Abstract
Venous thromboembolism (VTE) is a common and potentially life threatening condition. It continues to be under diagnosed and undertreated. Awareness among Indians regarding this potentially life-threatening disease is low. Contrary to earlier belief, the incidence of VTE in Asia and India is comparable to that in Western countries. The risk of VTE is especially high in hospitalized patients, in a majority of whom it is clinically silent. It is one of the commonest causes of unplanned readmission and preventable death. In the United States, it is responsible for more deaths than accidents. Thromboprophylaxis is highly effective in reducing the incidence of VTE without any increase in clinically significant bleeding. It is worth emphasizing that prevention of VTE is much easier and cheaper than its treatment.
Keywords: Thromboembolism, urology, venous
INTRODUCTION
Quoted as a major health problem and one of the most common preventable causes of hospital deaths in the western world, venous thromboembolism (VTE) has rarely evoked such consideration in India. Not surprisingly, a Pubmed search of “Deep vein thrombosis + India” yields just 8 articles. Deep venous thrombosis (DVT) and pulmonary embolism (PE) are a single clinico-pathological entity - venous thromboembolism (VTE). The true incidence of VTE is hard to get because of the often silent nature of the condition. In the western world, the incidence is one case of DVT and 0.5 cases of PE per 1000 population/year.[1] Hospitalized patients are especially at risk for VTE as most have multiple risk factors. Autopsy studies have shown the incidence of VTE in hospitalized patients to be as high as 34.7% with fatal pulmonary embolism in 9.4%. Table 1 shows the absolute risk of VTE in hospitalized patients.[2,3]
Table 1.
Absolute risk of venous thromboembolism in hospitalized patients
| Patient group | VTE prevalence (%) |
|---|---|
| Medical patients | 10-20 |
| Cardiac patients | 15-40 |
| Major gynaecological surgery | 15-40 |
| Major urological surgery | 15-40 |
| Neurosurgery | 15-40 |
| Stroke | 20-30 |
| Hip and knee arthroplasty | 40-60 |
| Major trauma | 40-50 |
| Spinal cord injury | 60-80 |
| Critical care patients | 10-20 |
In this article, we give an overview of the epidemiology, pathophysiology, treatment, and prevention of VTE and its complications. Special emphasis is laid on recent studies from the Indian/Asian population. We also present data from studies done on urological patients to assess the risk of VTE and to make recommendations regarding VTE prophylaxis.
Most studies from India have looked at specific patient groups like postoperative orthopaedic patients[4,5] and there is no data on the overall incidence of VTE in the general population. The prevailing belief that VTE in the Asian population is less than in the Western population has essentially been disproved[6–9] and there appears no reason to believe that it should be any different in India.
PATHOPHYSIOLOGY AND RISK FACTORS
Virchow's far-sighted observation on the triad of factors leading to venous thrombosis has, with some refinement, stood the test of time. The triad of change in blood flow, change in blood constituents, and change in vessel wall has now been refined to venous stasis, activated coagulation pathways, and venous endothelial injury.
Venous thrombi are made up of fibrin, red cells, platelets, and leucocytes. Typically, these thrombi are believed to start in areas of slow or turbulent venous flow such as large venous sinuses or venous valve cusps and also in areas of direct venous trauma. Activation of the coagulation pathway is the crucial step in the initial formation of venous thrombi and this is believed to happen due to local injury or remote release of mediators. Activation of the pathway alone is inadequate in formation of a full-fledged venous thrombus as inhibitors of thrombosis such as antithrombin and thrombomodulin-protein C and S, tissue factor pathway inhibitor (TFPI) along with the fibrinolytic pathway would clear the clot. Hence, it is persistent activation due to endothelial stimulation along with poor flow failing to clear the activated factors that results in an imbalance in the pro and anti-thrombotic pathways which ultimately leads to progression of the thrombus.
Heit and colleagues have listed the following conditions as major risk factors for developing VTE: increasing age, male gender, surgery, trauma, confinement in hospitals or nursing homes, malignancy, neurologic disease, central venous catheter, prior superficial vein thrombosis, and varicose veins.[10] Pregnancy, oral contraceptive pill use, and hormone replacement therapy are independent risk factors in women.
The surgical patient has all three Virchow's factors present in the peri-operative period. They have venous stasis due to immobilization and surgical positioning. Direct venous injury or remote release of mediators of coagulation due to tissue trauma also increases the risk of venous thrombosis. The risk factors for a surgical patient developing VTE have been extensively studied and the important determinants appear to be age, type of surgery, length of procedure, and duration of immobilization. Hull et al.[11] have categorized post operative patients into low, moderate and high risk for VTE on the basis of these characteristics [Table 2].
Table 2.
Stratification of patients based on their risk for developing venous thromboembolism
| Category | Characteristics |
|---|---|
| Low | Age <40 years, no other risk factors, uncomplicated abdominal/thoracic surgery |
| Age >40 years, no other risk factors, minor elective abdominal/thoracic surgery <30 min | |
| Moderate | Age >40 years, abdominal/thoracic surgery >30 min |
| History of recent thromboembolism | |
| High | Abdominal or pelvic procedure for malignancy |
| Major lower extremity orthopaedic procedure |
NATURAL HISTORY
Acute DVT is followed by a complex process of attempted recanalization of the vessel lumen which is mediated by leukocyte infiltration and cell mediated thrombolysis. In animal models, the recanalization process has been found to begin within a few days of the initial thrombosis and complete recanalization of the vessel lumen is the most common outcome.
Rethrombosis would naturally impede with the recanalization process and recurrent thromboembolism of up to 47% has been reported in patients inadequately anti-coagulated in the first 3 months after an initial proximal DVT.[12] The clinical behavior of acute DVT depends on the location of thrombosis. In the lower limb, proximal iliofemoral DVTs tend to cause more acute and chronic complications than distal calf vein DVTs. Calf vein DVTs tend to recanalize faster than proximal ones.
Pulmonary embolism is the most dangerous complication of acute DVT. As with acute DVT, this usually remains clinically silent. 25-50% of all patients with documented DVT and absence of pulmonary symptoms have been shown to have evidence of PE on lung perfusion scans.[13] Symptomatic pulmonary embolism is strongly associated with inadequately treated DVT and underlying cardio-pulmonary reserve of the patient. The mortality rate of PE is 11% within an hour of presentation and a further 30% among survivors if not recognized.[14]
DIAGNOSIS
The clinical diagnosis of DVT is generally inaccurate, especially in the inpatient setting. Of patients undergoing ultrasound “to rule out DVT”, only about 15% are found to have one.[15] To better select patients for screening, Wells et al.[16] have suggested the following clinical model to assess pre-test probability of DVT [Table 3].
Table 3.
Clinical model to assess pre-test probability of deep vein thrombosis
| Clinical feature | Score |
|---|---|
| Active cancer (treatment ongoing or within previous 6 months or palliative) | 1 |
| Paralysis, paresis, or recent plaster immobilization of the legs | 1 |
| Recently bedridden for more than 3 days or major surgery within 4 weeks | 1 |
| Localized tenderness along the distribution of the deep venous system | 1 |
| Entire leg swollen | 1 |
| Calf swelling by more than 3 cm compared with the asymptomatic leg (measured 10 cm below the tibial tuberosity) | 1 |
| Pitting oedema (greater in the symptomatic leg) | 1 |
| Collateral superficial veins (non-varicose) | 1 |
| Alternative diagnosis as likely or wider than that of deep vein thrombosis | -2 |
Low probability: score of 0 or less; Moderate probability: 1-2; High probability: 3 or more.
The value of a clinical probability score along with D-dimer assessment is useful to eliminate the possibility of DVT. Plasma D-dimers are derivatives of fibrin degradation products and are found to be raised in patients with thromboembolism. Plasma D-dimer assay has very high sensitivity but poor specificity for venous thrombosis and hence a patient with low probability of DVT with a negative D-dimer test has almost no chance of having venous thrombosis.
Duplex ultrasonography has replaced venography as the investigation of choice in diagnosing DVT. It is cheap, easily available, and non-invasive. However, it does have some disadvantages that need to be kept in mind - diagnosis of isolated calf vein DVT and evaluation of iliac veins is often dependent on operator experience, patient habitus, and the clinical situation. Despite these limitations, a negative report from a single, technically adequate ultrasound examination is sufficient grounds to withhold anticoagulation therapy. A complete ultrasound performed by trained sonologists with a proper protocol, resulted in only 4 (1.1%) instances of misdiagnosis in more than 400 patients.[17] Computerized tomography (CT) pulmonary angiography is the most useful test for detecting a pulmonary embolus. It can be combined with CT venography as a single scan to diagnose both PE and DVT.
TREATMENT
Once the diagnosis of DVT has been established, treatment involves anticoagulation to encourage clot lysis and recanalization and prevent re-thrombosis and embolization. Traditionally, this involved a continuous infusion of unfractionated heparin followed by oral anticoagulation with warfarin for a period of 3-6 months. Unfractionated heparin consists of a heterogeneous mixture of polysaccharide chains and hence, its therapeutic action can be extremely variable. This necessitates the need to monitor therapy using either activated partial thromboplastin time (aPTT) or heparin blood levels and titrating the dose based on these.
Low molecular weight heparins (LMWH) are derivatives of unfractionated heparin formed by depolymerization. The advantage they have over unfractionated heparin is that the anticoagulant response to a standard dose of LMWH is consistent and predictable and hence does not require monitoring during therapy. Also, the half life of these drugs is longer and once-daily dosage is possible. Several trials have shown that LMWH are at least as effective as unfractionated heparin in the treatment of DVT[18–21] and the risk of bleeding related complications appears to be less.[22] Continued anticoagulation is usually maintained using oral warfarin.
The major complication of anticoagulant therapy is bleeding. Heparin can also result in an immune mediated thrombocytopenia called Heparin Induced thrombocytopenia (HIT) syndrome which can result in wide spread arterial and venous thrombosis. If HIT syndrome is diagnosed then heparin in all forms has to be stopped and anticoagulation using other agents such as hirudin and argatroban will have to be instituted.
The Sixth ACCP Consensus Conference on Antithrombotic Therapy has made the following recommendations for duration of anticoagulation: patients with reversible or temporary risk factors for VTE may be treated for 3-6 months, while patients with irreversible risk factors such as thrombophilic states and malignancy are to be treated indefinitely.[23]
PROPHYLAXIS
There are two ways in which pulmonary embolism, the most serious complication of DVT can be prevented - primary prevention of the initial DVT and secondary prevention by early detection and treatment of DVT. Given the poor sensitivity of clinical diagnosis of DVT, primary prevention has become the preferred mode of prophylaxis. Methods of primary prevention include early and persistent mobilization, pharmacological agents like unfractionated heparin and low molecular weight heparin (LMWH) and mechanical means like intermittent pneumatic compression and graduated compression stockings.
Patients at low risk should be encouraged early mobilization and do not require prophylaxis. Patients with moderate risk should have additional pharmacological prophylaxis. Patients in the high-risk group require pharmacological and mechanical prophylaxis. In patients undergoing surgery in which minor haemorrhage may be critical to the outcome, such as neurosurgery, ophthalmic surgery, and spine surgery, mechanical prophylaxis is recommended.
In surgical patients, the prothrombotic state begins intraoperatively and hence prophylaxis should ideally commence before induction of anaesthesia. Trials have shown that initiating prophylactic anticoagulation 2 h prior to surgery is both safe and effective in decreasing the risk of fatal PE.[2,24] If prophylaxis cannot be started peri-operatively because of the risk of bleeding, there is still a beneficial effect in starting it postoperatively. Prophylaxis in the postoperative period is generally maintained till the patient is discharged. However, in high-risk orthopaedic procedures such as hip replacements, extended prophylaxis up to 30 days is indicated.
Prophylactic anticoagulation in the setting of regional neuraxial anaesthesia poses the risk of a spinal hematoma which can potentially lead to permanent neurological impairment. Specific risk factors for this happening are prolonged epidural analgesia, traumatic introduction, and females over the age of 75 years. Specific guidelines on the usage of prophylactic anticoagulation, in this setting, have been outlined by the American Association of Regional Anesthesia Consensus Conference.[25]
Among the various pharmacological agents, meta-analyses have shown that LMWH and unfractionated heparin have equal efficacy in preventing VTE in general surgical patients. However, the group on unfractionated heparin had a higher incidence of minor bleeding episodes such as wound hematomas.[26,27] Intermittent pneumatic compression alone is a useful method of thromboprophylaxis in general surgical and urological patients at high risk of bleeding. However, it is limited by patient compliance and ease of use. Graduated compression stockings are simple to use and moderately effective. They have been shown to increase the velocity of venous blood flow. They are recommended in patients at low risk for VTE and can be combined with pharmacological prophylaxis in patients at medium to high risk. Its usage is contraindicated in patients with peripheral vascular disease.
PULMONARY EMBOLISM
Fatal PE is the most dramatic effect of VTE and remains the most common preventable cause of death in hospitalized patients. The best strategy is prevention by adequate prophylaxis for VTE. Early detection is essential for improved out come and requires a high degree of suspicion, when patients have:
Unexplained tachycardia
Unexplained fever
Chest pain
Dyspnea
Hemoptysis
Treatment of PE is decided by the cardiovascular status of the patient. If the patient has circulatory collapse then he is best managed by catheter directed thrombolysis or thrombectomy. If the patient is stable than adequate anticoagulation is sufficient.
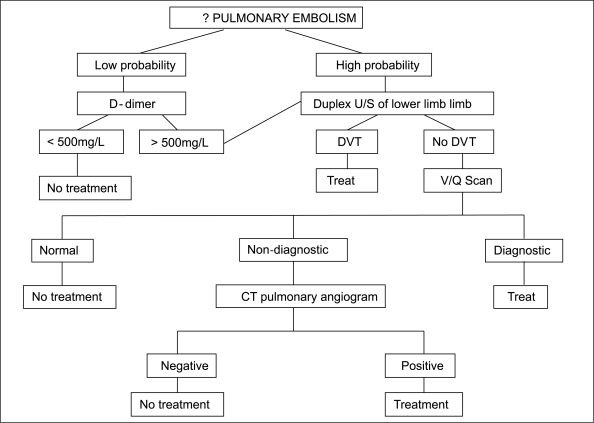
Figure 1.
Diagnostic strategy for the investigation of suspected pulmonary embolism
THE UROLOGICAL PATIENT
DVT is considered to be the most important nonsurgical complication following major urological procedures while PE is believed to be the most common cause of postoperative death.[28] Patients undergoing major urological surgery often have multiple risk factors for VTE like advanced age, malignancy, use of the lithotomy position, and pelvic surgery with or without lymph node dissection. Despite this, the role of thromboprophylaxis in urological surgery has not received much attention. A recent prospective observational study compared the incidence of VTE in patients undergoing surgery for urological cancers with general and gynaecological cancers. It suggests that while there is still significant risk in urological patients, the risk is less than general or gynaecological patients.[29] A survey of current urological practice in patients undergoing radical prostatectomy at various centers in 3 countries showed widely disparate usage of thromboprophylaxis.[30] Evidence from colorectal and gynaecological surgery would suggest that the risk of VTE in major pelvic urological surgery ought to be significant and prophylaxis with LMWH or low dose unfractionated heparin in conjunction with compression stockings is probably indicated. A retrospective analysis on the incidence of VTE in patients undergoing transurethral resection of the prostate (TURP) with limited prophylaxis, concluded that these patients are at low risk for clinically evident DVT and at intermediate risk of clinically evident PE.[31] This study seems to suggest that some form of pharmacological prophylaxis is essential to prevent PE in patients undergoing TURP. Obviously, further studies are required for making recommendations on the use of thromboprophylaxis in endo-urological procedures.
The current recommendation for VTE prophylaxis in Urological surgery is as follows:[32]
For patients undergoing transurethral or other low-risk urologic procedures, recommendation against the use of specific thromboprophylaxis other than early and frequent ambulation.
For all patients undergoing major, open urologic procedures, thromboprophylaxis be used routinely.
For patients undergoing major, open urologic procedures, routine thromboprophylaxis with low dose unfractionated heparin twice daily or three times daily, graduated compression stockings and/or intermittent pneumatic compression started just before surgery and used continuously while the patient is not ambulating, low molecular weight heparin, fondaparinux, or the combination of a pharmacologic method with the optimal use of a mechanical method.
For urologic surgery patients who are actively bleeding or who are at very high risk for bleeding, optimal use of mechanical thromboprophylaxis with graduated compression stockings and/or intermittent pneumatic compression at least until the bleeding risk decreases. When the high bleeding risk decreases, pharmacologic thromboprophylaxis be substituted for or added to the mechanical thromboprophylaxis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism: A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996;93:2212–45. doi: 10.1161/01.cir.93.12.2212. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA, Jr, et al. Prevention of venous thromboembolism. Chest. 2001;119:132S–75S. doi: 10.1378/chest.119.1_suppl.132s. [DOI] [PubMed] [Google Scholar]
- 3.Anderson FA, Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: The Worcester DVT Study. Arch Intern Med. 1991;151:933–8. [PubMed] [Google Scholar]
- 4.Bagaria V, Modi N, Panghate A, Vaidya S. Incidence and risk factors for development of venous thromboembolism in Indian patients undergoing major orthopaedic surgery: Results of a prospective study. Postgrad Med J. 2006;82:136–9. doi: 10.1136/pgmj.2005.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwala S, Bhagwat AS, Modhe J. Deep vein thrombosis in Indian patients undergoing major lower limb surgery. Indian J Surg. 2003;65:159–62. [Google Scholar]
- 6.Lee LH, Gu KQ, Heng D. Deep vein thrombosis is not rare in Asia--the Singapore General Hospital experience. Ann Acad Med Singapore. 2002;31:761–4. [PubMed] [Google Scholar]
- 7.Dhillon KS, Askander A, Doraismay S. Postoperative deep-vein thrombosis in Asian patients is not a rarity: A prospective study of 88 patients with no prophylaxis. J Bone Joint Surg Br. 1996;78:427–30. [PubMed] [Google Scholar]
- 8.Ko PS, Chan WF, Siu TH, Khoo J, Wu WC, Lam JJ. Deep venous thrombosis after total hip or knee arthroplasty in a “low-risk” Chinese population. J Arthroplasty. 2003;18:174–9. doi: 10.1054/arth.2003.50040. [DOI] [PubMed] [Google Scholar]
- 9.Leizorovicz A, Turpie AG, Cohen AT, Wong L, Yoos MC, Dans A, et al. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis: The SMART study. J Thromb Haemost. 2005;3:28–34. doi: 10.1111/j.1538-7836.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 10.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 11.Hull RD, Raskob GE, Hirsh J. Prophylaxis of venous thromboembolism: An overview. Chest. 1986;89:374S–83S. [PubMed] [Google Scholar]
- 12.Hull R, Delmore T, Genton E, Hirsh J, Gent M, Sackett D, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979;301:855–8. doi: 10.1056/NEJM197910183011602. [DOI] [PubMed] [Google Scholar]
- 13.Huisman MV, Büller HR, ten Cate JW, van Royen EA, Vreeken J, Kersten MJ, et al. Unexpected high prevalence of silent pulmonary embolism in patients with deepvenous thrombosis. Chest. 1989;95:498–502. doi: 10.1378/chest.95.3.498. [DOI] [PubMed] [Google Scholar]
- 14.Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17:259–70. doi: 10.1016/s0033-0620(75)80017-x. [DOI] [PubMed] [Google Scholar]
- 15.Criado E, Burnham CB. Predictive value of clinical criteria for the diagnosis of deep vein thrombosis. Surgery. 1997;122:578–83. doi: 10.1016/s0039-6060(97)90131-8. [DOI] [PubMed] [Google Scholar]
- 16.Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795–8. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 17.Wolf B, Nichols DM, Duncan JL. Safety of a single duplex scan to exclude deep venous thrombosis. Br J Surg. 2000;87:1525–8. doi: 10.1046/j.1365-2168.2000.01567.x. [DOI] [PubMed] [Google Scholar]
- 18.Hull RD, Raskob GE, Pineo GF, Green D, Trowbridge AA, Elliott CG, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med. 1992;326:975–82. doi: 10.1056/NEJM199204093261502. [DOI] [PubMed] [Google Scholar]
- 19.Prandoni P, Lensing AW, Büller HR, Carta M, Cogo A, Vigo M, et al. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet. 1992;339:441–5. doi: 10.1016/0140-6736(92)91054-c. [DOI] [PubMed] [Google Scholar]
- 20.Lopaciuk S, Meissner AJ, Filipecki S, Zawilska K, Sowier J, Ciesielski L, et al. Subcutaneous low molecular weight heparin versus subcutaneous unfractionated heparin in the treatment of deep vein thrombosis: A Polish multicenter trial. Thromb Haemost. 1992;68:14–8. [PubMed] [Google Scholar]
- 21.Simonneau G, Charbonnier B, Decousus H, Planchon B, Ninet J, Sie P, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenousunfractionated heparin in the treatment of proximal deep vein thrombosis. Arch Intern Med. 1993;153:1541–6. [PubMed] [Google Scholar]
- 22.Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis: A meta-analysis of randomized, controlled trials. Ann Intern Med. 1999;130:800–9. doi: 10.7326/0003-4819-130-10-199905180-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hyers TM, Agnelli G, Hull RD, Morris TA, Samama M, Tapson V, et al. Antithrombotic therapy for venous thromboembolic disease. Chest. 2001;119:176S–193S. doi: 10.1378/chest.119.1_suppl.176s. [DOI] [PubMed] [Google Scholar]
- 24.Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162–73. doi: 10.1056/NEJM198805053181805. [DOI] [PubMed] [Google Scholar]
- 25.Horlocker TT, Wedel DJ, Benzon H, Brown DL, Enneking FK, Heit JA, et al. Regional anesthesia in the anticoagulated patient: Defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation) Reg Anesth Pain Med. 2003;28:172–97. doi: 10.1053/rapm.2003.50046. [DOI] [PubMed] [Google Scholar]
- 26.Nurmohamed MT, Rosendaal FR, Büller HR, Dekker E, Hommes DW, Vandenbroucke JP, et al. Low-molecular-weight heparin versus standard heparin in general and orthopaedicsurgery: A meta-analysis. Lancet. 1992;340:152–6. doi: 10.1016/0140-6736(92)93223-a. [DOI] [PubMed] [Google Scholar]
- 27.Palmer AJ, Koppenhagen K, Kirchhof B, Weber U, Bergemann R. Efficacy and safety of low molecular weight heparin, unfractionated heparin and warfarin for thrombo-embolism prophylaxis in orthopaedic surgery: A meta-analysis of randomised clinical trials. Haemostasis. 1997;27:75–84. doi: 10.1159/000217437. [DOI] [PubMed] [Google Scholar]
- 28.Kibel AS, Loughlin KR. Pathogenesis and prophylaxis of postoperative thromboembolic disease in urological pelvic surgery. J Urol. 1995;153:1763–74. [PubMed] [Google Scholar]
- 29.Scarpa RM, Carrieri G, Gussoni G, Tubaro A, Conti G, Pagliarulo V, et al. Clinically overt venous thromboembolism after urologic cancer surgery: Results from the @RISTOS Study. Eur Urol. 2007;51:130–5. doi: 10.1016/j.eururo.2006.07.014. discussion 136. [DOI] [PubMed] [Google Scholar]
- 30.Galvin DJ, Mulvin D, Quinlan DM. Thromboprophylaxis for radical prostatectomy: A comparative analysis of present practice between the USA, the UK, and Ireland. Prostate. 2004;60:338–42. doi: 10.1002/pros.20063. [DOI] [PubMed] [Google Scholar]
- 31.Donat R, Mancey-Jones B. Incidence of thromboembolism after transurethral resection of the prostate (TURP): A study on TED stocking prophylaxis and literature review. Scand J Urol Nephrol. 2002;36:119–23. doi: 10.1080/003655902753679409. [DOI] [PubMed] [Google Scholar]
- 32.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]