Abstract
Recently, we demonstrated that intact nitric oxide (NO) signaling is essential for the development of cocaine behavioral sensitization in adulthood [2]. Given the requirement of dopamine (DA) transmission in cocaine-induced behavioral sensitization and the interactions between NO and DA systems, the present study investigated the role of the neuronal nitric oxide synthase (nNOS) gene and the effect of cocaine on the expression of tyrosine hydroxylase (TH)-immunoreactive (-ir) neurons. Adult (postnatal day 80) wild type (WT) and nNOS knockout (KO) mice received saline or a sensitizing regimen of cocaine (20mg/kg) for 5 days. After 24h, TH immunoreactivity was assessed in the ventral tegmental area (VTA) and the dorsal striatum (dST) using stereology and western blotting, respectively. We report that a) nNOS KO mice express lower levels of TH-ir neurons in the VTA compared to WT counterparts, b) cocaine administration to WT mice significantly increased striatal TH expression, and c) the same cocaine administration to nNOS KO mice significantly decreased striatal TH expression. Thus, the nitrergic system may contribute to cocaine-induced behavioral sensitization by regulating dopaminergic neurotransmission.
Keywords: cocaine, neuronal nitric oxide synthase (nNOS), tyrosine hydroxylase (TH), behavioral sensitization, dorsal striatum (dST), ventral tegmental are (VTA), stereology, western blotting
Introduction
In the central nervous system, nitric oxide (NO) is thought to be a highly reactive neuronal signaling molecule [8]. NO is produced from arginine via neuronal nitric oxide synthase (nNOS) in response to calcium influx caused by stimulation of N-methyl-D-aspartate (NMDA) subtype of glutamate receptors [10, 33]. Compelling evidence from neuroanatomical studies suggests the existence of interaction between dopaminergic and nitrergic systems. In nearly all mesolimbic, corticostriatal, and nigrostriatal regions such as the ventral tegmental area (VTA), nucleus accumbens (NAC), dorsal striatum (dST), substantia nigra, and frontal cortex, populations of tyrosine hydroxylase (TH)-immunoreactive (ir) and NOS-ir neurons interact with each other as manifested by the presence of NOS-ir endings on TH-ir neurons and vice versa [4, 9, 12, 20, 25]. The close appositions provide the locus at which NO can affect the release and uptake of dopamine (DA). NO exerts facilitatory influence on both tonic extracellular DA levels and phasic DA neuron spike activity [11, 38, 39]. Striatal NO transmission is facilitated via nigrostriatal DA and frontal cortical glutamate afferents [19, 30, 31]. Among other mechanisms, NO has been shown to inhibit the function of DA transporters [17, 19, 23, 28, 37] thereby facilitating dopaminergic transmission. The effect of NO on monoamine transporters is believed to represent a new form of interneuronal communication, that is, a nonsynaptic interaction that does not involve classical receptors [18].
The interactions between DA, glutamate, and NO in mesolimbic and corticostriatal circuits [7, 17] suggest that NO may contribute to the effects of cocaine. Acute systemic administration of cocaine significantly increased NO efflux in the medial prefrontal cortex (PFC) in a time-dependent manner [32]. Furthermore, cocaine administration has been shown to regulate nNOS. Chronic cocaine administration followed by 1h of withdrawal increases NOS activity in the cerebral cortex, cerebellum, midbrain, hypothalamus, hippocampus, amygdala, and spinal cord [5]. A significant cocaine-induced up-regulation of nNOS expression was observed at 24h but not 72h or 14 days of withdrawal in the frontal and parietal cortices [22].
Recently we reported that repeated, but not acute, cocaine administration resulted in a significant increase in the expression of nNOS-ir neurons in the dST 24h [2] but not 10 days (unpublished data) after cocaine administration was discontinued. Also, while WT males developed long-lasting sensitization to cocaine, nNOS knockout (KO) counterparts failed to do so. Together, these findings suggest that the nNOS gene has a role in the development of behavioral sensitization to cocaine [2]. There is also strong evidence that DA transmission, particularly in the VTA, is necessary for the development of cocaine sensitization [36]. The present study investigated the role of the nNOS gene in the expression of TH-ir neurons, and the effect of cocaine on TH-ir neurons. We report that a) nNOS KO mice express lower levels of TH-ir neurons in the VTA compared to WT counterparts, b) cocaine administration to WT mice significantly increased striatal TH expression, and c) the same cocaine administration to nNOS KO mice significantly decreased striatal TH expression.
Materials and Methods
Animals
Mice purchased from Jackson Laboratories (Bar Harbor, Maine) were bred in our facilities at the University of Miami, Miller School of Medicine, Miami, FL as we described previously [1]. Both genotypes, WT and nNOS KO, were generated on a mixed B6;129S genetic background [14]. Animals were housed in a temperature- (22±0.5°C) and humidity- (50%) controlled room and maintained on a 12-h light/dark schedule with free access to food and water. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, 1996) and approved by the University of Miami Animal Care and Use Committee.
Schedule of cocaine administration
Cocaine-HCl (Sigma, St. Louis, MO) was dissolved in 0.9% NaCl. All injections were given intraperitoneally (IP) in a volume of 0.1ml/10g weight.
Immunohistochemistry of TH
Adult (PD80) WT and nNOS KO male mice (n=4–5/group) were administered saline and cocaine (20mg/kg) for 5 days. The purpose of this schedule was to replicate the cocaine sensitizing-regimen we used previously in behavioral experiments [2]. Twenty four hours after the last saline or cocaine injection, mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). After loss of the foot-pinch response, mice were perfused transcardially via the left ventricle with sodium phosphate-buffered saline (PBS) followed by p-formaldehyde (4%) in PBS. The brains were removed and post-fixed overnight in the same fixative at 4°C. Serial coronal sections (50 μm) were cut with a Vibratome 1000 (TPI Inc., St. Louis, MO), collected in PBS and blocked for 1h at room temperature with normal goat serum (10%) in PBS containing Triton X-100 (0.3%). Sections were then incubated (72h; 4°C) with a rabbit polyclonal antibody to TH (1:3000, Chemicon, Temecula, CA) diluted in a vehicle of 2% normal goat serum with 0.3% Triton X-100 in PBS. After 3×10min washes the sections were then incubated for 1h at room temperature in biotinylated goat anti-rabbit IgG diluted 1:100 in vehicle. The signal was then amplified using the Vectastain Elite ABC immunoperixodase kit (Vector Laboratories, Burlingame, CA) and visualized with 0.05% DAB and 0.02% H2O2 in 50mM Tris Buffer. Sections were then rinsed 2×5min in PBS, mounted onto slides, and coverslipped. Negative control sections were treated in the same way as described, except that the primary antibody was omitted from the staining procedure.
Stereology
To quantify the total number of TH-ir neurons in the VTA, the optical fractionator method, a stereological technique, was used as we described previously [2]. For cell counts, sections were stained with DAB as the chromogen. Eight coronal sections (50 μm) were selected, taken at equally spaced intervals (100μm) through the full rostrocaudal extent of the midbrain and analyzed using a Zeiss Axiophot microscope equipped with software that includes an optical fractionator probe (StereoInvestigator, Microbrightfield). To perform cell number estimation in the structure volume, the optical fractionator method and optical disector probe were used. Dimensions of the optical disector were designed based upon the cell distribution in the section, and optical fractionator grid size was determined based upon the results of preliminary counts of the naive brain sample to allow 100–200 counts per region of the VTA. Thus, the standard counting method used was a counting frame (dissector; 65×65μm) and (fractionator; 150×150μm) to meet the high standards of systematic random sampling. The appropriateness of the sampling scheme chosen was evaluated by calculating the precision of the estimates in each animal, expressed as the coefficient of error [40]. In all cases, the coefficient of error was less than 0.10, suggesting that a minimal amount of variance in the counts can be attributed to the technique.
Western Blotting Experiments of TH
Adult (PD80) WT and nNOS KO male mice (n=4–5/group) were administered saline or cocaine (20mg/kg) for 5 days. Twenty four hours after the last saline or cocaine injection, striatum were microdissected and homogenized twice in 100 volumes of ice cold RIPA Buffer (50mM Tris-HCl pH7.5, 5mM EDTA, 150 mM NaCl, 0.5% NP-40 supplemented with 1mM PMSF, 1ug/ml pepstatin, 1ug/ml aprotinin, and 2ug/ml leupeptin) and incubated for 15 minutes. The homogenate was centrifuged and the supernatants collected. Protein concentrations were determined using the Lowry protein assay with bovine serum albumin as a standard. Proteins were separated using 7.5% SDS-PAGE and transferred to PDVF membranes. Membranes were incubated in blocking buffer (5% nonfat dry milk in Tris buffered saline (TBS) [20mM Tris-HCl (pH 7.4), and 150mM NaCl]) containing 0.1% Tween-20 (TBS-T) for 1h at RT. Membranes were incubated with a rabbit polyclonal antibody to TH (1:4500) (Santa Cruz Biotechnology, CA). Membranes were then washed 30 minutes with 3 intermediate changes of TBS-T. The blots were then incubated (1hr at RT) with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody at a dilution of 1:2000 and washed with TBS-T 3×10minutes. Visualization of the signal was enhanced by chemiluminescence using a Phototope-HRP Detection Kit (Pierce, Rockford, IL). To control for protein loading, immunoblots were stripped with Restore Western blot stripping buffer (Pierce, Rockford, IL), and blotted for a mouse monoclonal to β-tubulin (1:15,000, Upstate, NY). Quantification of band density was performed using UN-SCAN-IT gel quantifying software (Silk Scientific Inc, Utah) and data were normalized to β-tubulin.
Statistical Analysis
Statistical analysis was performed with SPSS 16 software. The numbers of VTA TH-ir neurons were analyzed using a two-way ANOVA (treatment × genotype) followed by post hoc analysis using Bonferroni correction to determine differences between multiple groups. Striatal TH protein levels in saline- and cocaine-treated mice were compared using unpaired, two-tailed Student’s t test. P values less than 0.05 were considered significant for all tests. Data are expressed as ±SEM.
Results
Stereological estimation of the total number of TH-ir neurons in the VTA
To quantify the changes in the number of TH-ir neurons in the VTA, DAB stained sections were counted using stereology. Fig. 1 shows the effect of repeated saline administration compared to the effect of repeated cocaine administration (20 mg/kg×5 days) in WT and nNOS KO mice. A two-way ANOVA (treatment × genotype) revealed a significant genotype effect F(1, 11)=27.60; p<0.001 and a non-significant treatment effect F(1,11)= 0.6793; p=0.4274. Post hoc analysis using Bonferroni correction revealed a significant reduction in the number of TH-ir neurons in the VTA of nNOS KO animals when compared to their WT counterparts when treated with either saline or cocaine (p<0.01 and p<0.05). These findings suggest that the absence of nNOS leads to decreased expression of TH in the VTA. Notably, however, cocaine administration had no effect on VTA TH expression in either WT or nNOS KO mice.
Figure 1. Stereological analysis of TH expression in the VTA of WT and nNOS KO mice after saline and cocaine administration.
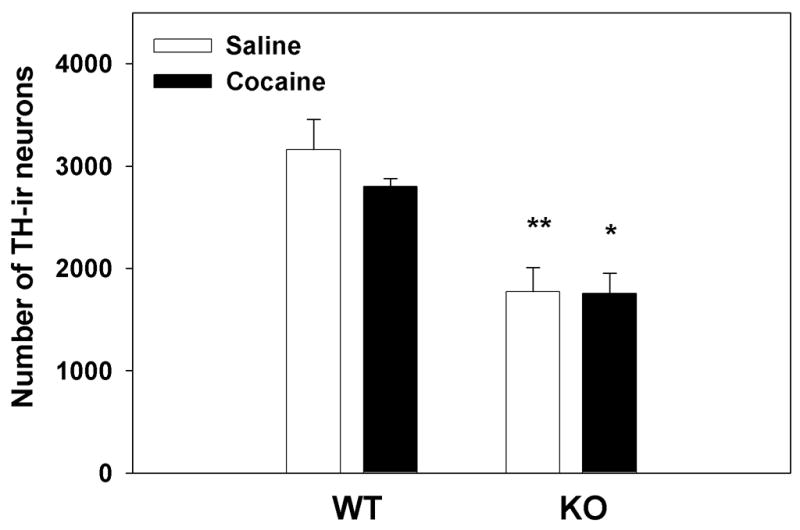
WT and nNOS KO mice received saline or cocaine (20mg/kg) for 5 consecutive days. After 24 hr animals were perfused and brain tissue was prepared for staining of TH-ir neurons, as described in Materials and Methods. A significant genotype-dependent effect was observed (**p<0.01 and *p<0.05) as the number of TH-ir neurons in the VTA of nNOS KO mice was lower than that in their WT counterparts, regardless of cocaine treatment which had no significant effect on the number of VTA TH-ir neurons.
Western blot analysis of TH-ir terminals in the dST
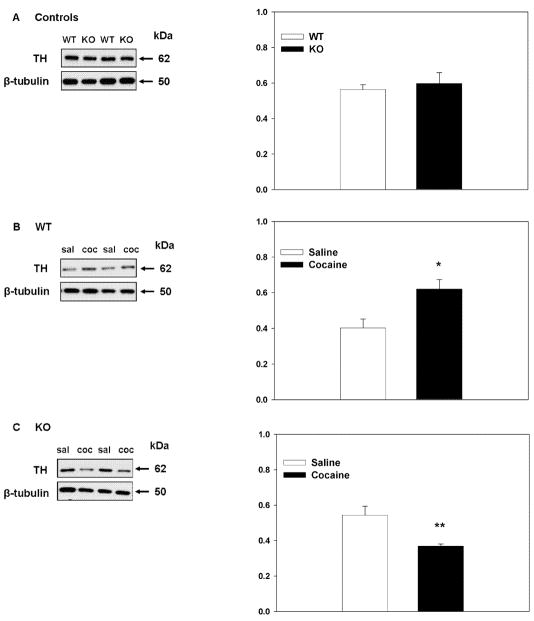
To quantify the changes in dopaminergic (TH-ir) nerve terminals in the dST we performed western blot analysis. Fig. 2A shows the effect of genotype on TH protein levels in control (saline-treated) mice. Student’s t-tests (unpaired, two-tail) revealed that there was no significant difference in TH protein levels between WT and nNOS KO mice under saline treated conditions. Fig. 2B and 2C shows the effect of genotype on cocaine mediated alterations in striatal TH. Student’s t-test (unpaired, two tail) showed that repeated cocaine administration induced a significant increase in striatal TH protein levels in WT mice when compared to saline-treated controls (t=2.91, p<0.05) (Fig. 2B). Conversely, in nNOS KO mice (Fig. 2C), repeated cocaine administration resulted in a reduction in striatal TH protein levels when compared to saline treated controls (t=3.171, p<0.05) suggesting that, in the absence of nNOS, TH is down-regulated after cocaine exposure.
Figure 2. Effects of genotype and cocaine on striatal TH-ir terminals.
Representative immunoblots demonstrating the effect of genotype on the levels of TH under control (saline-treated) conditions (A) and after repeated cocaine administration (B; WTs and C; KOs). dST lysates were immunoblotted with an antibody against TH. β-Tubulin was used as control for protein loading. WT and nNOS KO mice do not differ in the levels of dST TH when treated with saline (A). Repeated cocaine treatment significantly increases levels of dST TH in WT mice when compared to saline treated controls (* p<0.05) (B). Conversely, cocaine administration significantly decreases levels of striatal TH in nNOS KO mice when compared to saline treated controls (**p<0.01) (C).
Discussion
We have recently shown that administration of cocaine (20mg/kg) for 5 days to WT and nNOS KO mice resulted in: 1) long-lasting sensitization of WT mice but not nNOS KO mice, and 2) an increase in expression of nNOS-ir neurons in the dST of WT mice [2]. Given the interactions between nitrergic and dopaminergic transmission (Introduction), the present study investigated the role of the nNOS gene in the expression of TH-ir neurons, and the effect of cocaine on TH-ir neurons. The major findings are: 1) nNOS KO mice express reduced levels of TH-ir neurons in the VTA compared to WT counterparts, 2) a sensitizing regimen of cocaine significantly increased striatal TH expression in WT mice, and 3) the same regimen of cocaine significantly decreased striatal TH expression in nNOS KO mice.
VTA TH-ir neurons: Effects of genotype and cocaine
Cocaine-induced behavioral sensitization is largely a DA-dependent phenomenon. Repeated microinjection of cocaine in the VTA (DA cell body region) and not into the NAC or dST (DA terminal fields) produces behavioral sensitization to subsequent systemic drug challenge, while microinjection of a DA antagonist directly into the VTA region prevents the development of behavioral sensitization to peripherally administered drugs [36]. These findings suggest that DA transmission in the VTA is necessary for the induction of cocaine sensitization [36]. Thus, the diminished expression of TH-ir neurons in the VTA of nNOS KO mice compared to WT counterparts (Fig. 1) may contribute, in part, to the resistance of nNOS KO mice to cocaine-induced behavioral sensitization [2, 16]. We conclude that psychomotor sensitization relies on an intact nitrergic system possibly due to modulation of dopaminergic neurons. Fewer TH-ir neurons in the VTA of nNOS KO mice is most likely a direct consequence of the lack of the nNOS gene throughout development suggesting an essential role of nNOS in regulating the mesolimbic DA system. However, future studies are needed to determine if the deficiency in nNOS results in the death or a loss of dopaminergic phenotype in the VTA neurons. Nevertheless, the reduced expression of VTA TH-ir neurons we observed in nNOS KO mice is in agreement with the reduced TH protein levels observed in the adrenal glands and hypothalamus of these mice [26, 42].
Finally, the number of TH-ir neurons in VTA were unaffected by repeated cocaine treatment, regardless of genotype (Fig. 1). These results are in agreement with previous reports demonstrating that TH protein and mRNA levels in the VTA were also unaffected by repeated cocaine administration [13, 34], but may be at odds with other reports showing increases in TH protein and activity [3, 24]. However, thus far, the direction of cocaine-induced modulation of VTA dopaminergic transmission remains controversial most likely due to differences in species, technical assessment (Western Blot vs in situ hybridization), cocaine regimen, and the time elapsed after cocaine treatment and assaying.
Striatal TH-ir terminals: Effects of genotype and cocaine
DA projections most often linked with psychostimulant-induced behavioral sensitization are the mesoaccumbens projections from the VTA to the NAC [21]. However, DA projections from the substantia nigra pars compacta (SNc) to the dST have also been implicated [27]. In the dST, medium spiny neurons have connectivity to the substantia nigra pars reticulata and internal segment of the globus pallidus, which, in turn, control thalamocortical neurons and thus motor activity [6]. It is thought that the ventral striatum primarily controls the reinforcing effect of psychostimulants while the dST controls the psychomotor effects and stimulus–response habit formation [41]. A sensitized release of DA in the dST could contribute to the expression of behavioral sensitization to cocaine. This neurochemical sensitization in the dST is thought to be involved in habitual cocaine seeking behavior [15, 35]. Thus, both the mesolimbic and the nigrostriatal pathways have been implicated in behavioral sensitization.
While no differences were detected in the TH-ir fibers of the dST under basal conditions (Fig. 2A), significant genotypic differences were noted after repeated cocaine administration (Fig. 2B and 2C). Our cocaine-sensitizing regimen resulted in a significant increase in TH protein levels in the dST of WT mice (Fig. 2B). This cocaine-induced overexpression of dST TH is reminiscent of the cocaine-induced overexpression of dST nNOS-ir neurons we observed previously [2], suggesting mutual regulation of dST nitrergic/dopaminergic transmission by cocaine. Conversely, cocaine induced a decrease in dST TH-ir fibers in nNOS KO mice (Fig. 2C). The findings that cocaine treatment leads to 1) a concomitant increase in both nNOS and TH in the dST of WT mice that develop sensitization, and 2) a reduction in TH in nNOS KO animals that are resistant to sensitization, suggests that nNOS plays a role in the cocaine-mediated increase in TH. Thus, it appears that the overexpression of dST nNOS and TH is associated with cocaine-induced behavioral sensitization. In summary, results of the present study suggest that the nNOS gene, the level of TH expression in VTA, and the regulation of TH in the dST are associated with a sensitizing-regimen of cocaine.
Acknowledgments
This work was supported by awards RO1 DA019107 (YI) and F30 DA022847-01 (MB) from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 2.Balda MA, Anderson KL, Itzhak Y. Differential role of the nNOS gene in the development of behavioral sensitization to cocaine in adolescent and adult B6;129S mice. Psychopharmacology (Berl) 2008;200:509–519. doi: 10.1007/s00213-008-1228-2. [DOI] [PubMed] [Google Scholar]
- 3.Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem. 1991;57:344–347. doi: 10.1111/j.1471-4159.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 4.Benavides-Piccione R, DeFelipe J. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb Cortex. 2003;13:297–307. doi: 10.1093/cercor/13.3.297. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava HN, Kumar S. Sensitization to the locomotor stimulant activity of cocaine is associated with increases in nitric oxide synthase activity in brain regions and spinal cord of mice. Pharmacology. 1997;55:292–298. doi: 10.1159/000139541. [DOI] [PubMed] [Google Scholar]
- 6.Borgkvist A, Fisone G. Psychoactive drugs and regulation of the cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neurosci Biobehav Rev. 2007;31:79–88. doi: 10.1016/j.neubiorev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 8.Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujiyama F, Masuko S. Association of dopaminergic terminals and neurons releasing nitric oxide in the rat striatum: an electron microscopic study using NADPH-diaphorase histochemistry and tyrosine hydroxylase immunohistochemistry. Brain Res Bull. 1996;40:121–127. doi: 10.1016/0361-9230(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 10.Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- 11.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 12.Hidaka S, Totterdell S. Ultrastructural features of the nitric oxide synthase-containing interneurons in the nucleus accumbens and their relationship with tyrosine hydroxylase-containing terminals. J Comp Neurol. 2001;431:139–154. doi: 10.1002/1096-9861(20010305)431:2<139::aid-cne1061>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Hope BT, Crombag HS, Jedynak JP, Wise RA. Neuroadaptations of total levels of adenylate cyclase, protein kinase A, tyrosine hydroxylase, cdk5 and neurofilaments in the nucleus accumbens and ventral tegmental area do not correlate with expression of sensitized or tolerant locomotor responses to cocaine. J Neurochem. 2005;92:536–545. doi: 10.1111/j.1471-4159.2004.02891.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 15.Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itzhak Y, Ali SF, Martin JL, Black MD, Huang PL. Resistance of neuronal nitric oxide synthase-deficient mice to cocaine-induced locomotor sensitization. Psychopharmacology (Berl) 1998;140:378–386. doi: 10.1007/s002130050779. [DOI] [PubMed] [Google Scholar]
- 17.Kiss JP. Role of nitric oxide in the regulation of monoaminergic neurotransmission. Brain Res Bull. 2000;52:459–466. doi: 10.1016/s0361-9230(00)00282-3. [DOI] [PubMed] [Google Scholar]
- 18.Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24:211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- 19.Kiss JP, Zsilla G, Vizi ES. Inhibitory effect of nitric oxide on dopamine transporters: interneuronal communication without receptors. Neurochem Int. 2004;45:485–489. doi: 10.1016/j.neuint.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Klejbor I, Domaradzka-Pytel B, Ludkiewicz B, Wojcik S, Morys J. The relationships between neurons containing dopamine and nitric oxide synthase in the ventral tegmental area. Folia Histochem Cytobiol. 2004;42:83–87. [PubMed] [Google Scholar]
- 21.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 22.Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- 23.Lonart G, Johnson KM. Inhibitory effects of nitric oxide on the uptake of [3H]dopamine and [3H]glutamate by striatal synaptosomes. J Neurochem. 1994;63:2108–2117. doi: 10.1046/j.1471-4159.1994.63062108.x. [DOI] [PubMed] [Google Scholar]
- 24.Masserano JM, Baker I, Natsukari N, Wyatt RJ. Chronic cocaine administration increases tyrosine hydroxylase activity in the ventral tegmental area through glutaminergic- and dopaminergic D2-receptor mechanisms. Neurosci Lett. 1996;217:73–76. [PubMed] [Google Scholar]
- 25.Matthews RT, Beal MF, Fallon J, Fedorchak K, Huang PL, Fishman MC, Hyman BT. MPP+ induced substantia nigra degeneration is attenuated in nNOS knockout mice. Neurobiol Dis. 1997;4:114–121. doi: 10.1006/nbdi.1997.0141. [DOI] [PubMed] [Google Scholar]
- 26.Orlando GF, Langnaese K, Schulz C, Wolf G, Engelmann M. Neuronal nitric oxide synthase gene inactivation reduces the expression of vasopressin in the hypothalamic paraventricular nucleus and of catecholamine biosynthetic enzymes in the adrenal gland of the mouse. Stress. 2008;11:42–51. doi: 10.1080/10253890701449867. [DOI] [PubMed] [Google Scholar]
- 27.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 28.Pogun S, Baumann MH, Kuhar MJ. Nitric oxide inhibits [3H]dopamine uptake. Brain Res. 1994;641:83–91. doi: 10.1016/0006-8993(94)91818-x. [DOI] [PubMed] [Google Scholar]
- 29.Sammut S, Dec A, Mitchell D, Linardakis J, Ortiguela M, West AR. Phasic dopaminergic transmission increases NO efflux in the rat dorsal striatum via a neuronal NOS and a dopamine D(1/5) receptor-dependent mechanism. Neuropsychopharmacology. 2006;31:493–505. doi: 10.1038/sj.npp.1300826. [DOI] [PubMed] [Google Scholar]
- 30.Sammut S, Park DJ, West AR. Frontal cortical afferents facilitate striatal nitric oxide transmission in vivo via a NMDA receptor and neuronal NOS-dependent mechanism. J Neurochem. 2007;103:1145–1156. doi: 10.1111/j.1471-4159.2007.04811.x. [DOI] [PubMed] [Google Scholar]
- 31.Sammut S, Bray KE, West AR. Dopamine D2 receptor-dependent modulation of striatal NO synthase activity. Psychopharmacology (Berl) 2007;191:793–803. doi: 10.1007/s00213-006-0681-z. [DOI] [PubMed] [Google Scholar]
- 32.Sammut S, West AR. Acute cocaine administration increases NO efflux in the rat prefrontal cortex via a neuronal NOS-dependent mechanism. Synapse. 2008;62:710–713. doi: 10.1002/syn.20537. [DOI] [PubMed] [Google Scholar]
- 33.Snyder SH. Nitric oxide. More jobs for that molecule. Nature. 1994;372:504–505. doi: 10.1038/372504a0. [DOI] [PubMed] [Google Scholar]
- 34.Sorg BA, Chen SY, Kalivas PW. Time course of tyrosine hydroxylase expression after behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;266:424–430. [PubMed] [Google Scholar]
- 35.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 37.Volz TJ, Schenk JO. L-arginine increases dopamine transporter activity in rat striatum via a nitric oxide synthase-dependent mechanism. Synapse. 2004;54:173–182. doi: 10.1002/syn.20075. [DOI] [PubMed] [Google Scholar]
- 38.West AR, Grace AA. Striatal nitric oxide signaling regulates the neuronal activity of midbrain dopamine neurons in vivo. J Neurophysiol. 2000;83:1796–1808. doi: 10.1152/jn.2000.83.4.1796. [DOI] [PubMed] [Google Scholar]
- 39.West AR, Galloway MP, Grace AA. Regulation of striatal dopamine neurotransmission by nitric oxide: effector pathways and signaling mechanisms. Synapse. 2002;44:227–245. doi: 10.1002/syn.10076. [DOI] [PubMed] [Google Scholar]
- 40.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 41.White NM. Reward or reinforcement: what’s the difference? Neurosci Biobehav Rev. 1989;13:181–186. doi: 10.1016/s0149-7634(89)80028-4. [DOI] [PubMed] [Google Scholar]
- 42.Yamova L, Atochin D, Glazova M, Chernigovskaya E, Huang P. Role of neuronal nitric oxide in the regulation of vasopressin expression and release in response to inhibition of catecholamine synthesis and dehydration. Neurosci Lett. 2007;426:160–165. doi: 10.1016/j.neulet.2007.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]