Abstract
Despite encounter of novel brain antigens by the systemic immune system following stroke, autoimmune responses to these antigens do not seem to occur. A systemic inflammatory insult at the time of stroke, however, provokes changes increase the likelihood of developing detrimental autoimmunity. These findings may help to explain why infections in the post-stroke period are associated with worse outcome. In addition, data suggest that the immune response can be manipulated in an antigen specific fashion to improve stroke outcome. Together these data argue that the nature of the post-ischemic immune response influences neurological recovery from stroke.
Keywords: autoimmune, Th1, Th3/Treg, MBP, mucosal tolerance
Introduction
An antigen non-specific inflammatory response occurs within the brain and peripheral circulation following stroke. This inflammatory response is characterized by activation of microglia and the influx of neutrophils and monocytes into the brain, as well as by the elaboration of leukocyte adhesion molecules and inflammatory cytokines that can be detected within the brain parenchyma and the systemic circulation (Kriz, 2006). In addition, ischemic compromise of the blood-brain barrier (BBB) allows for the infiltration of lymphocytes into brain and for injured neurons and glial cells to “leak” antigens into the peripheral circulation (Schroeter et al., 1994, Jander et al., 1995, Cunningham et al., 1996, Abraha et al., 1997, Missler et al., 1997, Bertsch et al., 2001, Becker, 2004, Foerch et al., 2004). Both of these situations allow lymphocytes, the key cells of the adaptive immune system, to encounter antigens that are normally sequestered from it by the BBB; this encounter leads to the possibility of an (auto)immune response developing to those antigens. In fact, antibodies to brain antigens (myelin basic protein, neurofilaments and the NR2A/2B subtype of the N-methyl-D-aspartate receptor) are documented in persons after stroke (Wang et al., 1992, Bornstein et al., 2001, Dambinova et al., 2003). Based on these observations, we undertook a series of experiments to assess whether a cellular immune response occurs to brain antigens following stroke, and if so, the consequences of that response. Furthermore, we explored the therapeutic utility of modulating the post-ischemic immune response capitalizing on the fact that brain antigens are exposed to the systemic immune system following stroke.
CNS Autoimmune Responses in Experimental Stroke
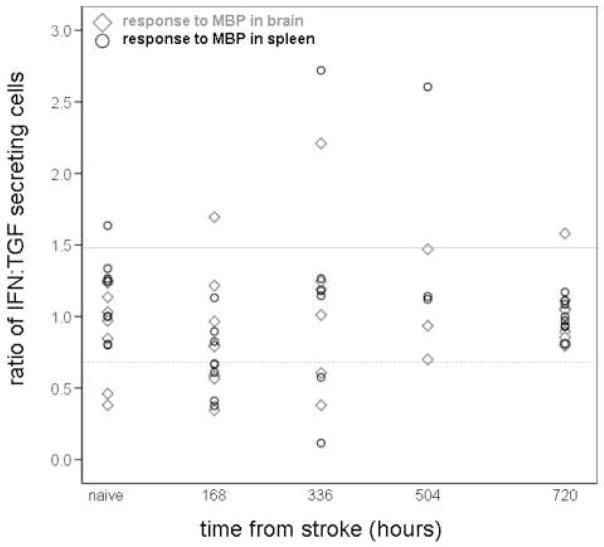
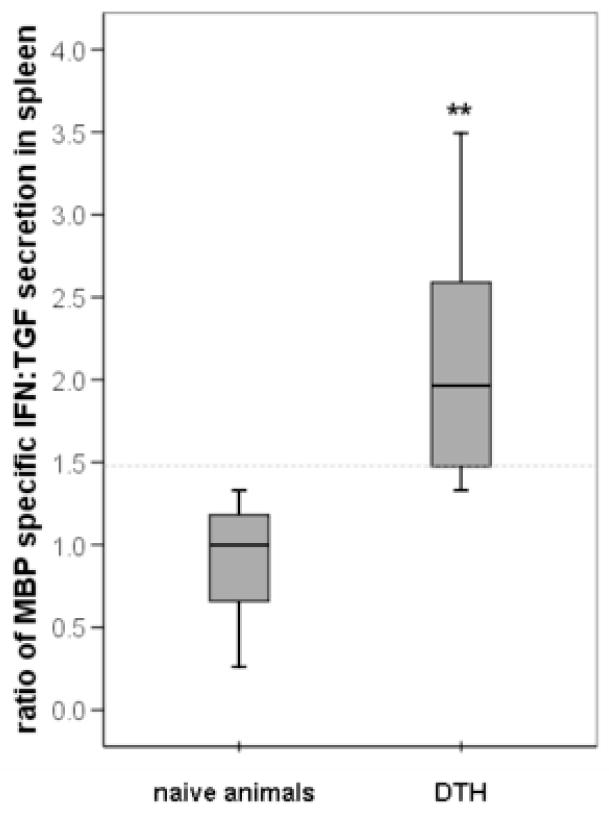
For all of the experiments described in this manuscript, Lewis rats were subjected to 3 hours of middle cerebral artery occlusion (MCAO) followed by varying periods of reperfusion; the cellular immune response to myelin basic protein (MBP), a prototypical central nervous system (CNS) antigen, was analyzed by performing ELISPOT assays on mononuclear cells extracted from the spleen and the ischemic hemisphere of the brain. The number of cells secreting interferon (IFN)-γ in response to stimulation with MBP (relative to the number of unstimulated cells secreting IFN-γ) was used as an indicator of a Th1 response; the number of cells secreting transforming growth factor (TGF)-β1 in response to stimulation with MBP (relative to the number of unstimulated cells secreting TGF-β1) was used as an indicator of the Th3/Treg response. The ratio of the relative increase in the number of MBP-specific IFN-γ secreting cells to that of the relative increase in the number of MBP-specific TGF-β1 secreting cells was used as a measure of the Th1 response to MBP. Figure 1 depicts the Th1 response to MBP among brain lymphocytes and splenocytes in individual animals. We considered animals to be “sensitized” to MBP if the ratio of the relative increase in the number of MBP specific IFN-γ secreting cells to the relative increase in MBP specific TGF-β1 secreting cells was at least 1.48. This value was chosen to dichotomize those with and without a Th1(+) response to MBP based on a previous study in our laboratory where animals were injected with MBP in complete Freund’s adjuvant, which is a typical method of inducing experimental autoimmune encephalomyelitis (EAE). In Figure 2, it can be seen that 1.48 represents the lower quartile of values for the ratio of the IFN:TGF response in animals “sensitized” to MBP and effectively excludes naïve/control animals. Based on these data, we considered animals to have a Th1(+) response to MBP if the ratio of the IFN:TGF response was ≥ 1.48; conversely, a Th3/Treg was considered to be induced if this ratio was ≤ 0.68 (ie. 1.00÷1.48). Based on the data presented in Figure 1, it does not appear that animals develop a cellular Th1 type immune response to following stroke; in fact, the predominant cellular immune response to MBP, at least in the first weeks after stroke, is that of a Th3/Treg response (Becker et al., 2005).
Figure 1. Immune response to MBP in brain and spleen following MCAO.
Individual animal data are presented. For each animal, the response of brain lymphocytes to MBP is depicted by open black circles; the response of splenocytes to MBP is depicted by open gray triangles. The ratio of the number of MBP-specific IFN-γ secreting cells to the number MBP-specific TGF-β1 secreting cells is depicted on the Y-axis; the time from MCAO is depicted on the X-axis. A ratio ≥ 1.48 is considered indicative of a Th1(+) response (solid gray line); a ratio ≤ 0.68 is considered indicative of a Th3/Treg response (dotted gray line). There is no demonstrable immune response to MBP following MCAO.
Figure 2. The immune response to MBP in spleen among naïve animals and animals “sensitized” to MBP.
The ratio of the number of MBP-specific IFN-γ secreting cells to the number MBP-specific TGF-β1 secreting cells in spleen is depicted on the Y-axis. The box plots display the median and interquartile ranges; the lower interquartile range of MBP sensitized animals (1.48) effectively excludes naïve animals. *P<0.01 using the t-test.
That we were unable to demonstrate a Th1(+) response to MBP after stroke may be related to a number of different factors. Firstly, lymphocyte activation/initiation of an adaptive immune response requires that antigen be presented to T cells in the context of the major histocompatibility class (MHC) II molecule and receive an additional costimulatory signal (June et al., 1994, Croft and Dubey, 1997). Within the CNS, however, there are a relative paucity of antigen presenting cells (APCs) and the expression of costimulatory molecules is limited (De Simone et al., 1995, Lovett-Racke et al., 1998, Perry, 1998, Suter et al., 2003). Secondly, the type of lymphocyte effector response that occurs upon antigen encounter depends upon the “milieu” of the local environment at the site of antigen encounter (expression of adhesion molecules, cytokines, etc.), and the “immunologic” milieu of the CNS tends to inhibit the development of an immune response (Hailer et al., 1998, Chang et al., 2001, Suter et al., 2003). Finally, cerebral ischemia induces a systemic immunodepression that inhibits the function of effector T cells (Prass et al., 2003 and Offner et al., this issue); this immunodepression would negatively impact the ability to initiate an adaptive immune response in both the CNS and in the periphery. From a teleological perspective, all of these mechanisms could be viewed as essential to preventing a detrimental autoimmune response to damaged brain tissue (Dirnagl et al., 2007).
While the microenvironment of the brain does not support the development of an immune response under usual circumstances, the expression of adhesion and costimulatory molecules, as well as the cytokines necessary for lymphocyte activation, can be induced. For instance, lipopolysaccharide (LPS), which contains a molecular motif known as a pathogen associated molecular pattern (PAMP), is a potent stimulator of the innate (antigen non-specific) immune response. When administered systemically, LPS provokes a number of changes within the brain and in the periphery that could support the induction of an antigen specific (adaptive) immune response (Satoh et al., 1995, de Vries et al., 1996, Henninger et al., 1997, Menendez Iglesias et al., 1997, Maciejewski-Lenoir et al., 1999, Bohatschek et al., 2001, Fraticelli et al., 2001, Kloss et al., 2001). The actions of LPS are mediated primarily through Toll-like receptor (TLR)-4 (Janeway and Medzhitov, 2002); other PAMPs activate innate immunity through other TLRs (Dalpke and Heeg, 2002). Given that infection is common after stroke, with at least 20% of hospitalized ischemic stroke patients developing a pneumonia or urinary tract infection, the possibility that systemic inflammation promotes the development of a CNS (auto)immune response needs to be considered (Davenport et al., 1996, Georgilis et al., 1999, Grau et al., 1999, Langhorne et al., 2000, Hilker et al., 2003, Aslanyan et al., 2004, Kwan and Hand, 2007). While activation of TLRs appears to be detrimental in stroke, modulation of TLR signaling can be used to induce endogenous neuroprotection (Stenzel-Poore, et al., this issue).
In order to address the effect of infection/systemic inflammation on the cellular immune response to MBP in our stroke model (3 hours MCAO), we injected a subset of animals with LPS 3 hours after the onset of ischemia (at the time of reperfusion). Given that LPS is a component of the Gram negative bacterial cell wall, and Gram negative bacteria commonly cause infection following stroke, this experimental paradigm is clinically relevant (Puri et al., 2002, Hilker et al., 2003). In these studies, we found that animals treated with LPS had increased microglial expression of the costimulatory molecule B7.1 (Becker et al., 2005). Members of the B7 family are among the most important costimulatory molecules and deliver their signal to lymphocytes through CD28 (Liang and Sha, 2002). Concomitant with the increased expression of B7.1 seen in the brains of our LPS treated animals, LPS treatment was associated with infiltration of more CD4+ lymphocytes into the CNS early after ischemia and more inflammation in the brain (characterized by increased numbers of CD8+ cells) one month after stroke (Becker et al., 2005). As might be predicted by this change in histology, LPS treated animals also had a greater likelihood of developing a Th1(+) response to MBP, and this response was associated with worse neurological outcome up to one month after MCAO (Becker et al., 2005). These results suggest that the post-ischemic immune response influences outcome from stroke and that the effect on outcome is durable.
Antigen Specific Modulation of the Immune Response Improves Outcome
In a previous study we showed that induction of “mucosal tolerance” to MBP (characterized as a Th3/Treg response to the antigen) could improve outcome in an animal model of stroke (Becker et al., 1997, Becker et al., 2003). In these experiments, regulatory T cells to MBP were induced by mucosal administration of MBP prior to MCAO. If an animal is “tolerized” to a given CNS antigen prior to stroke, re-encounter with that antigen after stroke leads to secretion of cytokines (TGF-β1 or IL-10) that modulate the local immune response (Chen et al., 2003, Frenkel et al., 2005). While this regulatory response is induced in an antigen specific manner, the secreted cytokines modulate the immune response in an antigen non-specific fashion, a phenomenon referred to as bystander suppression (Miller et al., 1991). Induction of a Th3/Treg response to a given antigen may therefore be of therapeutic benefit by modulating the immune response wherever that antigen is present, even if that antigen is not pathologic. By inducing a Th3/Treg response to MBP to modulate the immune response following stroke, we demonstrated that infarct size could be reduced at 24 hours and 96 hours after MCAO (Becker et al., 1997, Becker et al., 2003). This “neuroprotective” benefit was associated with decreased inflammation in the brain and could be transferred to naïve animals through MBP tolerized lymphocytes (Becker et al., 2003). Other investigators have shown that induction of mucosal tolerance to additional CNS (myelin oligodendrocyte glycoprotein) and vascular (E-selectin) antigens similarly reduces infarct volume and improves stroke outcome (Chen et al., 2003, Frenkel et al., 2003). The benefit of mucosal tolerance in these animal studies is evident within 24 hours of stroke onset, a time too early for the induction of an adaptive immune response. Following antigenic challenge, it generally takes weeks before a cellular immune response to the antigen can be detected (Filion et al., 1988, Rimmelzwaan et al., 2000, Mayer et al., 2002). Given the kinetics of the adaptive immune response, the early benefit of mucosal tolerance seen in these studies must occur independent of any affect on adaptive immunity; the “neuroprotection” associated with mucosal tolerance in these studies is thus more likely related to modulation of the innate immune response (through the bystander effect). That modulation of this early post-ischemic inflammatory response can improve outcome is suggested by multiple lines of evidence, although this experimental benefit has not yet translated into clinical success (Muir et al., 2007).
Systemic Inflammation is Associated with Worse Stroke Outcome
In our animal model, a Th1(+) response to MBP induced by systemic inflammation (ie. administration of LPS) is associated with worse outcome up to one month after MCAO (Becker et al., 2005). Given that neurological outcome is worse in patients who develop an infection following stroke, the possibility that the infection contributes to this worsened outcome (and is not just a marker for stroke severity) must be entertained (Davenport et al., 1996, Georgilis et al., 1999, Grau et al., 1999, Langhorne et al., 2000, Hilker et al., 2003, Aslanyan et al., 2004, Kwan and Hand, 2007). Clinical data to support the detrimental affects of inflammation following stroke are provided by a trial that investigated the therapeutic benefit of a murine monoclonal antibody to intercellular adhesion molecule (ICAM)-1 in acute ischemic stroke; in this study, patients who received the investigational antibody experienced increased morbidity and mortality compared to patients who received placebo (Investigators, 2001). Antibody treated patients were found to have an increased incidence of infection as well as aseptic inflammatory processes, including meningitis. In retrospect, these results may have been anticipated since the experimental antibody actually induces an inflammatory response and the formation of human anti-murine antibodies (Vuorte et al., 1999, Furuya et al., 2001). The premise of this trial was that inflammation contributes to cerebral ischemic injury (and that limiting the inflammatory response would improve outcome); ironically, the trial offered resounding support for this premise (see Teeling et al. and McColl et al., this issue). It could be argued that it is the activation of the innate immune system by infection that is responsible for the worse outcome seen in infected stroke patients, and that the cellular immune response to MBP seen in our model is merely an epiphenomenon of this activation (Urra et. al. in this issue). The fact that the Th1 response to MBP is more predictive of outcome from stroke than whether an animal has received an inflammatory challenge with LPS in our experimental model, and that the degree of the Th1 response correlates with performance on behavioral tasks at 1 month after MCAO, suggest that the development of an adaptive immune to MBP is pathologic (Becker et al., 2005, Gee et al., 2008)
The Nature of CNS Autoimmune Response Influences Outcome
Based on our experimental data and the fact that infection (and the attendant inflammatory response that occurs in response to infection) is so common following stroke, the circumstances that favor the development of an immune response to previously sequestered brain antigens exist in many stroke patients. Preventing infection could potentially limit the chances of developing an autoimmune response, but strategies to prevent infection following stroke have not yet been demonstrated to be effective (Chamorro et al., 2005 and Klehmet et. al. this issue). Immunomodulatory strategies could also be considered to limit the chances of developing a detrimental post-ischemic CNS autoimmune response. We thus performed another series of experiments in which we attempted to prevent the Th1(+) response to MBP using the paradigm of mucosal tolerance. Animals were “tolerized” to MBP or ovalbumin (OVA), an irrelevant antigen, prior to MCAO and administration of LPS. Pre-ischemic treatment with mucosal MBP resulted in fewer animals developing a Th1(+) response to MBP and was associated with improved outcome, as assessed by a number of measures, including performance on the rotarod (Figure 3a) (Gee et al., 2008). Animals with a Th1(+) response to MBP had a shorter latency to fall from the rotarod (worse performance) (Figure 3b), while those that developed a Th3/Treg response to MBP tended to perform better than those without such a response (Figure 3c). These data suggest that the nature of the immune response to brain antigens following stroke influences outcome and imply that a strategy of immunodulation may be viable for treating patients with stroke.
Figure 3. Rotarod performance following MCAO based on tolerization status and immune response to MBP.
Animals tolerized to MBP prior to MCAO performed better than those tolerized to OVA (a). Animals that developed a Th1(+) response to MBP fell more quickly from the rotarod than those that did not (b). Animals with a Treg response to MBP performed better than those with either a Th1(+) response or no response (c). The box plots display the median and interquartile ranges. *P≤0.05, ** P≤0.01 using the t-test or ANOVA, as appropriate.
That the cellular immune response to MBP following stroke is more than an epiphenomenon is further demonstrated by the fact that the degree of the Th1 response to MBP correlates to the neurological outcome at one month (using Pearson’s correlation); the more robust the Th1 response, the quicker the animals were to fall from the rotarod (P=0.020). Additionally, animals with a Th1(+) response to MBP in spleen had higher titers of anti-MBP antibodies than those without (P=0.001), and the antibody titer was correlated to the degree of the cellular immune response to MBP (P=0.001).
Other experimental studies also suggest that CNS autoreactive T cells contribute to CNS injury. For instance, transgenic animals in which greater than 95% of CD4+ T cells express receptors specific for MBP recover less following spinal cord injury than wild type controls, and animals immunized to MBP prior to stroke have increased stroke related mortality (Becker et al., 1997, Jones et al., 2002). Furthermore, T lymphocytes obtained from animals shortly after spinal cord injury can precipitate histopathological changes similar to experimental allergic encephalomyelitis (EAE) when injected into naïve animals (Popovich et al., 1996). Conversely, focal cerebral injury enhances the inflammation associated with EAE, and when animals with EAE are subjected to focal cerebral injury, adoptive transfer of lymphocytes from these animals into naïve animals results in more significant inflammation of the brain than does adoptive transfer of lymphocytes from animals with EAE but no cerebral injury (Phillips et al., 1995, Lake et al., 1999).
Pathological Effects of CNS Autoimmunity
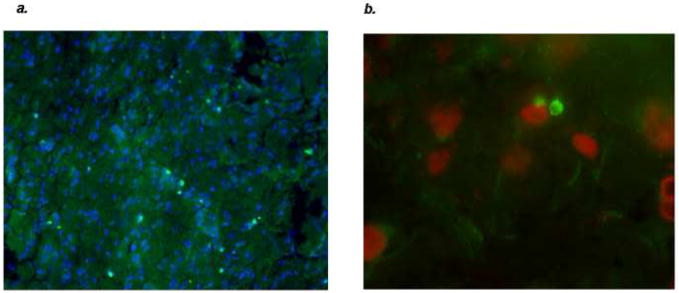
Our data thus show that induction of mucosal tolerance to a brain antigen (MBP) can modulate the post-ischemic inflammatory response to improve outcome; it also limits the chance of developing a detrimental Th1(+) response to brain antigens in animals subjected to a systemic inflammatory response following stroke. The mechanisms by which a Th1(+) immune response to MBP worsens outcome from stroke, however, are not completely clear. Potential contributing factors include the fact that activated lymphocytes secrete a number of cytokines that are either directly or indirectly neurotoxic; effector lymphocytes are also able to kill “target cells” through direct lysis or by inducing apoptosis (Hanisch et al., 1996, Lee et al., 1996, Barone et al., 1997, Hanisch et al., 1997, Hu et al., 1997, Rothwell et al., 1997, Shresta et al., 1998, Downen et al., 1999). Specifically, cytotoxic T lymphocytes (CTLs) release perforin, which integrates into the target cell membrane leading to osmotic lysis of the target cell (Tschopp et al., 1986). Once perforin is integrated into the target cell membrane, granzyme (a serine protease) can be “injected” into the target cell to trigger apoptosis (Nakajima and Henkart, 1994, Kajino et al., 1998). In vitro, NK cells are able to lyse neurons in a perforin dependent fashion, and pathological data suggest that CD8+ lymphocytes may kill neurons through a granzyme dependent pathway (Backstrom et al., 2000, Bien et al., 2002). Lymphocytes also induce apoptosis in target cells through Fas ligand (FasL) and release of tumor necrosis factor (TNF); neurons express cognate receptors for both and are thus susceptible to lymphocyte mediated injury (Botchkina et al., 1997, Kajino et al., 1998, Raoul et al., 2000). In experimental models of stroke, apoptotic neurons appear in close approximation to invading leukocytes, suggesting that inflammatory cells may contribute to ischemic neuronal death (Braun et al., 1996). In Figure 4, perforin positive cells (FITC positive) are seen in close contact to neurons within the infarcted tissue following MCAO. One month after stroke in our experimental model, animals treated with LPS evidenced more brain atrophy and had more apoptotic neurons than animals that did not receive an inflammatory stimulus (Becker et al., 2005).
Figure 4.
There are multiple perforin positive cells (stained with FITC) within the infarcted tissue of this LPS treated animals sacrificed one month after MCAO (a). These perforin positive cells are seen in close juxtaposition to DAPI positive cells (40x). The DAPI positive cells were shown to be neurons using a Texas-red conjugated antibody for neuron specific enolase (100x) (b).
While there is clear evidence that the immune response contributes to post-ischemic neuropathology, as outlined above, the effects of the immune response appear to depend on the model used. As is discussed by Schwarz et. al. and Kerchensteiner et. al. in this issue, the CNS immune response can also promote recovery and repair.
Conclusions
Our data demonstrate that an inflammatory insult (ie. LPS) after stroke onset is associated with worse outcome. LPS initiates the innate immune response through stimulation of TLR4 and sets the stage for the adaptive immune response by creating an environment that promotes lymphocyte sensitization to antigens; if one of these antigens is a brain antigen, a CNS autoimmune response may occur. The long-term consequences of this autoimmune response are unclear, but it appears that it is associated with worse outcome for at least one month following experimental stroke. Whether a similar cellular immune response to brain antigens occurs in persons with stroke is unknown, as are the potential consequences of such a response. In persons with vascular dementia, however, there is evidence of a humoral response to brain antigens (glial fibrillary acidic protein [GFAP], S100, tubulin and neurofilament), suggesting that CNS autoimmunity may not be benign (Mecocci et al., 1995, Terryberry et al., 1998). Given that induction of Treg cells to brain antigens (ie. MBP) can prevent the Th1(+) response to these antigens (and is associated with better outcome from stroke independent of this effect), antigen specific immunomodulation may be a viable therapeutic intervention for stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraha HD, Butterworth RJ, Bath PM, Wassif WS, Garthwaite J, Sherwood RA. Serum S-100 protein, relationship to clinical outcome in acute stroke. Ann Clin Biochem. 1997;34:366–370. doi: 10.1177/000456329703400405. [DOI] [PubMed] [Google Scholar]
- Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11:49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- Backstrom E, Chambers BJ, Kristensson K, Ljunggren HG. Direct NK cell-mediated lysis of syngenic dorsal root ganglia neurons in vitro. J Immunol. 2000;165:4895–4900. doi: 10.4049/jimmunol.165.9.4895. [DOI] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- Becker KJ, editor. The adaptive immune response in cerebral ischemic injury. Stuttgart: medpharm Scientific Publishers; 2004. [Google Scholar]
- Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, Hallenbeck JM. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 1997;94:10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch T, Casarin W, Kretschmar M, Zimmer W, Walter S, Sommer C, Muehlhauser F, Ragoschke A, Kuehl S, Schmidt R, Eden BP, Nassabi C, Nichterlein T, Fassbender K. Protein S-100B: a serum marker for ischemic and infectious injury of cerebral tissue. Clin Chem Lab Med. 2001;39:319–323. doi: 10.1515/CCLM.2001.050. [DOI] [PubMed] [Google Scholar]
- Bien CG, Bauer J, Deckwerth TL, Wiendl H, Deckert M, Wiestler OD, Schramm J, Elger CE, Lassmann H. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann Neurol. 2002;51:311–318. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- Bohatschek M, Werner A, Raivich G. Systemic LPS injection leads to granulocyte influx into normal and injured brain: effects of ICAM-1 deficiency. Exp Neurol. 2001;172:137–152. doi: 10.1006/exnr.2001.7764. [DOI] [PubMed] [Google Scholar]
- Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- Botchkina GI, Meistrell ME, 3rd, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3:765–781. [PMC free article] [PubMed] [Google Scholar]
- Braun JS, Jander S, Schroeter M, Witte OW, Stoll G. Spatiotemporal relationship of apoptotic cell death to lymphomonocytic infiltration in photochemically induced focal ischemia of the rat cerebral cortex. Acta Neuropathol (Berl) 1996;92:255–263. doi: 10.1007/s004010050516. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Horcajada JP, Obach V, Vargas M, Revilla M, Torres F, Cervera A, Planas AM, Mensa J. The Early Systemic Prophylaxis of Infection After Stroke study: a randomized clinical trial. Stroke. 2005;36:1495–1500. doi: 10.1161/01.STR.0000170644.15504.49. [DOI] [PubMed] [Google Scholar]
- Chang RC, Chen W, Hudson P, Wilson B, Han DS, Hong JS. Neurons reduce glial responses to lipopolysaccharide (LPS) and prevent injury of microglial cells from over-activation by LPS. J Neurochem. 2001;76:1042–1049. doi: 10.1046/j.1471-4159.2001.00111.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ruetzler C, Pandipati S, Spatz M, McCarron RM, Becker K, Hallenbeck JM. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci U S A. 2003;100:15107–15112. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M, Dubey C. Accessory molecule and costimulation requirements for CD4 T cell response. Crit Rev Immunol. 1997;17:89–118. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- Cunningham RT, Watt M, Winder J, McKinstry S, Lawson JT, Johnston CF, Hawkins SA, Buchanan KD. Serum neurone-specific enolase as an indicator of stroke volume. Eur J Clin Invest. 1996;26:298–303. doi: 10.1046/j.1365-2362.1996.129282.x. [DOI] [PubMed] [Google Scholar]
- Dalpke A, Heeg K. Signal integration following Toll-like receptor triggering. Crit Rev Immunol. 2002;22:217–250. [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke. 1996;27:415–420. doi: 10.1161/01.str.27.3.415. [DOI] [PubMed] [Google Scholar]
- De Simone R, Giampaolo A, Giometto B, Gallo P, Levi G, Peschle C, Aloisi F. The costimulatory molecule B7 is expressed on human microglia in culture and in multiple sclerosis acute lesions. J Neuropathol Exp Neurol. 1995;54:175–187. doi: 10.1097/00005072-199503000-00004. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- Downen M, Amaral TD, Hua LL, Zhao ML, Lee SC. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia. 1999;28:114–127. [PubMed] [Google Scholar]
- Filion LG, Saginur R, Szczerbak N. Humoral and cellular immune responses by normal individuals to hepatitis B surface antigen vaccination. Clin Exp Immunol. 1988;71:405–409. [PMC free article] [PubMed] [Google Scholar]
- Foerch C, Otto B, Singer OC, Neumann-Haefelin T, Yan B, Berkefeld J, Steinmetz H, Sitzer M. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. 2004;35:2160–2164. doi: 10.1161/01.STR.0000138730.03264.ac. [DOI] [PubMed] [Google Scholar]
- Fraticelli P, Sironi M, Bianchi G, D’Ambrosio D, Albanesi C, Stoppacciaro A, Chieppa M, Allavena P, Ruco L, Girolomoni G, Sinigaglia F, Vecchi A, Mantovani A. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA, Weiner HL. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J Immunol. 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Furuya K, Takeda H, Azhar S, McCarron RM, Chen Y, Ruetzler CA, Wolcott KM, DeGraba TJ, Rothlein R, Hugli TE, del Zoppo GJ, Hallenbeck JM. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke. 2001;32:2665–2674. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgilis K, Plomaritoglou A, Dafni U, Bassiakos Y, Vemmos K. Aetiology of fever in patients with acute stroke. J Intern Med. 1999;246:203–209. doi: 10.1046/j.1365-2796.1999.00539.x. [DOI] [PubMed] [Google Scholar]
- Grau AJ, Buggle F, Schnitzler P, Spiel M, Lichy C, Hacke W. Fever and infection early after ischemic stroke. J Neurol Sci. 1999;171:115–120. doi: 10.1016/s0022-510x(99)00261-0. [DOI] [PubMed] [Google Scholar]
- Hailer NP, Heppner FL, Haas D, Nitsch R. Astrocytic factors deactivate antigen presenting cells that invade the central nervous system. Brain Pathol. 1998;8:459–474. doi: 10.1111/j.1750-3639.1998.tb00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Neuhaus J, Quirion R, Kettenmann H. Neurotoxicity induced by interleukin-2: involvement of infiltrating immune cells. Synapse. 1996;24:104–114. doi: 10.1002/(SICI)1098-2396(199610)24:2<104::AID-SYN2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Neuhaus J, Rowe W, Van Rossum D, Moller T, Kettenmann H, Quirion R. Neurotoxic consequences of central long-term administration of interleukin-2 in rats. Neuroscience. 1997;79:799–818. doi: 10.1016/s0306-4522(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Henninger DD, Panes J, Eppihimer M, Russell J, Gerritsen M, Anderson DC, Granger DN. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997;158:1825–1832. [PubMed] [Google Scholar]
- Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, Heiss WD. Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke. 2003;34:975–981. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- Hu S, Peterson PK, Chao CC. Cytokine-mediated neuronal apoptosis. Neurochem Int. 1997;30:427–431. doi: 10.1016/s0197-0186(96)00078-2. [DOI] [PubMed] [Google Scholar]
- Investigators EAST. Use of anti-ICAM-1 therapy in ischemic stroke: Results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kajino K, Kajino Y, Greene MI. Fas- and perforin-independent mechanism of cytotoxic T lymphocyte. Immunol Res. 1998;17:89–93. doi: 10.1007/BF02786434. [DOI] [PubMed] [Google Scholar]
- Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–157. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- Kwan J, Hand P. Infection after acute stroke is associated with poor short-term outcome. Acta Neurol Scand. 2007;115:331–338. doi: 10.1111/j.1600-0404.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- Lake J, Weller RO, Phillips MJ, Needham M. Lymphocyte targeting of the brain in adoptive transfer cryolesion-EAE. J Pathol. 1999;187:259–265. doi: 10.1002/(SICI)1096-9896(199901)187:2<259::AID-PATH212>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, Dick F, Taylor GS, Murray G. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- Lee RK, Spielman J, Zhao DY, Olsen KJ, Podack ER. Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J Immunol. 1996;157:1919–1925. [PubMed] [Google Scholar]
- Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–390. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Trotter JL, Lauber J, Perrin PJ, June CH, Racke MK. Decreased dependence of myelin basic protein-reactive T cells on CD28- mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells [published erratum appears in J Clin Invest 1998 Apr 1;101(7):1542] J Clin Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol. 1999;163:1628–1635. [PubMed] [Google Scholar]
- Mayer S, Laumer M, Mackensen A, Andreesen R, Krause SW. Analysis of the immune response against tetanus toxoid: enumeration of specific T helper cells by the Elispot assay. Immunobiology. 2002;205:282–289. doi: 10.1078/0171-2985-00131. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Parnetti L, Romano G, Scarelli A, Chionne F, Cecchetti R, Polidori MC, Palumbo B, Cherubini A, Senin U. Serum anti-GFAP and anti-S100 autoantibodies in brain aging, Alzheimer’s disease and vascular dementia. J Neuroimmunol. 1995;57:165–170. doi: 10.1016/0165-5728(94)00180-v. [DOI] [PubMed] [Google Scholar]
- Menendez Iglesias B, Cerase J, Ceracchini C, Levi G, Aloisi F. Analysis of B7-1 and B7-2 costimulatory ligands in cultured mouse microglia: upregulation by interferon-gamma and lipopolysaccharide and downregulation by interleukin-10, prostaglandin E2 and cyclic AMP-elevating agents. J Neuroimmunol. 1997;72:83–93. doi: 10.1016/s0165-5728(96)00155-5. [DOI] [PubMed] [Google Scholar]
- Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler U, Wiesmann M, Friedrich C, Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. doi: 10.1161/01.str.28.10.1956. [DOI] [PubMed] [Google Scholar]
- Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr Opin Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Henkart PA. Cytotoxic lymphocyte granzymes trigger a target cell internal disintegration pathway leading to cytolysis and DNA breakdown. J Immunol. 1994;152:1057–1063. [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Weller RO, Kida S, Iannotti F. Focal brain damage enhances experimental allergic encephalomyelitis in brain and spinal cord. Neuropathol Appl Neurobiol. 1995;21:189–200. doi: 10.1111/j.1365-2990.1995.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri J, Mishra B, Mal A, Murthy NS, Thakur A, Dogra V, Singh D. Catheter associated urinary tract infections in neurology and neurosurgical units. J Infect. 2002;44:171–175. doi: 10.1053/jinf.2002.0968. [DOI] [PubMed] [Google Scholar]
- Raoul C, Pettmann B, Henderson CE. Active killing of neurons during development and following stress: a role for p75(NTR) and Fas? Curr Opin Neurobiol. 2000;10:111–117. doi: 10.1016/s0959-4388(99)00055-0. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Nieuwkoop N, Brandenburg A, Sutter G, Beyer WE, Maher D, Bates J, Osterhaus AD. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine. 2000;19:1180–1187. doi: 10.1016/s0264-410x(00)00310-8. [DOI] [PubMed] [Google Scholar]
- Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest. 1997;100:2648–2652. doi: 10.1172/JCI119808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J, Lee YB, Kim SU. T-cell costimulatory molecules B7-1 (CD80) and B7-2 (CD86) are expressed in human microglia but not in astrocytes in culture. Brain Res. 1995;704:92–96. doi: 10.1016/0006-8993(95)01177-3. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10:581–587. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- Suter T, Biollaz G, Gatto D, Bernasconi L, Herren T, Reith W, Fontana A. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur J Immunol. 2003;33:2998–3006. doi: 10.1002/eji.200323611. [DOI] [PubMed] [Google Scholar]
- Terryberry JW, Thor G, Peter JB. Autoantibodies in neurodegenerative diseases: antigen-specific frequencies and intrathecal analysis. Neurobiol Aging. 1998;19:205–216. doi: 10.1016/s0197-4580(98)00049-9. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Masson D, Stanley KK. Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. Nature. 1986;322:831–834. doi: 10.1038/322831a0. [DOI] [PubMed] [Google Scholar]
- Vuorte J, Lindsberg PJ, Kaste M, Meri S, Jansson SE, Rothlein R, Repo H. Anti-ICAM-1 monoclonal antibody R6.5 (Enlimomab) promotes activation of neutrophils in whole blood. J Immunol. 1999;162:2353–2357. [PubMed] [Google Scholar]
- Wang WZ, Olsson T, Kostulas V, Hojeberg B, Ekre HP, Link H. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88:157–162. doi: 10.1111/j.1365-2249.1992.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]