Abstract
The integrin α5β1 has been previously implicated in tumor angiogenesis, but its role in the remodeling of both blood vessels and lymphatics during inflammation is at an early stage of understanding. We examined this issue using a selective, small-molecule inhibitor of α5β1 integrin, 2-aroylamino-3-{4-[(pyridin-2-ylaminomethyl)heterocyclyl]phenyl}propionic acid (JSM8757), in a model of sustained airway inflammation in mice with Mycoplasma pulmonis infection, which is known to be accompanied by robust blood vessel remodeling and lymphangiogenesis. The inhibitor significantly decreased the proliferation of lymphatic endothelial cells in culture and the number of lymphatic sprouts and new lymphatics in airways of mice infected for 2 weeks but did not reduce remodeling of blood vessels in the same airways. In inflamed airways, α5 integrin immunoreactivity was present on lymphatic sprouts, but not on collecting lymphatics or blood vessels, and was not found on any lymphatics of normal airways. Macrophages, potential targets of the inhibitor, did not have α5 integrin immunoreactivity in inflamed airways. In addition, macrophage recruitment, assessed in infected airways by quantitative reverse transcription-polymerase chain reaction measurements of expression of the marker protein ionized calcium-binding adapter molecule 1 (Iba1), was not reduced by JSM8757. We conclude that inhibition of the α5β1 integrin reduces lymphangiogenesis in inflamed airways after M. pulmonis infection because expression of the integrin is selectively increased on lymphatic sprouts and plays an essential role in lymphatic growth.
Integrins, a multifunctional family of heterodimeric transmembrane receptors,1,2 play an important role in embryogenesis, hemostasis, immune responses, and tumor progression3,4,5 by binding to the extracellular matrix and other cellular adhesion receptors.3,6,7,8 Integrin engagement leads to activation of signaling processes that are essential for regulating cell survival, proliferation, and migration. Some integrins act in concert with soluble growth factors.9,10
Multiple integrins, including αvβ3, αvβ5, and α5β1, have been implicated in angiogenesis, and α5β1, α1β1, α2β1, α4β1, and α9β1 are thought to participate in lymphangiogenesis.1,11,12,13,14,15 The α5β1 integrin, also known as very late antigen-5, is the only integrin heterodimer that contains the α5 integrin subunit3,16,17 α5β1 integrin functions as a receptor for fibronectin and certain other extracellular matrix proteins.16
a5β1 integrin has little or no expression in most quiescent endothelial cells but is up-regulated in blood vessels of tumors.1,5,18 Exceptions are normal hepatic sinusoids and high endothelial venules of lymph nodes, which have strong α5 integrin immunoreactivity.19
The α5β1 integrin has an essential role in blood vessel development. Loss of the gene encoding α5 integrin subunit leads to embryonic lethality in mice.7,20 Inhibition of α5β1 integrin reduces growth factor-induced angiogenesis, tumor angiogenesis in chick chorioallantoic membrane assays,8 tumor growth rate,21 and choroidal neovascularization in animal models.22,23
Lymphatic vessels form a system of tubes with unidirectional flow to return extravasated protein-rich fluids in tissues back into the bloodstream.24,25 Lymph fluid and cells enter initial lymphatics, which are blind-ended vessels with valve-like openings between endothelial cells joined by unique button-like intercellular junctions.25 Collecting lymphatics, which have tight, zipper-like endothelial cell junctions and have smooth-muscle cells to propel lymph and valves to prevent backflow, deliver lymph fluid via lymph nodes into the venous circulation.24,26 In addition to their role in maintenance of tissue homeostasis, lymphatics are important in immune surveillance and inflammation27,28 and serve as routes for metastatic spread of cancer cells.26,29,30
Growth of new lymphatics from existing lymphatic vessels (lymphangiogenesis) is an integral part of many pathological conditions. The action of vascular endothelial growth factor (VEGF)-C and VEGF-D on VEGF receptor-3 (VEGFR-3) plays a central role in lymphangiogenesis.31,32,33 Lymphatic vessel growth under many conditions can be blocked by inhibition of VEGFR-3 signaling.24,34
Several lines of evidence are consistent with a role of α5β1 integrin in lymphangiogenesis mediated through VEGFR-3 signaling. Human lymphatic endothelial cells in culture express α5 integrin,15,35 and α5 integrin immunoreactivity has been found on some lymphatics in mouse eyes.11 Integrin α5β1 is important for activation of VEGFR-3 signaling in vitro, and integrin subunits α5 or β1 are associated with activated VEGFR-3.35 Selective inhibition of α5β1 integrin reduces lymphangiogenesis in a mouse model of suture-induced corneal inflammation.11 Furthermore, endostatin, which can inhibit endothelial cell migration by binding to α5β1 integrin,36 reduces lymphangiogenesis in skin tumors.37
The mouse tracheal mucosa has a distinctive segmented vascular architecture, in which repetitive arrangements of blood vessels and lymphatics are aligned with the framework of cartilage rings.38 Blood capillaries span the rings in a ladder-like pattern, and arterioles, venules, and lymphatics are restricted to the regions between the rings.31 This stereotype architecture makes it straightforward to detect and quantify the growth and remodeling of blood vessels and lymphatics in inflammatory conditions.31
Respiratory tract infection caused by Mycoplasma pulmonis results in conspicuous growth and remodeling of blood vessels and lymphatics in the airways.31,39,40,41 We used the vascular changes accompanying M. pulmonis infection to determine whether α5β1 integrin plays an essential role in blood vessel remodeling and lymphangiogenesis in airway inflammation. The role of α5β1 integrin was tested by using 2-aroylamino-3-{4-[(pyridin-2-ylaminomethyl)heterocyclyl]phenyl}propionic acid (JSM8757), a novel selective small molecule inhibitor of α5β1 integrin function. JSM8757 is an orally available analogue of JSM6427, which was previously shown to inhibit choroidal neovascularization and inflammatory lymphangiogenesis in the cornea.11,22,42
Our approach was first to determine the distribution of α5β1 integrin in the trachea by using α5 integrin subunit immunoreactivity as a readout. Next, we examined the efficacy of JSM8757 in inhibiting proliferation of cultured lymphatic endothelial cells and then on vascular remodeling and lymphangiogenesis after M. pulmonis infection. We found that the α5β1 integrin blockade reduced lymphatic sprouting and growth in airway inflammation but did not reduce blood vessel remodeling or macrophage recruitment.
Materials and Methods
Mice
Specific pathogen-free 8-week-old female C57BL/6 mice (Charles River, Hollister, CA) were housed under barrier conditions. Mice were anesthetized by intramuscular injection of ketamine (83 mg/kg) and xylazine (13 mg/kg). All experimental procedures were approved by the Institutional Animal Care and Use Committees of the University of California at San Francisco. All reagents were purchased from Sigma unless indicated otherwise.
Integrin Inhibitor JSM8757
JSM8757, which was synthesized at Jerini AG (Berlin, Germany) with a purity of >99%, is a small-molecule antagonist of integrin α5β1. JSM8757 mimics the RGD (Arg-Gly-Asp) recognition motif in fibronectin, where it interacts with α5 and β1 integrin subunits with subnanomolar IC50 values. The agent inhibits α5β1/ligand binding with an IC50 value of 0.9 nmol/L assessed by enzyme-linked immunosorbent assay and an IC50 value of 30 nmol/L in blocking the adhesion of α5β1-positive HEK293 cells to fibronectin. JSM8757 has much higher affinity for α5β1 integrin than for other RGD-binding integrins, with IC50 values >670-fold greater for αvβ3 integrin, >13,300-fold greater for αvβ5 integrin, and >34,000-fold greater for αIIbβ3 integrin. The enzyme-linked immunosorbent assay and IC50 determination was performed as described previously.42
Mouse Model of Inflammation
Mice at 8 weeks of age were anesthetized and inoculated intranasally on day 0 with 50 μl of broth containing 106 colony-forming units of M. pulmonis organisms (strain CT7) as described previously.31 M. pulmonis organisms activate an immune response with a time course similar to other airway infections.43 Mice were concurrently injected intraperitoneally with vehicle or JSM8757 (100 mg/kg) twice a day for 14 days. Previous pharmacokinetic studies show that this dose of JSM8757 results in plasma concentrations (Cmax) of approximately 40 μmol/L. At 14 days after M. pulmonis infection, mice were anesthetized again and tissues were harvested for further studies.
Immunohistochemistry
Mice were perfused for 2 minutes with fixative (1% paraformaldehyde in phosphate-buffered saline; PBS, pH 7.4) from a cannula inserted through the left ventricle into the aorta.31 Tracheas were removed and immersed in fixative for 1 hour at 4°C. Tissues were washed and stained immunohistochemically by incubating whole mounts or 60-μm cryostat sections with one or more primary antibodies diluted in PBS containing 0.3% Triton X-100, 0.2% bovine serum albumin, 5% normal goat serum, and 0.1% sodium azide, as described previously.31 The following antibodies were used at the indicated concentrations: lymphatics: lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), 1:500 (rabbit polyclonal 07–538; AngioBio, Del Mar, CA); endothelial cells: CD31, 1:500 (hamster anti-mouse PECAM-1, clone 2H8; Thermo, Waltham, MA); monocyte/macrophages: CD11b, 1:500 (rat anti-mouse CD11b, clone M1/70; eBioscience, San Diego, CA), ionized calcium-binding adapter molecule 1 (Iba1), 1:500 (rabbit polyclonal 019-19741; Wako Pure Chemical Industries, Osaka, Japan); α5 integrin/CD49e: 1:500 (rat anti-mouse CD49e, clone 5H10-27; BD Biosciences PharMingen, San Diego, CA). Because α5 integrin subunits (CD49e) pair solely with β1 subunits, anti-α5 integrin antibody was regarded as binding exclusively to α5β1 integrin.6,16,17 Secondary antibodies were labeled with fluorescein isothiocyanate, Cyanine3 (Cy3), or Cy5, 1:500 (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Specimens were viewed with an Axiophot fluorescence microscope (Carl Zeiss) with a 3-CCD low light RGB video camera (CoolCam; SciMeasure Analytical Systems, Atlanta, GA) or a LSM510 confocal microscope (Carl Zeiss) using AIM confocal software (version 3.2.2).
Morphometric Measurements
Measurements were performed using digitizing software interfaced to the Axiophot microscope and CoolCam camera. The number of lymphatic sprouts was measured in projected real-time fluorescent images of tracheal whole mounts. Lymphatic sprouts were counted and defined as tapered LYVE-1-positive projections visible at a screen magnification of 180×, in five regions per trachea, each 1.5 mm2 in area.31 Area densities (percentage of total tissue area) of LYVE-1-positive lymphatics and CD31-positive blood vessels viewed in real-time fluorescent images of tracheal whole mounts were measured by stereological point counting of 10 regions per trachea, each 1.7 mm2 in area.31
Fluorescence Intensity Measurements
The fluorescence intensity was measured as an estimate of the expression of α5 integrin in the mucosa overlying cartilage rings. The fluorescence intensity was measured on digital images stained for α5 integrin immunoreactivity using ImageJ software.44,45 A fluorescence intensity of 15 (range, 0–255) was established as the threshold for distinguishing pixels of α5 integrin immunoreactive tissues from those of the background. The fluorescence intensity represented the average brightness of all α5 integrin immunoreactive tissues in the mucosa overlying cartilage rings. This value was calculated from all pixels with fluorescence intensities ≥15 as described previously.46 The mean value was calculated from 10 images of the regions of interest in each trachea. The distribution of intensity values in the regions of interest was visualized using the surface plot function of ImageJ software.44
Lymphatic Endothelial Cell Proliferation Assay
Human lymphatic microvascular endothelial cells (HMVEC-dLy-Ad-der; Cambrex Bio Science, Walkersville, MD) were cultured in EGM2-MV medium (Cambrex Bio Science) according to instructions of the manufacturer. Ninety-six-well plates were precoated with 10 μg/ml fibronectin (F1904, Chemicon Europe, Hampshire, UK) and blocked with 1% bovine serum albumin. HMVECs were seeded at a density of 4 × 103 cells per well in the presence of indicated concentrations of the integrin α5 small-molecule antagonist (JSM8757). Vehicle dimethyl sulfoxide concentrations were adjusted and did not exceed 0.1%. Cells were grown for 3 days in growth medium containing serum and supplements. Plates were washed once with PBS and adherent cells were fixed with 5% glutaraldehyde for 30 minutes, rinsed three times with PBS, and stained with 0.1% crystal violet for 1 hour. After rinsing with PBS, 10% acetic acid was added and plates were analyzed at 570 nm with a Spectra Max M5 microplate reader (Molecular Devices, Sunnyvale, CA). To determine net cell proliferation, some cells were fixed 3 hours after seeding on day 0. The difference of absorbance on day 0 and on day 3 is the net proliferation. Experiments were performed in triplicate.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Mice were anesthetized and briefly perfused via the aorta with sterile PBS. Tracheas were removed and stored in RNAlater reagent, homogenized, and total RNA was extracted using RNeasy fibrous tissue kit (Qiagen, Hilden, Germany). cDNA was generated using random primers (Invitrogen, Carlsbad, CA) and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Samples of 1 ng of cDNA were subjected to reverse transcription-polymerase chain reaction using SYBR Green protocols and ER qPCR SuperMix Universal (Invitrogen). Primers were designed using data from PrimerBank (http://pga.mgh.harvard.edu/primerbank, last accessed April 23, 2008) or published sequences from the literature. The forward and reverse primers were: β-actin, 5′-GAAGCTGTGCTATGTTGCTCTA-3′ and 5′-GGAGGAAGAGGATGCGGCA-3′; and Iba1, 5′-ATCAACAAGCAATTCCTCGATGA-3′ and 5′-CAGCATTCGCTTCAAGGACATA-3′. Reverse transcription-polymerase chain reaction analysis was done using a MyiQ reverse transcription-polymerase chain reaction machine (Bio-Rad, Hercules, CA). Samples were tested in duplicate and relative gene expression data were normalized to β-actin. Results are presented as fold increases of mRNA relative to β-actin.
Statistical Analysis
Values are presented as means ± SEM with four or five mice per group unless otherwise indicated. The significance of differences between means was assessed by analysis of variance followed by the Dunn-Bonferroni test for multiple comparisons with P values <0.05 considered significant.
Results
Distribution of α5 Integrin Immunoreactivity in Normal Tracheal Microvasculature
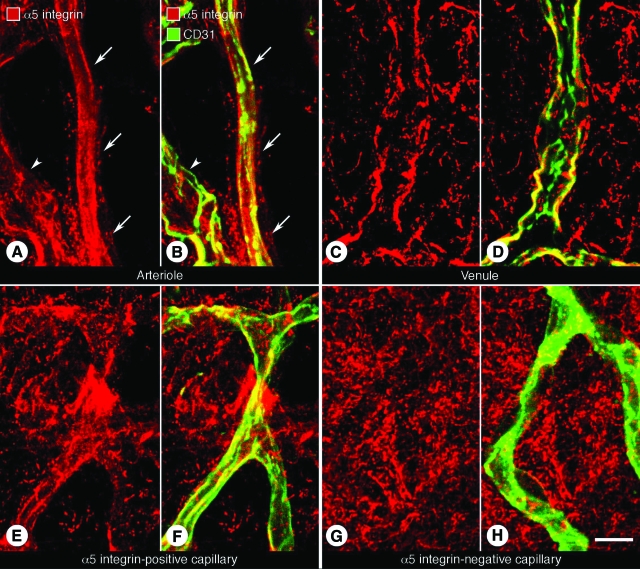
In tracheal whole mounts of pathogen-free mice, CD31-immunoreactive arterioles had mostly diffuse α5 integrin immunoreactivity (Figure 1, A and B). Venules also had α5 integrin immunoreactivity (Figure 1, C and D). Although some capillaries had α5 integrin immunoreactivity (Figure 1, E and F), most had none (Figure 1, G and H). Fibronectin immunoreactivity was widely distributed around the tracheal vasculature (data not shown).
Figure 1.
Distribution of α5 integrin immunoreactivity in normal tracheal blood vessels. Confocal microscopic images of tracheal whole mounts stained for CD31 (green, blood vessels) and α5 integrin (red) immunoreactivities of a small arteriole (A and B, arrows), venule (C and D), and unusual capillary (E and F) from a pathogen-free mouse. G and H: Tracheal capillary, like most tracheal capillaries, without detectable α5 integrin immunoreactivity. Arrowheads in A and B mark a small venule. Scale bar = 10 μm.
Inhibition of Human Lymphatic Endothelial Cell Proliferation by JSM8757
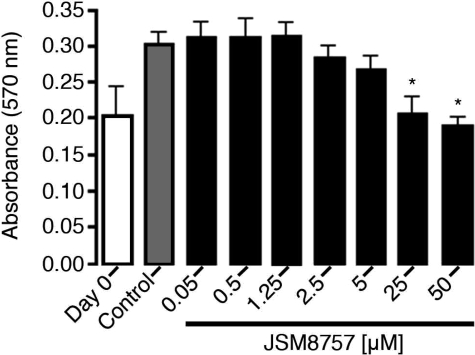
To determine whether α5β1 integrin was essential for proliferation of lymphatic endothelial cells, we examined the effect of JSM8757 on cultured human lymphatic endothelial cells. We found that JSM8757 significantly reduced the proliferation of lymphatic endothelial cells and at concentrations of 25 and 50 μmol/L fully inhibited the net expansion of the cells (Figure 2).
Figure 2.
Inhibition of lymphatic endothelial cell proliferation by JSM8757. Human lymphatic endothelial cells were cultured on fibronectin for 3 days, fixed with 5% glutaraldehyde, stained with crystal violet, and measured for absorbance at 570 nm. The difference between absorbance at day 0 (onset value for cells fixed on day 0) and absorbance of control cells (grown for 3 days in control culture medium containing 0.1% dimethyl sulfoxide) reflected the amount of endothelial cell proliferation. Values were significantly lower when grown in the presence of JSM8757 at concentrations of 25 or 50 μmol/L (*P < 0.01 versus control). Data are from one of three independent experiments. Values are means ± SD.
Effect of JSM8757 on Lymphangiogenesis and Blood Vessel Remodeling in Inflamed Tracheas
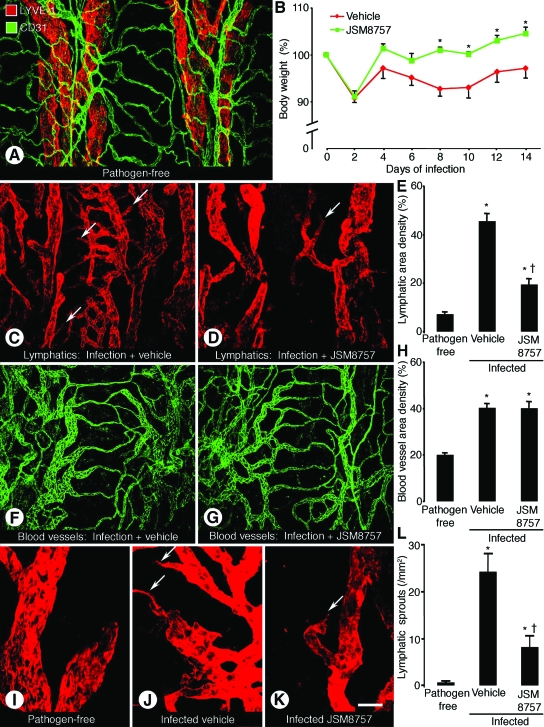
Having confirmed the effect of JSM8757 on lymphatic endothelial cell growth in vitro, we next examined effect of JSM8757 on blood vessel remodeling and lymphangiogenesis in mouse airways after M. pulmonis infection. Blood vessels (strong CD31 immunoreactivity) and lymphatics (strong LYVE-1 immunoreactivity) in tracheas of pathogen-free mice were segmented and aligned with the framework of the cartilage rings. Most arterioles and venules were between the rings, and most capillaries were in the mucosa over the rings (Figure 3A).
Figure 3.
Contrasting effects of JSM8757 on lymphatic growth and blood vessel remodeling. Confocal microscopic images of tracheal whole mounts stained for CD31 (green, blood vessels) and LYVE-1 (red, lymphatics) immunoreactivities in pathogen-free mouse (A) or after M. pulmonis infection for 14 days with concurrent treatment with vehicle or JSM8757 (C, D, F, G, I–K). A: Overview of arrangement of blood vessels and lymphatics in trachea of a pathogen-free mouse. B: Body weights of infected mice treated with vehicle or JSM8757 from day 0 to day 14. Initial body weight was considered 100%. *P < 0.05 compared with corresponding vehicle-treated mice. C and D: Comparison of lymphatics in the trachea of infected mice treated with vehicle (C) or JSM8757 (D). Arrows indicate newly grown lymphatics in the mucosa overlying cartilage rings. E: Area density of lymphatics in tracheal mucosa over cartilage rings in pathogen-free mice or infected mice treated with vehicle or JSM8757. F and G: Similarity of blood vessel caliber in infected mice treated with vehicle (F) or JSM8757 (G). H: Blood vessel area density in the mucosa over cartilage rings in pathogen-free mice or in infected mice treated with vehicle or JSM8757. No sprouts are present on lymphatics in pathogen-free mouse (I), but lymphatics in infected mouse treated with vehicle have numerous sprouts (J, arrows). Fewer sprouts are present on lymphatics of infected mouse treated with JSM8757 (K, arrow). L: Number of lymphatic sprouts in tracheas of pathogen-free or infected mice treated with vehicle or JSM8757. P < 0.05 compared with pathogen-free mice (*) or to vehicle-treated infected mice (†). Values are means ± SEM, five mice per group. Scale bar in K applies to all panels: 80 μm in A, C, D, F, and G; 30 μm in I--K.
After M. pulmonis infection, all mice lost about 10% of their body weight over the first 2 days and then JSM8757-treated mice returned to their original weight and continued to grow. By comparison, after infection, vehicle-treated mice stayed below their original weights (Figure 3B). At 14 days after infection, lymphatics not only were located between cartilage rings but also were abundant over the rings, where none were normally present (Figure 3C). However, mice treated with JSM8757 during the infection had few lymphatics in regions of the tracheal mucosa over the rings (Figure 3D). The area density of lymphatics over rings was significantly greater in M. pulmonis-infected mice than in pathogen-free mice, but was 57% less in JSM8757-treated mice than in vehicle-treated mice (Figure 3E).
Consistent with the vascular remodeling that occurs after M. pulmonis infection,39,40 blood vessels were enlarged. The amount of enlargement was similar in both groups of mice (Figure 3, F and G), as reflected by the increase in area density of CD31-positive blood vessels (Figure 3H). JSM8757 had no significant effect on the amount of enlargement (Figure 3H). Similarly, the mean diameter of tracheal blood vessels was not significantly different in the two groups (12.6 ± 0.6 μm after vehicle compared with 11.9 ± 0.7 μm after JSM8757).
The surface of lymphatics in pathogen-free airways was smooth and lacked sprouts (Figure 3I). After infection, many lymphatics had sprouts (Figure 3J), but sprouts were much more abundant in vehicle-treated mice than in JSM8757-treated mice (Figure 3K). The number of lymphatic sprouts was significantly greater in infected mice than in pathogen-free mice, but JSM8757-treated mice had 66% fewer lymphatic sprouts than vehicle-treated mice (Figure 3L).
α5 Integrin Immunoreactivity of Initial Lymphatics in Inflamed Airways
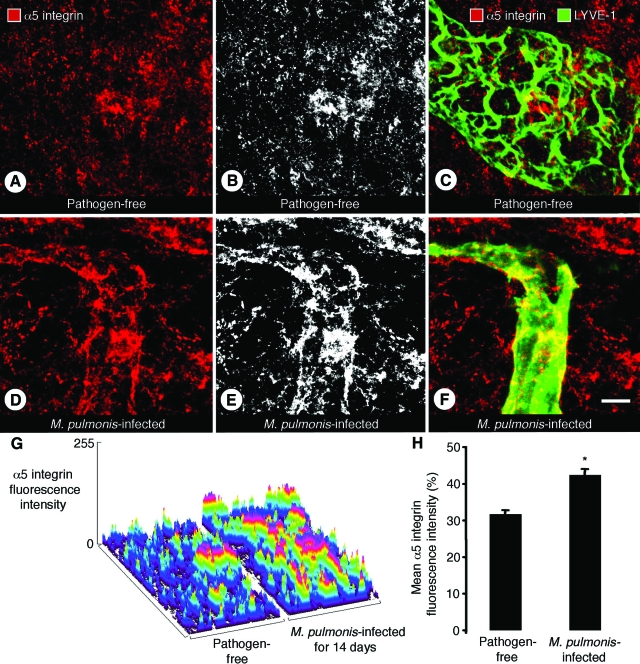
Little or no α5 integrin immunoreactivity was detected on initial lymphatics or collecting lymphatics in tracheas of pathogen-free mice (Figure 4, A–C). However, after infection for 14 days, newly formed lymphatics identified by LYVE-1 immunoreactive vessels over cartilage rings had patchy but clear α5 integrin immunoreactivity, both on sprouts and on initial lymphatics (Figure 4, D–F). The stronger α5 integrin immunoreactivity after infection and the patchy nature of the staining were evident in surface plots of fluorescence intensity (Figure 4G). High-intensity spots were more numerous in infected mice (Figure 4G). The mean intensity of α5 integrin immunofluorescence was 34% greater in infected mice (Figure 4H).
Figure 4.
α5 integrin immunoreactivity of lymphatics in infected tracheas. Confocal microscopic images of tracheal whole mounts, stained for LYVE-1 (green) and α5 integrin (red) immunoreactivities, from pathogen-free mice (A--C) or mice infected with M. pulmonis for 14 days (D–F). A–C: Absence of α5 integrin immunoreactivity (red) on a collecting lymphatic in a pathogen-free mouse shown by lack of colocalization with LYVE-1 (green). B: Gray scale image of A to highlight staining of the lymphatic. D–F: An initial lymphatic in an infected mouse showing patchy α5 integrin immunoreactivity. E: Gray scale image of D. G: Surface plot of the intensity of α5 integrin immunofluorescence in tracheal mucosa over a cartilage ring. H: Mean fluorescence intensity of α5 integrin in mucosa over cartilage rings in infected and pathogen-free mice. Values are means ± SEM *P < 0.05 compared with pathogen-free mice. Five mice per group. Scale bar = 10 μm.
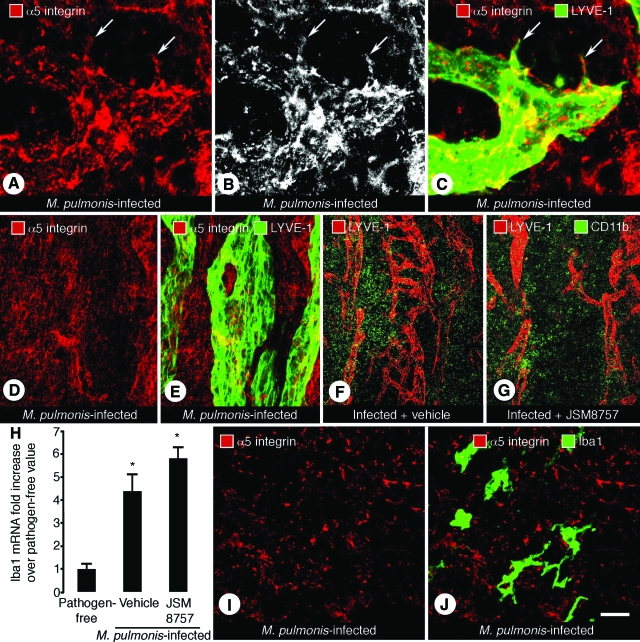
Effect of JSM8757 on Lymphatic Sprouts but Not on Macrophages in Infected Airways
Lymphatic sprouts had patchy but clear α5 integrin immunoreactivity (Figure 5, A–C). No α5 integrin immunoreactivity was found in collecting lymphatics of infected mice (Figure 5, D and E). Tissues around lymphatics also had patchy α5 integrin immunoreactivity (Figure 5, A–F).
Figure 5.
α5 integrin immunoreactivity of lymphatic sprouts but not macrophages in infected airways. Confocal microscopic images of tracheas stained for LYVE-1 (green) and α5 integrin (red) immunoreactivities from mice infected with M. pulmonis for 14 days and concurrently treated with vehicle or JSM8757. A–C: Lymphatic sprouts (arrows) in infected trachea from vehicle-treated mouse. D and E: Collecting lymphatic of infected mouse trachea showing lack of colocalization of α5 integrin (red) and LYVE-1 (green) immunoreactivities. F and G: Lymphatics stained for LYVE-1 (red) and a monocyte/macrophage marker CD11b (green) after vehicle or JSM8757 treatment. The number of CD11b immunoreactive cells was similar between infected tracheas treated with vehicle (F) or JSM8757 (G). H: Quantitative reverse transcription-polymerase chain reaction measurements of Iba1 mRNA expression in macrophages in tracheas of pathogen-free mice and infected mice treated with vehicle or JSM8757. Values are means ± SEM, five mice per group. *P < 0.05 compared with pathogen-free values. I and J: Trachea of infected mouse showing lack of colocalization of α5 integrin (red) and Iba-1 (green) immunoreactivities. Scale bar in J applies to all panels: 10 μm in A–C; 30 μm in D and E; 120 μm in F and G; 15 μm in I and J.
To assess the possible contribution of macrophages as a target of JSM8757 in blocking lymphangiogenesis after M. pulmonis infection, we compared the number of CD11b immunoreactive cells in whole mounts of infected tracheas after vehicle or JSM8757 treatment for 14 days and found no treatment-related difference between the two groups (Figure 5, F and G). We also measured mRNA expression of the macrophage marker protein, ionized calcium-binding adapter molecule 1 (Iba1).46,47 Iba1 mRNA was greater in M. pulmonis-infected mice than in pathogen-free mice (Figure 5H), but the increase was about the same in vehicle-treated mice and JSM8757-treated mice (Figure 5H). Iba1-immunoreactive macrophages in inflamed airways did not have α5 integrin immunoreactivity (Figure 5, I and J).
Discussion
These experiments provided multiple lines of evidence that favor the involvement of α5β1 integrin in lymphangiogenesis after M. pulmonis infection. Sprouting and growth of lymphatics in the airways after infection were significantly reduced by JSM8757, a selective small-molecule inhibitor of α5β1 integrin. Similarly, proliferation of lymphatic endothelial cells in culture was reduced by JSM8757. Unlike lymphatics in pathogen-free airways, lymphatic sprouts and initial lymphatics that formed after infection had α5 integrin immunoreactivity. The effect of JSM8757 on lymphangiogenesis was not a reflection of general toxicity, as the treated mice increased in body weight after infection while their vehicle-treated counterparts lost weight. Indeed, JSM8757 seemed to have had a protective effect after infection. Because JSM8757 did not affect macrophage influx after infection, the antilymphangiogenic action was apparently through a direct effect on tracheal lymphatics.
The presence of α5 integrin immunoreactivity in lymphatic sprouts in inflamed airways, but not in pathogen-free airways, suggests that the integrin is up-regulated in growing lymphatics. To our knowledge, this is the first report of the selective induction of integrin expression in inflamed lymphatics. Although fibroblast-like connective tissue cells in the airway mucosa also had α5 integrin immunoreactivity, colocalization with LYVE-1 and surface plots of α5 integrin immunofluorescence confirmed the presence of the integrin on lymphatics.
In our study, α5 integrin immunoreactivity had a patchy distribution on lymphatics, suggestive of focal adhesion sites. Sprouts on lymphatic endothelial cells were particularly common sites of staining. The immunoreactivity also had a patchy distribution in some regions of the surrounding tissue. Signaling complexes involving integrin α5β1 have also been reported to have a patchy distribution on cultured cells.8
The reduction in sprouting and proliferation of lymphatics that occur after M. pulmonis infection in the presence of JSM8757 is consistent with inhibition of lymphangiogenesis through inhibition of binding of α5β1 integrin to fibronectin, which reduces endothelial cell proliferation and sprouting. α5β1 integrin-mediated signaling has been linked to endothelial cell survival and proliferation. Adhesion via α5 integrin has been shown to support cell proliferation by activation of mitogen-activated protein kinases and transcriptional regulation of cell cycle proteins.48 Antagonists of α5β1 integrin can cause apoptosis of endothelial cells by induction of protein kinase A and activation of caspase-8.49
In addition to blocking binding to fibronectin, JSM8757 may also reduce binding to fibrillin,50 which is another integrin ligand that is a main component of lymphatic anchoring filaments.51 Anchoring filaments are a characteristic feature of initial lymphatics and connect lymphatic endothelial cells to elastic fibers in the perivascular extracellular matrix.51 Integrins α5β1 and αvβ3 are reported to be receptors for fibrillin in some cultured cells.50 If this applies to airway lymphatics, α5β1 integrin blockade could inhibit lymphatic growth by blocking the binding of anchoring filaments to lymphatic endothelial cells. Part of the antilymphangiogenic action of JSM8757 could also result from the association of α5β1 integrin with activation of VEGFR-3.35
Macrophages are believed to be among the immune cells that drive lymphangiogenesis.31,52 As macrophages express α5β1 integrin in some inflammatory conditions,53 a potential mechanism for inhibition of lymphangiogenesis by JSM8757 is prevention of macrophage recruitment. In this context, JSM6427, an α5β1 integrin inhibitor similar to JSM8757, had no effect on macrophage recruitment in a model of corneal inflammation.11 Similarly, we found that macrophages were recruited to infected airways, but the amount of recruitment, assessed by mRNA of the macrophage marker Iba1,46,47 was not significantly reduced by JSM8757. Moreover, we did not detect α5 integrin immunoreactivity on recruited macrophages. These results suggest that in airway inflammation produced by M. pulmonis infection, JSM8757 inhibits lymphangiogenesis by mechanisms other than blocking of macrophage recruitment and favor effects on lymphatic endothelial cells.
In conclusion, in mouse airways, α5β1 integrin was distributed on most blood vessels larger than capillaries, but selective blockade of the integrin did not affect blood vessel remodeling after M. pulmonis infection. In contrast, α5β1 integrin was not found on lymphatics in normal airways but was strongly up-regulated on lymphatic sprouts and new lymphatics, where it had a patchy distribution suggestive of focal adhesion sites. Blockade of α5β1 integrin by JSM8757 significantly reduced lymphangiogenesis after M. pulmonis. Based on these findings, we propose a mechanism by which α5β1 integrin is involved in lymphangiogenesis in inflammation by promoting sprouting and proliferation of lymphatic endothelial cells. Our results suggest a novel approach for inhibiting lymphangiogenesis by exploiting the selective expression of α5β1 integrin on activated lymphatics. Inhibition of lymphangiogenesis could be beneficial in chronic inflammatory airway disease, psoriasis, tumor cell spread via lymphatics, kidney transplant rejection, or other clinical conditions where lymphatic proliferation or abnormalities play a role.24,54
Acknowledgments
We thank Hiroya Hashizume for critical review of the manuscript, Roland Stragies and Ariane Zwintscher (Jerini AG) for contributions to the medicinal chemistry and pharmacokinetics of JSM8757, and Seike Gericke (Jerini AG) for technical assistance in cell assays.
Footnotes
Address reprint requests to Donald M. McDonald, M.D., Ph.D., Department of Anatomy, S1363, University of California, 513 Parnassus Avenue, San Francisco, CA 94143-0452. E-mail: donald.mcdonald@ucsf.edu.
Supported in part by a grant from Jerini AG Berlin to the University of California, San Francisco (to D.M.M.). This research was supported in part by National Institutes of Health grants HL24136 and HL59157 from the National Heart, Lung, and Blood Institute, CA82923 from the National Cancer Institute, and funding from AngelWorks Foundation (to D.M.M.) Research funding was also provided by Jerini AG. T.O. was supported in part by a postdoctoral fellowship from the Uehara Memorial Foundation of Japan.
D.V., G.Zi., G.Za., J.K., and C.C. are employees of Jerini AG.
References
- Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghisi GC, Ruegg C. Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium. 2006;13:113–135. doi: 10.1080/10623320600698037. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Chen J, Feng W, Somanath PR, Razorenova OV, Byzova TV. Integrin affinity modulation in angiogenesis. Cell Cycle. 2008;7:335–347. doi: 10.4161/cc.7.3.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer D, Stragies R, Zahn G, Cursiefen C. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171:361–372. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, Hirashima M, Morisada T, Oike Y, Araie M, Niwa H, Kubo H, Suda T, Miyazono K. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18:1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Makale M, Fuster M, Varner JA. Methods to study lymphatic vessel integrins. Methods Enzymol. 2007;426:415–438. doi: 10.1016/S0076-6879(07)26018-5. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar CC. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- Magnussen A, Kasman IM, Norberg S, Baluk P, Murray R, McDonald DM. Rapid access of antibodies to alpha5beta1 integrin overexpressed on the luminal surface of tumor blood vessels. Cancer Res. 2005;65:2712–2721. doi: 10.1158/0008-5472.CAN-04-2691. [DOI] [PubMed] [Google Scholar]
- Parsons-Wingerter P, Kasman IM, Norberg S, Magnussen A, Zanivan S, Rissone A, Baluk P, Favre CJ, Jeffry U, Murray R, McDonald DM. Uniform overexpression and rapid accessibility of alpha5beta1 integrin on blood vessels in tumors. Am J Pathol. 2005;167:193–211. doi: 10.1016/s0002-9440(10)62965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development. 1997;124:4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- Stupp R, Ruegg C. Integrin inhibitors reaching the clinic. J Clin Oncol. 2007;25:1637–1638. doi: 10.1200/JCO.2006.09.8376. [DOI] [PubMed] [Google Scholar]
- Umeda N, Kachi S, Akiyama H, Zahn G, Vossmeyer D, Stragies R, Campochiaro PA. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol. 2006;69:1820–1828. doi: 10.1124/mol.105.020941. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V, Bhaskar V, Law DA, Wong MH, DuBridge RB, Breinberg D, O'Hara C, Powers DB, Liu G, Grove J, Hevezi P, Cass KM, Watson S, Evangelista F, Powers RA, Finck B, Wills M, Caras I, Fang Y, McDonald D, Johnson D, Murray R, Jeffry U. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5:273–286. [PubMed] [Google Scholar]
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MH, Jones K, Wilting J, Dictor M, Selg M, McHale N, Gershenwald JE, Jackson DG. Structure function relationships in the lymphatic system and implications for cancer biology. Cancer Metastasis Rev. 2006;25:159–184. doi: 10.1007/s10555-006-8496-2. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Streeter PR, Breve J, Duijvestijn AM, Kraal G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J Cell Biol. 1991;115:85–95. doi: 10.1083/jcb.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: new insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006;25:677–694. doi: 10.1007/s10555-006-9026-y. [DOI] [PubMed] [Google Scholar]
- Tobler NE, Detmar M. Tumor and lymph node lymphangiogenesis–impact on cancer metastasis. J Leukoc Biol. 2006;80:691–696. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J Cell Physiol. 2005;202:205–214. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
- Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci USA. 2003;100:4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K, Pihlajaniemi T, Heljasvaara R. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res. 2007;67:11528–11535. doi: 10.1158/0008-5472.CAN-07-1458. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Angiogenesis and vascular remodeling in inflammation and cancer. Figg WD, Folkman J, editors. New York: Springer,; Biology and architecture of the vasculature. 2008:pp 15–31. [Google Scholar]
- Thurston G, Murphy TJ, Baluk P, Lindsey JR, McDonald DM. Angiogenesis in mice with chronic airway inflammation: strain-dependent differences. Am J Pathol. 1998;153:1099–1112. doi: 10.1016/S0002-9440(10)65654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Maas K, Labarbara A, McLean JW, McDonald DM. Microvascular remodelling in chronic airway inflammation in mice. Clin Exp Pharmacol Physiol. 2000;27:836–841. doi: 10.1046/j.1440-1681.2000.03342.x. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- Stragies R, Osterkamp F, Zischinsky G, Vossmeyer D, Kalkhof H, Reimer U, Zahn G. Design and synthesis of a new class of selective integrin alpha5beta1 antagonists. J Med Chem. 2007;50:3786–3794. doi: 10.1021/jm070002v. [DOI] [PubMed] [Google Scholar]
- Aurora AB, Baluk P, Zhang D, Sidhu SS, Dolganov GM, Basbaum C, McDonald DM, Killeen N. Immune complex-dependent remodeling of the airway vasculature in response to a chronic bacterial infection. J Immunol. 2005;175:6319–6326. doi: 10.4049/jimmunol.175.10.6319. [DOI] [PubMed] [Google Scholar]
- Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Kohler C. Allograft inflammatory factor-1/Ionized calcium-binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell Tissue Res. 2007;330:291–302. doi: 10.1007/s00441-007-0474-7. [DOI] [PubMed] [Google Scholar]
- Roovers K, Davey G, Zhu X, Bottazzi ME, Assoian RK. Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol Biol Cell. 1999;10:3197–3204. doi: 10.1091/mbc.10.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest. 2002;110:933–941. doi: 10.1172/JCI14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, Humphries MJ, Kielty CM. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J Biol Chem. 2003;278:34605–34616. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- Weber E, Rossi A, Solito R, Sacchi G, Agliano M, Gerli R. Focal adhesion molecules expression and fibrillin deposition by lymphatic and blood vessel endothelial cells in culture. Microvasc Res. 2002;64:47–55. doi: 10.1006/mvre.2002.2397. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holers VM, Ruff TG, Parks DL, McDonald JA, Ballard LL, Brown EJ. Molecular cloning of a murine fibronectin receptor and its expression during inflammation. Expression of VLA-5 is increased in activated peritoneal macrophages in a manner discordant from major histocompatibility complex class II. J Exp Med. 1989;169:1589–1605. doi: 10.1084/jem.169.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]