Abstract
The calcium-binding protein calretinin has emerged as a useful marker for the identification of mesotheliomas of the epithelioid and mixed types, but its putative role in tumor development has not been addressed previously. Although exposure to asbestos fibers is considered the main cause of mesothelioma, undoubtedly, not all mesothelioma patients have a history of asbestos exposure. The question as to whether the SV40 virus is involved as a possible co-factor is still highly debated. Here we show that increased expression of SV40 early gene products in the mesothelial cell line MeT-5A induces the expression of calretinin and that elevated calretinin levels strongly correlate with increased resistance to asbestos cytotoxicity. Calretinin alone mediates a significant part of this protective effect because cells stably transfected with calretinin cDNA were clearly more resistant to the toxic effects of crocidolite than mock-transfected control cells. Down-regulation of calretinin by antisense methods restored the sensitivity to asbestos toxicity to a large degree. The protective effect observed in clones with higher calretinin expression levels could be eliminated by phosphatidylinositol 3-kinase (PI3K) inhibitors, implying an important role for the PI3K/AKT signaling (survival) pathway in mediating the protective effect. Up-regulation of calretinin, resulting from either asbestos exposure or SV40 oncoproteins, may be a common denominator that leads to increased resistance to asbestos cytotoxicity and thereby contributes to mesothelioma carcinogenesis.
Malignant mesotheliomas are tumors of the serosal lining of the pleural, pericardial, and peritoneal cavities causing more than 2000 deaths per year in the US alone.1,2 Current therapy is ineffective in slowing the time course of the disease and median survival from the time of diagnosis is rarely greater than 1 year. Mesothelioma is most often associated with asbestos exposure.3,4 Because exposure to fibers of the amphibole subgroup (crocidolite, amosite) is linked to mesothelioma and lung cancer development,5 crocidolite is often used in experimental studies to investigate asbestos-related damage.3 Although asbestos is not considered as a classical mutagenic agent,2 the way it causes malignant transformation is still under debate. Approximately 80% of mesothelioma patients have a history of asbestos exposure, whereas ∼20% or more cases remain idiopathic.6 Thus, additional factors (man-made mineral fibers, ionizing radiation, SV407) have been proposed to either induce or possibly act as co-carcinogens for mesotheliomas.8 The most discussed one is SV40, with many reports supporting a role for SV40 in mesotheliomas,9,10,11,12,13,14 but also many questioning it15,16,17 or proposing to perform more, well-designed epidemiological studies.18 A review panel initiated by the Institute of Medicine that re-evaluated all epidemiological studies on a putative link between the distribution of SV40 contaminated vaccines and mesotheliomas or other types of SV40-associated cancers concluded that there was no evidence to rule either in favor or against a role of SV40-contaminated vaccines in mesothelioma and other tumors.19
Mesothelial cells are uniquely susceptible to SV40 infection and transformation,1 which is linked to mesothelioma development because intrapleural injection results in the development of mesotheliomas in 100% of injected hamsters in 3 to 6 months.20 In humans, SV40 has been detected in the same type of tumors as the ones observed in animals (mesotheliomas, ependymomas, choroid plexus tumors, and osteosarcomas21). Initial polymerase chain reaction (PCR) analyses of human mesotheliomas showed that SV40 sequences were present in 60% of the samples10 and the overall consensus is that ∼50% of malignant mesotheliomas in the US contain SV40,22 whereas the absence of SV40 in Finnish specimens was likely attributable to the absence of SV40 contaminations in the used vaccine.23 In another study, the authors observed the presence of SV40 sequences in 6% of specimens; their prior detection in a higher percentage of specimens was interpreted as resulting from laboratory contamination by SV40 plasmids.16
Evidence for SV40 acting as a co-carcinogen came from studies demonstrating that SV40 large T antigen (Tag) confers a protective effect on cells exposed to crocidolite. Although addition of crocidolite to cultured human mesothelial cells normally results in massive cell death, concomitant transfection with SV40 Tag leads to foci formation and increased cell survival.1 Tag is a 90-kDa protein that is directly mutagenic by altering the karyotype and the stability of the host genome; it acts in conjunction with the small t antigen (tag), a 17-kDa protein localized in the cytoplasm that inhibits phosphatase 2A, stimulates MAP kinase, and helps Tag in binding/inactivating cellular tumor suppressors including the retinoblastoma family members pRb, p107, p130, and also the tumor suppressor p53.2 In transgenic mice expressing Tag selectively in mesothelial cells, asbestos-induced mesothelioma formation shows a dose dependency: in a transgenic line with a high copy number of integrated transgene, tumors develop faster and are more invasive than in a single-copy line.24 Besides sequestering and thereby inactivating tumor suppressors, novel mechanisms involving Tag in the process of transformation have been recently reported. Tag in a complex with p53, pRb, and p300 binds and activates the insulin-like growth factor I promoter and thus regulates transcription of insulin-like growth factor I25 and it is proposed that this multiprotein complex promotes growth of malignant cells through its ability to activate the insulin-like growth factor I signaling pathway. Furthermore, Tag also binds to insulin receptor substrate 126 and insulin receptor substrate 1 binding to Tag appears necessary for Tag-mediated phosphorylation of AKT. Thus, Tag is suggested to inhibit apoptosis via the activation of PI3K/AKT signaling.
A protein detected in essentially all mesotheliomas of the epithelioid and mixed type, while present only in a small percentage (≈10%) of lung adenocarcinomas is calretinin, a calcium-binding protein of the EF-hand family.27,28 The authors reporting this initial finding came to the conclusion that calretinin was an extremely useful positive marker for the distinction between these two tumor types; consequently, calretinin antibodies together with a large panel of other antibodies either serving as positive or negative markers have gained wide acceptance for the diagnosis of mesotheliomas. Yet, no published study has directly addressed the question whether the expression of calretinin in mesotheliomas is linked (directly or indirectly) with the process of transformation. In healthy individuals, this protein is mainly expressed in a specific subset of neurons.29,30,31 The precise physiological function of calretinin is still unknown; a role in intracellular Ca2+ homeostasis and/or Ca2+ buffering has been suggested in nerve cells.32 In nonexcitable colon cancer cells, calretinin interacts with cytoskeletal elements.33 A correlation exists between the expression of calretinin and cell proliferation in certain colon cancer cells; down-regulation of calretinin in WiDr cells blocks the cell cycle and increases apoptosis.34 Calretinin is not expressed in normal enterocytes; it is also absent in normal simple squamous epithelium of the peritoneal cavity consisting of mesothelial cells in situ,27 but is present in reactive mesothelial cells35 and benign multicystic mesothelioma.36 However, its putative role in carcinogenesis is still unclear.
Here we tested the hypothesis that up-regulation of calretinin in mesothelial cells contributes to the escape from cell death as proposed for calretinin-expressing colon carcinoma cells,34 which then favors additional genetic alterations leading to malignant transformation.
Materials and Methods
Cell Culture
Two different MeT-5A cell lines (mesothelial origin, human, immortalized with SV40 early region genes) were used for the experiments: one directly obtained from the American Type Cell Collection (ATCC; Rockville, MD) named MeT-5A-ATCC, the other was a clone received from Geneva Hospital, Geneva, Switzerland (MeT-5A-GE). The cells were grown at 37°C/5% CO2 in Dulbecco’s modified Eagle’s medium/F-12 1:1 plus GlutaMax (Gibco, Basel, Switzerland) supplemented with 10% fetal calf serum (Gibco) and antibiotics (100 U/ml penicillin; 100 μg/ml streptomycin).
Isolation of Genomic DNA from MeT-5A Clones and Semiquantitative Determination of Transgene Copy Number
Genomic DNA (0.5 μg) from MeT-5A cells (ATCC and GE clones) was isolated using standard methods and used to either amplify exon 1 of SV40 Tag or intron 9 of the human calretinin (CALB2) gene. The latter signal was used for normalization. Sequences of primers for Tag and calretinin were: SV40Ex1-5 (5′-CAGAGAGGAATCTTTGCAGCTAATGG-3′), SV40Ex1-3 (5′-AAGCCTCCAAAGTCAGGTTGATGAGC-3′) and CR-It9410F (5′-GGTGGTTTTCTTCATAACCAGTGTTGG-3′), CR-It9665R (5′-TAAGAGTCTAGCCTCTCCATTACTCTG-3′). A limited number of PCR cycles were performed (annealing: 66°C, 25 cycles) and the PCR amplicons were separated on a 2% agarose gel. The signal intensities were quantitatively analyzed by the Gene Tools (Syngene, Cambridge, UK) software.
Immunohistochemistry
Cells were grown on laminin-treated glass coverslips and fixed with 4% paraformaldehyde, 15% (v/v) saturated picric acid, and 0.38% glutaraldehyde in a 0.2 mol/L sodium phosphate buffer, pH 7.3. They were then incubated with either calretinin antibody CR7696 (1:1000)37 or Tag antibody (Pab101, 1:500; Santa Cruz, Biotechnology, Santa Cruz, CA) for 16 hours at room temperature in 0.1 mol/L Tris-buffered saline, pH 7.3, containing 10% bovine serum. The secondary biotinylated antibody (1:200; Vector Laboratories, Burlingame, CA) was added for 2 hours at room temperature in 0.1 mol/L Tris-buffered saline, pH 7.3, containing 10% bovine serum, followed by incubation with the avidin-biotin complex (Vector) diluted 1:200. The antibody complex was visualized by incubation with 3,3′-diaminobenzidine-HCl-hydrogen peroxide.
Western Blot Assays for Calretinin and Tag
Cells were washed with Ca2+- and Mg2+-free (CMF)-phosphate-buffered saline, removed from the culture flasks with a cell scraper, and resuspended in 10 mmol/L Tris-HCl, 2 mmol/L ethylenediaminetetraacetic acid, pH 8.0. Cells were disrupted by ultrasonication for 20 seconds, the suspension was centrifuged (13,000 × g, 4°C, 30 minutes) and the supernatant was recovered. Protein concentrations were determined by the Bradford method using the reagent from Bio-Rad (Hercules, CA) and bovine serum albumin as standard. Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5%) and transferred onto nitrocellulose membranes (BDH Laboratory Supplies, Dorset, UK) using a semidry blotting apparatus (Bio-Rad). Membranes were stained with Ponceau S (Sigma, Buchs, Switzerland) to check for even loading of the gels and then further processed as described before38 using CR7696 antibody (1:1000) or anti-Tag antibody (1:500). For normalization of the Western blots, densitometric analysis of the Ponceau S-stained membranes using the GeneTools software was performed. This decreases the bias toward a specific protein (eg, GAPDH, β-actin) often used for normalization that might also be affected by the experimental manipulations.39,40 Calretinin expression was quantified as the blackening of the enhanced chemiluminescence films in several separate experiments using the GeneTools software; the data were log-transformed for the analyses. Because only a subset of clones were assayed in any particular replication of the experiment, to avoid a bias, we estimated calretinin expression levels of each clone as least-square means from a two-way analysis of variance with clone and experiments as factors. For graphical presentation the least-square means were back-transformed and expressed on a relative scale such that the calretinin expression for the SV40 clone SV8 = 100.
Asbestos Toxicity Assays
We quantified the resistance of control and transfected clones to asbestos toxicity, and the effects of the PI3K-inhibitors PI103 and ZSTK474,41 the inhibitor of mTOR (rapamycin) and the inhibitor of ERK1/2 (PD98059). A standard type of asbestos, NIEHS crocidolite obtained from Dr. Brooke T. Mossman, Department of Pathology, University of Vermont, Burlington, VT, was used. Crocidolite asbestos fibers were suspended in Hanks’ balanced salt solution (1 mg/ml), triturated 10 times through a 22-gauge needle to obtain a homogenous suspension and added directly to the medium.42 Cells were seeded in 96-well plates (1000 to 5000 cells/well) and allowed to attach for 24 hours before treatment. The number of viable cells in culture was determined with the MTT assay.43 Initial growth curves of various transfected clones were obtained in the absence of any treatment. In assays of crocidolite toxicity, crocidolite (10.0 or 7.5 μg/cm2) was added 24 hours after plating, and the cell number was assessed 5 days later; parallel, untreated cultures served as controls. For the assay of crocidolite resistance in the presence of inhibitors of signaling pathways, cells were incubated for 1 hour with PI103 (750 nmol/L), ZSTK474 (1 μmol/L), rapamycin (10 nmol/L; all kind gifts of Matthias Wymann, University of Basel, Basel, Switzerland) or PD98059 (20 μmol/L; Cell Signaling Technology, Bioconcept, Allschwil, Switzerland) before the addition of crocidolite (5 μg/cm2); the number of viable cells was determined 48 hours later. All clones tested in a given assay were grown in 96-well plates, with several replicate wells per clone and treatment (generally n = 7); the entire assay was replicated four times. Pilot experiments verified the linearity between cell number (determined by cell counting) and optical density (OD) 540-nm measurements (see Supplemental Figure S1 at http://ajp.amjpathol.org). Resistance to crocidolite toxicity was quantified as the OD (reflecting the number of viable cells) in cultures treated with crocidolite, relative to the OD of untreated cultures of the same clone. As is appropriate for ratio data, the analysis was performed on the log scale. OD values were log-transformed and averaged over replicate wells containing a given clone within each microtiter plate. Crocidolite resistance was compared among different types of clones (such as mock-transfected, calretinin-transfected, and SV40-transfected) with an analysis of variance, whereby individual clones were treated as a random factor nested within clone types and replicate assays were treated as blocks. Analogous approach was used to analyze the effects of treatment with PI103, ZSTK474, rapamycin, and PD98059. The analysis was performed with PROC GLM of SAS (Cary, NC) statistical software. For graphical representation, the mean OD values for a given cell line and treatment were back-transformed and expressed as percent of the OD of untreated control.
Plasmids
To allow for the production of stably transfected cells, the plasmid pCMV-Tag (kind gift of Sandro Rusconi, University of Fribourg, Fribourg, Switzerland) containing the SV40 early region genes was modified. The neor cassette was synthesized by PCR using the primers NEO-TOT5/NHE (5′-TACAGCTAGCTTGAATTCTACCGGGTA GG-3′), NEO- TOT3/NHE (5′-CGGTAGCTAGCTTCTGATGGAATTAGAAC-3′) and the previously described neor cassette44 as template. The PCR fragment contained terminal NheI sites that allowed insertion into the unique NheI site in pCMV-Tag. The PCR amplicon was digested with NheI and ligated into pCMV-Tag. The final construct pCMV-Tag-NEO was linearized using the unique site AccI. For the production of stably calretinin-transfected MeT-5A-GE cells, the previously described SacI-linearized plasmid RSV-CR-NEO was used.45
Stable Gene Transfer
Plasmids were diluted in OptiMem (Gibco) and transfections were performed according to the manufacturer’s protocol using Lipofectamine 2000 (Invitrogen, Life Technologies, Basel, Switzerland). Twenty-four hours after transfection, cells were treated with 0.75 mg/ml of G418 for 10 days and surviving cells were diluted in 96-well plates to isolate clones deriving from a single cell. The dilution procedure was repeated once.
Isolation of RNA and Reverse Transcription (RT)-PCR
Total RNA (5 μg) isolated by the guanidinium thiocyanate-phenol-chloroform method was used for RT-PCR using random primers (Revert Aid H Minus Strand cDNA synthesis kit; Fermentas, Glen Burnie, MD). Amplification of glyceraldhyde-phosphate dehydrogenase (GAPDH) using the two oligodeoxynucleotides 5′-GAGCTGAACGGGAAGCTCACTGG-3′ and 5′-CAACTGTGAGGAGGGGAGATTCAG-3′ (annealing: 55°C, 25 cycles) served as positive control. The primers used for detecting calretinin mRNA were 5′-GACAGGAGTGGCTACATCGAAGCCAATGAG-3′ and 5′-GGCATCCAGCTCATGCTCGTCAATGTAGCC-3′ (annealing: 66°C, 25 cycles). Aliquots of each RT-PCR reaction containing the amplified fragments were separated on a 2% agarose gel.
Cell-Based Enzyme-Linked Immunosorbent Assay for AKT
Protein phosphorylation of signaling molecules was directly measured in 96-well cultured cells (CASE; SuperArray Bioscience, Frederick, MD). Cells (12,000 cells/well) were seeded into 96-well plates and 24 hours later treated with crocidolite (5 μg/cm2, 1 hour). The cells were then fixed with 4% formaldehyde to preserve any activation-specific protein modification and further processed according to the manufacturer’s protocol. The amount of phosphorylated protein, once normalized to the amount of total protein, is directly related to the extent of downstream pathway activation. The ratios pAKT/AKT were log-transformed and analyzed in an analysis of variance.
Down-Regulation of Calretinin Expression in CR Clones Using Antisense Oligonucleotides (ASOs) and CR siRNA
For the calretinin down-regulation experiments, cells were seeded into 96-well plates (2000 cells/well) and grown for 24 hours. The antisense oligo CR-AS9 (5′-TCTCGATTTTCCCATCTGA-3′) was added (300 nmol/L) using Lipofectamine 2000 (Invitrogen) as transfection reagent. Controls were transfected with an unrelated nonsense oligo (300 nmol/L; NSO 5′-AAGAAACTGGGGAGGCTTCGCAGAGGGATG-3′). After incubation for 24 hours, crocidolite (2.5 μg/cm2) was added and 48 hours later the MTT assay was performed. The siRNA (PRED siRNA Hs CALB2; Qiagen, Hombrechtikon, Switzerland) was used at a final concentration of 10 nmol/L and was delivered into the cells also using Lipofectamine 2000. Control cells were treated with 10 nmol/L of the same control oligo as above and crocidolite toxicity was determined as for the ASO.
Results
Up-Regulation of Calretinin in MeT-5A-GE Cells after Transfection with pCMV-SV40-NEO Plasmid
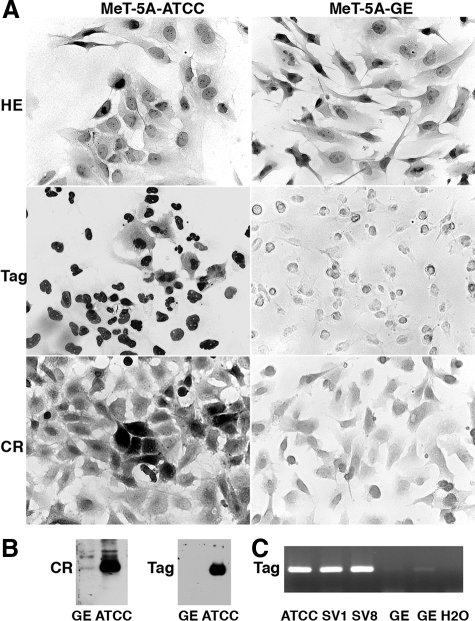
Two clones of SV40 early region genes-immortalized mesothelial cells, MeT-5A-ATCC and MeT-5A-GE, were analyzed. Morphologically they were very similar, with an adherent phenotype and growing as a monolayer as previously described for the MeT-5A-ATCC clone (Figure 1).46 Cells of the GE clone were somewhat flatter, more spread out than the ATCC cells and filiform processes were more pronounced. Although immunoreactivity was very weak for calretinin and almost undetectable for Tag in the GE clone (Figure 1A), the ATCC clone showed strong staining for both calretinin (diffuse cytosolic staining) and Tag (nuclear staining); findings that were supported by Western blot analyses (Figures 1B and 2A). The weaker Tag expression in the GE clone is likely related to the smaller copy number of inserted transgenic elements. Semiquantitative PCR analysis of the 5′ region (exon 1) of Tag sequences in the genomic DNA isolated from both clones revealed the signal to be ∼20- to 50-fold stronger in the ATCC clone (Figure 1C).
Figure 1.
Immunohistochemistry and Western blot analysis for calretinin (CR) and T large antigen (Tag) in the two mesothelial cell clones MeT-5A-ATCC and MeT-5A-GE. A: Fixed cells were either H&E-stained (top), or immunostained for T large antigen (Tag, middle) or calretinin (CR, bottom). Immunostaining for both CR and Tag was stronger in the ATCC clone than in the GE clone. CR immunoreactivity was seen throughout the cytoplasmic compartment and also the nucleus, whereas Tag expression was essentially nuclear. B: The Western blot signals for both CR and Tag were much stronger in the ATCC clone compared with the GE clone. C: PCR for Tag exon 1 using genomic DNA resulted in strong signals for the ATCC clone and the SV40-Tag-transfected GE clones SV1 and SV8 (for details, see Results), whereas the signal for the parental GE clone (two independent DNA isolations) resulted in much weaker signals. Semiquantitative analysis of the PCR signal under nonsaturating conditions (signal not reaching the plateau) for the ATCC clone revealed the GE signal to be 20- to 50-fold weaker reflecting the smaller copy number of Tag plasmid inserts in the GE clone. A control PCR reaction with only bidistilled H2O as input did not yield a detectable PCR signal.
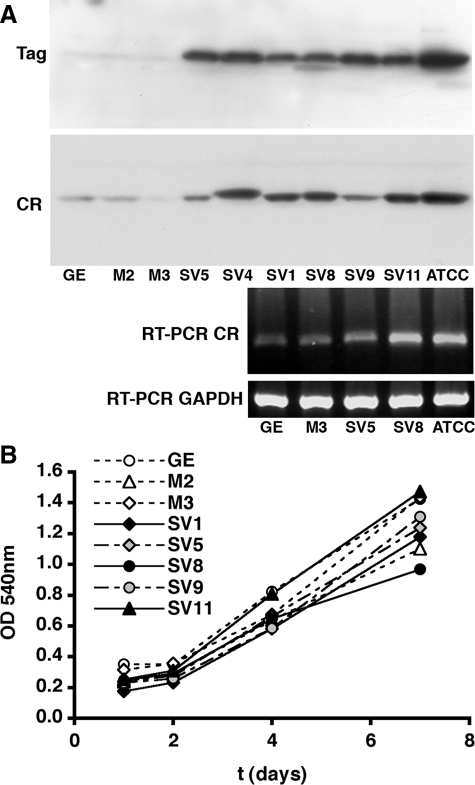
Figure 2.
Western blot and RT-PCR analysis of SV40-transfected MeT-5A-GE cells and growth curves of various MeT-5A-GE clones. A: Relative protein expression levels determined by Western blot analysis of Tag (top) and calretinin (CR, middle) were strongly linked. The Ponceau S-stained membrane used for the normalization of the signals is shown in Supplemental Figure S3 at http://ajp.amjpathol.org). Tag levels in SV40-transfected MeT5A-GE clones SV5, SV4, SV1, SV8, SV9, and SV11 were considerably higher than in control cells GE, M2, and M3. CR levels in clones SV1, SV8, and SV11 were similar as in the ATCC clone, whereas CR levels of SV5 and SV9 were lower, yet still higher than in the three controls. The protein recognized by the calretinin antiserum in clone SV4 had a higher molecular weight indicative of aberrant integration and/or expression and was not used in further experiments. Semiquantitative RT-PCR (25 cycles) using total RNA from the various clones revealed a close correlation between mRNA and protein levels (bottom) suggestive of transcriptional control of expression. For the normalization, the GAPDH signal obtained under identical experimental conditions (25 cycles) was used. B: Growth curves of selected MeT-5A-GE clones determined by MTT assay showed no correlation with either CR or Tag expression levels (for details, see Results section). Cells (1500 per well) were seeded in 96-well plates (eight wells per clone, three experiments) and incubated for 1, 2, 4, or 7 days. Scale bar = 20 μm.
The parallel differences in Tag and calretinin expression levels between the two MeT-5A clones suggested that Tag and/or tag could induce expression of calretinin. To test this hypothesis, we stably transfected MeT-5A-GE cells with the plasmid pCMV-Tag-NEO, containing the SV40 early genes under the control of the CMV promoter. Western blot analysis of transfected cells that survived the G418 selection revealed the calretinin signal to be approximately twofold higher than in the parental MeT-5A-GE cells (see Supplemental Figure S2 at http://ajp.amjpathol.org). Eighteen clones were obtained (SV1 to SV18), each derived from a single transfected cell that showed strong immunoreactivity for Tag. In addition, two Tag-negative clones (M2, M3) served as additional control (mock-transfected) clones besides the untransfected GE line. The genomic DNA from all clones contained the neor cassette used as a selection marker confirming the integration of the plasmid DNA into the genome (data not shown) and furthermore Tag copy numbers of clones SV1 and SV8 were similar as in the ATCC clone (Figure 1C). Expression levels of Tag and calretinin were analyzed semiquantitatively by Western blot analysis (Figure 2A, for normalization of Western blot signals, see Supplemental Figure S3 at http://ajp.amjpathol.org). Although both Tag and calretinin protein levels were very low in the untransfected GE cells, all clones with high Tag expression also had considerably higher calretinin expression levels than the GE cells. No increase in calretinin was detected in clones with low Tag expression (M2, M3) used as controls. Clones with high Tag expression (SV1, SV8, and SV11) had calretinin levels comparable with the ATCC clone. A close correspondence was observed between signal intensities in the calretinin RT-PCR reactions and the calretinin Western blots in a subset of clones indicating control at the transcriptional level (Figure 2A, bottom gel). The stability of calretinin expression in the SV40-transfected clones was tested 10 passages later. Results from Western blot analysis were virtually identical (not shown) and thus, all further experiments were performed with cells with no more than 10 additional passages. We therefore conclude that Tag expression stably up-regulates calretinin expression in transfected mesothelial cells.
Cell Growth of SV40-Transfected MeT-5A-GE Cells Is Not Correlated with Calretinin Expression Levels
The cell growth of SV40-transfected MeT-5A-GE cells was compared with mock-transfected and control cells and averaged growth curves from three experiments are shown in Figure 2B. The number of viable cells was determined 1, 2, 4, and 7 days after plating. None of the transfected clones exhibited faster growth than the untransfected control cells. Although most cell lines were growing somewhat slower, the calretinin-negative control clone M3 and the high calretinin-expression clone SV11 were growing as fast as the untransfected control cells. No correlation existed between calretinin/Tag expression levels and proliferation rate. Nonetheless, the small differences in proliferation rates among the various clones were taken into consideration for the cytotoxicity experiments (see below).
Effect of Crocidolite Treatment on Cell Survival of SV-40-Transfected MeT-5A-GE Cells
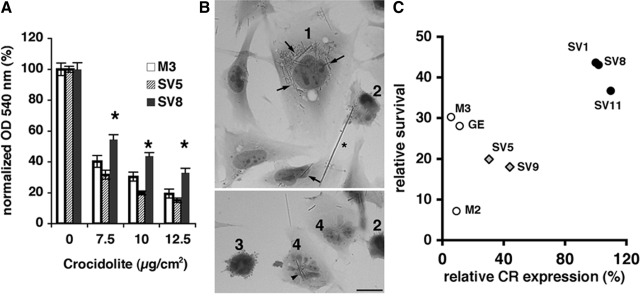
It has been proposed that SV40 Tag might act synergistically with asbestos fibers on the pathogenesis of malignant mesothelioma, by counteracting the cytotoxic effects of asbestos fibers, thus allowing the resistant clones to slowly accumulate additional protective mutations.2,24,47 Therefore, the susceptibility of SV40 early genes-transfected MeT-5A-GE clones to asbestos fiber-induced cell death was investigated at various crocidolite concentrations (7.5, 10, and 12.5 μg/cm2), similarly as described before for rat pleural mesothelial cells.48 Three clones were initially selected: the control clone M3, clone SV5 (low calretinin expression), and clone SV8 (high calretinin expression). For each clone, the MTT signal at day 5 of cell culture in the absence of crocidolite was defined as 100% to account for the slight variations in cell growth between clones as shown before (Figure 2B). Crocidolite treatment led to a strong, dose-dependent reduction of viable cells in all clones (Figure 3A); at all doses tested survival of the high-calretinin expressing clone SV8 was better compared with the two other clones (analysis of variance, followed by Tukey test; P < 0.05 at all concentrations). Thus, we conjectured that up-regulation of calretinin in MeT5A-GE clones observed after transfection with early region SV40 genes might protect the cells against crocidolite toxicity. The cell morphology of MeT-5A cells was considerably affected by the crocidolite treatment irrespective of the clone type; cells were either more flattened compared with untreated cells or were more rounded and often shrunk. The latter were characterized by dark pycnotic nuclei and sometimes by cytoplasmic blebbing characteristic of apoptotic cells. In some cells strongly lobulated and fragmented nuclei were observed. Asbestos fibers were either engulfed in the cells, often surrounding the nucleus or even localized within the nucleus or present as large rod-like structures loosely attached to the outer cell surface (Figure 3B).
Figure 3.
Crocidolite toxicity and morphological changes in asbestos-treated MeT-5A clones. A: The dose-dependant effect of crocidolite-mediated cytotoxicity was assessed in MTT assays. The number of viable cells decreased by incubating cells with higher crocidolite concentrations. Although the number of viable cells was similar for the control cells M3 and clone SV5 with relatively low calretinin expression levels, at all crocidolite doses tested (7.5, 10, and 12.5 μg/cm2), clone SV8 with high calretinin expression levels was significantly more resistant to asbestos-mediated cytotoxicity (values are mean ± SE, analysis of variance on relative OD with crocidolite versus untreated cells: 7.5 μg/cm2: F2,33 = 10.7, P < 001, Tukey test, *P < 0.05: SV8 > M3 = SV5; 10 μg/cm2: F2,33 = 28.9, P < 0.001; Tukey test, *P < 0.05: SV8 > M3 > SV5 and 12.5 μg/cm2: F2,33 = 12.4, P < 0.001, Tukey test, *P < 0.05: SV8 > M3 = SV5). B: Altered morphology of crocidolite-treated MeT-5A cells includes cell flattening, ie, increased surface (1), condensed pycnotic nuclei (2), blebbing of the plasma membrane (3), and lobulation combined with fragmentation of the nucleus (4). Asbestos fibers were engulfed in cells, often surrounding the nucleus (arrows), within the nucleus (arrowhead), or present as large rod-like structures (asterisk) loosely attached to the outer cell surface. C: SV40-transfected MeT-5A GE clones with higher calretinin (CR) expression levels (filled circles) were more resistant to crocidolite toxicity than either low-expressing clones (gray diamonds), mock-transfected clones M2 and M3, or the parental untransfected GE cells (open circles). Crocidolite resistance of the eight analyzed clones was positively correlated with CR expression (Pearson’s r = 0.75, P = 0.032). The percentage of viable cells was determined by MTT assay 5 days after exposure to crocidolite (10 μg/cm2). Scale bar = 20 μm.
To address further the relationship between SV40 transfection, calretinin expression, and crocidolite toxicity, we studied the effect of a toxic dose of crocidolite (10.0 μg/cm2, 5 days exposure) on cell culture growth in eight clones with different calretinin expression: five SV40-tranfected clones, two mock-transfected clones, and the original MeT-5A-GE line. Thus, this measure of resistance reflects the net effect of the treatment on cell mortality and cell division. Across these eight clones, the resistance to crocidolite was positively correlated with calretinin expression (Figure 3C; Pearson’s r = 0.75, P = 0.032). In particular, three SV40-transfected clones with a high calretinin expression (SV1, SV8, SV11) survived the crocidolite treatment considerably better than the two SV40 clones with low calretinin expression (SV5, SV9; F1,3 = 108.0, P = 0.002). In the latter two SV40 clones the crocidolite resistance was similar to that of the untransfected control (GE) or the mock-transfected clones M2 and M3. Thus, transfection of MeT-5A-GE cells with SV40 early genes enhances crocidolite resistance only if accompanied by calretinin up-regulation.
Increased Calretinin Expression Protects MeT-5A from Crocidolite-Induced Cytotoxicity
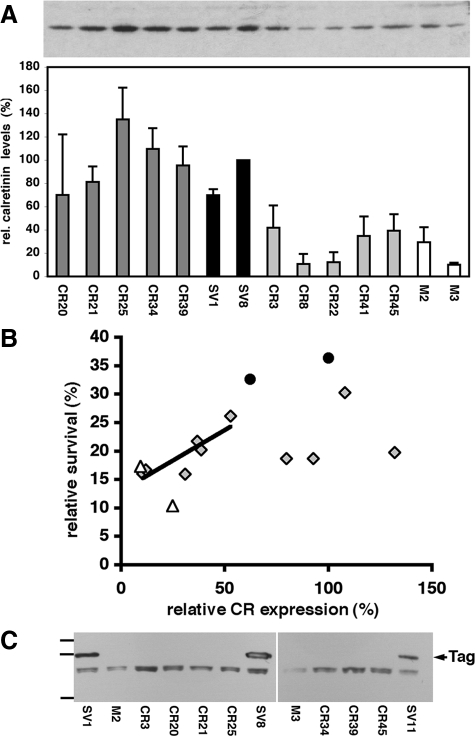
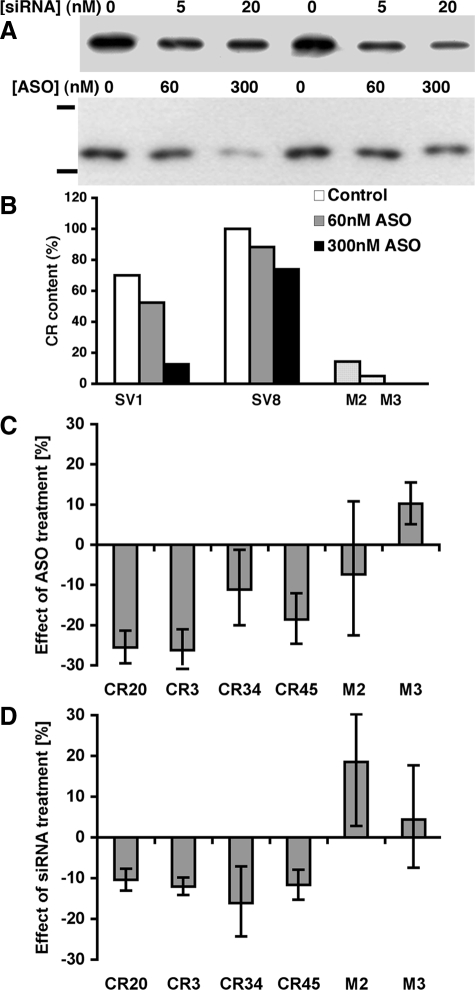
The positive correlation between calretinin expression in SV40-transfected cell lines and their resistance to crocidolite suggested a protective role of calretinin. Alternatively, increased calretinin expression could be a by-product of other SV40-induced changes; SV40-transfection is known to lead to altered expression of several other genes implicated in cell-cycle control/cell growth.49 To discern between these alternatives, we tested whether elevated calretinin expression suffices to enhance resistance to crocidolite in the absence of elevated Tag levels. For this, MeT-5A-GE cells were stably transfected with the plasmid RSV-CR-NEO that yielded 45 G418-resistant clones. Ten of these clones spanning the whole range of calretinin expression levels were chosen for the crocidolite cytotoxicity assays (Figure 4A); the most resistant SV40-transfected clones SV1 and SV8 served as positive controls, whereas the mock-transfected clones M2 and M3 were used as negative controls. Cell cultures were treated with 7.5 μg/cm2 of crocidolite for 3 days and the number of viable cells was determined by the MTT assay. The resistance of those cell lines is plotted in Figure 4B against their calretinin expression levels (estimated by Western blots). Despite significant variation in the resistance among the calretinin-transfected clones (F9,18 = 2.9, P = 0.028), as a group these clones were significantly more resistant than the two mock-transfected clones (F1,11 = 5.6, P = 0.038), although not as resistant as the two highly resistant SV40 clones (F1,11 = 10.2, P = 0.009). Although some of the calretinin-transfected clones had low crocidolite resistance despite high calretinin expression, none of the clones with low expression showed high resistance. Among the six calretinin-transfected clones with the lowest calretinin expression there was a significant correlation between crocidolite resistance and calretinin expression (Pearson’s r = 0.87, P = 0.025). Thus, among those cell lines, enhanced calretinin expression seemed necessary, although not entirely sufficient for improved resistance to crocidolite, although their resistance was not as strong as observed in the two SV40 clones. Because increased Tag expression was associated with elevated calretinin levels (Figure 2A), which was in part responsible for the cytoprotective effect, we wanted to ascertain that the protective effect seen in the CR clones was not mediated by reciprocal up-regulation of Tag in the CR clones. Western blot analysis revealed a strong Tag signal only in the Tag-transfected SV1, SV8, and SV11 clones used as positive controls, whereas all CR clones were essentially negative, similar as the control clones M2 and M3 (Figure 4C). As described above, neither the mock clone nor the CR clones were completely Tag-negative, but below the detection level of Western blot analysis, when loading identical amounts of total protein as in the Tag-transfected SV1, SV8, and SV11 clones. Therefore, the protective effect seems to have been mediated by calretinin itself, independent of Tag. To further verify this hypothesis, we down-regulated calretinin expression in CR clones using two different antisense technologies. In the first one, we used CR antisense oligonucleotides (ASOs) previously demonstrated to down-regulate calretinin expression in colon cancer cell lines (S. Vonlanthen and B. Schwaller, unpublished).34 The most efficient oligonucleotide CR9-ASO was tested in clones SV1 and SV8. The addition of CR9-ASO led to a concentration-dependent decrease in calretinin levels after 48 hours, and an ≈80% reduction of calretinin expression was achieved by 300 nmol/L CR9-ASO in clone SV1 (Figure 5, A and B); this effect was less pronounced in clone SV8, which had a higher initial calretinin expression (Figure 5B). However, calretinin expression levels after addition of CR9-ASO were still higher than in control clones M2 and M3. The control oligo (NSO; 300 nmol/L) did not affect calretinin expression levels (not shown). A similar down-regulation of calretinin was also observed in clone SV8 treated with CR siRNA (5, 20 nmol/L) for 24 hours and 48 hours (Figure 5A, top). The asbestos cytotoxicity assay was performed with four CR and two control clones. In the presence of crocidolite, the MTT signal of CR clones treated with ASO compared with control NSO-treated cells was on average reduced by 11 to 26% (treatment F1,3 = 28.0, P = 0.013), and was similar for all CR clones (treatment × clone interaction F3,21 = 0.1, P = 0.95 ; Figure 5C). The effect was of similar magnitude (≈20%) as the protection initially brought about by calretinin: compare with Figure 4B and with Figure 6A; middle bars (crocidolite-treated) in the control and CR clones. The ASO-mediated effect was minimal or absent in control clones M2 and M3 (F1,1 = 0.0, P = 0.92), clones with already very low calretinin expression levels (Figure 2A). The siRNA treatment resulted in a similar (10 to 16%) increase in the sensitivity to crocidolite toxicity in the CR clones (treatment F1,3 = 87.8, P = 0.003; treatment × clone interaction F3,21 = 0.1, P = 0.99) (Figure 5D). As for ASO, this effect of siRNA was absent in the control clones M2 and M3 (F1,1 = 2.8, P = 0.34). When combining the results of control clones M2 and M3 exposed to CR9-ASO or CR siRNA, the net effect as compared with treatment with control oligo, was close to zero. Thus, calretinin down-regulation primarily eliminated the protective phenotype of CR clones—antisense-treated cells became again more susceptible to the toxic effects of crocidolite. This further supports calretinin’s protective effect.
Figure 4.
Comparison of calretinin (CR) expression levels and cell survival of MeT-5A-GE cells stably transfected with the expression plasmid RSV-CR-NEO. A: A representative Western blot is shown in the upper panel and the relative quantitative results are depicted in the bottom panel (average of three Western blots and three samples for each clone). Five clones with high CR expression (dark gray bars) and five with low CR expression (light gray bars) were compared with pCMV-Tag-transfected ones with high CR expression (SV40 clones SV1 and SV8, black bars) and to the negative controls (M2 and M3, white bars) by Western blot analyses. Relative CR expression levels in the high CR group were comparable with levels in the SV40 group, whereas levels in the low CR group were on average only slightly higher than in control clones M2 and M3. B: The resistance to asbestos cytotoxicity was determined by the MTT assay and results are the mean from three experiments. The relationship between CR expression and the survival of cells after crocidolite treatment is shown for 10 CR-transfected clones (gray diamonds), two mock-transfected controls (open triangles), and two SV40-transfected clones (black circles). A regression line is shown for the six CR clones with the lowest CR expression levels (Pearson’s r = 0.87, P = 0.025). C: Western blot of Tag (arrow) in SV40 clones (SV1, SV8, and SV11) in comparison with CR-transfected clones and the control clones M2 and M3. No signal for Tag was detectable in any of the CR clones indicating that calretinin up-regulation does not induce Tag expression. The lower nonspecific band in all samples is attributable to an endogenous biotinylated protein. Bars on the left indicate the position of molecular marker proteins (from top to bottom: 100 kDa, 75 kDa, 50 kDa).
Figure 5.
Down-regulation of calretinin by antisense methods decreases survival of crocidolite-treated CR clones. A: Western blot of cytosolic proteins isolated from clone SV8 (top) after treatment with calretinin siRNA (0, 5, and 20 nmol/L) for 24 hours (left) or 48 hours (right) or clones SV1 (left) and SV8 (right) incubated with antisense oligonucleotides (CR9-ASO; 0, 60, and 300 nmol/L; 48 hours; bottom); the bars indicate the position of marker proteins of 50 kDa (upper bar) and 25 kDa (lower bar). B: Calretinin expression levels were reduced by ≈80% in clone SV1 after exposure to 300 nmol/L CR9-ASO for 48 hours. On average, expression levels were still somewhat higher than in control clones M2 and M3. The relative values for clones SV1 and SV8 (untreated) and M2 and M3 were obtained from Western blots as shown in Figure 4A; clone SV8 was set to 100%. C: Quantitative analysis of the effect of calretinin down-regulation by CR9-ASO on crocidolite cytotoxicity in comparison with NSO-treated (control) cells. For the analysis, the OD values were log-transformed, averaged over replicate wells with the same clone and treatment within an experiment, and subjected to an analysis of variance, with treatment, clone, and their interaction as factors, and experiment (multiwell plate) treated as block. Clone was treated as a random factor, and so treatment × clone interaction was used as the denominator in the F-test for the effect of treatment. For the graphical representation the means were back-transformed and differences are presented as percentage changes. The MTT signal was decreased in the four CR9-ASO-treated CR clones on average by 11 to 26% (treatment F1,3 = 28.0, P = 0.013), but not in the CR9-ASO-treated control clones M2 and M3 (F1,1 = 0.0, P = 0.92). D: In siRNA-treated clones, the variability was larger with respect to clones (F3,21 = 8.2, P < 0.001) and replicate experiments (F3,21 = 74.2, P < 0.001), but compared with NSO controls, siRNA treatment consistently reduced the MTT signal of all four CR9-ASO-treated CR clones by 10 to 16% (the effect of treatment F1,3 = 87.8, P = 0.003, treatment × clone interaction F3,21 = 0.1, P = 0.99). This effect was absent in the control clones M2 and M3 (F1,1 = 2.8, P = 0.34).
Figure 6.
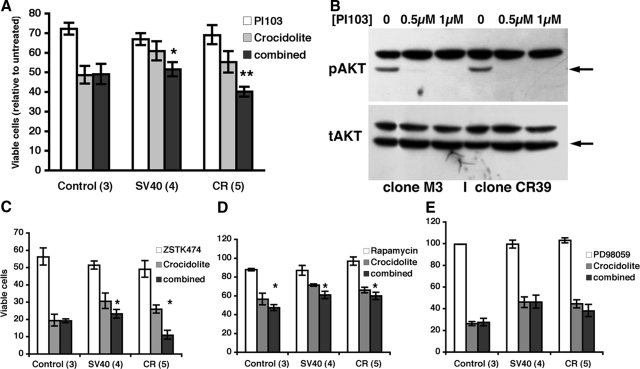
Activation of the AKT signaling pathway is involved in mediating the increased resistance against asbestos-mediated cytotoxicity exclusively in calretinin-expressing clones A: Both PI103 (0.75 μmol/L) and crocidolite (5 μg/cm2) reduced the number of viable cells after 48 hours of treatment in all clones. The effect of PI103 was similar in all clones. A protection against crocidolite treatment was evident in the four SV40-transfected (SV1, SV2, SV8, SV11) and the five CR-transfected (CR20, CR21, CR25, CR34, CR39) clones (see also Figures 3C and 4B). Crocidolite-induced toxicity in the presence of PI103 was further increased only in the high CR-expressing clones of the SV40 (F1,3 = 35.7, *P < 0.01) and the CR (F1,3 = 14.66, **P < 0.02) groups. In the control group, the inhibition of the PI3K/AKT signaling pathway by PI103 treatment did not aggravate the asbestos-induced toxicity. B: Western blot analysis of MeT-5A clones M3 (left) and CR39 (right) treated with 0.5 μmol/L or 1 μmol/L PI103 for 24 hours; pAKT signals (top, arrow) were considerably reduced at 0.5 μmol/L PI103 (>95% for clone M3) and completely absent at 1 μmol/L. For clone CR39 already at 0.5 μmol/L PI103 no specific pAKT signal was detected. Total AKT levels (bottom, arrow) were unaffected by PI103 at the doses tested. The upper band on both blots is a nonspecific signal resulting from an endogenous biotinylated protein. C and D: Treatment of the same clones as in B with the PI3K-specific inhibitor ZSTK474 (C, 1 μmol/L) or rapamycin (D, 10 nmol/L), the specific inhibitor of mTOR. Results with ZSTK474 were almost identical to the ones with PI103 (*P < 0.01). Co-incubation of crocidolite and rapamycin resulted in a decreased MTT signal when compared with crocidolite alone, but the reduction was not different in the three groups (F2,9 = 0.6, P = 0.59). Thus, co-incubation of rapamycin with asbestos did slightly aggravate asbestos cytotoxicity in all clones (control, SV40, and mock) indicating that neither Tag nor calretinin was involved in this effect. E: Inhibition of the ERK1/2 signaling pathway with the inhibitor PD 98059 (20 μmol/L) did not heighten crocidolite-mediated cytotoxicity in all groups of clones (main effect F1,9 = 1.4, P = 0.27).
Activation of Signaling Pathways After Asbestos Exposure and Their Involvement in Protection Against Cytotoxicity
Exposure of mesothelial cells to asbestos fibers activates signaling pathways involved in cell survival and cell death, including the AKT pathway.50 Prolonged exposure of human mesothelial cells to SV40 increases cell survival by AKT activation and finally induces transformation evidenced by loss of cell contact inhibition and generation of several foci.51 We determined the relative phosphorylation level of AKT (Ser473) in the control cell lines (GE, M2, M3), in four SV40-transfected clones (SV1, SV2, SV8, SV11) and in five calretinin-transfected clones, which showed a high calretinin expression (CR20, CR21, CR25, CR34, CR39; Figure 4A). This cell-based enzyme-linked immunosorbent assay was performed both under basal conditions and after short-term asbestos exposure (5 μg/cm2 for 1 hour). The short-term asbestos exposure led to an increase of the pAKT/AKT ratio by a factor of 1.5 (F1,9 = 60.3, P < 0.0001); this increase was similar in all three types of clones (cell type × exposure interaction F2,9 = 0.4, P = 0.7). Basal pAKT levels differed quite considerably from one clone to another, the difference between the lowest and the highest value was almost twofold. However, analysis of variance revealed that there were no differences among the three groups (SV40, CR, and control), neither for basal pAKT levels, induced levels after crocidolite treatment, nor for the fold induction; among individual clones there was no correlation between pAKT levels and resistance toward crocidolite cytotoxicity.
To assess whether pAKT signaling plays a role in resistance to asbestos and whether it depends on calretinin expression, we used the PI3K-inhibitors PI103 and ZSTK474 to block the AKT-signaling pathway.52 The Western blot signal for pAKT was dramatically reduced (to less than 5%) in the presence of 0.5 μmol/L PI103, and was undetectable in the presence of 1 μmol/L inhibitor, yet total AKT levels were not affected (Figure 6B). Therefore we chose the concentration of 750 nmol/L to study the effect of PI103 on crocidolite resistance in a series of 12 clones: the original GE line and the two mock clones (which all show low CR expression), four SV40 clones with high CR expression, and five CR-transfected clones, likewise with high CR expression. In the absence of crocidolite, 2 days of PI103 exposure reduced the number of viable cells by ∼30% compared with untreated controls (Figure 6A, white bars); this effect did not differ significantly among the three categories of clones (F2,9 = 3.0, P = 0.10), nor did it vary among clones of the same category (F9,69 = 0.9, P = 0.53). However, the three categories of clones were affected differently by the PI103 treatment in the presence of crocidolite (Figure 6A; PI103 treatment × clone category interaction, F2,9 = 5.2, P = 0.031). In the clones with low CR expression (mock clones and untransfected GE cells) the combination of asbestos and PI103 resulted in an almost identical survival as in cells only treated with asbestos alone. In contrast, blocking the pAKT signaling with PI103 significantly reduced resistance to crocidolite in the two sets of clones with high calretinin expression: in the SV40-transfected cells (F1,3 = 35.7, P < 0.01) and even more so in the CR-transfected cells (F1,3 = 14.66, P < 0.02). Because it had been shown before that PI103 not only inhibits PI3K, but also mTOR,53 we applied a novel PI3K inhibitor ZSTK474 (1 μmol/L) that even at higher concentrations (up to 100 μmol/L) only weakly affects mTOR.41 Results with ZSTK474 and PI103 were almost identical (Figure 6C). The resistance to crocidolite in ZSTK474-treated cells was decreased in the SV40 (F1,3 = 18.0, P = 0.024) and CR clones (F1,4 = 14.4, P = 0.019), but not in the control clones (F1,2 = 0.0, P = 0.95). That the two calretinin-expressing groups (SV40 and CR) indeed responded differently from the control group was supported by the corresponding interaction contrast (F1,9 = 5.2, P = 0.049). This difference in response to ZSTK474 was not observed in the absence of crocidolite (F1,9 = 0.7, P = 0.42). On the other hand, the mTOR inhibitor rapamycin also reduced the magnitude of the MTT signal in the presence of crocidolite (F1,9 = 20.3, P = 0.0015; Figure 6D), but the effect did not differ among the groups of clones (F2,9 = 0.6, P = 0.59) and more importantly not between the control and the two groups with elevated calretinin expression (SV40 and CR; interaction contrast F1,9 = 0.4, P = 0.55). Thus, neither SV40 Tag nor calretinin were involved in the mTOR-mediated effect on crocidolite cytotoxicity. Finally, also the involvement of the ERK1/2 pathway was investigated using the inhibitor PD98059. The inhibitor alone (in the absence of crocidolite) had no effect on the MTT signal in any group of clones (white bars in Figure 6E, main effect of PD98059 F1,9 = 0.4, P = 0.54, PD98059 × group interaction F2,9 = 0.6, P = 0.55). Similarly, no effect of the inhibitor was detected in cells treated with crocidolite (gray versus black bars in Figure 6E, main effect of PD98059 F1,9 = 1.4, P = 0.27, PD98059 × group interaction F2,9 = 1.9, P = 0.20, interaction contrast F1,9 = 1.4, P = 0.27; Figure 6E).
Taken together, the above results indicate that specific blocking of PI3K/AKT signaling, but not the mTOR or ERK1/2 signaling, eliminates calretinin’s protective effect against asbestos-induced cytotoxicity only in cells with high calretinin expression (ie, the SV40 and CR clones). Thus, the PI3K/AKT signaling pathway acts as a survival signaling pathway in the presence of crocidolite.
Discussion
Since 1996, more than 170 reports were published on calretinin as a marker for the identification of mesotheliomas of the epithelioid and mixed (biphasic) type, irrespective whether tumorigenesis was linked to asbestos exposure or caused by other means. Hence, the six mesothelioma cases without asbestos exposure described in the study by Cristaudo and co-workers12 were also diagnosed using antibodies against calretinin, cytokeratin, CEA, BerEP4, and CD15. Furthermore, a large-scale transcriptional profiling of more than 22,000 genes has resulted in 1405 candidate genes differentially expressed among i) normal tissue (lung and pleura), ii) mesothelioma cell lines, and iii) mesothelioma samples.54 Based on the expression profiles, mesotheliomas could be further grouped into two classes (C1 and C2; see Figure 2 in Gordon et al54); all four groups showed distinct up- or down-regulation of particular gene products. Among the very few genes up-regulated in both mesothelioma groups C1 and C2, as well as in mesothelioma cell lines, while unchanged in normal tissue is calretinin (human gene name: CALB2). This has prompted us to investigate the putative role of calretinin in tumorigenesis of mesotheliomas and the SV40-immortalized cell line MeT-5A served as a useful model to studying in vitro calretinin’s role. Cell culture models are considered useful to identifying proteins that participate in transformation (for a review on the role of Tag on cellular transformation see Ahuja and colleagues55). However, the exact role(s) played by a given protein during tumorigenesis in vivo needs to be verified, eg, in animal models, to assess the protein’s effects in the context of a tissue. We had access to two MeT-5A clones that differed in their immunoreactivity for calretinin and SV40 Tag and the expression appeared highly correlated: the ATCC clone with high calretinin expression levels also showed strong Tag expression, whereas the GE clone was almost negative for both. Stable transfection of the GE cells with the pSV40 plasmid resulted in significantly elevated Tag and concomitantly, elevated calretinin levels, in the best case to levels similar as in the ATCC clone, demonstrating that expression of SV40 early gene products (Tag and tag) suffices to induce up-regulation of calretinin in mesothelial cells. To test the hypothesis that SV40 Tag counteracts the cytotoxic effects of asbestos fibers, and thus acts as a co-carcinogen,2 we exposed the SV40-transfected cells to various crocidolite concentrations. Clearly, SV40 clones with higher calretinin and Tag expression levels were better protected than the untransfected GE clone and the mock-transfected clones, which had neither significantly elevated SV40 Tag nor calretinin expression levels. Also, transfected clones with only slightly elevated calretinin expression levels were not better protected against asbestos cytotoxicity than the control clones.
Evidently, the calretinin gene CALB2 is not the only gene affected by SV40 transfection. Aberrant methylation of genes after SV40 infection of mesothelial cells includes the tumor suppressor gene RASSF1A and other genes such as RRAD and TMS1.13 Increased methylation status of these genes in cell lines and mesotheliomas is correlated with the presence of SV40 sequences. Consequently, increased methylation of TMS1 and hypermethylated in cancer (HIC-1) in mesothelioma patients strongly correlate with decreased survival time. SV40 transformation of human mesothelial cells (HMCs) also induces cell survival via AKT activation and AKT activity is further increased in HMCs exposed to asbestos. SV40-transformed cells are also more resistant to Onconase, one of the few chemotherapeutic agents used in patients with malignant mesothelioma.56 Thus, the combined effects of asbestos and SV40 on AKT-dependent signaling pathways have been proposed to progressively induce transformation of HMCs.51
Hence, to directly assess the putative cytoprotective effect of calretinin, MeT-5A GE cells were stably transfected with a calretinin expression plasmid. Although the calretinin-transfected clones were not conferring a similar degree of asbestos resistance compared with the SV40-transfected group, the resistance among the calretinin-transfected clones as a group was significantly higher than in the mock-transfected clones and the cytoprotective effect in clone CR34 was almost as potent as in the SV clones. Thus, up-regulation of calretinin alone cannot fully account for the protective effect attributable to SV40 early gene expression, but calretinin appears as a major factor contributing to the resistance to asbestos toxicity. In support of our hypothesis, CR clones could be rendered more sensitive again to the cytotoxic effects of crocidolite by calretinin down-regulation using either CR antisense oligonucleotides or CR siRNA. The effect of the reversal (11 to 26% by ASO treatment) was of similar magnitude as the initial protection by calretinin (≈20 to 30%) consistent with a direct role for calretinin in this process. The fact that the reversal was not complete can be likely attributed to the maximal attainable down-regulation, which rarely exceeds 80 to 90% using antisense methods. However, the net difference between the CR clones and control clones subjected to either CR ASO- or siRNA treatment were essentially the same, in the order of 20%.
The exact mechanism how calretinin might contribute to tumorigenesis of mesotheliomas remains unknown. In colon carcinoma cells down-regulation of calretinin leads to a blockage of the cell cycle finally leading to increased apoptosis.34 Furthermore, butyrate, an inducer of differentiation in colonocytes, which also blocks the cell cycle, down-regulates calretinin expression in colon cancer cells.57 Although principally distributed in the cytosol, calretinin can also associate with cytoskeletal structures33 and is found in particulate fractions of brain homogenates.58 A developmentally regulated calretinin accumulation beneath the plasma membrane in neurons59 and a nuclear localization in tumor cells under specific conditions57 is indicative of additional roles besides Ca2+-buffering. Little is known on CALB2 gene regulation: an AP2-like sequence was reported to drive neuron-specific calretinin expression60 by binding a yet unidentified nuclear protein resulting in an increased CALB2 gene promoter activity. In contrast, the AP2-like sequence appears not to be involved in the regulation of CALB2 gene transcription in adenocarcinoma and mesothelioma cells, indicating that CALB2 gene regulation in neurons and cancer cells occurs via different mechanisms.61 Calretinin expression in tumor cells correlates with their proliferative state and possibly increases their resistance toward differentiating or pro-apoptotic signals. This might be possibly clinically exploited in the case of mesotheliomas. Mesothelioma cells transfected with a plasmid containing part of the CALB2 promoter followed by the thymidine kinase gene were 100-fold more sensitive to the drug ganciclovir in vitro than the untransfected cells,62 and the authors suggested that the CALB2 promoter might be a promising candidate as a specific and efficient promoter in malignant mesotheliomas. As discussed above, calretinin’s protective effect was mediated via the AKT signaling pathway, because blocking it by PI3K inhibitors abrogated calretinin’s protective effect. Also, tumor necrosis factor-α protects mesothelial cells from asbestos cytotoxicity via a nuclear factor-κB-mediated pathway and was proposed to play a role in the mechanism of mesotheliomagenesis.63 Thus, the observed cytoprotective effect induced by either tumor necrosis factor-α or calretinin points out that the likely key to asbestos carcinogenesis is induction of resistance mechanisms to the cytotoxic effects of asbestos or possibly other asbestos-like materials such as carbon nanofibers.64 It remains to be shown whether tumor necrosis factor-α affects calretinin expression and whether AKT signaling and nuclear factor-κB signaling possibly act synergistically and/or merge on a final common pathway. Our results further indicate that part of the protective effect against crocidolite cytotoxicity may also be mediated by the mTOR pathway, in line with findings by Wilson and colleagues65 who reported that mTOR signaling mediates survival of small primary mesothelioma samples grown ex vivo. However the protection reported in our study was similar in all clones suggesting that neither Tag nor calretinin were involved in the mTOR-mediated protection. Finally, the ERK signaling pathway has also been reported to play a role in asbestos fiber-mediated transformation of mesothelial cells66 and pERK was proposed as a new molecular therapeutic target.67 Conversely, the absence of differences in ERK activation in reactive mesothelium and malignant mesothelioma was used as an argument against an involvement in the transformation of benign to malignant mesothelium.68 Our results support the latter viewpoint: they indicate that inhibition of ERK phosphorylation has no adverse effect on the acute cytotoxicity investigated in our study.
In summary, the overexpression of calretinin in immortalized mesothelial cells (CR clones) can to a large extent replicate the cytoprotective effects observed in SV40-transfected clones. In both cases, the activation of the PI3K/AKT signaling pathway is involved in the increased survival after asbestos exposure. Because calretinin expression is observed in practically all asbestos-related mesotheliomas,27,28 and we demonstrated here that transfection with an SV40 early gene region is sufficient to increase calretinin expression, elevated levels of this protein in mesothelial cells may be the common underlying cause leading to the increased resistance to signals (eg, asbestos fibers) normally leading to cell death. In such a way, affected mesothelial cells may escape senescence, accumulate additional mutations finally leading to a fully transformed state. Mechanisms of selectively blocking calretinin function and/or down-regulating its expression may be envisaged as a strategy to combat the development of mesotheliomas. In the light of the fact that mesotheliomagenesis is a very slow process, intervention at a time point much passed of the initial exposure may still be of therapeutic relevance.
Supplementary Material
Footnotes
Address reprint requests to Prof. Dr. Beat Schwaller, Unit of Anatomy, Department of Medicine, University of Fribourg, Route Albert-Gockel 1, CH-1700 Fribourg, Switzerland. E-mail: beat.schwaller@unifr.ch.
Supported by the Swiss National Science Foundation (grants 3100-063448.00/1 and 310000-113518/1 to B.S.) and Novartis (grant 03B38 to B.S.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR, Pass HI, Rizzo P, Carbone M. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci USA. 2000;97:10214–10219. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo P, Bocchetta M, Powers A, Foddis R, Stekala E, Pass HI, Carbone M. SV40 and the pathogenesis of mesothelioma. Semin Cancer Biol. 2001;11:63–71. doi: 10.1006/scbi.2000.0347. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Kamp DW, Weitzman SA. Mechanisms of carcinogenesis and clinical features of asbestos-associated cancers. Cancer Invest. 1996;14:466–480. doi: 10.3109/07357909609018904. [DOI] [PubMed] [Google Scholar]
- Manning CB, Vallyathan V, Mossman BT. Diseases caused by asbestos: mechanisms of injury and disease development. Int Immunopharmacol. 2002;2:191–200. doi: 10.1016/s1567-5769(01)00172-2. [DOI] [PubMed] [Google Scholar]
- LaDou J. The asbestos cancer epidemic. Environ Health Perspect. 2004;112:285–290. doi: 10.1289/ehp.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead JE, Mossman BT. The pathogenesis of asbestos-associated diseases. N Engl J Med. 1982;306:1446–1455. doi: 10.1056/NEJM198206173062403. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Imbernon E, Rolland P, Gilg Soit Ilg A, Saves M, de Quillacq A, Frenay C, Chamming's S, Arveux P, Boutin C, Launoy G, Pairon JC, Astoul P, Galateau-Salle F, Brochard P. The French National Mesothelioma Surveillance Program. Occup Environ Med. 2006;63:390–395. doi: 10.1136/oem.2005.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Nino ME, Testa JR, Altomare DA, Pass HI, Carbone M, Bocchetta M, Mossman BT. Cellular and molecular parameters of mesothelioma. J Cell Biochem. 2006;98:723–734. doi: 10.1002/jcb.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF, Butel JS, Carbone M. SV40 and human tumours: myth, association or causality? Nat Rev Cancer. 2002;2:957–964. doi: 10.1038/nrc947. [DOI] [PubMed] [Google Scholar]
- Carbone M, Pass HI, Rizzo P, Marinetti M, Di Muzio M, Mew DJ, Levine AS, Procopio A. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9:1781–1790. [PubMed] [Google Scholar]
- Elmishad AG, Bocchetta M, Pass HI, Carbone M. Polio vaccines, SV40 and human tumours, an update on false positive and false negative results. Dev Biol (Basel) 2006;123:109–132. [PubMed] [Google Scholar]
- Cristaudo A, Foddis R, Vivaldi A, Buselli R, Gattini V, Guglielmi G, Cosentino F, Ottenga F, Ciancia E, Libener R, Filiberti R, Neri M, Betta P, Tognon M, Mutti L, Puntoni R. SV40 enhances the risk of malignant mesothelioma among people exposed to asbestos: a molecular epidemiologic case-control study. Cancer Res. 2005;65:3049–3052. doi: 10.1158/0008-5472.CAN-04-2219. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Toyooka S, Shivapurkar N, Shigematsu H, Miyajima K, Takahashi T, Stastny V, Zern AL, Fujisawa T, Pass HI, Carbone M, Gazdar AF. Aberrant methylation profile of human malignant mesotheliomas and its relationship to SV40 infection. Oncogene. 2005;24:1302–1308. doi: 10.1038/sj.onc.1208263. [DOI] [PubMed] [Google Scholar]
- Vilchez RA, Butel JS. Emergent human pathogen simian virus 40 and its role in cancer. Clin Microbiol Rev. 2004;17:495–508. doi: 10.1128/CMR.17.3.495-508.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah KV. Causality of mesothelioma: SV40 question. Thorac Surg Clin. 2004;14:497–504. doi: 10.1016/S1547-4127(04)00112-4. [DOI] [PubMed] [Google Scholar]
- López-Ríos F, Illei PB, Rusch V, Ladanyi M. Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet. 2004;364:1157–1166. doi: 10.1016/S0140-6736(04)17102-X. [DOI] [PubMed] [Google Scholar]
- Manfredi JJ, Dong J, Liu WJ, Resnick-Silverman L, Qiao R, Chahinian P, Saric M, Gibbs AR, Phillips JI, Murray J, Axten CW, Nolan RP, Aaronson SA. Evidence against a role for SV40 in human mesothelioma. Cancer Res. 2005;65:2602–2609. doi: 10.1158/0008-5472.CAN-04-2461. [DOI] [PubMed] [Google Scholar]
- Magnani C. SV40, genetic polymorphism and mesothelioma. pathological and epidemiological evidence. Med Lav. 2005;96:347–353. [PubMed] [Google Scholar]
- IOM Stratton K, Almario DA, McCormick MC, editors. Washington DC: The National Academies Press,; Immunization Safety ReviewSV40 Contamination of Polio Vaccine and Cancer. 2002 [PubMed] [Google Scholar]
- Cicala C, Pompetti F, Carbone M. SV40 induces mesotheliomas in hamsters. Am J Pathol. 1993;142:1524–1533. [PMC free article] [PubMed] [Google Scholar]
- Lowe DB, Shearer MH, Jumper CA, Kennedy RC. SV40 association with human malignancies and mechanisms of tumor immunity by large tumor antigen. Cell Mol Life Sci. 2007;64:2391–2403. doi: 10.1007/s00018-007-6414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Pass HI, Miele L, Bocchetta M. New developments about the association of SV40 with human mesothelioma. Oncogene. 2003;22:5173–5180. doi: 10.1038/sj.onc.1206552. [DOI] [PubMed] [Google Scholar]
- Hirvonen A, Mattson K, Karjalainen A, Ollikainen T, Tammilehto L, Hovi T, Vainio H, Pass HI, Di Resta I, Carbone M, Linnainmaa K. Simian virus 40 (SV40)-like DNA sequences not detectable in Finnish mesothelioma patients not exposed to SV40-contaminated polio vaccines. Mol Carcinog. 1999;26:93–99. [PubMed] [Google Scholar]
- Robinson C, van Bruggen I, Segal A, Dunham M, Sherwood A, Koentgen F, Robinson BW, Lake RA. A novel SV40 TAg transgenic model of asbestos-induced mesothelioma: malignant transformation is dose dependent. Cancer Res. 2006;66:10786–10794. doi: 10.1158/0008-5472.CAN-05-4668. [DOI] [PubMed] [Google Scholar]
- Bocchetta M, Eliasz S, De Marco MA, Rudzinski J, Zhang L, Carbone M. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 2008;68:1022–1029. doi: 10.1158/0008-5472.CAN-07-5203. [DOI] [PubMed] [Google Scholar]
- Yu Y, Alwine JC. Interaction between simian virus 40 large T antigen and insulin receptor substrate 1 is disrupted by the K1 mutation, resulting in the loss of large T antigen-mediated phosphorylation of Akt. J Virol. 2008;82:4521–4526. doi: 10.1128/JVI.02365-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzos V, Vogt P, Celio MR. The calcium binding protein calretinin is a selective marker for malignant pleural mesotheliomas of the epithelial type. Pathol Res Pract. 1996;192:137–147. doi: 10.1016/S0344-0338(96)80208-1. [DOI] [PubMed] [Google Scholar]
- Doglioni C, Tos APD, Laurino L, Iuzzolino P, Chiarelli C, Celio MR, Viale G. Calretinin: a novel immunocytochemical marker for mesothelioma. Am J Surgical Pathol. 1996;20:1037–1046. doi: 10.1097/00000478-199609000-00001. [DOI] [PubMed] [Google Scholar]
- Winsky L, Nakata H, Martin BM, Jacobowitz DM. Isolation, partial amino acid sequence, and immunohistochemical localization of a brain-specific calcium-binding protein. Proc Natl Acad Sci USA. 1989;86:10139–10143. doi: 10.1073/pnas.86.24.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Résibois A, Rogers JH. Calretinin in rat brain: an immunohistochemical study. Neuroscience. 1992;46:101–134. doi: 10.1016/0306-4522(92)90012-q. [DOI] [PubMed] [Google Scholar]
- Rogers JH. Immunohistochemical markers in rat cortex: co-localization of calretinin and calbindin-D28k with neuropeptides and GABA. Brain Res. 1992;587:147–157. doi: 10.1016/0006-8993(92)91439-l. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1:241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- Marilley D, Schwaller B. Association between the calcium-binding protein calretinin and cytoskeletal components in the human colon adenocarcinoma cell line WiDr. Exp Cell Res. 2000;259:12–22. doi: 10.1006/excr.2000.4942. [DOI] [PubMed] [Google Scholar]
- Gander JC, Gotzos V, Fellay B, Schwaller B. Inhibition of the proliferative cycle and apoptotic events in WiDr cells after down-regulation of the calcium-binding protein calretinin using antisense oligodeoxynucleotides. Exp Cell Res. 1996;225:399–410. doi: 10.1006/excr.1996.0191. [DOI] [PubMed] [Google Scholar]
- Kitazume H, Kitamura K, Mukai K, Inayama Y, Kawano N, Nakamura N, Sano J, Mitsui K, Yoshida S, Nakatani Y. Cytologic differential diagnosis among reactive mesothelial cells, malignant mesothelioma, and adenocarcinoma: utility of combined E-cadherin and calretinin immunostaining. Cancer. 2000;90:55–60. doi: 10.1002/(sici)1097-0142(20000225)90:1<55::aid-cncr8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Assaly M, Bongiovanni M, Kumar N, Egger JF, Pelte MF, Genevay M, Finci V, Tschanz E, Pache JC. Cytology of benign multicystic peritoneal mesothelioma in peritoneal washings. Cytopathology. 2008;19:224–228. doi: 10.1111/j.1365-2303.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Buchwald P, Blumcke I, Celio MR, Hunziker W. Characterization of a polyclonal antiserum against the purified human recombinant calcium binding protein calretinin. Cell Calcium. 1993;14:639–648. doi: 10.1016/0143-4160(93)90089-o. [DOI] [PubMed] [Google Scholar]
- Chen G, Racay P, Bichet S, Celio MR, Eggli P, Schwaller B. Deficiency in parvalbumin, but not in calbindin D-28k upregulates mitochondrial volume and decreases smooth endoplasmic reticulum surface selectively in a peripheral, subplasmalemmal region in the soma of Purkinje cells. Neuroscience. 2006;142:97–105. doi: 10.1016/j.neuroscience.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo AC, Moreno-Igoa M, Manzano R, Ordovas L, Yague G, Olivan S, Munoz MJ, Zaragoza P, Osta R. Determination of protein and RNA expression levels of common housekeeping genes in a mouse model of neurodegeneration. Proteomics. 2008;8:4338–4343. doi: 10.1002/pmic.200701091. [DOI] [PubMed] [Google Scholar]
- Kong D, Yamori T. ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms. Cancer Sci. 2007;98:1638–1642. doi: 10.1111/j.1349-7006.2007.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2001;24:405–413. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- Marilley D, Vonlanthen S, Gioria A, Schwaller B. Calretinin and calretinin-22k increase resistance towards sodium butyrate-induced differentiation in CaCo-2 colon adenocarcinoma cells. Exp Cell Res. 2001;268:93–103. doi: 10.1006/excr.2001.5261. [DOI] [PubMed] [Google Scholar]
- Colbére-Garapin F, Horodniceanu F, Kourilsky P, Garapin A. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981;150:1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- D'Orlando C, Fellay B, Schwaller B, Salicio V, Bloc A, Gotzos V, Celio MR. Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res. 2001;909:145–158. doi: 10.1016/s0006-8993(01)02671-3. [DOI] [PubMed] [Google Scholar]
- Saydan N, Salicio V, Cappelli-Gotzos B, Gotzos V. Expression of calretinin in human mesothelioma cell lines and cell cycle analysis by flow cytometry. Anticancer Res. 2001;21:181–188. [PubMed] [Google Scholar]
- Kroczynska B, Cutrone R, Bocchetta M, Yang H, Elmishad AG, Vacek P, Ramos-Nino M, Mossman BT, Pass HI, Carbone M. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci USA. 2006;103:14128–14133. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levresse V, Moritz S, Renier A, Kheuang L, Galateau-Salle F, Mege JP, Piedbois P, Salmons B, Guenzburg W, Jaurand MC. Effect of simian virus large T antigen expression on cell cycle control and apoptosis in rat pleural mesothelial cells exposed to DNA damaging agents. Oncogene. 1998;16:1041–1053. doi: 10.1038/sj.onc.1201627. [DOI] [PubMed] [Google Scholar]
- Testa JR, Giordano A. SV40 and cell cycle perturbations in malignant mesothelioma. Semin Cancer Biol. 2001;11:31–38. doi: 10.1006/scbi.2000.0344. [DOI] [PubMed] [Google Scholar]
- Berken A, Abel J, Unfried K. Beta1-integrin mediates asbestos-induced phosphorylation of AKT and ERK1/2 in a rat pleural mesothelial cell line. Oncogene. 2003;22:8524–8528. doi: 10.1038/sj.onc.1207195. [DOI] [PubMed] [Google Scholar]
- Cacciotti P, Barbone D, Porta C, Altomare DA, Testa JR, Mutti L, Gaudino G. SV40-dependent AKT activity drives mesothelial cell transformation after asbestos exposure. Cancer Res. 2005;65:5256–5262. doi: 10.1158/0008-5472.CAN-05-0127. [DOI] [PubMed] [Google Scholar]
- Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, Bjerke LM, Kelland L, Valenti M, Patterson L, Gowan S, de Haven Brandon A, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Patel S, Saghir N, Parker P, Waterfield M, Workman P. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GJ, Rockwell GN, Jensen RV, Rheinwald JG, Glickman JN, Aronson JP, Pottorf BJ, Nitz MD, Richards WG, Sugarbaker DJ, Bueno R. Identification of novel candidate oncogenes and tumor suppressors in malignant pleural mesothelioma using large-scale transcriptional profiling. Am J Pathol. 2005;166:1827–1840. doi: 10.1016/S0002-9440(10)62492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- Ramos-Nino ME, Vianale G, Sabo-Attwood T, Mutti L, Porta C, Heintz N, Mossman BT. Human mesothelioma cells exhibit tumor cell-specific differences in phosphatidylinositol 3-kinase/AKT activity that predict the efficacy of Onconase. Mol Cancer Ther. 2005;4:835–842. doi: 10.1158/1535-7163.MCT-04-0243. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Herrmann B. Regulated redistribution of calretinins in WiDr cells. Cell Death Differ. 1997;4:325–333. doi: 10.1038/sj.cdd.4400240. [DOI] [PubMed] [Google Scholar]
- Winsky L, Kuznicki J. Distribution of calretinin, calbindin D28k and parvalbumin in subcellular fractions of rat cerebellum: effects of calcium. J Neurochem. 1995;65:381–388. doi: 10.1046/j.1471-4159.1995.65010381.x. [DOI] [PubMed] [Google Scholar]
- Hack NJ, Wride MC, Charters KM, Kater SB, Parks TN. Developmental changes in the subcellular localization of calretinin. J Neurosci. 2000;20:RC67. doi: 10.1523/JNEUROSCI.20-07-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing-Marczak K, Buzanska L, Winsky L, Nowotny M, Rudka T, Isaacs K, Belin MF, Kuznicki J. AP2-like cis element is required for calretinin gene promoter activity in cells of neuronal phenotype differentiated from multipotent human cell line DEV. Biochim Biophys Acta. 2002;1577:412–420. doi: 10.1016/s0167-4781(02)00443-8. [DOI] [PubMed] [Google Scholar]
- Billing-Marczak K, Zieminska E, Lesniak W, Lazarewicz JW, Kuznicki J. Calretinin gene promoter activity is differently regulated in neurons and cancer cells. Role of AP2-like cis element and zinc ions. Biochim Biophys Acta. 2004;1678:14–21. doi: 10.1016/j.bbaexp.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Inase N, Miyake S, Yoshizawa Y. Calretinin promoter for suicide gene expression in malignant mesothelioma. Anticancer Res. 2001;21:1111–1114. [PubMed] [Google Scholar]
- Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, Bubici C, Mossman BT, Pass HI, Testa JR, Franzoso G, Carbone M. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci USA. 2006;103:10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Barbone D, Yang TM, Jablons DM, Bueno R, Sugarbaker DJ, Nishimura SL, Gordon GJ, Broaddus VC. mTOR mediates survival signals in malignant mesothelioma grown as tumor fragment spheroids. Am J Respir Cell Mol Biol. 2008;39:576–583. doi: 10.1165/rcmb.2007-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res. 2002;62:6065–6069. [PubMed] [Google Scholar]
- de Melo M, Gerbase MW, Curran J, Pache JC. Phosphorylated extracellular signal-regulated kinases are significantly increased in malignant mesothelioma. J Histochem Cytochem. 2006;54:855–861. doi: 10.1369/jhc.5A6807.2006. [DOI] [PubMed] [Google Scholar]
- Vintman L, Nielsen S, Berner A, Reich R, Davidson B. Mitogen-activated protein kinase expression and activation does not differentiate benign from malignant mesothelial cells. Cancer. 2005;103:2427–2433. doi: 10.1002/cncr.21014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.