Abstract
Melanocytic nevi frequently harbor oncogenic BRAF mutations, but only a minority progress to melanoma. In human melanocytes, persistent BRAFV600E expression triggers oncogene-induced senescence, which implies that bypass of oncogene-induced senescence is necessary for malignant transformation of melanocytes. We show that a subpopulation of primary human melanocytes with persistent expression of BRAFV600E do not enter oncogene-induced senescence, but instead survive despite heightened MAPK activity. Disruption of the p53 pathway using short-hairpin RNA initiated rapid growth of these V600E+ melanocytes in vitro. The resultant V600E+/p53sh melanocytes grew anchorage-independently in soft agar, formed pigmented lesions reminiscent of in situ melanoma in artificial skin reconstructs, and were weakly tumorigenic in vivo. Array comparative genomic hybridization analysis demonstrated that the transformed melanocytes acquired a substantial deletion in chromosome 13, which encodes the Rb1 tumor suppressor gene. Gene expression profiling study of nevi and melanomas showed that p53 target genes were differentially expressed in melanomas compared with nevi, suggesting a dysfunctional p53 pathway in melanoma in vivo. In summary, these data demonstrate that a subpopulation of melanocytes possesses the ability to survive BRAFV600E-induced senescence, and suggest that p53 inactivation may promote malignant transformation of these cells.
Melanocytic nevi (moles) are common benign human tumors. Eighty percent of nevi harbor BRAF mutations, with the most frequent mutation being a valine-to-glutamic acid substitution at position 600 (V600E).1 BRAFV600E has highly-elevated kinase activity and confers constitutive activation of the MAPK pathway.2 Although BRAF mutations are thought to be an early and critical step in the initiation of melanocytic neoplasia, the exact role of mutant BRAF in human melanocytic tumor initiation is unclear. Sustained expression of BRAFV600E induces nevus-like growth of melanocytes in transgenic zebrafish3 and benign growth of epithelial cells in transgenic mice,4 indicating that BRAFV600E may induce abnormal cell growth during development. However, introduction of BRAFV600E into primary human melanocytes induces cell cycle arrest and senescence.5 A more recent study, conversely, has shown that a minor population of primary human melanocytes may survive sustained expression of BRAFV600E; however, those observations were not the focus of that particular study.6
The p53 protein is a master transcription factor and a key responder to a variety of stresses, including DNA damage, genomic stability, hypoxia, and oncogenic aberrations.7 TP53 mutations or deletions have been detected in approximately 50% of cancers. However, TP53 mutations in human melanoma are not common, with reports ranging from nearly absent up to 10%8 and melanomas express normal or augmented levels of p53.9,10,11 Since melanomas are resistant to apoptosis,12 the function of p53 in melanomas is puzzling. Studies in transgenic mice expressing a melanocyte specific activated HRAS allele on a p53-null background provide strong evidence for p53 inactivation during melanoma genesis.13 More recently, an ‘epithelial-like’ melanoma subtype was shown to have significantly higher frequency of p53 inactivation.14 We have shown that melanomas exhibit impaired p53-dependent apoptosis15 and p53-target genes are often dissociated from p53 regulation,16 suggesting the p53 pathway is impaired in melanomas. A better understanding of the mechanisms of p53 pathway dysregulation in melanoma will be necessary to understand the molecular steps during transformation and to develop better chemotherapeutic strategies.
Melanomas commonly arise from nevi,17 suggesting that transformation of melanocytes requires a bypass of the senescent response induced by mutant BRAF. Here, we show that sustained expression of BRAFV600E does not induce oncogene-induced senescence in all infected human melanocytes; rather, a subpopulation of melanocytes survives. The acquisition of an additional insult, namely disruption of p53, immortalizes these cells and promotes an early transformed cell phenotype both in vitro and in vivo. Loss of p53 function in these cells may also result in genetic instability and loss of retinoblastoma (Rb)1 locus. These data support a tumor suppressor role for p53 in melanoma. Gene expression profiling analyses show clear disparities between p53 target gene expression in melanomas and nevi, suggesting a dysfunctional p53 pathway in vivo. Together, these data suggest that the BRAFV600E mutation is a founder event for melanocytic lesion formation, and disruption of p53 pathway may be critical for melanocyte transformation.
Materials and Methods
Plasmids
We used the pBABE plasmid (courtesy of D. Herlyn; The Wistar Institute, Philadelphia, PA) to clone human BRAFV600E into the pLenti6 lentiviral plasmid (Invitrogen, Carlsbad, CA) and verified sequences by sequencing both strands. The p53 small interfering RNA lentiviral plasmid with green fluorescent protein (GFP) tag was a generous gift from M. Soengas (University of Michigan, Ann Arbor, MI).
Cell Culture, Retroviral Transduction, Cell Proliferation, and β-Galactosidase Activity
Primary human melanocytes were isolated from the epidermis of neonatal foreskin as previously described.18 The melanocytes were maintained in melanocyte 254CF medium and human melanocyte growth supplement-2 (Cascade Biologics, Portland, OR). Transformed cells were also cultured in Tu 2% medium (MCDB153/L15 medium (v/v: 4/1) supplemented with 2% fetal bovine serum, insulin (5 units/ml), CaCl2 (2 mmol/L), 100 U/ml penicillin, and 100 mg/ml streptomycin). Production of pseudotyped lentivirus was achieved as previously described.19 Lentiviral vectors pLenti6/BRAFV600E, p53 short hairpin (sh)RNA-GFP, and GFP vector were used to infect melanocytes. BRAFV600E-infected cells were then selected in 6 μg/ml blasticidin-containing melanocyte culture medium. Cultured cells were stained for SA-β-galactosidase (SA-β-Gal) activity (Promega, Fitchburg, WI).
Western Blot, Immunocytochemistry, Immunohistochemistry, and Quantitative PCR
Western blots were performed as previously described.20 The antibodies used for Western blotting were pERK (1:1000, Cell Signaling), p53 (1: 50 Cell Signaling), and β-actin (1:5000; Sigma). Immunohistochemistry and immunocytochemistry were performed as previously described.21 The antibodies used were anti-S-100 (1:500; Dakocytomation), Melan-A (1:75; Novocasta Lab Ltd), tyrosinase (1:75; Novocasta Lab Ltd), and phosphor-H2AX (1:50; EMD Chemicals). 4,6-Diamidino-2-phenylindole staining was used to visualize nuclei.
Soft Agar and Matrigel Migration Assays
For soft agar assay, cells (5000 to 10,000 per well) were seeded in 0.5% low-melting-point agarose in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum, layered onto 0.8% agarose in Dulbecco’s Modified Eagle Medium/10% fetal bovine serum. Colonies were enumerated and photographed at ×200 magnification 8 weeks after seeding. The Matrigel in vitro migration assay was performed using BD BioCoat Tumor invasion system (BD Biosciences, Franklin Lakes, NJ).
Human Skin Reconstructs
Human skin reconstructs were generated as described.21,22 Briefly, dermal reconstructs consisted of bovine collagen type I with embedded dermal fibroblasts. Control or melanocytes infected with various vectors were seeded into tissue reconstruct trays together with keratinocytes onto dermal reconstructs at a 1:5 ratio of melanocytic cells to keratinocytes. Two weeks later, reconstructs were harvested and fixed in 10% neutral buffered formalin for 2 to 3 hours, processed by routine histological methods, embedded in paraffin, and sectioned. After sectioning, the reconstructs were stained with Melan-A or S-100 to depict melanocytes.
Xenograft Experiments
We injected 2 × 106 normal melanocytes or melanocytes with BRAFV600E and p53 knockdown (V600E+/p53sh) subcutaneously into the flanks of non-obese diabetic-severe combined immunodeficiency mice (10 mice each group) in 100 μl Matrigel (BD Biosciences, San Jose, CA). After tumors were palpable (∼2 weeks), caliper measurements were obtained every 72 hours. Tumor volume was monitored closely for 9 weeks, at which time the animals were sacrificed. All of the organs were examined for metastasis and preserved. The tumors were excised and processed for histology.
Human Tissue Samples and Microarray Study
Excessive tissues from surgical resection specimens of nevi or melanomas were used in this study. The protocol has been approved by University of Pennsylvania Institutional Review Board. All of the specimens were fresh frozen and stored in −80°C before use. Total RNA was extracted from homogenized tissues and 10 μg total RNA was used for cDNA Synthesis (Invitrogen, Carlsbad, CA). Labeled cRNA was fragmented and hybridized to the Human Genome U133A Array (Affymetrix, Santa Clara, CA) that contains 14,500 genes. The hybridization and data collection were performed at the University of Pennsylvania microarray core facility.
Gene Expression Microarray Data Analysis
Gene expression data from Affymetrix microarrays from two databases were used. The first dataset23 was obtained from Gene Expression Omnibus (GSE 3189) that had Affymetrix 133A microarray data available on 63 samples (18 nevi, and 45 melanomas). MAS5.0 was used to derive gene expression values by the original authors. The second dataset included data from Affymetrix microarrays done at University of Pennsylvania microarray facility from 33 samples (14 nevi, 19 melanomas). Robust multichip analysis was used to derive gene expression values.24
Genes were included in this analysis if there was expression and sufficient variability across all samples in a dataset. In the first dataset, a gene was excluded when either its mean expression or the range of its expression values (for all samples) was less than 75. In the second dataset, 30 was used instead of 75. Hierarchical cluster analysis using Euclidean distance was used to cluster genes and samples for the heatmaps. A two-sample t-test was performed for each probeset, assuming equal or unequal variances as indicated by the test for equal variances. Adjusted P values were computed using the Benjamini-Hochberg procedure to adjust for multiple hypothesis tests (results in supplemental Table S1 at http://ajp.amjpathol.org). Genespring was used to process the gene expression data and produce the heatmaps. Statistical analyses were done using SAS Version 9.03.
Array-Based Comparative Genomic Hybridization
Array based comparative genomic hybridization (aCGH) was performed onto the platform with ∼5000 human bacterial artificial chromosomes spaced at an average of 1Mb across the genome developed at the University of Pennsylvania.25 For hybridization, 1 μg of test DNA and 1 μg of sex-match pooled normal human DNA (obtained from a set of ten healthy female or male volunteers) were labeled with either Cy3-dCTP or Cy5-dCTP (Amersham Biosciences, UK) incorporated by random priming (Bioprime Labeling Kit, Invitrogen, Carlsbad, CA). Equal amounts of test and reference DNA (1 μg each) were mixed and precipitated with Cot1-DNA, 3M NaOAc (pH 7.0) and ethanol. Arrays were hybridized with 50% deionized formamide, 2× SSC, 2% SDS, 10% dextran sulfate, and 100 μg/μl of yeast tRNA for 72 hours at 37°C in a moist chamber on a slowly rocking table. Slides were washed and arrays were scanned on a GenePix 4000B dual scanner (Axon Instruments, Downingtown, PA).
Array-CGH Data Analysis
Fluorescent data from hybridization images were processed and analyzed with Gene Pix Pro 5.0 (Axon Instruments, Union City, CA) to obtain the log2ratios (tumor/reference) of each slide. Array-CGH data were processed through print-tip loess normalization, using the DNMAD application, which also allowed us to merge and filter clones between replicates in a slide and in the dye-swap experiment. We filtered out inconsistent replicates (those ones with a log2 ratio distant to the median log2 ratio of the replicates greater than 0.3) and clones that did not have available data in more than 70% of the cases. For visualization and detection of copy number alteration we used “Analysis of Copy number Errors” algorithm in CGH-Explorer (V 3.1b), estimates copy number calls based on genomic regions rather than on individual bacterial artificial chromosomes. Log2 ratios for each of the bacterial artificial chromosomes are plotted according to chromosome position using a “moving average smoother” with a three-clone window. The significance is expressed by the estimation of the positive false discovery rate; in our case the positive false discovery rate chosen was 0.1.
Statistical Analysis
One-way analysis of variance (analysis of variance), followed by Tukey’s multiple comparison test was used to analyze the data comparing groups with different treatments. Statistical significance was determined if the two-sided P value of a test was less than 0.05.
Results
Generation of BRAFV600E Positive Human Melanocytes
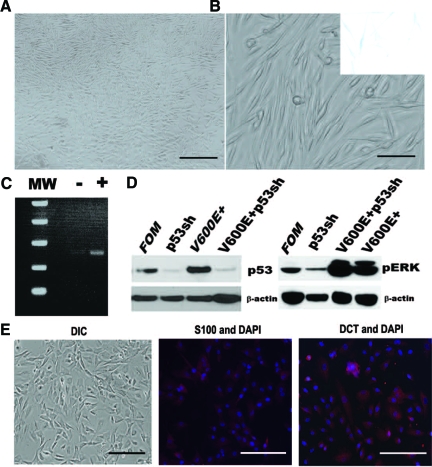
We infected foreskin-derived primary melanocytes with bicistronic lentiviral vectors co-expressing BRAFV600E and a blasticidin resistance cassette. During selection in blasticidin-containing medium, most BRAFV600E-infected melanocytes (∼95%) gradually stopped proliferating and exhibited intense SA-β-Gal activity (data not shown) similar to previous studies.5,26 However, a subpopulation of BRAFV600E-positive melanocytes (∼5% of cells seeded) survived and proliferated in the selection medium. These cells grew to confluence within one month and they retained typical melanocyte morphology (Figure 1A). These cells did not show significant SA-β-Gal activity (Figure 1B, right corner insert). Similar results were obtained from primary melanocytes derived from at least nine different individuals. Normal melanocytes infected with lentivirus carrying GFP were not able to survive in blasticidin selection medium after 2 days of selection (data not shown). Using a primer pair specific for BRAFV600E,5 reverse transcription-PCR was performed to verify that these surviving melanocytes expressed the exogenous oncogene product (Figure 1C). These cells had a similar lifespan as normal melanocytes and they can be passaged 10 to 12 times in vitro. These data suggest that a subpopulation may respond to BRAFV600E differently than the overwhelming majority of melanocytes.
Figure 1.
Isolation and characterization of V600E+ primary human melanocytes. BRAFV600E-infected human melanocytes were selected and expanded in the blasticidin containing medium. A: Confluent growth of V600E+ primary human melanocytes in culture. Scale bar = 100 μm. Representative images from at least nine independent experiments. B: V600E+ melanocytes retained typical melanocyte morphology. Scale bar = 20 μm. Inset: V600E+ melanocytes did not show significant SA-β-Gal activity. C: V600E+ melanocytes expressed BRAFV600E gene by reverse transcription PCR, while control melanocytes did not express BRAFV600E. D: V600E+ melanocytes were infected with lentivirus with p53 shRNA (p53sh). Western blot analysis for p53 and pERK in control (FOM) and infected cells. The expression of p53 protein was significantly decreased in cells infected with p53 shRNA, while pERK levels were significantly increased after BRAFV600E infection. The blots represent typical results from three independent experiments. E: Representative image of V600E+/p53sh melanocytes at passage 11. Differential interference contrast showed that cells became more epithelioid with abundant cytoplasm. Scale bar = 40 μm. Cells were stained with antibody against either S-100 or dopachrome tautomerase, and nuclei were visualized with 4,6-diamidino-2-phenylindole. Scale bar = 20 μm.
Knockdown of p53 Increases Proliferation of V600E+ Melanocytes
p53 function in melanoma is thought to be largely inactive, so we began to examine the role of p53 in melanocytes harboring the BRAFV600E gene product. To disrupt p53 function in V600E+ melanocytes, p53 shRNA was expressed from a lentiviral vector co-expressing GFP. Immunoblotting was performed to confirm the knockdown of p53 protein expression in these cells. As shown in Figure 1D, p53 shRNA effectively decreased the expression of p53 protein in infected cells but had no significant effect on pERK levels; as expected, pERK was heightened in V600E+ melanocytes. After about 10 passages, V600E+/p53sh melanocytes acquired an epithelioid cellular morphology, reminiscent of the morphology of melanoma cells in culture (Figure 1E). To ensure that these cells were indeed of melanocytic origin, they were assayed for specific markers; as measured by quantitative reverse transcription PCR, they expressed microphthalmia-associated transcription factor and Tyrp1, suggesting that contaminant cell types were not responsible for the observed phenotype (data not shown). Subsequent immunocytochemical stains demonstrated that they continued to express other melanocytic markers such as dopachrome tautomerase, S-100, and HMB45 (S-100 and dopachrome tautomerase: Figure 1E; HMB-45, data not shown).
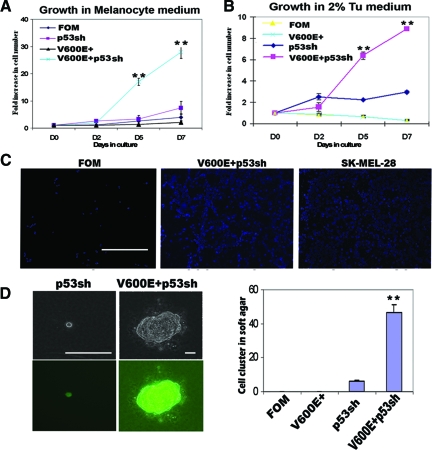
We compared the growth rate of control, V600E+, p53 knockdown (p53sh), and V600E+/p53sh melanocytes in melanocyte growth medium. V600E+ melanocytes grew slightly slower than control melanocytes in the medium, but the difference was not statistically significant. While p53 knockdown moderately increased melanocyte proliferation rate, V600E+/p53sh melanocytes displayed dramatically increased cell growth. Indeed, the V600E+/p53sh cell number increased 30-fold after 7 days (Figure 2A). These results indicate that p53 knockdown dramatically increases the proliferation capacity of the V600E+ human melanocytes.
Figure 2.
V600E+/p53sh melanocytes show transformed phenotype in vitro. A: Cells were cultured in the melanocyte growth medium and their growth rates were measured. V600E+ melanocytes grew slowest, while V600E+/p53sh melanocytes grew most quickly with a 30-fold increase in cell number by day 7. B: Cells were cultured in 2% Tu medium, which does not support growth of normal melanocytes, and their growth rates were measured. Control and V600E+ melanocytes were not able to grow in this medium. p53sh melanocytes were able to proliferate initially but then reached a growth plateau after 2 days. V600E+/p53sh melanocytes proliferated well in this medium. C: Matrigel migration assay. Normal melanocytes and SK-Mel-28 melanoma cells were used as negative and positive controls, respectively. Nuclei of the cells that penetrated the transwell were stained with 4,6-diamidino-2-phenylindole. V600E+/p53sh melanocytes can migrate efficiently through the transwell similar to melanoma cells. Scale bar = 100 μm. D: Soft agar assay. Control or V600E+ melanocytes were not able to survive in the soft agar. A few single cells or 2- to 3-cell clusters of p53sh melanocytes were present in the soft agar after 6 weeks, while V600E+/p53sh melanocytes were able to proliferate and form large colonies. Since p53 shRNA vectors also contained GFP, these colonies showed green fluorescence. Scale bars = 200 μm. The number of cell clusters in each well were counted and averaged, V600E+/p53sh melanocytes formed significantly more cell clusters. The experiments were repeated at least three times. **P < 0.01 compared to control.
V600E+/p53sh Melanocytes Acquire a Transformed Cell Phenotype in Vitro
Growth factor-independent proliferation is often associated with malignant transformation.27 For optimal growth, human melanocytes normally require basic fibroblast growth factor, stem cell factor, or 12-O-tetradecanoyl phorbol 13-acetate.28 To test whether V600E+/p53sh melanocytes have acquired growth factor-independence, we cultured these cells in a minimal medium (Tu 2%) that supports the growth of melanoma cells, but not normal melanocytes. While control melanocytes and V600E+ melanocytes were not able to survive in this medium, p53sh melanocytes proliferated for the first 2 days and subsequently reached a growth plateau. In contrast, V600E+/p53sh melanocytes continued to proliferate indefinitely, suggesting that these cells have acquired the ability to grow in the absence of growth factors that are essential for normal melanocytes (Figure 2B).
Because the morphology of the V600E+/p53sh melanocytes resembled melanoma cells, we sought to determine whether they also possessed biological properties often associated with transformed cells. In a Matrigel migration assay, V600E+/p53sh melanocytes migrated through Matrigel at a similar rate as malignant SK-Mel-28 melanoma cells (Figure 2C). To test whether these cells are capable of anchorage-independent growth, soft agar assays were performed. As expected, control melanocytes and V600E+ melanocytes did not survive in soft agar. Only single cells or 2 to 3 cell clusters were observed for p53sh melanocytes after 6 weeks in soft agar. However, V600E+/p53sh melanocytes were able to form large and many colonies within 6 weeks (Figure 2D). These results indicate that V600E+/p53sh melanocytes have acquired a transformed cell phenotype in vitro.
V600E+/p53sh Melanocytes Acquire Features of in Situ Melanoma Cells in Artificial Human Skin Reconstructs
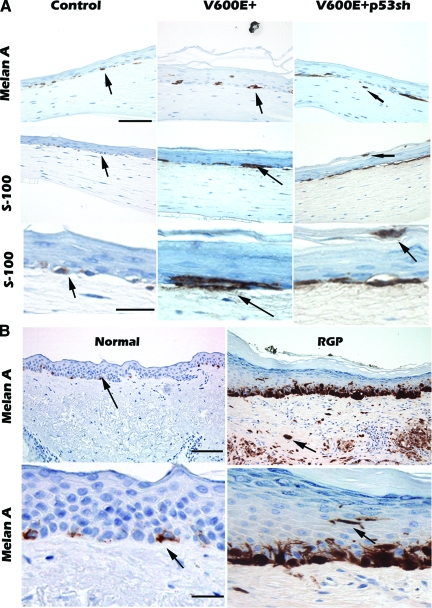
To study the interaction of V600E+ and V600E+/p53sh melanocytes with epidermal keratinocytes and dermal fibroblasts, we introduced these melanocytes into artificial human skin reconstructs that mimic human skin architecture.18 As expected, normal melanocytes migrated to the dermal-epidermal (d-e) junction with non-continuous distribution (Figure 3A, control). V600E+ melanocytes also migrated to the d-e junction with non-continuous distribution, however, the cell size was increased and occasional aggregates of melanocytes were present at the d-e junction; aggregation is a characteristic feature of nevus cells (Figure 3A, V600E+). In contrast, V600E+/p53sh melanocytes demonstrated focal continuous distribution with occasional pagetoid proliferation (Figure 3A, V600E+p53sh). In addition, rare cells were observed in the dermis, a morphology that is similar to in situ melanoma with microinvasion. Normal melanocytes in human skin tissue show non-continuous distribution at the dermal epidermal junction (d-e junction) (Figure 3B, normal), while in situ melanoma often shows continuous distribution of enlarged melanoma cells at the d-e junction with pagetoid proliferation (upward movement of melanocytes to the granular layer of the epidermis) (Figure 3B, radial growth phase). Therefore, V600E+/p53sh melanocytes have acquired at least some features of radial growth phase melanoma cells, such as continuous proliferation near the d-e junction, pagetoid proliferation, and increased cell size.
Figure 3.
V600E+/p53sh melanocytes form in situ melanoma in skin reconstructs. V600E+/p53sh control, V600E+ and V600E+/p53sh were mixed with keratinocytes and laid on top of dermal collagen and fibroblasts. The skin reconstructs were harvested and tissue sections were stained with Melan-A or S-100 to visualize the melanocytes or melanoma cells. A: Normal melanocytes migrated to the d-e junction with non-continuous growth pattern in skin reconstructs. Arrows point to positive single cells. V600E+ melanocytes migrated to the d-e junction with non-continuous growth patterns and occasionally formed nests. The cell size was increased compared with normal melanocytes. Arrows point to single and nested positive cells. V600E+ p53sh melanocytes migrated to the d-e junction with focal continuous growth pattern and pagetoid proliferation (arrows); and rare tumor cells are in the dermis (arrow). The experiments were repeated three times. B: Normal human skin and radial growth phase melanoma are shown for comparison. In normal human skin, melanocytes are present at the d-e junction with non-continuous growth pattern. Arrows point to positive melanocytes. Spontaneous human melanoma in situ with microinvasion, melanoma cells are present at the d-e junction with continuous growth pattern, pagetoid proliferation, and invasive tumor cells in the dermis (arrow). Scale bars in low power view = 100 μm; scale bars in high power view = 20 μm.
V600E+/p53sh Melanocytes Are Weakly Tumorigenic in Vivo
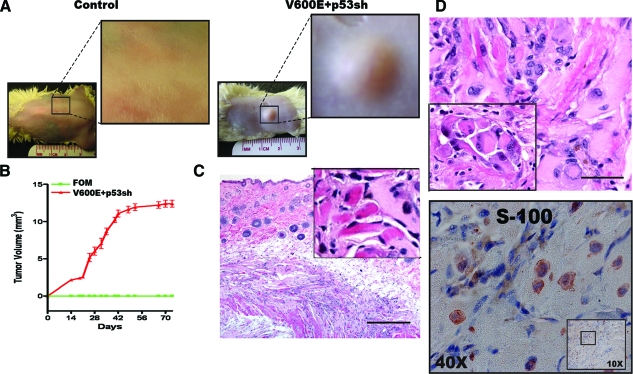
To determine whether the transformed melanocytes were tumorigenic in vivo, 2 × 106 normal or V600E+/p53sh melanocytes were subcutaneously injected into the flanks of non-obese diabetic-severe combined immunodeficiency mice (10 mice each group). Tumor volume was monitored closely for 9 weeks, at which time the animals were sacrificed, tumors were excised, and tissue was preserved for analysis. As expected, normal melanocytes were not able to form tumors in these mice (Figure 4A, control), but V600E+/p53sh melanocytes formed palpable masses after 2 weeks in 100% of animals (Figure 4A, V600E+/p53sh). However, the tumor growth reached a plateau after 6 weeks and these mice were sacrificed shortly thereafter (Figure 4B). Histology of the xenografts showed that tumor cells had infiltrated the muscle (Figure 4C), and these cells had enlarged nuclei and prominent nucleoli, some with nuclear inclusions (Figure 4D). The morphology is similar to that of melanoma cells in tissues (Figure 4D, inset). However, evidence of metastatic melanoma was not seen in these mice. The tumor cells were positive for both S-100 (Figure 4D) and tyrosinase (not shown). Immunohistochemical stain of Ki-67 was performed and only rare tumor cells were positive and few blood vessels were present in the xenografts (data not shown). These results indicate that V600E+/p53sh melanocytes are tumorigenic in vivo, but the tumorigenecity is weak and these cells are not yet capable of metastasis.
Figure 4.
V600E+/p53sh melanocytes are tumorigenic in vivo. A: Control and V600E+p53sh melanocytes were injected subcutaneously into non-obese diabetic-severe combined immunodeficiency mice. Control melanocytes were not able to form tumors. V600E+p53sh melanocytes were able to form xenografts in 100% of injected mice (10/10). B: Tumor growth curve. The tumors became palpable after 2 weeks and continued to grow for 6 weeks and reach a plateau. C: Xenograft-derived tumor cells infiltrated the muscle fibers. Inset: tumor cells infiltrating eosinophilic skeletal muscle fibers. D: V600E+/p53sh melanocytes in the xenografts showed severe nuclear atypia and had morphological features of human melanoma cells. Inset: histology of human melanoma cells for comparison. V600E+/p53sh melanocytes within the xenografts were positive for S100 (below). Scale bar in (C) = 100 μm; scale bar in (D) = 20 μm.
Dysregulation of p53 Pathway during Progression from Nevi to Melanomas
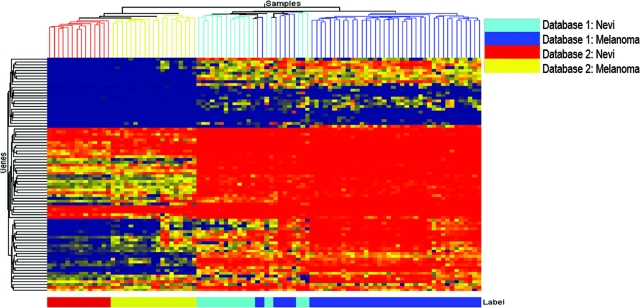
The expression of normal or high levels of p53 protein in melanoma cells is intriguing, as melanoma cells are highly resistant to apoptosis. Although the exact mechanism is unknown, this paradoxical observation may be explained by dysregulated p53 functionality in melanoma cells. Previous studies have demonstrated that activation of p53 observed in melanoma cell lines does not correlate with the regulation of its target genes, suggesting functional inactivation of p53 in melanomas.16 To demonstrate alteration in the p53 transcriptional activity during melanocytic tumor progression, we compared p53-target gene expression using gene expression profiling data from two independent studies. The first database included 18 nevi and 45 melanomas, and the second included 14 nevi and 19 melanomas. Because p53-target genes are not discriminate from one tissue to another,29 we included genes that have been identified as p53-target genes in the literature30,31,32,33,34 for analysis. This set included 97 Affymetrix probesets (some genes had multiple probesets; see supplemental Table S1 at http://ajp.amjpathol.org for the probe sets included for each set). Using these confirmed p53-target genes, we found that a majority of benign nevi can be separated from melanomas (59 of 63 samples were correctly classified) in the first data set. These p53-target genes were able to completely stratify nevi from melanomas in the second data set (Figure 5). These data demonstrate that there are indeed alterations in the p53-target gene expression during the progression from nevi to melanomas and suggest that, despite a lack of genetic mutations, the p53 pathway becomes dysfunctional in malignant melanoma.
Figure 5.
Disruption of p53 target gene expression in melanomas. Gene expression of a panel of p53 transcriptional targets in two independent datasets (left heatmap describes database 2, 14 nevi and 19 melanomas and right heatmap describes database 1, 18 nevi and 45 melanomas). This p53 gene signature set was able to separate a majority of nevi from melanomas in the first dataset (right) and 100% of nevi from melanoma in the second dataset (left).
Genomic Instability and Inactivation of Rb Pathway in Transformed V600E+/p53 sh Melanocytes
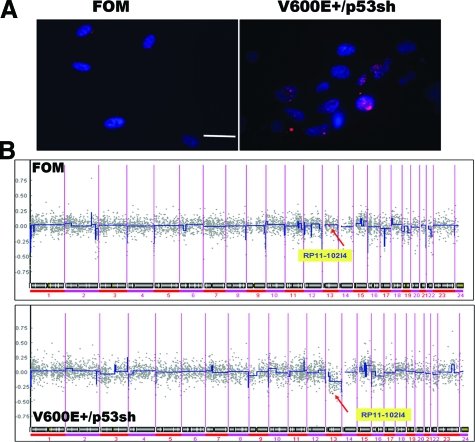
Inactivation of p53 is known to induce genomic instability.35 H2AX, a variant form of histone H2A, is phosphorylated on a serine four residues from the carboxyl terminus in response to the introduction of DNA double-strand breaks,36 and high endogenous levels of phosphorylated H2AX are a consequence of chromatin instability.37 To examine whether p53 knockdown in melanocytes induces genomic instability, we studied H2AX phosphorylation in V600E+/p53sh melanocytes and showed that over 50% of cells are highly-positive for phospho-H2AX, as opposed to less than 10% for control melanocytes, indicating genomic instability in these cells (Figure 6A). We then performed aCGH on the transformed cells and compared genome-wide abnormalities to control melanocytes. Representative whole-genome DNA copy number profile for V600E+/p53sh and parental control melanocytes are shown in Figure 6B. A major genomic deletion in chromosome 13p14, where the tumor suppressor gene Rb1 is located, was observed in V600E+/p53sh melanocytes. Although the exact mechanism underlying the deletion is unclear, these data suggest that mutant BRAF and p53 inactivation significantly increase genetic instability and subsequent genetic amplifications/deletions may occur to contribute to the transformation of V600E+/p53sh melanocytes, in this case, loss of the Rb1 locus.
Figure 6.
Genomic instability and disruption of Rb1 locus during V600E+/p53sh melanocyte transformation. A: Control and V600E+/p53sh melanocytes were stained an antibody against phosphorylated H2AX, and nuclei were visualized with 4,6-diamidino-2-phenylindole. Scale bar = 10 μm. There is significantly increased H2AX phosphorylation in V600E+/p53sh melanocytes. B: Representative whole-genome DNA copy number profile for V600E+/p53sh melanocytes and control FOM melanocytes. Log2 ratios for each of the bacterial artificial chromosomes are plotted according to chromosome position using a “moving average smoother” with a three-clone window. A substantial deletion in chromosome 13p14, where Rb1 is located, is shown in the V600E+/p53sh melanocytes, but not parental control cells (arrows point to the described mutations).
Discussion
In this study, we present surprising findings that a subpopulation of primary human melanocytes can survive mutant BRAF-induced senescence and that those cells acquire early transformed cell phenotypes after disruption of the p53 pathway. The transformed melanocytes are weakly tumorigenic in vivo, and they are not capable of metastasis. This is, to our knowledge, the first report detailing a strategy for transforming human primary melanocytes without the use of human telomerase reverse transcriptase or SV40, often used to immortalize primary cells; these data provide a biological foundation to help elucidate the roles of the MAPK and p53 pathway in the etiology of melanoma without artificial factors, such as SV40.
We have previously shown that a combination of growth factors and UV radiation can transform human melanocytes in vivo.18,22 Others have demonstrated that human melanocytes can be fully transformed to melanoma cells without UV radiation using a combination of SV40, human telomerase reverse transcriptase, and Ras or C-Met; a separate study detailed the use of human telomerase reverse transcriptase, p53, the Rb pathway, and Ras or phosphoinositide-3 kinase.38,39 The results presented here are supported by these earlier studies because activation of the MAPK pathway and inhibition of p53 are central features of each of these studies, albeit through varying methods (SV40 or dominant-negative mutants against p53). However, unlike the previous studies, the transformed V600E+/p53sh melanocytes are weakly tumorigenic and the tumor growth reaches a plateau after 6 weeks. Transgene down-regulation in vivo has been well documented,40 hence we hypothesize that the cessation of tumor growth is at least partially due to down-regulation of V600E+ transgene in the xenografts. Nevertheless, additional genetic alterations are likely needed for these cells to become fully metastatic in vivo.
It is intriguing to speculate about the differences between the subpopulation of melanocytes that are more susceptible to transformation and those cells that enter oncogene-induced senescence. Our results are in agreement with a recent study by Green and colleagues in which approximately 5% of melanocytes remained alive and retained bromodeoxyuridine labeling after BRAFV600E infection; however, their study did not focus on these surviving cells.6 The possibility exists that this subpopulation is comprised primarily of melanocyte precursor cells, which are more resistant to oncogene-induced senescence. The surviving cells expressed heightened levels of nestin (data not shown), a type VI intermediate filament protein, which is often expressed by neural stem/progenitor cells.41 We have previously shown that nestin-positive hair bulge neural crest stem cells can be differentiated to melanocytes.21 Since melanocytes are neural crest-derived, we cautiously postulate that primary human foreskin melanocytes contain a subpopulation of progenitor cells and, unlike mature melanocytes, they are less susceptible to mutant BRAF-induced senescence. However, this remains speculative and only prospective isolation of these progenitor cells may allow us to test this hypothesis.
Melanomas often arise from pre-existing nevi,17 suggesting that cell cycle arrest in nevi may be overcome by additional genetic or epigenetic changes. The possibility that loss of p53 function is involved in promoting melanoma from a benign nevus is plausible. Indeed, transgenic animal models have demonstrated that growth arrest in BRAFV600E-induced benign melanocytic or epithelial tumors can be overcome through concomitant mutation of TP53.3,4 p53 inactivation can cooperate with activated RAS to promote the development of cutaneous melanomas that are clinically indistinguishable from those arisen on the INK4a(Delta2/3) null background.13 Thus, disruption of the p53 pathway promotes a detour of BRAFV600E–induced senescence during tumor development. Related studies have shown that activation of MEK permanently arrests primary murine fibroblasts, but enhances mitogenesis and transformation in cells also lacking p53.42 Still other studies contradict these data by showing that loss of TP53 does not enable escape from oncogene-induced senescence in human fibroblasts.43,44 However, these studies used alternative oncogenes and did not involve primary human melanocytes. Weinberg and his colleagues have shown that expression of melanocyte-specific factors present before neoplastic transformation play a pivotal role in governing the outcome of melanocytes after oncogenic insult38 and, recently, it has been noted that TP53 is indeed one of several genes that are required for bypass of BRAFV600E-mediated senescence in human melanocytes.6 Thus, the role of p53 during oncogene-induced senescence appears to be both species- and cell type-specific, and functional p53 appears to be critical for BRAFV600E-induced senescence in human melanocytes.
p53 has a central role in skin pigmentation, and may impact on melanoma initiation and progression; however, the role of p53 in melanoma has been somewhat under-appreciated due to it’s low mutation frequency.8 The function of p53 in melanoma may be dysregulated by a myriad of mechanisms, including dysregulation of its target genes such as APAF-145 and p53-up-regulated modulator of apoptosis.46 Using two independent gene expression profiling data sets of human melanocytic lesions, we showed that there are indeed alterations in p53-target gene expression during progression from nevi to melanomas. The results support a dysfunctional p53 pathway in melanoma in vivo.
Inactivation of p53 increased endogenous H2AX phosphorylation and genomic instability in V600E+/p53sh melanocytes. The transformed phenotype of V600E+/p53sh melanocytes appears to result from concomitant deletion in a key locus on chromosome 13 that encodes the Rb1 gene. This genomic aberration is likely a consequence of genetic instability incurred through increased stress at DNA replication forks via BRAFV600E overexpression47,48,49,50 and the lack of p53 to inhibit cell cycle progression and initiate apoptosis. Acquisition of this genomic deletion may affect the p16/Rb pathway in such a manner that cyclin D1 and CDK4 are able to drive cell cycle progression without entering the G1 restriction point.35 Inactivation of Rb and p53 pathways in melanoma is frequently achieved through genetic loss of the CDKN2A locus.51 Analyses of three critical human melanoma loci—CDKN2A, TP53, and CDK4—have shown that nearly 80% of melanoma cell lines harbor genetic evidence of impaired Rb and p53 pathway functionality.52 The observation that interference with both Rb and p53 pathway function is oncogenic in the context of disruption of the BRAF pathway is intriguing and allows for speculation regarding novel drug formulations that may target these pathways for therapeutic benefit in melanoma.
Therefore, our data suggest that the BRAFV600E mutation is a founder event in melanocytic neoplasia and inactivation of both p53 and Rb pathways in a subpopulation of human melanocytes harboring BRAFV600E appears sufficient to transform these cells. The interaction between the mutant BRAF and dysfunctional p53 pathway may represent an important mechanism underpinning the genesis of melanoma. Additional biomechanistic studies are currently underway to further delineate the interaction of mutant BRAF and p53 pathways in our system and those results will surely help elucidate the roles of these signaling cascades in melanoma genesis.
Supplementary Material
Acknowledgments
We thank Drs. Dorothy Herlyn and Maria Soengas for providing DNA constructs and Dr. J. Li for suggestions on the manuscript.
Footnotes
Address reprint requests to Xiaowei Xu, M.D., Ph.D., Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 3400 Spruce Street, Philadelphia, PA 19104, E-mail: xug@mail.med.upenn.edu; or Meenhard Herlyn, DMV, The Wistar Institute, 3601 Spruce Street, Philadelphia, PA, 19104, E-mail: herlynm@wistar.org.
Supported by Specialized Program of Research Excellence (SPORE) on Skin Cancer (CA-093372) 5R21CA116103 and Melanoma Research Foundation.
H.Y. and R.M. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD, Berghmans S, Mayhall EA, Traver D, Fletcher CD, Aster JC, Granter SR, Look AT, Lee C, Fisher DE, Zon LI. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- Box NF, Terzian T. The role of p53 in pigmentation, tanning and melanoma. Pigment Cell Melanoma Res. 2008;21:525–533. doi: 10.1111/j.1755-148X.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Blaszyk H, Cunningham JS, McGovern RM, Schroeder JS, Helander SD, Pittelkow MR, Sommer SS, Kovach JS. Overexpression and mutations of p53 in metastatic malignant melanomas. Int J Cancer. 1996;67:313–317. doi: 10.1002/(SICI)1097-0215(19960729)67:3<313::AID-IJC1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Weiss J, Schwechheimer K, Cavenee WK, Herlyn M, Arden KC. Mutation and expression of the p53 gene in malignant melanoma cell lines. Int J Cancer. 1993;54:693–699. doi: 10.1002/ijc.2910540427. [DOI] [PubMed] [Google Scholar]
- Sparrow LE, Soong R, Dawkins HJ, Iacopetta BJ, Heenan PJ. p53 gene mutation and expression in naevi and melanomas. Melanoma Res. 1995;5:93–100. doi: 10.1097/00008390-199504000-00004. [DOI] [PubMed] [Google Scholar]
- Perlis C, Herlyn M. Recent advances in melanoma biology. Oncologist. 2004;9:182–187. doi: 10.1634/theoncologist.9-2-182. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Bastian BC, Hezel A, Pinkel D, Depinho RA, Chin L. Dual inactivation of RB and p53 pathways in RAS-induced melanomas. Mol Cell Biol. 2001;21:2144–2153. doi: 10.1128/MCB.21.6.2144-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Thomas NE, Cregger M, Berger AJ, Leslie M, Torrice C, Hao H, Penland S, Arbiser J, Scott G, Zhou T, Bar-Eli M, Bear JE, Der CJ, Kaufmann WK, Rimm DL, Sharpless NE. Lack of extracellular signal-regulated kinase mitogen-activated protein kinase signaling shows a new type of melanoma. Cancer Res. 2007;67:1502–1512. doi: 10.1158/0008-5472.CAN-06-3311. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Contractor R, Haass NK, Kulp AN, Tilla-Gokcumen GE, Williams DS, Bregman H, Flaherty KT, Soengas MS, Meggers E, Herlyn M. An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67:209–217. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- Haapajarvi T, Pitkanen K, Laiho M. Human melanoma cell line UV responses show independency of p53 function. Cell Growth Differ. 1999;10:163–171. [PubMed] [Google Scholar]
- Bevona C, Goggins W, Quinn T, Fullerton J, Tsao H. Cutaneous melanomas associated with nevi. Arch Dermatol. 2003;139:1620–1624. doi: 10.1001/archderm.139.12.1620. [DOI] [PubMed] [Google Scholar]
- Berking C, Takemoto R, Satyamoorthy K, Shirakawa T, Eskandarpour M, Hansson J, VanBelle PA, Elder DE, Herlyn M. Induction of melanoma phenotypes in human skin by growth factors and ultraviolet B. Cancer Res. 2004;64:807–811. doi: 10.1158/0008-5472.can-03-3438. [DOI] [PubMed] [Google Scholar]
- Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005;115:3166–3176. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SM, Yu H, Edwards R, Chen L, Kazianis S, Brafford P, Acs G, Herlyn M, Xu X. Mutant V600E BRAF increases hypoxia inducible factor-1alpha expression in melanoma. Cancer Res. 2007;67:3177–3184. doi: 10.1158/0008-5472.CAN-06-3312. [DOI] [PubMed] [Google Scholar]
- Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–1888. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking C, Takemoto R, Satyamoorthy K, Elenitsas R, Herlyn M. Basic fibroblast growth factor and ultraviolet B transform melanocytes in human skin. Am J Pathol. 2001;158:943–953. doi: 10.1016/S0002-9440(10)64041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, Atkins D, Wang Y. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11:7234–7242. doi: 10.1158/1078-0432.CCR-05-0683. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greshock J, Naylor TL, Margolin A, Diskin S, Cleaver SH, Futreal PA, deJong PJ, Zhao S, Liebman M, Weber BL. 1-Mb resolution array-based comparative genomic hybridization using a BAC clone set optimized for cancer gene analysis. Genome Res. 2004;14:179–187. doi: 10.1101/gr.1847304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, bdel-Malek ZA, Marais R, Wynford-Thomas D, Bennett DC. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wossning T, Herzog S, Kohler F, Meixlsperger S, Kulathu Y, Mittler G, Abe A, Fuchs U, Borkhardt A, Jumaa H. Deregulated Syk inhibits differentiation and induces growth factor-independent proliferation of pre-B cells. J Exp Med. 2006;203:2829–2840. doi: 10.1084/jem.20060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, Kim NH, Choi WI, Youm YH. Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. J Invest Dermatol. 2005;124:976–983. doi: 10.1111/j.0022-202X.2005.23667.x. [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Sax JK, El-Deiry WS. p53 downstream targets and chemosensitivity. Cell Death Differ. 2003;10:413–417. doi: 10.1038/sj.cdd.4401227. [DOI] [PubMed] [Google Scholar]
- Rozan LM, El-Deiry WS. p53 downstream target genes and tumor suppression: a classical view in evolution. Cell Death Differ. 2007;14:3–9. doi: 10.1038/sj.cdd.4402058. [DOI] [PubMed] [Google Scholar]
- Laptenko O, Prives C. Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 2006;13:951–961. doi: 10.1038/sj.cdd.4401916. [DOI] [PubMed] [Google Scholar]
- Cheah PL, Looi LM. p53: an overview of over two decades of study. Malays J Pathol. 2001;23:9–16. [PubMed] [Google Scholar]
- Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–858. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- Yu T, MacPhail SH, Banath JP, Klokov D, Olive PL. Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA Repair (Amst) 2006;5:935–946. doi: 10.1016/j.dnarep.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, Naber SP, Weinberg RA. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37:1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudnovsky Y, Adams AE, Robbins PB, Lin Q, Khavari PA. Use of human tissue to assess the oncogenic activity of melanoma-associated mutations. Nat Genet. 2005;37:745–749. doi: 10.1038/ng1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Rosenblad C, Andsberg K, Moller A, Lundberg C, Bjorlund A, Johansen TE. Evaluation of Tet-on system to avoid transgene down-regulation in ex vivo gene transfer to the CNS. Gene Ther. 2002;9:1291–1301. doi: 10.1038/sj.gt.3301778. [DOI] [PubMed] [Google Scholar]
- Gilyarov AV. Nestin in central nervous system cells. Neurosci Behav Physiol. 2008;38:165–169. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- Lin AW, Barradas M, Stone JC, van AL, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C, Lowe SW. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Haemel AK, Wood GS. Apoptosis and melanoma: molecular mechanisms. J Pathol. 2003;199:275–288. doi: 10.1002/path.1300. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre' M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d'Adda di Fagagna F. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsutake N, Knauf JA, Mitsutake S, Mesa C, Jr, Zhang L, Fagin JA. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. 2005;65:2465–2473. doi: 10.1158/0008-5472.CAN-04-3314. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Kannan K, Xu J, Bosenberg MW, Chin L. Both products of the mouse Ink4a/Arf locus suppress melanoma formation in vivo. Oncogene. 2003;22:5055–5059. doi: 10.1038/sj.onc.1206809. [DOI] [PubMed] [Google Scholar]
- Yang G, Rajadurai A, Tsao H. Recurrent patterns of dual RB and p53 pathway inactivation in melanoma. J Invest Dermatol. 2005;125:1242–1251. doi: 10.1111/j.0022-202X.2005.23931.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.