wild-type microglia (Figure 5B); this is distinct from EP2−/− primary microglia that do not induce COX-2 or iNOS in response to LPS.6 Finally, medium concentrations of selected cytokines (IL-1β, tumor necrosis factor-α, IL-6, and MCP-1) secreted by primary microglia following activation by LPS were determined. Medium concentrations of IL-6 were lower in EP1−/− primary microglia cultures at 8 (Figure 6A) and 24 (not shown) hours after LPS compared with wild type. Medium concentrations of MCP-1, which were undetectable at 8 hours, also were significantly lower in EP1−/− primary microglia compared with wild type after 24 hours of incubation with LPS (Figure 6B). Medium concentrations of IL-1β and tumor necrosis factor-α were not different between wild-type and EP1−/− cultures at 8 and 24 hours (not shown), similar to EP2−/− primary microglia.6 We conclude that EP1 does play an innate immune-modulatory role in murine microglia but that it is different from EP2.
Figure 5.
A: Time course for induction of enzymes involved in PGE2 production and iNOS in primary wild-type (wt) mouse microglia following incubation with LPS. Data are expressed as percent change in Western blot band density for LPS exposure versus PBS exposure. Results showed expected increase in expression of COX-2, iNOS, and mPGES-1 and expected lack of change in expression of COX-1 or cPGES in wild-type microglia exposed to LPS. B: Primary wild-type and EP1−/− microglia were exposed to LPS as in A; data are presented as Western blot band density. Among the five proteins investigated in A, only changes in mPGES-1 were significantly different between wild-type and EP1−/− microglia. Two-way analysis of variance for mPGES-1 at 24 hours had P < 0.0001 for exposure, P < 0.0001 for genotype, and P < 0.01 for interaction. Bonferroni-corrected post-tests had *P < 0.001 for LPS exposed wild type versus EP1−/− at 24 hours.
Figure 6.
Data are medium concentrations of secreted IL-6 (A) or MCP-1 (B) by primary wild-type (wt) or EP1−/− microglia determined 8 and 24 hours of incubation with LPS or PBS. LPS-stimulated secretion of both cytokines was suppressed by ablation of EP1. A: Two-way analysis of variance for medium IL-6 at 8 hours had P < 0.0001 for exposure, P < 0.0001 for genotype, and P < 0.0001 for interaction; similar results were obtained at 24 hours (not shown). Bonferroni-corrected post-tests had *P < 0.001 for LPS exposed wild type versus EP1−/−. B: Two-way analysis of variance for medium MCP-1 at 24 hours had P < 0.0001 for exposure, P < 0.001 for genotype, and P < 0.01 for interaction; MCP-1 was undetectable at 8 hours (not shown). Bonferroni-corrected post-tests had *P < 0.001 for LPS exposed wild type versus EP1−/−.
Discussion
Adult neurogenesis has generated excitement by introducing potentially new therapeutic targets for regeneration and repair of neural pathways in a number of neurological diseases including neurodegenerative diseases, stroke, epilepsy, traumatic brain injury, and cognitive impairment after radiation therapy.33,47,48,49,50,51,52,53 All of these conditions share activation of innate immunity, and others have shown that innate immune activation with LPS leads to NS398- or indomethacin-dependent depletion of newly born neurons in the SGZ.35,50 Moreover, indomethacin also enhances neurogenesis following focal cerebral ischemia.48 Since COX isozymes initiate a cascade that generates multiple PGs and eicosanoids, and since these nonsteroidal anti-inflammatory drugs have actions in addition to COX isozyme inhibition, our studies tested the hypothesis that activation of specific PGE2 receptors were responsible for innate immunity-mediated suppression of hippocampal neurogenesis. The results demonstrated that EP1 or EP2 expression is necessary for innate immunity-mediated depletion of SGZ Tbr2+ IPCs in vivo. Ablation of either EP receptor subtype provided protection to IPCs from innate immune activation. Results from primary cultures suggest a two-step model in which microglial EP2 (and perhaps EP1) expression is necessary for innate immune activation and IPC EP1 expression is necessary for paracrine toxicity.
Several laboratories have used LPS-activated innate immunity in the investigation of microglial contribution to neurogenic niches and have reported that survival of newly born neurons in the SGZ is reduced following exposure to this stressor.35,50 Classically, LPS activates a receptor complex that necessarily includes CD14 and TLR4 coreceptors.45 Indeed, we have shown previously that paracrine damage to mature hippocampal pyramidal neurons from innate immune activation with our ICV LPS protocol is completely CD14- and TLR4-dependent,8 and we now show here that depletion of SGZ Tbr2+ IPCs by ICV LPS also is TLR4-dependent. Other endogenous molecules that activate innate immunity, at least in part, via CD14- or TLR4-dependent mechanisms have been indentified recently. Indeed, with respect to models of brain disease, neuronal apoptosis induced by Aβ peptides or lipid peroxidation products,54,55 microglial activation by heat shock protein 60 released by degenerating neurons,56 enhanced cytokine secretion in a transgenic model of cerebral Aβ deposition,57,58 neuronal damage from ischemic stroke,59,60,61 and progression of prion disease62 all have been shown to be, at least in part, CD14- or TLR4-dependent. Thus, results that derive from our ICV LPS model may be relevant to these other diseases of brain that share TLR4-dependent activation. As with mature neurons, the deleterious effects observed following a single exposure to ICV LPS are reversible because the inflammogen is cleared from tissue.39 Many of the diseases with CD14- and TLR4-dependent processes present a chronic inflammatory stress and likely a more sustained depletion of SGZ IPCs, which can cause impaired cognition.63,64,65
Another group has reported TLR4 expression by murine neural stem/progenitor cells in culture and showed that exposure of these cultures to LPS reduces expression of the neuronal marker β-III tubulin.46 We confirmed expression of TLR4 in aHPCs and showed that exposure to LPS did not alter Tbr2 expression in mouse hippocampus or induce cell death or proliferation of aHPCs in culture. Moreover, although a control experiment and not a focus of this study, ICV PBS-exposed TLR4−/− mice had SGZ Tbr2+ cell counts similar to wild-type mice. Importantly, TLR4 is not expressed on mature neurons in cerebrum.66 Together with the results of others, these data suggest that SGZ TLR4 expression may be developmentally regulated and influence neurogenesis at some step distal to Tbr2 expression.
EP receptor subtypes are linked to different second messenger systems, so it is not surprising that our results indicated that ablation of EP1 or EP2 protected IPCs to varying extents or through different mechanisms. Indeed, the in vivo data showed that Tbr2+ IPCs were completely protected in EP1−/− mice and suggested that Tbr2+ IPCs were partially protected in EP2−/− mice following ICV LPS. Moreover, data with aHPCs supports a direct protective effect by EP1, but not EP2, ablation. Activation of EP1 leads to increased intracellular calcium that presents a direct stress to mature neurons.12 The data showed that EP1 was functionally expressed by aHPCs in culture since its ablation suppressed toxicity from a metabolically stable analog of PGE2. In combination with the in vivo data, these findings support blockade of EP1 as a means to provide direct protection to IPCs from processes unleashed by TLR4-dependent innate immune activation. Colocalization of EP1 with Tbr2+ cells was not pursued, since the available antibodies for EP receptor subtypes are not specific; we have reviewed this topic recently.67 In contrast to EP1, EP2 expression or activity was not detected in aHPC cultures. Since EP2 is expressed by mature neurons and appears to be localized to synapses,68 these results raise the possibility EP2 expression is developmentally regulated in neurons.
In an abbreviated series of experiments, others reported that primary EP1−/− mouse microglial respond to LPS exposure as wild-type microglia.12 The studies presented here undertook to validate these findings. Indeed, they confirmed that COX-2 and iNOS induction are no different between EP1−/− and wild-type microglia. However, suppression of mPGES-1 induction and reduced secretion of some cytokines with microglia from EP1−/− mice compared with wild type was observed. We have proposed a reinforcing cycle between EP2 and PGE2 synthesis, since Aβ42- or LPS-induced expression of COX-2 is EP2-dependent in primary murine microglia.5,6,7 The current results indicate that microglial EP1 might also participate in this cycle by regulating mPGES-1 induction while not altering induction of COX-2. Together, these results support the proposal that a component of Tbr2+ cytoprotection in EP1−/− mice was indirect from suppression of microglial activation, while all of the protective effect from EP2 ablation appears to be indirect. It is not possible to use these current mouse models to distinguish the relative importance of direct from indirect toxic actions of EP1 activation on IPCs following innate immune activation.
In summary, LPS-induced depletion of SGZ IPCs was confirmed using immunohistochemistry for Tbr2. Furthermore, LPS-induced depletion of SGZ IPCs was shown to be largely (EP2) to completely (EP1) dependent on expression of EP receptor subtypes. In addition to validating the results of others who showed that ibuprofen protects SGZ IPCs from innate immune activation, these findings critically extend our knowledge of the mechanism behind nonsteroidal anti-inflammatory drug-mediated protection, since these drugs suppress production of all COX products, including multiple PGs and other eicosanoids, as well as inhibit other pathways. Moreover, our cell culture data provide new insight into the cellular aspects of protection. Lack of EP1 appeared to protect progenitor cells directly from PGE2-mediated toxicity as well as indirectly by partially suppressing microglial activation. In contrast, EP2 was not detectably expressed by aHPCs and lack of EP2 expression did not protect from direct toxic effects of PGE2, consistent with our previous studies of mature neurons.8 These results show that suppression of PGE2 signaling protects IPCs through direct and indirect mechanisms and suggest additional therapeutic targets to protect this important pool of precursor cells from the damaging effects of TLR4-dependent innate immune activation.
Footnotes
Address reprint requests to Thomas J. Montine, M.D., Ph.D., Department of Pathology, University of Washington, Box 359791, Seattle, WA 98104. E-mail: tmontine@u.washington.edu.
Supported by National Institutes of Health grants ES007032, AG24011, NS46724, MH080766, GM15431, and DK37097 and the Nancy and Buster Alvord Endowment.
References
- Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Keene CD, Cimino PJ, Breyer RM, Montine KS, Montine TJ. E prostanoid receptors in brain physiology and disease. Lajtha A, editor. New York: Springer,; Handbook of Neurochemistry and Molecular NeurobiologyNeurotransmitter Systems. 2008:pp 385–401. [Google Scholar]
- Shie FS, Breyer RM, Montine TJ. Microglia lacking E prostanoid receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005;166:1163–1172. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 as a new target to increase amyloid beta phagocytosis and decrease amyloid beta-induced damage to neurons. Brain Pathol. 2005;15:134–138. doi: 10.1111/j.1750-3639.2005.tb00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine KS, Shie FS, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immune response in vivo. J Neuroinflammation. 2004;1:20. doi: 10.1186/1742-2094-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Milatovic D, Gupta RC, Valyi-Nagy T, Morrow JD, Breyer RM. Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem. 2002;83:463–470. doi: 10.1046/j.1471-4159.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- Liang X, Wang Q, Shi J, Lokteva L, Breyer RM, Montine TJ, Andreasson K. The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol. 2008;64:304–314. doi: 10.1002/ana.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, Andreasson K. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005;25:10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol. 2005;57:758–761. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Mallinson RA, O'Malley JP, Wallen LD, Frei B, Morrow JD, Petito CK, Roberts CT, Jr, Murdoch GH, Montine TJ. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58:108–120. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. New York: Oxford University Press,; Adult NeurogenesisStem Cells and Neuronal Development in the Adult Brain. 2006 [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, Blasco-Ibanez JM, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J Neurosci Res. 2005;81:753–761. doi: 10.1002/jnr.20596. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Takashima N, Arai Y, Nomura T, Inokuchi K, Yuasa S, Osumi N. Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neurogenesis. Genes Cells. 2005;10:1001–1014. doi: 10.1111/j.1365-2443.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6. Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF. Intermediate progenitors in adult hippocampal neurogenesis: tbr2 expression and coordinate regulation of neuronal output. J Neurosci. 2008;28:3707–3717. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA: 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos GN, Moriya T, Inui F, Katura T, Nakahata N. Involvement of cyclooxygenase-2 in lipopolysaccharide-induced impairment of the newborn cell survival in the adult mouse dentate gyrus. Neuroscience. 2008;155:454–462. doi: 10.1016/j.neuroscience.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Inflammation regulates functional integration of neurons born in adult brain. J Neurosci. 2008;28:12477–12488. doi: 10.1523/JNEUROSCI.3240-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98:736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine KS, Horner PJ, Montine TJ. Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J Neurochem. 2003;87:1518–1526. doi: 10.1046/j.1471-4159.2003.02120.x. [DOI] [PubMed] [Google Scholar]
- Fink AJ, Englund C, Daza RA, Pham D, Lau C, Nivison M, Kowalczyk T, Hevner RF. Development of the deep cerebellar nuclei: transcription factors and cell migration from the rhombic lip. J Neurosci. 2006;26:3066–3076. doi: 10.1523/JNEUROSCI.5203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Englund C, Kohtz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–618. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S. Neural stem cells: guardians of the brain. Nat Cell Biol. 2007;9:1031–1034. doi: 10.1038/ncb0907-1031. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Emsley JG, Magavi SS, Arlotta P, Macklis JD. Constitutive and induced neurogenesis in the adult mammalian brain: manipulation of endogenous precursors toward CNS repair. Dev Neurosci. 2004;26:101–117. doi: 10.1159/000082131. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Gage FH. Mobilization of neural precursors in the adult central nervous system. Rao MS, editor. Totowa NJ: Humana Press,; Neural Development and Stem Cells. 2006:pp 343–369. [Google Scholar]
- Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, Kokaia Z, Lindvall O. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–1587. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Lathia JD, Selvaraj PK, Jo DG, Mughal MR, Cheng A, Siler DA, Markesbery WR, Arumugam TV, Mattson MP. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol. 2008;213:114–121. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H, Fassbender K. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain. 2005;128:1778–1789. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, Li C. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner DS, Cho IS, Park SY, Kim JI, Meeker HC, Ye X, Lafauci G, Kerr DJ, Flory MJ, Kim BS, Kascsak RB, Wisniewski T, Levis WR, Schuller-Levis GB, Carp RI, Park E, Kascsak RJ. Accelerated prion disease pathogenesis in Toll-like receptor 4 signaling-mutant mice. J Virol. 2008;82:10701–10708. doi: 10.1128/JVI.00522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Cimino PJ, Keene CD, Breyer RM, Montine KS, Montine TJ. Therapeutic targets in prostaglandin E2 signaling for neurologic disease. Curr Med Chem. 2008;15:1863–1869. doi: 10.2174/092986708785132915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]