Abstract
Genetic and pharmacological inhibition of inducible nitric oxide synthase (iNOS) decreases atherosclerosis development. Potential proatherogenic effects of iNOS include iNOS mediated oxidative stress and iNOS expression in different cellular compartments. Lesional iNOS can potentially produce nitric oxide radicals (NO), superoxide radicals (O2−), or both; these radicals may then react to form peroxynitrite. Alternatively, O2− radicals from oxidases co-expressed with iNOS could react with NO to produce peroxynitrite. Therefore, the expression profiles of the genes that modulate the redox system in different iNOS-expressing cell compartments may determine the role of iNOS in atherosclerosis. We used apoE (apoE−/−) and apoE/iNOS double knockout (apoE−/−/ iNOS−/−) mice to assess vascular NO, O2−, and peroxynitrite formation by electron spin resonance spectroscopy, high performance liquid chromatography, and 3-nitrotyrosine staining. The relevance of the iNOS expressing cell compartment was tested by bone marrow transplantation. We show that iNOS significantly contributes to vascular NO production and itself produces O2−, leading to peroxynitrite formation in atherosclerotic lesions. Our bone marrow transplantation experiments show that bone marrow derived cells exclusively mediate the proatherosclerotic effects of iNOS in males, while both parenchymal and bone marrow derived iNOS equally contribute to atherosclerosis in females. Moreover, iNOS expression affects vascular remodeling. These findings establish for the first time that the proatherosclerotic effects of iNOS vary with sex in addition to the compartment of its expression.
Atherosclerosis is considered a chronic inflammatory disease.1 The cellular and molecular mechanisms that initiate and propagate atherosclerosis are complex and involve the expression of inducible nitric oxide synthase (iNOS) in early and advanced atherosclerotic plaques.2 In healthy vessels iNOS is usually not expressed. However, in response to stimulation with inflammatory cytokines/bacterial by-products or low pH (pH 7.0) in the microenvironment of inflammatory lesions, a wide range of tissues and cell types can express iNOS.3,4 Aside from its transcriptional regulation, multiple post-translational modifications of iNOS have been identified that may allow complex regulations of the catalytic activity of iNOS.5,6,7,8,9 iNOS protein produces high amounts of nitric oxide (NO), which is highly reactive with other free radicals. NO reacts with superoxide (O2−) to form peroxynitrite, which in turn leads to protein nitration, DNA damage, and poly (ADP-ribose) polymerase activation.10 Peroxynitrite reacts with other molecules to form a variety of oxygen and nitrogen free radicals ie, nitrogen dioxide, peroxynitrous acid, and hydroxyl radical.11 Therefore, the predominant role of iNOS is thought to relate to oxidative stress mediated host defense against tumor cells and microorganisms.3
However, iNOS can also act as an anti-inflammatory molecule. For example, iNOS−/− mice showed an impaired ability to resolve colonic inflammation and increased neutrophil infiltration in a model of acetic acid induced acute colitis12 and lipopolysaccharide-treated iNOS−/− mice show increased neutrophil endothelial interactions in post capillary venules of the cremaster muscle assessed by intravital microscopy.13 Whether iNOS also influences leukocyte endothelial-interactions in atherosclerosis, where mononuclear cells are the predominant subset of leukocytes interacting with the endothelium of large arteries is currently unknown.
Several reasons may explain the beneficial or detrimental effects of iNOS. The role of iNOS may depend on the phase of inflammation. For example, studies of experimental traumatic brain injury suggest that early after injury, iNOS may be detrimental via peroxynitrite formation while long term iNOS expression following brain injury seems to be protective via antioxidant effects.14 The antioxidant effects of NO are explained by its ability to scavenge oxygen free radicals and to inhibit lipid peroxidation. It has been suggested that the balance between local concentrations of NO and O2− may determine whether peroxynitrite forms and lipid peroxidation occurs.15 Conditions that favor peroxynitrite formation would favor lipid peroxidation, whereas excess NO without O2− may block lipid peroxidation by scavenging peroxyl radicals.
In a model of chronic atherosclerosis, the apoE−/− mouse, iNOS deficiency potently reduces plaque development on a high fat diet, suggesting that iNOS is proatherogenic.16,17 Moreover, chronic treatment of hypercholesterolemic rabbits with a pharmacological iNOS inhibitor decreases atherosclerosis development18,19 and gene transfer mediated increased vascular iNOS expression results in impaired NO dependent vasodilation.20,21 In contrast, transient gene transfer mediated expression of iNOS decreases smooth muscle cell proliferation and prevents neointima formation following balloon angioplasty (vascular injury model) in rats and pigs.22 Addition of NO donors, as well as iNOS mediated NO production in vascular smooth muscle cells, inhibits cell proliferation.23 In mice, iNOS protects from developing transplant arteriosclerosis by inhibiting neointimal smooth muscle accumulation.24 The seemingly opposing results may be due to differences in the cellular composition of the vessel wall and the compartment of iNOS expression in chronic atherosclerosis versus transplant arteriosclerosis or smooth muscle cell proliferation following balloon angioplasty. iNOS expression relevant to atherosclerosis development was detected in vascular smooth muscle cells, mononuclear cells, and lymphocytes. These various cellular sources are capable of generating different amounts of iNOS and subsequently target iNOS expression to various compartments of the plaque. Moreover, the cellular source determines a specific array of genes co-expressed with iNOS, which may influence their redox status.25 For example, leukocytes can produce substantial amounts of NO and O2− from iNOS and NADPH oxidase, resulting in the formation of peroxynitrite.10 Peroxynitrite can oxidize low density lipoprotein and cause nitrosylation of proteins, which influences protein function.26 Moreover, under conditions of substrate (l-arginine) or cofactor (tetrahydrobiopterin) deficiency NOS can “uncouple” to generate O2− instead of NO.27 Even more intriguing iNOS is likely capable of producing NO and O2− simultaneously, which could directly lead to peroxynitrite formation.20 Whether iNOS is “uncoupled” in atherosclerosis and produces substantial amounts of O2− in addition to NO is currently unknown.
To design treatment strategies that decrease iNOS expression in chronic atherosclerosis without interfering with potentially beneficial effects of iNOS, ie, prevention of neointima proliferation following balloon angioplasty or host defense against infectious or neoplastic disease, a detailed understanding of the role of iNOS in plaque development is required.
Therefore, the current study was designed to assess possible mechanisms involved in the proatherogenic effects of iNOS. We used apoE−/− and apoE−/−/iNOS−/− mice, which allowed testing the effects of iNOS deficiency on spontaneous atherosclerosis development. Since theoretically iNOS is capable of producing both NO and O2−, we investigated the contribution of iNOS to vascular NO production by electron spin resonance (ESR) spectroscopy, oxidative stress by ESR and high performance liquid chromatography (HPLC) measurements of O2− and nitrosative stress by quantification of 3-nitrotyrosine. ESR and HPLC methods provide direct detection of NO and O2− production with utmost specificity and sensitivity. To assess the role of the iNOS expressing cellular compartment in atherosclerosis we studied plaque formation following bone marrow transplantation (BMT) between apoE−/− and apoE−/−/iNOS−/− animals. Using this approach, we investigated atherosclerosis development in animals selectively expressing iNOS in bone marrow derived versus parenchymal cells of the vessel wall.
Materials and Methods
All procedures performed conformed with the policies of the University of Würzburg, the NIH guidelines and an independent governmental committee for care and use of laboratory animals.
Animals
ApoE−/− and iNOS−/− animals were obtained from The Jackson Laboratories. All mice were backcrossed for ten generations to the C57Bl6 strain. Ly5.2/Cr mice on a C57Bl6 genetic background, carrying a mutation of their common leukocyte antigen (designated CD45.1) were obtained from the National Cancer Institute. iNOS−/− and apoE−/− animals were crossed to generate double knockout apoE−/−/iNOS−/− mice. Mice were genotyped by PCR using protocols supplied by The Jackson Laboratories. ApoE−/−/iNOS−/− or apoE−/−/iNOS+/+ were used as bone marrow donors or recipients to generate the following two “chimeric” mice strains. Bone marrow cells of apoE−/−/iNOS+/+ were transplanted into apoE−/−/ iNOS−/− mice to generate mice that lack parenchymal iNOS but express iNOS in blood cells (apoE−/−/iNOS+/+ in apoE−/−/iNOS−/−). Additionally, bone marrow cells of apoE−/−/iNOS−/− were transplanted into apoE−/−/iNOS+/+ mice to generate mice expressing iNOS in parenchymal cells, but lack iNOS in circulating blood cells (apoE−/−/iNOS−/− in apoE−/−/iNOS+/+). The animals were 10 weeks old when used for BMT experiments. As controls, apoE−/−/iNOS−/− received apoE−/−/iNOS−/− marrow, generating mice that lack iNOS completely. Moreover, apoE−/−/iNOS+/+ mice were transplanted with apoE−/−/iNOS+/+ bone marrow cells to generate controls expressing iNOS in both compartments (Table 1). A second line of mice was generated crossing apoE−/− with Ly5.2/Cr (Ly5.1+/+) animals. These mice were genotyped for apoE by PCR and for the Ly5.1 locus by fluorescence-activated cell sorting analysis of peripheral blood cells. ApoE−/−/Ly5.1+/+ mice were used as donors to evaluate transplantation efficiency. Four weeks after BMT a high fat “western-type” diet (Harlan Teklad, Madison, Wisconsin) was started and maintained for 16 weeks. Animals used in experiments that did not involve BMT were weaned at 3 weeks of age and fed with “western-type” diet for 18 weeks.
Table 1.
Characteristics of BMT Animals
| Genotype | Body weight
|
Total area aorta (mm2) male
|
Total area aorta (mm2) female
|
Total cholesterol (mg/dl)
|
||||
|---|---|---|---|---|---|---|---|---|
| n | n | n | n | |||||
| C57Bl6 | 28 ± 0.5 | 12 | 78 ± 1.4 | 12 | ||||
| apoE−/− | 25 ± 0.6 | 23 | 77 ± 2.9 | 10 | 67 ± 1.2 | 16 | 940 ± 55 | 22 |
| apoE−/− in apoE−/−/iNOS−/− | 27 ± 0.9 | 15 | 74 ± 2.4 | 6 | 70 ± 1.5 | 11 | 994 ± 53 | 18 |
| apoE−/−/iNOS−/−in apoE−/− | 26 ± 0.9 | 20 | 67 ± 1.3* | 8 | 60 ± 1.1* | 8 | 1187 ± 84 | 16 |
| apoE−/−/iNOS−/− | 25 ± 0.7 | 13 | 79 ± 2.1 | 8 | 76 ± 2.3 | 5 | 927 ± 139 | 10 |
P < 0.05 vs. all groups; n, number of animals analyzed.
Measurement of Vascular NO Production by Electron Paramagnetic Spintrapping
NO production of aortic rings was measured in an organ bath using colloid iron (II) diethyldithiocarbamate (Fe[DETC]2) as a spin trap and ESR detection (e-scan ESR spectrometer, Bruker BioSpin GmbH, Karlsruhe, Baden Wurttemburg). The method was adapted for detection of baseline NO production in apoE−/− mice.28 Briefly, animals were anesthetized with Avertin (80 μg/kg i.p.). The aorta was removed quickly and cleaned off adherent tissue at 4°C in chilled Krebs-Hepes Buffer (KHB) using a cold plate (Noxygen Science Transfer & Diagnostics, Denzlingen, Germany). The aortas were cut into 2 mm rings and were placed in one well of a 12-well plate containing 1500 μl KHB. The aortic NO produced was trapped with Fe-(DETC)2 for 1 hour in 37°C KHB. The protein concentration of the samples were quantified with a BCA protein assay kit (Pierce, Rockford, IL) and used to normalize the ESR signal intensity. The instrumental settings are mentioned in Table 2.
Table 2.
Instrumental Settings Used for ESR Measurements
| Instrumental setting | Experiment
|
|
|---|---|---|
| Vascular NO | Vascular superoxide | |
| Centre field | 3308 G | 3388 G |
| Sweep width | 80 G | 132 G |
| Microwave frequency | 9.495 GHz | 9.497 GHz |
| Microwave power | 50 mW | 1.25 mW |
| Modulation amplitude | 4.6 G | 1.63 G |
| Modulation frequency | 86 kHz | 86 kHz |
| Time constant | 81.92 ms | 40.96 ms |
| Conversion time | 20.48 ms | 10.24 ms |
| Number of scans | 100 | 50 |
Measurement of Vascular Oxygen Radical Production by ESR
Production of O2− was measured in aortic rings in an organ bath in 37°C KHB. The experimental procedure was performed according to a previously published protocol29 using the above mentioned e-scan spectroscope. Instrumental settings are reported in Table 2. O2− production was assessed by pre incubating aortic rings with PEG-SOD (100 U/ml) parallel to 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine for 1 hour. The intensity of the ESR signal was normalized to the sample’s protein content.
Measurement of Intracellular O2− Production by HPLC-Detection of Oxyethidium
HPLC measurements served as a second, independent method for O2− detection. O2− was measured in aortic rings by detection of oxyethidium, the fluorescent reaction product of O2− and dihydroethidium.30 Briefly, vessel rings were incubated in an organ bath with KHB containing 50 μmol/L dihydroethidium at 37°C. Subsequently, dihydroethidium was washed off and the rings were incubated for 1 hour. Aortic rings were then homogenized in ice cold methanol. The homogenate was filtered and separated by reverse phase HPLC using a C-18 column (Nucleosil 250, 4.5 mm; Sigma-Aldrich, Munich, Bayern) on a AKTA HPLC system (Amersham Biosciences, GE Health care, Munich, Bayern). Oxyethidium was quantified with a fluorescence detector (Jasco, Great Dunmov, Essex). The detected oxyethidium was normalized to the sample’s protein content.31
Bone Marrow Transplantation
Recipient animals were housed in autoclaved, single ventilated microisolator-cages. Animals were treated with acidified water (pH 2.0) containing 100 mg/L neomycin and 10 mg/ml polymyxin B sulfate (Sigma Aldrich) for ten days prior, until 2 weeks after lethal irradiation. Mice were irradiated with 1000 rads from a cesium γ source, followed by BMT 4 hours later.32 Bone marrow cells were prepared from the tibia and femur bones by flushing the bones with RPMI 1640 media (Gibco, Grand Island, NY) containing 2% fetal bovine serum and 400 U/ml DNase. Cells were filtered through a spleen mesh, counted and resuspended in media 199 containing 1% Hepes buffer, 400 U/ml DNase and 200 μg/ml gentamicin. Recipient animals received ∼5 × 106 bone marrow cells in 0.5 ml of BMT medium (formula) by tail vein injection.
Estimation of Transplantation Efficiency
To evaluate transplantation efficiency, apoE−/− animals were transplanted with ∼5 × 106 bone marrow cells from either apoE−/−/Ly5.2 or apoE−/−/Ly5.1, following the above protocol. Two weeks following BMT, animals were anesthetized with isofluorane and a 100 μl blood sample was drawn from the retro-orbital venous plexus. The red blood cells were subsequently lysed using red cell lysis buffer (BD Biosciences, Heidelberg, Baden Wurttemburg). The white cell pellet was resuspended in PBS and 1% fetal bovine serum, and stained with a monoclonal PE-anti-mouse CD45.1 and a fluorescence isothiocyanate-anti-mouse CD45.2 antibodies (BD Biosciences). The fluorescence of 5 × 104 cells was measured on a FACScan (BD Biosciences).
Lesion Assessment
The aorta was dissected and analyzed as previously described.33 Briefly, animals were anesthetized with Avertin (80 μg/kg i.p.), the aorta was perfused with PBS, pH 7.4, dissected from the aortic valve to the iliac bifurcation and fixed in 4% paraformaldehyde. Adventitial tissue was removed and the aorta was opened longitudinally and pinned onto a black wax surface using micro needles (Fine Science Tools, Heidelberg, Baden Wurttemburg). Serial images of the submerged vessels were captured with a black and white video camera (COHU, Poway, CA) mounted on the c-mount of a stereomicroscope (Leica, Wetzlar, Hessen). Lipid rich intraluminal lesions were subsequently stained with Sudan IV. Serial color pictures were captured using the same microscope equipped with a Leica 35 mm camera and used to identify lesions. Image analysis of digital images was performed using Image Pro Plus (Version 4.1; Media Cybernetics, Bethesda, MD). The amount of aortic lesion formation in each animal was measured as percent lesion area per total area of the aorta.
Tissue Preparation and Immunohistochemistry
Aortic arches were embedded in Tissue-Tek (Sakura Finetek, Heppenheim, Hesse) and snap-frozen in liquid nitrogen. Serial sections were fixed in acetone before staining. Immunostaining was performed using an anti-nitrotyrosine antibody (Upstate Biotechnology Inc., Lake Placid, NY). Biotinylated anti-rabbit IgG (Vector Laboratoties, Burlingame, CA) was used as the secondary antibody, and the nitrotyrosine staining was visualized using Vectastain ABC kit and DAB as the substrate (Vector laboratories).
Histomorphometry
Photomicrographs of the vessel sections were taken with a Leitz-camera mounted on a light microscope (Carl-Zeiss, Munich, Bayern). Pictures were digitalized and transferred to a computer for planimetry using Image Pro Plus (Media Cybernetics). All images were analyzed at 400-fold magnification. Results were expressed as positive staining area per total area of the plaque.
Western Blot Analysis
Aortic protein was isolated using RIPA buffer and western blots were performed using a polyclonal anti-iNOS antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). To confirm equal loading of protein an anti-α-actin-antibody (Santa Cruz Biotechnology, Inc.) was used. Blots were analyzed densitometrically using Quantity One software (Bio-Rad Laboratories, Munich, Bayern) and the density of protein bands for iNOS were normalized to the corresponding α-actin bands of the same blots.
Real-Time PCR
First-strand cDNA was synthesized from 2 μg of total aortic RNA, using random primers (Fermentas, St. Leon-Rot, Baden Wurttemburg). mRNA expression of nNOS and eNOS was quantified by real-time PCR (iCycler, Bio-Rad Laboratories). PCR amplification was performed for 40 cycles at primer annealing of 60°C. The expression of nNOS and eNOS was normalized to HPRT signal. The primer pairs used for real-time PCR are shown in Table 3.
Table 3.
Sequences of Primers and Probes Used for Real-Time PCR
| Gene | Primers and Flurogenic probes |
|---|---|
| nNOS | Sense: 5′-CCCACCAAAGCTGTCGATCT-3′ |
| Antisense: 5′-GGAGGTTGGCCTTGGTATTT-3′ | |
| 5′-6FAM-CACACCATTAGCCTGGGAGACTGAGCC-TMR | |
| eNOS | Sense: 5′-CTGGCAGCCCCAAGACCTA-3′ |
| Antisense: 5′-CGATGACGTCACCGGCTT-3′ | |
| 5′-6FAM-TCCTGAGGACAGAGCTAGCCGCGGAXT-Phosphate | |
| HPRT | Sense: 5′-GTTGGATACAGGCCAGACTTTGT-3′ |
| Antisense: 5′-CCACAGGACTAGAACACCTGC-3′ | |
| 5′-6FAM-CTCGTATTTGCAGATTCAACTTGCGCXT-PH |
Lipids and Lipoprotein Characterization
Lipoprotein cholesterol distribution of plasma samples was evaluated after fractionation by fast protein liquid chromatography gel filtration. Separate plasma samples (200 μl) from four animals per sex and genotype were fractionated on two serial superose-6-columns using an AKTA-BASIC system. Total cholesterol of plasma samples and fast protein liquid chromatography-fractions were measured using Infinity Cholesterol Reagent (Thermo Electron, Melbourne, Victoria) and a Spectra MAX 250 photometer (Molecular Devices, Sunnyvale, CA).
Statistical Analyses
Statistical analyses were performed using Stat View 4.51 (Abacus Concepts, Inc., Berkeley, CA) and Sigma Plot (Version 8, Systat Software Inc., San Jose, CA). Data were analyzed using Student’s t-test or two way analysis of variance, followed by Scheffe’s F-test. All data are expressed as mean ± SE. P < 0.05 was considered significant.
Results
iNOS Contributes Significantly to Vascular NO Production
To evaluate the contribution of iNOS to NO production we used ESR. Detection of vascular wall NO production using Fe-(DETC)2 spin trap showed significantly lower NO levels in apoE−/−/iNOS−/−, compared with apoE−/− in females (n = 13 vs. n = 6, P < 0.05, respectively) and males (n = 13 vs. n = 8, P < 0.05, respectively, Figure 1). Total NO production in apoE−/− was increased, compared with C57Bl6 animals (females: n = 6 vs. n = 5, P < 0.05; males: n = 8 vs. n = 7, P < 0.05, respectively, Figure 1). NO generation in female apoE−/−/iNOS−/− animals was significantly higher, compared with apoE−/−/ iNOS−/− males (P ≤ 0.01).
Figure 1.
ESR measurements of vascular NO production: Quantification of aortic NO production using Fe-(DETC)2 spin trap. Signal represents NO production from vessel rings over a one hour incubation period. ApoE−/− mice produced higher NO concentrations compared with C57Bl6 mice of both sexes (females: n = 6 vs. n = 5 *P < 0.05; males: n = 8 vs. n = 7, P < 0.05). Genetic deletion of iNOS in apoE−/−/iNOS−/− mice reduced NO formation to the levels of C57Bl6 mice. NO production was higher in female apoE−/− (n = 6) and apoE−/−/iNOS−/− (n = 13 ± SE) animals compared with males (apoE−/−: n = 8 and apoE−/−/iNOS−/−: n = 13) within each genotype. **P ≤ 0.01. All values are expressed as mean ± SE.
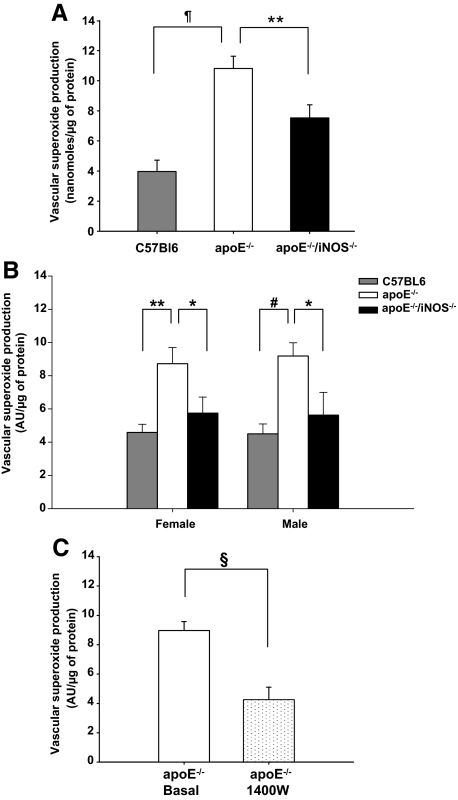
iNOS Significantly Contributes to Vascular O2− Production
ESR measurements of SOD inhibitable O2− levels showed significantly increased amounts of O2− in apoE−/− (n = 18) compared with C57Bl6 aortas (n = 12, P < 0.00001, Figure 2A). The levels of O2− were significantly lower in apoE−/−/iNOS−/− (n = 20) than in apoE−/− vessels (n = 18, P ≤ 0.01, Figure 2A). Additionally, O2− was measured by HPLC quantification of oxyethidium, the specific reaction product of dihydroethidium and O2−. In support of the ESR data, the HPLC assay showed that apoE−/− had higher O2− levels than apoE−/−/iNOS−/− (females: n = 7 vs. n = 7, P ≤ 0.05 and males: n = 8 vs. n = 9, P < 0.05, respectively, Figure 2B). O2− levels of C57Bl6 animals (females: n = 4, P ≤ 0.01; males: n = 5, P ≤ 0.001, Figure 2B) were significantly lower than the concentrations observed in apoE−/− mice. To determine whether acute pharmacological inhibition of iNOS reproduces these results, we incubated apoE−/− aortas with the iNOS specific inhibitor N-(3-aminomethyl) benzyl-acetamidine (1400W). Acute iNOS inhibition (n = 10) significantly reduced O2− levels in apoE−/− vessels, compared with basal levels (n = 15, P ≤ 0.0001, Figure 2C). Our results suggest that uncoupled iNOS itself is a significant source of O2− formation in apoE−/− vessels. No significant difference in O2− levels between sexes were observed.
Figure 2.
Quantitation of vascular O2− production. A: O2− levels were significantly higher in apoE−/− (n = 18) compared with C57Bl6 animals (n = 12), as assessed by ESR measurements of SOD inhibitable 3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine. signal. Significantly lower levels of O2− were observed in apoE−/−/iNOS−/− (n = 20) animals compared with apoE−/− controls (n = 18, ¶P < 0.00001, **P ≤ 0.01). B: Vascular O2− production assessed by HPLC was lower in apoE−/−/ iNOS−/− compared with apoE−/− animals in both sexes (females: n = 7 vs. n = 7, males: n = 9 vs. n = 8). ApoE−/− mice had significantly higher vascular O2− levels than C57Bl6 mice(females: n = 4, males: n = 5, *P ≤ 0.05, #P ≤ 0.001, **P ≤ 0.01). C: Acute inhibition of iNOS in vessels of apoE−/− mice using the iNOS specific inhibitor 1400W significantly reduced O2− production (n = 10 vs. basal, n = 15, §P ≤ 0.0001). All values are expressed as mean ± SE.
NO and O2− Generation from iNOS Results in Peroxynitrite Formation
Peroxynitrite, a reaction product of NO and O2− was quantified by measuring 3-nitrotyrosine positive atherosclerotic plaque areas (Figure 3A). Peroxynitrite staining was significantly reduced in apoE−/−/iNOS−/−, compared with apoE−/− in both sexes (female: n = 3 vs. n = 4, P < 0.01; male: n = 4 vs. n = 5, P < 0.05, respectively, Figure 3B). Interestingly, the 3-nitrotyrosine levels in female apoE−/− animals were significantly higher than in male apoE−/− (P < 0.01, Figure 3B). A similar sex difference was observed in apoE−/−/iNOS−/−, as residual 3-nitrotyrosine staining was more pronounced in females, compared with males (P < 0.05, Figure 3B).
Figure 3.
Quantification of peroxynitrite mediated nitrosative stress. A: Representative immunohistochemistry of 3-nitrotyrosine staining, a marker of peroxynitrite mediated oxidative stress in aortic plaques of apoE−/− and apoE−/−/iNOS−/− animals. The images were analyzed at 400-fold magnification (scale bar = ∼25 μm). B: Planimetry of % positive staining plaque area for nitrotyrosine revealed decreased nitrosative stress in lesion of apoE−/−/iNOS−/− compared with apoE−/− animals (female: n = 3 vs. n = 4; male: n = 4 vs. n = 5, *P < 0.05, **P < 0.01). All values are expressed as mean ± SE.
Sex-Dependent Difference of the iNOS Compartment on Atherosclerosis
To test the role of the iNOS expressing cellular compartment on plaque formation we generated chimeric animals that express iNOS selectively, either in bone marrow derived cells or in parenchymal cells. To evaluate transplantation efficacy we generated apoE−/−Ly5.2/Cr double homozygote mice, which carry a mutation of the common leukocyte antigen CD45 (designated CD45.1). Two weeks following BMT of apoE−/− recipients with apoE−/−Ly5.2/Cr bone marrow cells fluorescence-activated cell sorting analysis of peripheral blood monocytes revealed ≥98% CD45.1 positive cells, indicating nearly complete replacement of recipient (CD45.2 positive) leukocytes with donor cells (supplemental Figure S1, see http://ajp.amjpathol.org). We then performed BMT between apoE−/− and apoE−/−/iNOS−/− animals, serving as donors and recipients.
After 16 weeks of high fat diet there was no difference in bodyweight, total plasma cholesterol (Table 1) or lipoprotein profiles (Figure 4) in any of the four transplantation groups included in the study.
Figure 4.
Analysis of plasma lipoprotein profiles: Lipoprotein profiles of all four transplantation groups. No significant difference of lipoprotein distribution was observed between the groups. n = 4 per group.
Inspection of the longitudinal “en face” prepared aorta revealed that apoE−/− transplanted with apoE−/− bone marrow, expressing iNOS in the blood and the parenchymal compartment (apoE−/− in apoE−/−) developed large confluent lesions. In contrast, lesions in chimeric animals (apoE−/−/iNOS−/− in apoE−/− and apoE−/− in apoE−/−/iNOS−/−) and control apoE−/−/iNOS−/− recipients receiving apoE−/−/iNOS−/− bone marrow (apoE−/−/iNOS−/− in apoE−/−/iNOS−/−) were granular and less dense (Figure 5, A and B).
Figure 5.
Lesion assessment: Black and white images of the “en face” prepared aortic arch in female (A) and male (B) chimeric mice (left). Surface plot pictures of each respective image (right). Optical density of plaques increases in the order red<pink<blue<green. C: Lesion area of females was equally reduced in chimeric mice expressing iNOS selectively in parenchymal (n = 11) or bone marrow derived cells (n = 9 vs. apoE−/− in apoE−/−, n = 16). Deletion of iNOS in both compartments resulted in maximum lesion area reduction in females (n = 6). In contrast, only bone marrow derived iNOS is proatherogenic in male chimeras (apoE−/−/iNOS−/− in apoE−/−, n = 8 vs. apoE−/− in apoE−/−, n = 10). Parenchymal iNOS did not influence lesion development in males (apoE−/− in apoE−/−/iNOS−/−, n = 6 vs. apoE−/− in apoE−/−, n = 10, *P < 0.05, **P < 0.01). All values are expressed as mean ± SE.
The greatest reduction in % lesion area was observed in female and male apoE−/−/iNOS−/− recipients receiving apoE−/−/iNOS−/− bone marrow (apoE−/−/iNOS−/− in apoE−/−/iNOS−/−), compared with apoE−/− animals transplanted with apoE−/− bone marrow cells (apoE−/− in apoE−/−) (females: n = 6 vs. n = 16, P ≤ 0.01; males: n = 8 vs. n = 10, P ≤ 0.01, respectively, Figure 5C).
In female chimeric mice lacking iNOS selectively in bone marrow cells (apoE−/−/iNOS−/− in apoE−/−) percent aortic lesion area was significantly reduced compared with control apoE−/− recipients transplanted with apoE−/− donor cells (apoE−/− in apoE−/−, n = 9 vs. n = 16, P ≤ 0.01, Figure 5C). Similar results were obtained in female chimeric mice lacking iNOS selectively in parenchymal cells (apoE−/− in apoE−/−/iNOS−/−, n = 11 vs. n = 16, P ≤ 0.01, Figure 5C). These results show that both leukocyte and parenchymal iNOS mediate the proatherogenic effects in females. In male chimeric mice that lack iNOS in bone marrow cells (apoE−/−/iNOS−/− in apoE−/−), lesion area was significantly reduced compared with male apoE−/− controls expressing iNOS in both compartments (apoE−/− in apoE−/−, n = 8 vs. n = 10, P < 0.05, Figure 5C). In contrast, there was no difference in % lesion area in male mice expressing iNOS selectively in bone marrow cells (apoE−/− in apoE−/−/iNOS−/−), compared with the controls (apoE−/− in apoE−/−, n = 6 vs. n = 10, P = 0.9, NS, Figure 5C). This suggests that in males only bone marrow derived cells mediate the proatherogenic effects of iNOS.
The iNOS Compartment Affects Vascular Remodeling
Total area of the aortas in female and male chimeric animals selectively lacking iNOS in the bone marrow derived compartment (apoE−/−/iNOS−/− in apoE−/−) was significantly reduced compared with all other transplantation groups as well as age matched C57Bl6 mice (Table 1). These results show that unbalanced expression of iNOS in the parenchymal compartment affects vascular remodeling.
Contribution of the iNOS Cell Compartment to Total Vascular iNOS Expression
Western blot analysis of aortic iNOS protein expression revealed highest iNOS levels in apoE−/− expressing iNOS in both, the bone marrow and the parenchymal compartments and absence of vascular iNOS expression in apoE−/−/iNOS−/− (Figure 6). The iNOS protein levels in animals selectively expressing iNOS in parenchymal cells (apoE−/−/iNOS−/− in apoE−/−, n = 3 females and 6 males) was comparable with control mice expressing iNOS in both compartments (apoE−/−, n = 4 females and 3 males, NS, Figure 6). In contrast, iNOS protein expression in mice expressing iNOS selectively in leukocytes (apoE−/− in apoE−/−/iNOS−/−) (n = 5 females and 4 males) was significantly lower when compared with animals expressing iNOS selectively in parenchymal cells (apoE−/−/iNOS−/− in apoE−/−, females: P < 0.001, males: P < 0.05) or both compartments (apoE−/−, P < 0.01, Figure 6).
Figure 6.
Parenchymal cells contribute significantly to total vascular iNOS: Western blot analysis revealed lower contribution of the bone marrow compartment (apoE−/− in apoE−/−/iNOS−/−, n = 5 females and 4 males), compared with the parenchymal compartment (apoE−/−/iNOS−/− in apoE−/−, n = 3 females and 6 males), to total iNOS expression. The results were consistent in females and males. ApoE−/− (n = 4 females and 3 males) and apoE−/−/iNOS−/− (n = 3 females and 4 males) served as positive and negative controls, respectively (*P < 0.05, **P ≤ 0.01, #P ≤ 0.001). All values are expressed as mean ± SE.
iNOS Deletion Does Not Affect the Expression of Other NOS Isoforms
The expression levels of eNOS and nNOS mRNA measured by real-time PCR were unchanged in all four transplantation groups (Table 4).
Table 4.
Real-Time PCR Quantification of eNOS and nNOS
| Genotype | Fold expression of eNOS
|
Fold expression of nNOS
|
||
|---|---|---|---|---|
| n | n | |||
| apoE−/− | 1.0 ± 0.1 | 4 | 1.0 ± 0.1 | 3 |
| apoE−/− in apoE−/−/ iNOS−/− | 1.2 ± 0.2 | 5 | 0.6 ± 0.3 | 5 |
| apoE−/−/iNOS−/−in apoE−/− | 0.7 ± 0.09 | 5 | 0.6 ± 0.2 | 4 |
| apoE−/−/iNOS−/− in apoE−/−/iNOS−/− | 0.8 ± 0.1 | 6 | 0.8 ± 0.4 | 4 |
n, number of experiments from individual animals.
Discussion
Our ESR measurements show that iNOS significantly contributes to NO production in the vasculature of atherosclerotic apoE−/− mice. Interestingly, total aortic NO formation is increased in apoE−/−, compared with healthy wild-type C57Bl6 mice. Vascular NO production in apoE−/−/iNOS−/− mice was reduced to the levels of C57Bl6 mice, suggesting that the source of the increased NO production in apoE−/− mice is iNOS. Since iNOS expression is induced in smooth muscle cells and leukocytes in the plaque, these cell types mediate the additional aortic NO production.2 In healthy vessels constitutive NOS enzymes are the only isoforms detectable.2 In contrast, all three NOS isoforms (nNOS, iNOS, eNOS) are expressed in atherosclerotic plaque.2 iNOS is regulated transcriptionally and catalyzes the production of high concentrations of NO, once the enzyme is expressed. In contrast, eNOS and nNOS (both constitutively expressed, cNOS) produce low amounts of NO in a highly regulated way.
Indirect measurements of NO like the l-arginine to l-citrulline conversion assay and cGMP levels show that there is reduced constitutive NOS activity in atherosclerotic vessels.34 Impaired eNOS dependent NO production results in impaired endothelial vasodilator function, also termed endothelial dysfunction. Therefore, atherosclerosis is generally considered a state of decreased NO bioavailability. In contrast, our data shows that iNOS increases total NO production of the vessel wall in the apoE−/− model. Our study is consistent with the previously published report by Minor et al, who showed increased nitrogen oxides in the aorta of atherosclerotic rabbits.35 The latter study used an indirect method for NO detection and didn’t specify the source of NO production. We here used ESR spin trapping of NO, a highly sensitive method for direct NO detection that allows differentiating NO from other nitric oxide metabolites. NO production was higher in female, compared with male apoE−/−. Since a similar observation was made in apoE−/−/iNOS−/− mice, this sex difference is not mediated by iNOS. Given the well documented positive effects of estrogens on eNOS function, this observation is likely due to differences of eNOS activity between sexes.36,37,38,39
Increased O2− production can decrease NO bioavailability and result in proatherogenic oxidative stress. Aside from other enzymatic sources of O2−, iNOS itself may contribute directly to O2− production.27 Therefore, we evaluated O2− production in apoE−/− and apoE−/−/iNOS−/− mice. Using two independent direct methods we found a significant decrease in vascular wall O2− production in apoE−/−/iNOS−/−, compared with apoE−/−. In addition, total vascular reactive oxygen species production, assessed by measuring the conversion of 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine to nitroxide 3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine was significantly reduced in apoE−/−/iNOS−/−, compared with apoE−/− animals (data not shown). Since decreased oxidative stress in apoE−/−/iNOS−/− mice could also be due to secondary changes resulting from chronic gene knockout, we tested whether acute iNOS inhibition reduces O2− production. And indeed, pretreatment of the vessels with the iNOS specific inhibitor 1400W significantly reduced O2− levels, indicating that iNOS itself contributes to O2− production in atherosclerosis. To the best of our knowledge, this is the first direct evidence supporting O2− generation by iNOS itself in atherosclerosis. In contrast, ample evidence supports eNOS “uncoupling” in atherosclerotic vessels leading to O2−, instead of NO production by the enzyme.40,41 The mechanism of O2− generation by iNOS differs from eNOS and nNOS. The latter two enzymes produce O2− from the heme centers of their oxygenase domains,42,43 while iNOS produces O2− from the reductase domain.27 While 100 μmol/L of l-arginine was sufficient to block O2− generation from nNOS,43 there was no effect of 100 μmol/L l-arginine on iNOS mediated O2− generation.27 Higher concentrations of l-arginine (1 mmol/L to 5 mmol/L) only reduced, but did not abolish O2− production, indicating that O2− generation by iNOS may even occur when substrate is not limited.27 Xia et al reported that induction of iNOS in macrophages reduced l-arginine concentrations, indicating high substrate demand.20 These authors hypothesized that iNOS may catalyze NO from the oxygenase- and O2− from the reductase domain simultaneously, which could directly lead to peroxynitrite formation. Quantification of tissue 3-nitrotyrosine, considered a footprint of peroxynitrite mediated protein damage showed reduced levels of 3-nitrotyrosine in apoE−/−/iNOS−/− vessels, compared with apoE−/− vessels. Peroxynitrite oxidizes tetrahydrobiopterin, a cofactor of NOS,44 which in its oxidized form causes uncoupling of the constitutive NOS enzymes.45 iNOS mediated peroxynitrite formation and its high substrate demand may thus cause uncoupling of constitutive NOS isoforms, resulting in further O2− production. This hypothesis is supported by the finding of reduced BH2 levels in apoE−/−/ iNOS−/− mice.46 The residual peroxynitrite detected in apoE−/−/iNOS−/− might be due to the reaction of NO from constitutive NOS with superoxide generated from myeloperoxidase, NADPH oxidase, and xanthine oxidase.47
Since iNOS is expressed in lesional bone marrow derived and parenchymal cells we performed BMT experiments between apoE−/− and apoE−/−/iNOS−/− mice to identify the cellular compartment responsible for iNOS’s proatherogenic effects. Control experiments showed nearly complete replacement of recipient bone marrow with donor cells, confirming that the generated mice expressed iNOS either in the parenchymal or bone marrow-derived cell compartment. Our data shows that both, parenchymal and leukocyte derived iNOS is proatherogenic in female animals. In contrast, only bone marrow derived iNOS accelerated plaque development in male animals. Moreover, selective expression of iNOS in parenchymal cells coincided with significantly smaller aortic surface areas while total aortic area in all other BMT groups and C57Bl6 control animals were similar. Taken together, these findings establish a sex difference in the role of the iNOS expressing cellular compartment in atherosclerosis and suggest that parenchymal iNOS expression affects vascular remodeling.
In other disease models different iNOS expressing cellular compartments mediate deleterious or protective effects. For example both, iNOS in parenchymal and bone marrow derived cells play a regulatory role in a model of experimental autoimmune encephalomyelitis.48 Further, the iNOS expressing compartment differs between organs, which in turn affects systemic NO production. In tumor necrosis factor- or lipopolysacharide-induced septic shock, parenchymal cells were the only source of systemic nitroxides, whereas during granulomatous inflammation nitroxide production was mediated by hematopoietic cells.49 In endotoxemia, bone marrow derived cells account for iNOS expression in lungs, whereas parenchymal cells are the predominant source of iNOS in colon, liver, and cremaster muscle.50
Our immunoblotting results showed that iNOS expression was highest in chimeric animals expressing iNOS in parenchymal cells, while the expression levels of leukocyte derived iNOS were low. Since leukocyte derived iNOS accelerates atherosclerosis we hypothesize that iNOS expression in leukocytes is more transient than parenchymal iNOS expression. Interestingly, high iNOS expression in parenchymal cells did not increase atherosclerosis in this experimental group. A possible explanation may be that the amount of iNOS protein does not reflect the enzyme’s activity. In this respect Jones et al reported that post-translational modifications of iNOS in cultured smooth muscle cells regulates the enzyme’s activity.51 However, at present it is unknown whether this post-translational regulation differs between sexes. In addition, we observed an increased level of iNOS expression in female animals compared with males. While some studies have shown that estrogen induces the expression of iNOS in arteries,52,53 others have shown that estrogen down-regulates iNOS expression.54,55 However, the above mentioned studies did not employ atherosclerosis models and differed in the animal species studied (ewes and rats). Our results show higher iNOS expression in females, demonstrating a possible role of female hormones in regulating iNOS in atherosclerosis.
In summary, iNOS simultaneously increases NO and O2− production and nitrosative stress in the atherosclerotic plaque. Therefore, iNOS may directly increase proatherosclerotic oxidative stress. Our finding, that iNOS increases total vascular NO production indicates that increased vascular NO, although considered an important anti-atherosclerotic factor is not favorable when its source is iNOS. Leukocyte derived iNOS accelerates plaque development in female and male apoE−/− mice. In contrast, parenchymal iNOS only accelerated plaque formation in females, but not in male animals. Therefore, our study establishes a sex difference of the iNOS expressing cellular compartment on plaque development.
Supplementary Material
Acknowledgments
We thank Dr. Bruno Fink for suggestions and discussions regarding the ESR experiments (Noxygen Science Transfer & Diagnostics, Denzlingen, Germany) and Dr. Megan Sykes (Massachusetts General Hospital, Boston, MA) for sharing the BMT technique and helping us with the evaluation of the model. We also thank Bruker Biospin GmbH, Karlsruhe, Germany for the use of their ESR facility. The excellent technical assistance from Ms. Gabriele Riehl and Mrs. Alla Ganscher is well appreciated.
Footnotes
Address reprint requests to Peter J. Kuhlencordt, M.D., Medizinische Poliklinik, Standort Innenstadt, Ludwig Maximilians University, Pettenkoferstrasse-8a, 80336 Munich, Germany. E-mail: peter.kuhlencordt@med.uni-muenchen.de.
Supported by grants from the Deutsche-Forschungsgemeinschaft (KU-1206(1) and 1206(2) and the IZKF-Würzburg to P.K.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17:2479–2488. doi: 10.1161/01.atv.17.11.2479. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B, Cherqui G, Baud L. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. Evidence for involvement of nuclear factor-kappaB activation. J Biol Chem. 1998;273:5086–5092. doi: 10.1074/jbc.273.9.5086. [DOI] [PubMed] [Google Scholar]
- Giordano A, Tonello C, Bulbarelli A, Cozzi V, Cinti S, Carruba MO, Nisoli E. Evidence for a functional nitric oxide synthase system in brown adipocyte nucleus. FEBS Lett. 2002;514:135–140. doi: 10.1016/s0014-5793(02)02245-7. [DOI] [PubMed] [Google Scholar]
- Kolodziejska KE, Burns AR, Moore RH, Stenoien DL, Eissa NT. Regulation of inducible nitric oxide synthase by aggresome formation. Proc Natl Acad Sci USA. 2005;102:4854–4859. doi: 10.1073/pnas.0500485102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Lerida I, Corvi MM, Barrientos AA, Gavilanes F, Berthiaume LG, Rodriguez-Crespo I. Palmitoylation of inducible nitric-oxide synthase at Cys-3 is required for proper intracellular traffic and nitric oxide synthesis. J Biol Chem. 2004;279:55682–55689. doi: 10.1074/jbc.M406621200. [DOI] [PubMed] [Google Scholar]
- Pan J, Burgher KL, Szczepanik AM, Ringheim GE. Tyrosine phosphorylation of inducible nitric oxide synthase: implications for potential post-translational regulation. Biochem J. 1996;314:889–894. doi: 10.1042/bj3140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini R, Patel S, Saluja R, Sahasrabuddhe AA, Singh MP, Habib S, Bajpai VK, Dikshit M. Nitric oxide synthase localization in the rat neutrophils: immunocytochemical, molecular, and biochemical studies. J Leukoc Biol. 2006;79:519–528. doi: 10.1189/jlb.0605320. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide, Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–1027. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- Hickey MJ, Sharkey KA, Sihota EG, Reinhardt PH, Macmicking JD, Nathan C, Kubes P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J. 1997;11:955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, Billiar TR, Williams DL, Kochanek PM. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J Cereb Blood Flow Metab. 2005;25:673–684. doi: 10.1038/sj.jcbfm.9600068. [DOI] [PubMed] [Google Scholar]
- Darley-Usmar V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- Detmers PA, Hernandez M, Mudgett J, Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock J, Weikel R, Bergstrom JD, Shevell DE, Hermanowski-Vosatka A, Sparrow CP, Chao YS, Rader DJ, Wright SD, Pure E. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J Immunol. 2000;165:3430–3435. doi: 10.4049/jimmunol.165.6.3430. [DOI] [PubMed] [Google Scholar]
- Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation. 2001;103:3099–3104. doi: 10.1161/01.cir.103.25.3099. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Matsui-Hirai H, Fukatsu A, Sumi D, Kano-Hayashi H, Rani PJ, Iguchi A. Selective iNOS inhibitor. ONO1714 successfully retards the development of high-cholesterol diet induced atherosclerosis by novel mechanism, Atherosclerosis. 2006;187:316–324. doi: 10.1016/j.atherosclerosis.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Behr-Roussel D, Rupin A, Simonet S, Bonhomme E, Coumailleau S, Cordi A, Serkiz B, Fabiani JN, Verbeuren TJ. Effect of chronic treatment with the inducible nitric oxide synthase inhibitor N-iminoethyl-l-lysine or with l-arginine on progression of coronary and aortic atherosclerosis in hypercholesterolemic rabbits. Circulation. 2000;102:1033–1038. doi: 10.1161/01.cir.102.9.1033. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages, Proc Natl Acad Sci USA. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnett CA, Lund DD, Chu Y, Brooks RM, 2nd, Faraci FM, Heistad DD. NO-dependent vasorelaxation is impaired after gene transfer of inducible NO-synthase. Arterioscler Thromb Vasc Biol. 2001;21:1281–1287. doi: 10.1161/hq0801.093509. [DOI] [PubMed] [Google Scholar]
- Shears LL, 2nd, Kibbe MR, Murdock AD, Billiar TR, Lizonova A, Kovesdi I, Watkins SC, Tzeng E. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. J Am Coll Surg. 1998;187:295–306. doi: 10.1016/s1072-7515(98)00163-x. [DOI] [PubMed] [Google Scholar]
- Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koglin J, Glysing-Jensen T, Mudgett JS, Russell ME. Exacerbated transplant arteriosclerosis in inducible nitric oxide-deficient mice. Circulation. 1998;97:2059–2065. doi: 10.1161/01.cir.97.20.2059. [DOI] [PubMed] [Google Scholar]
- Kubes P. Inducible nitric oxide synthase: a little bit of good in all of us. Gut. 2000;47:6–9. doi: 10.1136/gut.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- Kleschyov AL, Munzel T. Advanced spin trapping of vascular nitric oxide using colloid iron diethyldithiocarbamate. Methods Enzymol. 2002;359:42–51. doi: 10.1016/s0076-6879(02)59170-9. [DOI] [PubMed] [Google Scholar]
- Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, Katusic ZS. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- Minor RL, Jr, Myers PR, Guerra R, Jr, Bates JN, Harrison DG. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990;86:2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Up-regulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett. 1995;360:291–293. doi: 10.1016/0014-5793(95)00124-r. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- Nuedling S, Kahlert S, Loebbert K, Doevendans PA, Meyer R, Vetter H, Grohe C. 17 Beta-estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovasc Res. 1999;43:666–674. doi: 10.1016/s0008-6363(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Prevot V, Beauvillain JC, Cadet P, Fimiani C, Welters I, Fricchione GL, Breton C, Lassalle P, Salzet M, Bilfinger TV. Cell-surface estrogen receptors mediate calcium-dependent nitric oxide release in human endothelia. Circulation. 2000;101:1594–1597. doi: 10.1161/01.cir.101.13.1594. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, Masada M, Yokoyama M. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest. 2002;110:331–340. doi: 10.1172/JCI15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Hattori S, Wang X, Satoh H, Nakanishi N, Kasai K. Oral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:865–870. doi: 10.1161/01.ATV.0000258946.55438.0e. [DOI] [PubMed] [Google Scholar]
- Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- Pou S, Keaton L, Surichamorn W, Rosen GM. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upmacis RK, Crabtree MJ, Deeb RS, Shen H, Lane PB, Benguigui LE, Maeda N, Hajjar DP, Gross SS. Profound biopterin oxidation and protein tyrosine nitration in tissues of ApoE-null mice on an atherogenic diet: contribution of inducible nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2007;293:H2878–H2887. doi: 10.1152/ajpheart.01144.2006. [DOI] [PubMed] [Google Scholar]
- Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arterioscler Thromb Vasc Biol. 2000;20:1716–1723. doi: 10.1161/01.atv.20.7.1716. [DOI] [PubMed] [Google Scholar]
- Zehntner SP, Bourbonniere L, Hassan-Zahraee M, Tran E, Owens T. Bone marrow-derived versus parenchymal sources of inducible nitric oxide synthase in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;150:70–79. doi: 10.1016/j.jneuroim.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Bultinck J, Sips P, Vakaet L, Brouckaert P, Cauwels A. Systemic NO production during (septic) shock depends on parenchymal and not on hematopoietic cells: in vivo iNOS expression pattern in (septic) shock. FASEB J. 2006;20:2363–2365. doi: 10.1096/fj.06-5798fje. [DOI] [PubMed] [Google Scholar]
- Hickey MJ, Sihota E, Amrani A, Santamaria P, Zbytnuik LD, Ng ES, Ho W, Sharkey KA, Kubes P. Inducible nitric oxide synthase (iNOS) in endotoxemia: chimeric mice reveal different cellular sources in various tissues. FASEB J. 2002;16:1141–1143. doi: 10.1096/fj.01-0764fje. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Jourd'heuil D, Salerno JC, Smith SM, Singer HA. iNOS regulation by calcium/calmodulin-dependent protein kinase II in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2634–H2642. doi: 10.1152/ajpheart.01247.2006. [DOI] [PubMed] [Google Scholar]
- Binko J, Majewski H. 17 beta-Estradiol reduces vasoconstriction in endothelium-denuded rat aortas through inducible NOS. Am J Physiol. 1998;274:H853–H859. doi: 10.1152/ajpheart.1998.274.3.H853. [DOI] [PubMed] [Google Scholar]
- Mershon JL, Baker RS, Clark KE. Estrogen increases iNOS expression in the ovine coronary artery. Am J Physiol Heart Circ Physiol. 2002;283:H1169–H1180. doi: 10.1152/ajpheart.00397.2000. [DOI] [PubMed] [Google Scholar]
- Kauser K, Sonnenberg D, Diel P, Rubanyi GM. Effect of 17beta-oestradiol on cytokine-induced nitric oxide production in rat isolated aorta. Br J Pharmacol. 1998;123:1089–1096. doi: 10.1038/sj.bjp.0701715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu FM, Ozbilgin K, Kuscu NK, Inan S, Vatansever S, Ceylan E. The effect of oestradiol and neta on immunohistochemical staining of iNOS and eNOS in coronary arteries of ovariectomized rats. Histol Histopathol. 2006;21:367–371. doi: 10.14670/HH-21.367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.