Abstract
Both Eph receptors and ephrin ligands have been implicated in blood vessel and neuronal development. Recent studies suggested that EphA2 inhibition reduces tumor angiogenesis, but its role in blood vessel development and inflammation is unclear. We examined these issues using either airways of pathogen-free, EphA2-deficient mice at various ages or EphA2-deficient mice whose airways were inflamed by either Mycoplasma pulmonis infection or ovalbumin sensitization and challenge. EphA2-deficient mice had fewer capillaries, a greater number of endothelial sprouts, and greater capillary diameters than age-matched, wild-type control mice. Moreover, capillaries in EphA2-deficient mice had significantly less pericyte coverage, suggesting abnormal interactions between endothelial cells and pericytes. These differences were apparent in early postnatal life but decreased during progression into adulthood. In inflamed airways, significantly more angiogenesis and lymphangiogenesis, a greater number of infiltrating leukocytes, and higher expression levels of inflammatory cytokine mRNA were present in EphA2-deficient mice after M. pulmonis infection. Additionally, in allergic airway inflammation with ovalbumin sensitization and challenge, a greater number of lymphatic sprouts and infiltrating leukocytes, higher mRNA expression levels of TH2 cytokines and chemokines related to allergic airway inflammation, and enhanced airway hyper-responsiveness were present in EphA2-deficient mice. We conclude that defective pericyte coverage causes capillary defects, abundant endothelial sprouts, and thick capillary diameters in EphA2-deficient mice, indicating that these animals have exaggerated responses to airway inflammation.
Formation of blood vessel networks is regulated by coordinated attractive and repulsive guidance cues. Among guidance cues, Eph receptors with Ephrin ligands are recognized as one of the major cues.1,2,3,4,5,6,7 The A and B families of Eph receptors and ephrin ligands are the two families that are known. Within each family of Eph receptors, one ligand can bind to multiple receptors and, conversely, one receptor can bind to multiple ligands.8,9 In addition, Eph/ephrin signaling is bidirectional, such that signal transduction pathways can be activated by ephrin ligands on binding to the receptor.8 Among the B family, targeted disruption of EphB4 and ephrinB2 in mice showed that these molecules are essential for angiogenic remodeling and embryonic survival.10,11 Among the Eph A family, endothelial cells in tumors express EphA2.8,12
For blood vessel development, the association of endothelial cells with mural cells—pericytes and smooth muscle cells—is important for forming stable, functional, and organized blood vessels.13 Defective association of endothelial cells with pericytes enhances formation of new endothelial sprouts,13 and association of endothelial cells with pericytes controls capillary diameter.14 Recent studies have suggested a critical role of Ephrin-Eph interactions in mural cells. EphrinB2 is expressed on mural cells and plays essential roles in vivo.7,13,15,16 Among A families, EphA2 and EphA4 are expressed on smooth muscle cells of blood vessels.17,18
In addition to the expression by endothelial cells in tumors, many tumor cells themselves express EphA219 and inhibition of EphA2 reduced tumor growth rate and tumor angiogenesis.8,20,21,22 However, little is known about the role of EphA2 in other angiogenic processes such as inflammation. Up-regulation of EphA2 mRNA expression was reported in the liver and lung during inflammation induced by systemic administration of lipopolysaccharide in rats.23 Although the role of EphA2 in airway inflammation is unknown, EphA2 has been shown to be expressed in endothelial, epithelial, and dendritic cells, but not on lymphocytes, monocytes, and granulocytes.23 One method of inducing airway inflammation is to infect mice with Mycoplasma pulmonis, which caused leukocyte influx, angiogenesis, blood vessel remodeling, lymphangiogenesis, mucosal edema, epithelial changes, and fibrosis typical of chronic airway inflammation.24,25,26,27,28,29,30
Asthma is also considered an inflammatory disorder of the airways and characterized by airway hyper-responsiveness, inflammatory infiltrates, and reversible airway obstruction.31 The airway inflammation in asthma consists mainly of eosinophils, mast cells, and CD4− lymphocytes, the latter of which are believed to play a central role in the pathogenesis of bronchial asthma by producing a number of key Th2 cytokines, which include interleukin (IL)-4, IL-5, and IL-13, that induce many of the features of the pathophysiology of the disease.32,33,34 IL-4 plays an important role in B cell class switch and has a unique role in the differentiation of naïve T helper precursor cells to the Th2 subset of cells.35 IL-5 is essential for eosinophil mobilization and differentiation, whereas IL-13 is required for the induction of mucus production and airway hyper-responsiveness.35,36,37 In addition to these cytokines, chemokines such as eotaxin-1 and eotaxin-2 are involved in recruitment of eosinophils and T cells to the inflammatory site.38,39 Blood vessel remodeling in airways has been suggested to be involved in asthma40 but little is known about specifically how it is involved in asthma.41,42 Moreover, even less is known about involvement of airway lymphangiogenesis in asthma. A commonly used mouse model of allergic asthma is where animals are immunized with ovalbumin (OVA) and later exposed to inhaled OVA, this induces an influx of Th2 lymphocytes, eosinophils, and the development of airway hyper-responsiveness.43
The mouse trachea has a discrete and segmented network of blood vessels aligned with the framework of cartilage rings. This characteristic allows clear observation and quantification of blood vessel changes such as in inflammation and development.26,44
Accordingly, the present study sought to determine the distribution of immunoreactivity for EphA2 on airway blood vessels, the effect of EphA2 deficiency on neonatal blood vessel development from early neonatal life to adulthood, and the response to inflammation. Our approach was first to use immunohistochemistry to determine the distribution of immunoreactivity for EphA2 and its ligands in tracheas isolated from 2- to 25-week-old control wild-type and EphA2-deficient mice to compare the development of the tracheal blood vessels during the neonatal ages. Our study revealed defective capillary development and pericyte association with endothelial cells in EphA2-deficient mice. We used two models of airway inflammation induced by M. pulmonis infection or by sensitization and inhalation of allergen (ovalbumin). EphA2-deficient mice revealed an exaggerated response to both types of airway inflammation.
Materials and Methods
Mice
EphA2-deficient mice (strain 006028, Jackson Laboratories, Bar Harbor, ME) were a mixture of 129xC57BL/6 (strain B6129F2/J) background, backcrossed with C57BL/6 genetic background for two generations. These mice were originally described.45 B6129F2/J mice (strain 101045, Jackson Laboratories) were used as control mice. Specific pathogen-free mice were housed under barrier conditions. Before being subjected to experimental procedures, mice were anesthetized by intramuscular injection of ketamine (83 mg/kg) and xylazine (13 mg/kg). All experimental procedures were approved by the Institutional Animal Care and Use Committees of the University of California, San Francisco, or MedImmune, Inc.
M. pulmonis Infection
Mice at 10 weeks of age were inoculated intranasally on day 0 with 50 μl of broth containing 3.3 × 105 colony-forming units of M. pulmonis organisms (strain CT7) as described previously.26 Mice were anesthetized before inoculation and then allowed to recover. At 14 days after M. pulmonis infection, mice were anesthetized again for further studies. Body weights of the mice were measured daily, and relative body weights were shown in percentages by setting the body weights on day 0 as 100%.
Immunohistochemistry
Mice were perfused for 2 minutes with fixative (1% paraformaldehyde in phosphate-buffered saline; PBS, pH 7.4) from a cannula inserted through the left ventricle into the aorta.26 Tracheas were removed and immersed in fixative for 1 hour. Tissues were washed and stained immunohistochemically by incubating whole mounts with one or more primary antibodies diluted in PBS containing 0.3% Triton X-100, 0.2% bovine serum albumin, 5% normal goat serum, and 0.1% sodium azide, as described previously.26 The following antibodies were used at indicated concentrations: lymphatics: lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), 1:500 (rabbit polyclonal 07-538; Upstate Biotechnology, Billerica, MA or AngioBio, Del Mar, CA); CD31, 1:500 (hamster anti-mouse PECAM-1, clone 2H8; Thermo Scientific, Waltham, MA); pericytes: desmin, 1:2000 (rabbit polyclonal 8592; Abcam, Cambridge, MA); EphA2, 1:500 (goat polyclonal AF639; R&D Systems, Minneapolis, MN). Secondary antibodies were labeled with fluorescein isothiocyanate, cyanine 3 (Cy3), or Cy5, 1:500 (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Specimens were viewed with a fluorescence microscope (Axiophot; Carl Zeiss MicroImaging, Inc.) with a 3-CCD low light RGB video camera (CoolCam, SciMeasure Analytical Systems, Atlanta, GA) or a confocal microscope (LSM-510; Carl Zeiss MicroImaging, Inc.) using AIM confocal software (version 3.2.2).
The specificity of the EphA2 antibody (catalog number AF639, R&D Systems) has been documented. In direct enzyme-linked immunosorbent assay and Western blots, this antibody shows less than 1% cross-reactivity with rhEphA1, rmEphA3, rmEphA4, rmEphA6, rmEphA7, rmEphA8, and rrEphA5 R&D Systems product circular (anti-mouse EphA2 antibody, R&D Systems). In addition, the use of the EphA2 antibody AF639 has been documented in DMBA/TPA-induced skin tumors.46
Induction of Airway Inflammation by Ovalbumin
Mice were immunized with 10 μg of ovalbumin (OVA) (Sigma, St. Louis, MO) in alum (1 mg of aluminum hydroxide gel, Sigma) in 0.2 ml of sterile saline intraperitoneally on days 0 and 10. Sham-immunized mice received the same amount of saline in alum. Since induction of airway lymphangiogenesis required longer term OVA challenge, we performed two terms of OVA challenge. For airway hyper-responsiveness, cell counts in bronchoalveolar lavage fluid, and quantitative reverse transcription-polymerase chain reaction (RT-PCR) experiments, mice were exposed to aerosolized OVA (5%, 30 minutes) or saline (sham) on days 17 to 22. Airway hyper-responsiveness and airway inflammation were determined 24 hours later (day 23). For tracheal whole-mount staining, mice were intranasally inoculated with OVA as previously shown with some modifications.38 Mice were anesthetized and intranasally inoculated with 50 μl of OVA (0.667 mg/ml) in saline or saline alone (sham) on days 17 to 22, 24, 26, 28, 31, and 33. Tracheas were isolated 24 hours later (day 34).
Assessment of Airway Hyper-Responsiveness
Airway hyper-responsiveness was determined using a modified version of previously described methods.47 Briefly, mice were anesthetized with pentobarbital sodium and connected to a small animal ventilator (FlexiVent, Scireq, Montreal, Canada). Next baseline recordings of all parameters were obtained. The mice were then challenged with an aerosol of PBS and recordings of all parameters were made every 10 seconds for 3 minutes, alternating between signals (Snapshot and Quick-Prime, the latter measurements being made by interrupting the ventilation for a 1-second passive expiration followed by 2-second broadband (1–19.625 Hz) volume perturbation). Finally, two more deep lung inflations were given and the above protocol repeated three more times with aerosols containing methacholine (Sigma) at sequentially increasing concentrations of 3.125, 12.5, and 50 mg/ml.
Morphometric Measurements
For morphometric measurements, the trachea was imaged using a Zeiss Axiophot fluorescence microscope equipped with a CoolCam fluorescence camera. Measurements were performed using digitizing software interfaced to the microscope and CoolCam camera. The number of capillaries per millimeter was measured by counting fluorescent images of CD31-positive blood vessels that crossed the center of cartilage rings.44 Capillary diameters were assessed by measuring CD31-positive blood vessels at the center of cartilage rings.44 Number of endothelial sprouts was measured by counting CD31-positive endothelial sprouts, defined as any thin, tapered CD31-positive projections. These CD31-positive vessel measurements were performed by observing 10 cartilage rings per trachea, each 0.2 mm2 in area. The number of lymphatic sprouts was measured viewing real-time fluorescent images of tracheal whole mount, we counted lymphatic sprouts, defined as tapered LYVE-1 positive projections visible at a screen magnification of x180, in 5 regions per trachea, each 1.5 mm2 in area.26 Pericyte coverage was counted by measuring the length of capillaries covered by desmin-positive pericytes. Pericyte coverage percentage represents the length of capillaries covered by desmin-positive pericytes divided by the total length of capillaries. Pericyte coverage measurements were performed by observing five cartilage rings per trachea.48 Pericyte cell bodies were identified as bulbous regions of desmin-positive cells closely associated with capillaries. Pericyte number was expressed as cell bodies per millimeter of length of CD31-positive capillary.49 Area densities (percentage of total tissue area) of LYVE-1-positive lymphatic vessels and CD31-positive blood vessels viewed in real-time fluorescent images of tracheal whole mounts were measured by stereological point counting of 10 regions per trachea, each 1.7 mm2 in area.26
Quantitative RT-PCR
Mice were anesthetized and briefly perfused via the aorta with sterile PBS. The lungs were removed and stored in RNAlater reagent, homogenized, and total RNA was extracted using RNAeasy Plus mini kit (Qiagen, Hilden, Germany). cDNA was synthesized using Sprint Power Script Double Preprimed 96 kit (Clontech, Mountain View, CA). Gene expression was measured by TaqMan real-time PCR (Applied Biosystems, Foster City, CA) following the manufacturer’s protocols. The probe sets were obtained from Applied Biosystems as TaqMan gene expression assays. TaqMan reactions contained either the reference gene GAPDH or the genes of interest (Applied Biosystems).
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) was performed as previously shown with some modifications.50 Briefly, the trachea was cannulated, and the airway lumen was washed with 0.6 ml of PBS three times to collect BAL fluid. The cells in BAL were spun onto glass slides and stained with Diff-Quik stain set (Dade Behring AG, Düdingen, Switzerland), and 300 cells were identified according to morphological criteria. The number of lymphocytes, neutrophils, and eosinophils therein was determined by multiplying the proportion of each leukocyte by the total number of cells counted by a cell counter.
Statistical Analysis
Values are presented as means ± SEM with four or five mice per group unless otherwise indicated. The significance of differences between means was assessed by analysis of variance followed by the Dunn-Bonferroni test for multiple comparisons with P values <0.05 considered significant.
Results
Distribution of EphA2 in Normal Tracheal Microvasculature
Blood vessels were identified by the immunoreactivity to endothelial cell marker CD31, and lymphatics were identified by a weaker immunoreactivity to CD31 than blood vessels and by their morphology. Airway blood vessels, lymphatics, and nerves were EphA2 immunoreactive in the trachea (Figure 1, A and B). Airway epithelium was also EphA2 immunoreactive (data not shown).
Figure 1.
Distribution of EphA2 in tracheal microvasculature; confocal microscopic images of tracheal whole mounts. A and B: Tracheal whole mounts stained for endothelial cell marker CD31 (green) and EphA2 (red) immunoreactivity. Lymphatics are weakly immunoreactive with CD31. A single arrow indicates blood vessels, double arrow indicates lymphatics, and an arrowhead indicates nerves. Scale bar = 20 μm.
Number of Capillaries and Endothelial Sprouts in Wild-Type and EphA2-Deficient Mouse Airways
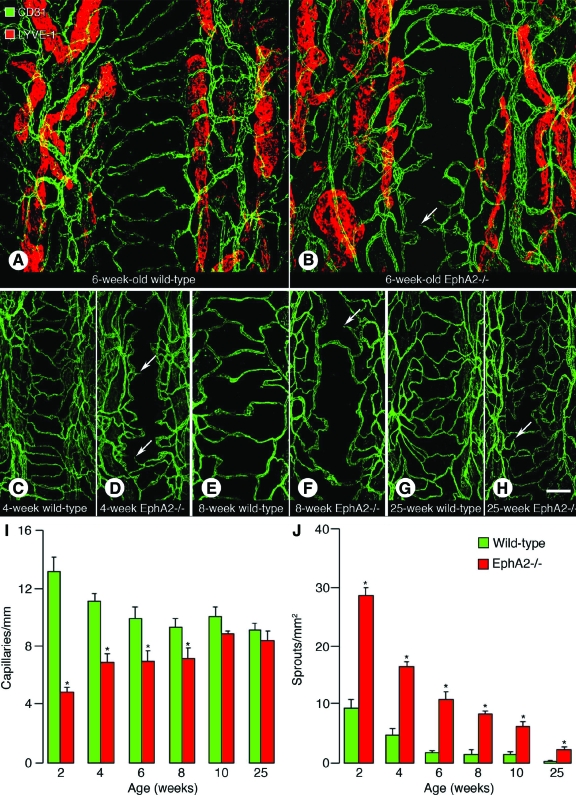
Since EphA2 was immunoreactive on blood vessels and lymphatics, tracheal whole mounts isolated from 6-week-old wild-type and EphA2-deficient mice were stained for CD31 and a lymphatic marker LYVE-1 immunoreactivity (Figure 2, A and B). In wild-type mice, crossing capillaries in the mucosa overlying the cartilage rings were abundant and endothelial sprouts were few (Figure 2A). However, in the EphA2-deficient mice, crossing capillaries were few and endothelial sprouts were abundant (Figure 2B). LYVE-1 immunoreactive lymphatics were restricted to the mucosa between cartilage rings in both wild-type and EphA2-deficient mice (Figure 2, A and B). Next, we sought to determine when the capillary characteristics were formed in EphA2-deficient mice by staining tracheal whole mounts from various ages of mice (from 2 to 25 weeks) for CD31 immunoreactivity. In 4-, 8-, and 25-week-old wild-type mice, crossing capillaries were abundant and endothelial sprouts were few (Figure 2, C, E, G). In 4-week-old EphA2-deficient mice, crossing capillaries were few and endothelial sprouts were abundant (Figure 2D). In 8-week-old EphA2-deficient mice, crossing capillaries were few and a few endothelial sprouts were present (Figure 2F). In 25-week-old EphA2-deficient mice, crossing capillaries were abundant and only a few endothelial sprouts were present (Figure 2H). When we measured the number of crossing capillaries at the center of cartilage rings, EphA2-deficient mice had significantly fewer number of capillaries than wild-type mice from 2 to 8 weeks old, which was 64% and 23% fewer in 2-week-old and 8-week-old EphA2-deficient mice, respectively (Figure 2I). The difference was not significant in 10- and 25-week-old mice (Figure 2I). The number of endothelial sprouts was significantly greater in EphA2-deficient mice than in wild-type mice at all ages, which was 206% greater in 2-week-old EphA2-deficient mice (Figure 2J). However, the number of sprouts decreased toward adulthood in EphA2-deficient mice (Figure 2J).
Figure 2.
Number of capillaries and endothelial sprouts in wild-type and EphA2-deficient mouse airways. A–H: Confocal microscopic images of tracheal whole mounts. Arrows indicate endothelial sprouts. A and B: Tracheas isolated from 6-week-old wild-type (A) and EphA2-deficient (B) mice stained for CD31 (green) and LYVE-1 (red, lymphatics) immunoreactivity. C–H: Tracheas stained for CD31 (green) immunoreactivity isolated from 4-week-old wild-type (C) and EphA2-deficient (D) mice, 8-week-old wild-type (E) and EphA2-deficient (F) mice, and 25-week-old wild-type (G) and EphA2-deficient (H) mice. I: The number of crossing capillaries in mucosa overlying cartilage rings. J: The number of endothelial sprouts in mucosa overlying cartilage rings. Values are presented as means ± SEM; n = 5–8 mice per group; *P < 0.05 versus wild-type. EphA2−/−, EphA2-deficient mice. Scale bar in H applies to all panels: 130 μm in A and B; 100 μm in C–H.
Pericyte Association with Capillaries and Capillary Diameters in Wild-Type and EphA2-Deficient Mice
Since the defective association of pericytes with endothelial cells enhances the formation of new endothelial sprouts, tracheal whole mounts were stained for CD31 and pericyte marker desmin immunoreactivity. In 4-week-old wild-type mice, most capillaries were covered with pericytes, whereas in EphA2-deficient mice, many capillaries were not covered (Figure 3, A and B). We next analyzed the association of endothelial cells and pericytes at various ages. In 6-week-old and 8-week-old wild-type mice, most capillaries were covered with pericytes, whereas in EphA2-deficient mice, some capillaries were not covered with pericytes (Figure 3, C–F). In 25-week-old wild-type mice, most capillaries were covered with pericytes, whereas in EphA2-deficient mice, a few capillaries were not covered with pericytes (Figure 3, G and H). When we measured pericyte coverage on capillaries, EphA2-deficient mice had significantly less pericyte coverage than wild-type mice from 2 to 25 weeks old, which was 20% and 5% less in 2- and 25-week-old EphA2-deficient mice, respectively (Figure 3I). However, the number of pericyte bodies was not significantly different between age matched wild-type and EphA2-deficient mice (Figure 3J). Since capillaries in 4-, 6-, and 8-week-old EphA2-deficient mice had thick diameters (Figure 3, A–F) and pericytes control capillary diameter, diameter of capillaries at the center of cartilage rings was measured (Figure 3K). EphA2-deficient mice had significantly greater capillary diameter than wild-type mice from 2 to 10 weeks old, which was 32% and 12% greater in 2- and 10-week-old EphA2-deficient mice, respectively. However, at 25 weeks old, the difference was not significant (Figure 3K).
Figure 3.
Pericyte association with capillaries and capillary diameters. A–H: Confocal microscopic images of tracheal whole mounts stained for CD31 (green) and desmin (red, pericytes) immunoreactivity isolated from 4-week-old wild-type (A) and EphA2-deficient (B) mice, 6-week-old wild-type (C) and EphA2-deficient (D) mice, 8-week-old wild-type (E) and EphA2-deficient (F) mice, 25-week-old wild-type (G) and EphA2-deficient (H) mice. Arrows indicate capillaries without pericyte coverage. I–K: Pericyte coverage on capillaries (I), the numbers of pericyte bodies per mm capillary length (J), and capillary diameter (K) in 2- to 25-week-old wild-type and EphA2-deficient mice. Values are presented as means ± SEM; n = 5–8 mice per group; *P < 0.05 versus wild-type. Scale bar = 50 μm.
Response to Airway Inflammation Induced by M. pulmonis Infection
Since the responses of EphA2-deficient mice to airway inflammation and inflammation-induced blood vessel remodeling and lymphangiogenesis are unknown, we induced airway inflammation by infecting mice with M. pulmonis for 14 days. While all of the wild-type mice survived during the 14 days of infection, EphA2-deficient mice began to die at day 2, and their survival rate was 40% at day 14 (Figure 4A). After 14 days of infection, tracheal whole mounts were stained for CD31 and LYVE-1 immunoreactivity to determine the extent of blood vessel remodeling and lymphangiogenesis typically seen in M. pulmonis-infected airways (Figure 4, B–E). In wild-type mice, the blood vessels were not so enlarged and had a simple network (Figure 4B). In EphA2-deficient mice, blood vessels were abundant, enlarged, and had a complex network (Figure 4D). Lymphatics in the mucosa overlying cartilage rings elongating from the collecting lymphatics in intercartilaginous regions were rare in wild-type mice, but these lymphatics were abundant in EphA2-deficient mice (Figure 4, C and E). To quantify these differences in the trachea, we measured CD31-positive blood vessel area density (Figure 4F) and LYVE-1-positive lymphatic area density (Figure 4G). The area densities of CD31-positive blood vessels or LYVE-1 positive lymphatics were not significantly different between pathogen-free wild-type and EphA2-deficient mice (Figure 4, F and G). In M. pulmonis-infected mice, the area density of CD31-positive blood vessels and LYVE-1-positive lymphatics was significantly greater by 61% and 174%, respectively, in EphA2-deficient mice than in wild-type mice (Figure 4, F and G). After 14 days of infection, EphA2-deficient mice lost significantly more body weight than wild-type mice. Setting the body weights on day 0 as 100%, 14 days after infection, mean body weight was 90% in EphA2-deficient mice and 103% in wild-type mice, P < 0.05 (data not shown).
Figure 4.
Response to airway inflammation induced by M. pulmonis infection. Wild-type and EphA2-deficient mice were infected with M. pulmonis on day 0 and sacrificed on day 14. A: Survival rate of the mice during infection for 14 days. B–E: Confocal microscopic images of tracheal whole mounts isolated from wild-type (B and C) or EphA2-deficient (D and E) mice stained for CD31 (green) and LYVE-1 (red) immunoreactivity. Arrows in C and E indicates lymphatics in mucosa overlying cartilage rings. F and G: CD31 immunoreactive blood vessel area density (F) or LYVE-1 immunoreactive lymphatic area density (G) in pathogen-free or M. pulmonis infected wild-type and EphA2-deficient mice. Values are presented as means ± SEM; n = 5–10 mice per group; *P < 0.05 versus wild-type mice. Scale bar = 200 μm.
Infiltrating Leukocytes and Inflammatory Cytokine mRNA Levels in Infected Airways
To assess the severity of inflammation, the number of infiltrating leukocytes in BAL was counted and mRNA expression levels of inflammatory cytokines in the airways were quantified by quantitative RT-PCR after 14 days of M. pulmonis infection. The number of total infiltrating cells, lymphocytes, and neutrophils in BAL was 13.9, 7.8, and 47.6 times greater, respectively, in infected EphA2-deficient mice than in infected wild-type mice, P < 0.05 (Figure 5, A–C). As for inflammatory cytokines, mRNA expression levels of tumor necrosis factor, IL-1α, IL-1β, IL-6, and KC was 33.3, 3.8, 3.2, 3.2, and 7.9 times greater, respectively, in infected EphA2-deficient mice than in infected wild-type mice, P < 0.05 (Figure 5, D–H).
Figure 5.
Infiltrating leukocytes and inflammatory cytokine mRNA levels in infected airways. Pathogen-free or 14-day M. pulmonis-infected wild-type and EphA2-deficient mice were sacrificed. A–C: The numbers of total cells (A), lymphocytes (B), and neutrophils (C) that infiltrated into bronchoalveolar lavage were counted. D–H: mRNA expression levels of inflammatory cytokines, tumor necrosis factor (D), IL-1α (E), IL-1β (F), IL-6 (G), and KC (H) in trachea were measured by quantitative RT-PCR. Values are presented as means ± SEM; n = 5–8 mice per group; *P < 0.05 versus wild-type. −/−, EphA2-deficient mice.
Lymphatics in Airways of Ovalbumin-Challenged Mice
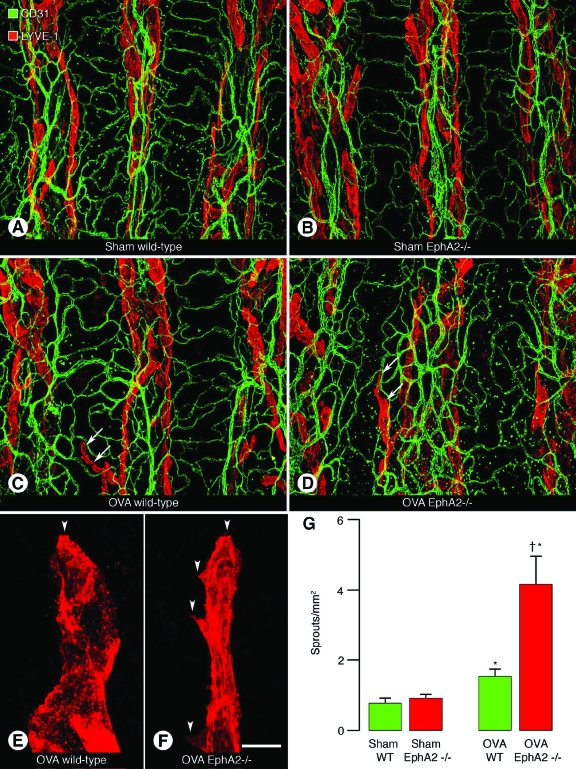
As EphA2-deficient mice displayed exaggerated response to airway inflammation induced by M. pulmonis infection, we assessed response of EphA2-deficient mice to another type of inflammation by inducing allergic airway inflammation. Mice were sensitized with OVA in alum (OVA-sensitized) or sham-treated (alum alone) and then exposed to OVA (OVA-challenged) or saline (sham-treated), respectively. One day after the last OVA challenge, mice were sacrificed, and tracheal whole mounts were isolated and stained for CD31 and LYVE-1 immunoreactivity to determine the extent of blood vessel remodeling and lymphangiogenesis. In OVA-challenged wild-type and EphA2-deficient mice, the diameters of capillaries were similar to those in sham-treated mice and the blood vessel network was simple in all groups (Figure 6, A–D). As for lymphatics, in OVA-challenged wild-type and EphA2-deficient mice, some collecting lymphatic in intercartilaginous regions had initial lymphatics in the mucosa overlying cartilage rings, but other collecting lymphatic did not, whereas in sham-treated wild-type and EphA2-deficient mice, collecting lymphatic in intercartilaginous regions did not have initial lymphatics in mucosa overlying cartilage rings (Figure 6, A–D). Initial lymphatics in wild-type mice had few lymphatic sprouts in addition to the tip of the lymphatics, whereas those in EphA2-deficient mice had several lymphatic sprouts (Figure 6, E and F). When we measured the number of lymphatic sprouts, OVA-challenged wild-type and EphA2-deficient mice had significantly greater numbers of lymphatic sprouts than sham-treated wild-type and EphA2-deficient mice (Figure 6G). However, OVA-challenged EphA2-deficient mice had significantly greater numbers of lymphatic sprouts than OVA-challenged wild-type mice by 171% (Figure 6G).
Figure 6.
Lymphatics in airways in OVA-challenged mice. A–F: Confocal microscopic images of tracheal whole mounts stained for CD31 (green) and LYVE-1 (red) immunoreactivity isolated from sham-treated wild-type (A) and EphA2-deficient (B) mice, or OVA-challenged wild-type (C) and EphA2-deficient (D) mice 24 hours after last sham treatment or OVA challenge. Arrows indicate lymphatics in the mucosa overlying cartilage rings (C and D). E and F: High-magnification images of lymphatics in mucosa overlying cartilage rings in OVA-challenged wild-type (E) or EphA2-deficient (F) mice. Arrowheads indicate lymphatic sprouts. G: The number of lymphatic sprouts in sham-treated or OVA-challenged wild-type and EphA2-deficient mice. Values are presented as means ± SEM; n = 5 mice per group; *P < 0.05 versus sham-treated mice; †P < 0.05 versus OVA-challenged wild-type mice. Scale bar in F applies to all panels: 200 μm in A–D; 12 μm in E and F.
Infiltrating Leukocytes, TH2 Cytokines, Chemokines, and Airway Hyper-Responsiveness in OVA-Challenged Mice
To assess the severity of allergic airway inflammation, the number of infiltrating leukocytes in BAL, mRNA expression levels of TH2 cytokines and allergic airway inflammation related chemokines, and physiological response to increasing concentrations of inhaled methacholine were measured 24 hours after the last OVA challenge. The number of total infiltrating cells, eosinophils, and lymphocytes in BAL was 4.4, 4.7, and 3.7 times greater, respectively, in OVA-challenged EphA2-deficient mice than in OVA-challenged wild-type mice, P < 0.05 (Figure 7, A–C). As for TH2 cytokines and allergic airway inflammation related chemokines, mRNA expression levels of IL-5, IL-13, eotaxin-1, and eotaxin-2 were 2.7, 3.6, 2.6, and 5.7 times greater, respectively, in OVA-challenged EphA2-deficient mice than in OVA-challenged wild-type mice, P < 0.05 (Figure 7, D–G). As for airway hyper-responsiveness, OVA-challenged EphA2-deficient mice displayed significantly greater airway resistance than OVA-challenged wild-type mice after methacholine inhalation (Figure 7H). Similarly, we saw a significant enhancement in elastance in OVA-challenged EphA2-deficient mice than in wild-type mice (see Supplementary Figure S1 at http://ajp.amjpathol.org).
Figure 7.
Infiltrating leukocytes, TH2 cytokines, chemokines, and airway hyper-responsiveness in OVA-challenged mice. Sham-treated wild-type and EphA2-deficient mice, or OVA-challenged wild-type and EphA2-deficient mice were subjected to the measurements 24 hours after last sham treatment or OVA challenge. A–C: The numbers of total cells (A), eosinophils (B), and lymphocytes (C) that infiltrated into BAL were counted. D–G: mRNA expression levels of TH2 cytokines, IL-5 (D) and IL-13 (E), and allergic airway inflammation related chemokines, eotaxin-1 (F) and eotaxin-2 (G) in airways were measured by quantitative RT-PCR. H: Assessment of airway hyper-responsiveness to inhaled methacholine in anesthetized mice. Airway responses of sham-treated wild-type (open circle) and EphA2-deficient (open square) mice or OVA-challenged wild-type (closed circle) and EphA2-deficient (closed square) mice to increasing concentrations of methacholine were assessed by a small animal ventilator. The dose-response curves for pulmonary resistance are shown. Values are presented as means ± SEM; n = 5–8 mice per group; *P < 0.05 versus OVA-challenged wild-type mice.
Discussion
Morphological characteristics of capillaries in EphA2-deficient mice were fewer number of crossing capillaries, greater number of endothelial sprouts, and greater diameter of capillaries, which was similar to the characteristics of blood vessels without pericyte coverage. EphA2-deficient mice also had defective pericyte coverage on tracheal capillaries. The severity of the characteristics in EphA2-deficient mice diminished as the neonatal mice aged toward adulthood. Furthermore, EphA2-deficient mice had exaggerated response to two types of airway inflammation presented in this study, either induced by M. pulmonis infection or OVA sensitization and challenge.
Genetic mouse studies have shown five important signaling pathways between endothelial cells and mural cells: transforming growth factor β, angiopoietin, platelet-derived growth factor B, sphingosine-1-phosphate, Notch, and their respective receptors.51,52,53,54,55 In addition to these signaling pathways, recent studies have suggested a critical role of Eph-ephrins in mural cells. Pericyte interaction with endothelial cells suppresses formation of new endothelial cell sprouts.13 This evidence suggests that defective pericyte coverage on endothelial cells in EphA2-deficient mice may result in a significantly greater number of endothelial sprouts in EphA2-deficient mice. Pericytes control capillary diameter.14 This evidence suggests that defective pericyte coverage on endothelial cells may cause the greater capillary diameter in EphA2-deficient mice. The observation that characteristics of blood vessels in EphA2-deficient mice were more apparent at neonatal age and diminished as the mouse approaches adulthood suggests that the defects are more of a developmental delay. The mechanism of this developmental delay might be due to the abnormal interactions between pericytes and endothelial cells. However, similar pericyte body numbers on capillaries in wild-type and EphA2-deficient mice suggests that EphA2 affects the shape of pericyte bodies and/or the formation of pericyte processes but not pericyte numbers. We also examined the tracheal blood vessels of mice younger than 2 weeks. We found dramatic morphological changes in tracheal capillaries from late embryonic stage to neonatal stage in both wild-type and EphA2-deficient mice. However, there was also a delay in neonatal capillary development in EphA2-deficient mice (A. Ni and E. Lashnits, personal communication).
In contrast to the capillary defects described in this study and reduced tumor angiogenesis reported by other studies,8,20,21,22 during inflammation induced by M. pulmonis infection, angiogenesis and blood vessel remodeling were exaggerated in EphA2-deficient mice. Lymphangiogenesis was also more exaggerated in EphA2-deficient mice than in wild-type mice. In addition to the exaggerated angiogenesis and lymphangiogenesis, other responses such as lower survival rate, more body weight loss, greater number of infiltrating cells in BAL, and greater inflammatory cytokine mRNA expression levels in the airways of EphA2-deficient mice suggest exaggerated overall response to the inflammation by M. pulmonis infection.
In allergic airway inflammation, OVA-challenged mice had initial lymphatics in mucosa overlying the cartilage rings and greater number of lymphatic sprouts than sham-treated mice, suggesting the presence of lymphangiogenesis in this model. Recently, a reduced distribution of lymphatics was reported in the airways of patients with fatal asthma.56 However, little has been reported about the relationship between lymphangiogenesis and allergic airway inflammation. Our data suggests that lymphangiogenesis occurs earlier than blood vessel remodeling or angiogenesis during allergic airway inflammation. A greater number of lymphatic sprouts in OVA-challenged EphA2-deficient mice than in wild-type mice suggests greater lymphangiogenesis in EphA2-deficient mice. Previous studies reported blood vessel remodeling in the mouse lung of allergic airway inflammation induced by OVA sensitization and challenge or chronic house dust mite extract exposure.41,57 However, blood vessel remodeling was not present in the trachea of OVA-treated mice. In contrast to our study, another study reported presence of blood vessel remodeling in a mouse model of allergic airway inflammation.42 These discrepancies might be due to the difference in mouse strain or the method of OVA treatment. In addition to lymphangiogenesis, EphA2-deficient mice had increased number of infiltrating cells in BAL, greater TH2 cytokine and allergic asthma-related chemokine mRNA expression levels in airways, and enhanced airway hyper-responsiveness to methacholine, which suggests exaggerated response to allergic airway inflammation in EphA2-deficient mice.
In conclusion, EphA2-deficient mice had fewer number of crossing tracheal capillaries and greater number of endothelial sprouts. Capillaries in EphA2-deficient mice had greater diameters and lower pericyte coverage than those in wild-type mice. Lower pericyte coverage on capillaries in EphA2-deficient mice suggests that the characteristics of capillaries are due to the abnormal interaction between pericytes and endothelial cells. These characteristics were more conspicuous in early neonatal life but decreased toward adulthood, suggesting the characteristics represent developmental delay in EphA2-deficient mice. EphA2-deficient mice displayed exaggerated response to two types of airway inflammation induced by M. pulmonis infection or OVA sensitization and challenge. Overall, this study showed novel and unexpected roles of EphA2 in normal capillary development and inflammation in airways.
Supplementary Material
Acknowledgments
We thank Weon Kyoo You for critical review of the manuscript and Ebony Benjamin for technical expertise.
Footnotes
Address reprint requests to Donald M. McDonald, M.D., Ph.D., Department of Anatomy, S1363, University of California, 513 Parnassus Avenue, San Francisco, CA 94143-0452. E-mail: donald.mcdonald@ucsf.edu.
Supported in part by National Institutes of Health grants HL24136 and HL59157 from the National Heart, Lung, and Blood Institute; grant CA82923 from the National Cancer Institute; and funding from AngelWorks Foundation (to D.M.M.). Research funding was also provided by MedImmune, Inc. T.O. was supported in part by a postdoctoral fellowship from the Uehara Memorial Foundation of Japan.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
J.K., A.J.C., and A.H. are employees of MedImmune, Inc.
References
- Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16:535–548. doi: 10.1016/j.cytogfr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Crawford Y, Shojaei F, Ferrara N. Endothelium-microenvironment interactions in the developing embryo and in the adult. Dev Cell. 2007;12:181–194. doi: 10.1016/j.devcel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Noble F, Klein C, Tintu A, Pries A, Buschmann I. Neural guidance molecules, tip cells, and mechanical factors in vascular development. Cardiovasc Res. 2008;78:232–241. doi: 10.1093/cvr/cvn058. [DOI] [PubMed] [Google Scholar]
- Erber R, Eichelsbacher U, Powajbo V, Korn T, Djonov V, Lin J, Hammes HP, Grobholz R, Ullrich A, Vajkoczy P. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. 2006;25:628–641. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Brantley DM, Chen J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002;13:75–85. doi: 10.1016/s1359-6101(01)00031-4. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–151. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MA, Jr, Anderson DJ. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Deroanne C, Vouret-Craviari V, Wang B, Pouyssegur J. EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J Cell Sci. 2003;116:1367–1376. doi: 10.1242/jcs.00308. [DOI] [PubMed] [Google Scholar]
- Ogita H, Kunimoto S, Kamioka Y, Sawa H, Masuda M, Mochizuki N. EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ Res. 2003;93:23–31. doi: 10.1161/01.RES.0000079310.81429.C8. [DOI] [PubMed] [Google Scholar]
- Walker-Daniels J, Hess AR, Hendrix MJ, Kinch MS. Differential regulation of EphA2 in normal and malignant cells. Am J Pathol. 2003;162:1037–1042. doi: 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J. 2005;19:1884–1886. doi: 10.1096/fj.05-4038fje. [DOI] [PubMed] [Google Scholar]
- Dobrzanski P, Hunter K, Jones-Bolin S, Chang H, Robinson C, Pritchard S, Zhao H, Ruggeri B. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004;64:910–919. doi: 10.1158/0008-5472.can-3430-2. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, Mangala LS, Merritt WM, Lin YG, Gao C, Schmandt R, Kamat AA, Li Y, Thaker P, Gershenson DM, Parikh NU, Gallick GE, Kinch MS, Sood AK. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Putative dual role of ephrin-Eph receptor interactions in inflammation. IUBMB Life. 2006;58:389–394. doi: 10.1080/15216540600756004. [DOI] [PubMed] [Google Scholar]
- Aurora AB, Baluk P, Zhang D, Sidhu SS, Dolganov GM, Basbaum C, McDonald DM, Killeen N. Immune complex-dependent remodeling of the airway vasculature in response to a chronic bacterial infection. J Immunol. 2005;175:6319–6326. doi: 10.4049/jimmunol.175.10.6319. [DOI] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist K, Umemoto EY, Brokaw JJ, Dupuis M, McDonald DM. Tissue macrophages associated with angiogenesis in chronic airway inflammation in rats. Am J Respir Cell Mol Biol. 1999;20:237–247. doi: 10.1165/ajrcmb.20.2.3081. [DOI] [PubMed] [Google Scholar]
- Ezaki T, Baluk P, Thurston G, La Barbara A, Woo C, McDonald DM. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol. 2001;158:2043–2055. doi: 10.1016/S0002-9440(10)64676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- Thurston G, Murphy TJ, Baluk P, Lindsey JR, McDonald DM. Angiogenesis in mice with chronic airway inflammation: strain-dependent differences. Am J Pathol. 1998;153:1099–1112. doi: 10.1016/S0002-9440(10)65654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling, Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Cohn L, Tepper JS, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J Immunol. 1998;161:3813–3816. [PubMed] [Google Scholar]
- Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- Tormanen KR, Uller L, Persson CG, Erjefalt JS. Allergen exposure of mouse airways evokes remodeling of both bronchi and large pulmonary vessels. Am J Respir Crit Care Med. 2005;171:19–25. doi: 10.1164/rccm.200406-698OC. [DOI] [PubMed] [Google Scholar]
- Su X, Taniuchi N, Jin E, Fujiwara M, Zhang L, Ghazizadeh M, Tashimo H, Yamashita N, Ohta K, Kawanami O. Spatial and phenotypic characterization of vascular remodeling in a mouse model of asthma. Pathobiology. 2008;75:42–56. doi: 10.1159/000113794. [DOI] [PubMed] [Google Scholar]
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- Baffert F, Thurston G, Rochon-Duck M, Le T, Brekken R, McDonald DM. Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ Res. 2004;94:984–992. doi: 10.1161/01.RES.0000125295.43813.1F. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004;117:2037–2049. doi: 10.1242/jcs.01061. [DOI] [PubMed] [Google Scholar]
- Guo H, Miao H, Gerber L, Singh J, Denning MF, Gilliam AC, Wang B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol. 2004;96:2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Nakao A, Nakano H, Takahashi F, Takahashi K, Shimozato O, Takeda K, Yagita H, Okumura K. Impairment of bleomycin-induced lung fibrosis in CD28-deficient mice. J Immunol. 2001;167:1977–1981. doi: 10.4049/jimmunol.167.4.1977. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metheny-Barlow LJ, Li LY. The enigmatic role of angiopoietin-1 in tumor angiogenesis. Cell Res. 2003;13:309–317. doi: 10.1038/sj.cr.7290176. [DOI] [PubMed] [Google Scholar]
- Sainson RC, Harris AL. Regulation of angiogenesis by homotypic and heterotypic notch signalling in endothelial cells and pericytes: from basic research to potential therapies. Angiogenesis. 2008;11:41–51. doi: 10.1007/s10456-008-9098-0. [DOI] [PubMed] [Google Scholar]
- Ebina M. Remodeling of airway walls in fatal asthmatics decreases lymphatic distribution; beyond thickening of airway smooth muscle layers. Allergol Int. 2008;57:1–10. doi: 10.2332/allergolint.O-07-497. [DOI] [PubMed] [Google Scholar]
- Rydell-Tormanen K, Johnson JR, Fattouh R, Jordana M, Erjefalt JS. Induction of vascular remodeling in the lung by chronic house dust mite exposure. Am J Respir Cell Mol Biol. 2008;39:61–67. doi: 10.1165/rcmb.2007-0441OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.