Abstract
Context
Hyperuricemia is a predictor for the development of hypertension and is commonly present in new-onset essential hypertension. Experimentally increasing uric acid levels using a uricase inhibitor causes systemic hypertension in animal models.
Objective
To determine whether lowering uric acid lowers blood pressure (BP) in hyperuricemic adolescents with newly diagnosed hypertension.
Design, Setting, and Patients
Randomized, double-blind, placebo-controlled, crossover trial (September 2004-March 2007) involving 30 adolescents (aged 11–17 years) who had newly diagnosed, never-treated stage 1 essential hypertension and serum uric acid levels ≥6 mg/dL. Participants were treated at the Pediatric Hypertension Clinic at Texas Children’s Hospital in Houston. Patients were excluded if they had stage 2 hypertension or known renal, cardiovascular, gastrointestinal tract, hepatic, or endocrine disease.
Intervention
Allopurinol, 200 mg twice daily for 4 weeks, and placebo, twice daily for 4 weeks, with a 2-week washout period between treatments. The order of the treatments was randomized.
Main Outcome Measures
Change in casual and ambulatory blood pressure.
Results
For casual BP, the mean change in systolic BP for allopurinol was −6.9 mm Hg (95% confidence interval [CI], −4.5 to −9.3 mm Hg) vs −2.0 mm Hg (95% CI, 0.3 to −4.3 mm Hg; P=.009) for placebo, and the mean change in diastolic BP for allopurinol was −5.1 mm Hg (95% CI, −2.5 to −7.8 mm Hg) vs −2.4 (95% CI, 0.2 to −4.1; P=.05) for placebo. Mean change in mean 24-hour ambulatory systolic BP for allopurinol was −6.3 mm Hg (95% CI, −3.8 to −8.9 mm Hg) vs 0.8 mm Hg (95% CI, 3.4 to −2.9 mm Hg; P=.001) for placebo and mean 24-hour ambulatory diastolic BP for allopurinol was −4.6 mm Hg (−2.4 to −6.8 mm Hg) vs −0.3 mm Hg (95% CI, 2.3 to −2.1 mm Hg; P=.004) for placebo. Twenty of the 30 participants achieved normal BP by casual and ambulatory criteria while taking allopurinol vs 1 participant while taking placebo (P<.001).
Conclusions
In this short-term, crossover study of adolescents with newly diagnosed hypertension, treatment with allopurinol resulted in reduction of BP. The results represent a new potential therapeutic approach, although not a fully developed therapeutic strategy due to potential adverse effects. These preliminary findings require confirmation in larger clinical trials.
Trial Registration
clinicaltrials.gov Identifier: NCT00288184
Hypertension is commonly associated with hyperuricemia.1,2 Early investigators proposed uric acid as having a causal role in hypertension.3–5 However, an elevation of uric acid in hypertension could be a consequence of reduced renal function, the use of diuretics, the presence of hyperinsulinemia and oxidative stress, or elevated renal vascular resistance, which are commonly present in this condition.6 As such, hyperuricemia is not considered a true risk factor for hypertension by the Joint National Committee,7 nor is it considered a cardiovascular risk factor by most expert organizations.8
Recent studies have challenged this long-standing paradigm. For example, numerous studies have reported that hyperuricemia independently predicts the development of hypertension,9–13 even in individuals lacking features of the metabolic syndrome.14 If hyperuricemia precedes the development of hypertension then it cannot simply be a secondary phenomenon. We also previously reported that elevated uric acid is present in nearly 90% of adolescents presenting with essential hypertension.15 Of 63 participants with essential hypertension, 89% had a uric acid level higher than 5.5 mg/dL (mean, 6.7 mg/dL; to convert milligrams per deciliter to micro-moles per liter, multiply by 59.485), whereas this was observed in only 30% with secondary hypertension (mean, 4.3 mg/dL; n=40) and none of the controls with blood pressure (BP) that was lower than the 90th percentile (mean, 3.6 mg/dL; n=40) or white-coat hypertension (mean, 3.5 mg/dL; n=22). The latter group was of particular interest because they had similar degrees of obesity as the patients with essential hypertension. The relationship was also linear and strong (r=0.8, P<.01)15 but did not prove a causal relationship.
Evidence supporting a causal role of uricacidin hypertension has come from experimental studies in laboratory animals. Humans do not express uricase, an enzyme that degrades uric acid to allantoin. As a consequence, humans have higher levels of uric acid and also cannot regulate blood levels as effectively as most mammals.16 To determine the effect of uric acid on BP in laboratory animals, uric acid levels in rats were increased by administering oxonic acid, which is a uricase inhibitor.17 Interestingly, raising uric acid levels in rats resulted in increased BP and the development of microvascular disease (resembling arteriolosclerosis) in the kidneys.17,18 The mechanism of hypertension was shown to be caused by a uric acid–mediated reduction in endothelial nitric oxide levels19,20 and stimulation of renin expression.18 Studies in humans have also correlated uric acid levels with both endothelial dysfunction21,22 and elevated plasma renin activity.23,24 Furthermore, several controlled clinical trials have reported that lowering uric acid with xanthine oxidase inhibitors improves endothelial function under a variety of conditions.25–27
We performed a randomized, double-blind, placebo-controlled, crossover trial of allopurinol in children with newly diagnosed essential hypertension to test the hypothesis that lowering uric acid levels with a xanthine oxidase inhibitor might lower BP. Although hypertension is less common in adolescents than in adults, the short duration of elevated BP, often known with certainty, and typical lack of confounding medical conditions make adolescents an ideal population in which to investigate possible, early causal steps in the development of hypertension.
METHODS
Participants
Participants were all recruited from the Hypertension Clinic at Texas Children’s Hospital in Houston between September 2004 and March 2007. Children referred for the evaluation of newly suspected hypertension underwent routine screening for the causes of their hypertension in accordance with the recommendations of the Fourth Report of the Task Force on the Diagnosis, Evaluation and Treatment of Hypertension in Children and Adolescents.28 Inclusion criteria were adolescents aged 11 through 17 years with confirmed stage 1 hypertension (BP >95th percentile for sex, age, and height percentile) who had a serum uric acid level of 6 mg/dL or higher, had no evidence for target organ damage, had never been treated with a hypertensive medication for any indication, and were not currently taking medications. We restricted the population to those with mild hypertension because we thought it unethical to randomize patients with marked hypertension. We selected 6 mg/dL based on our previous studies of children with essential hypertension in which a serum uric acid level higher than 5.5 mg/dL was commonly observed in children with essential hypertension and rare in those without elevated BP.15 By selecting an inclusion value slightly higher, we could ensure that participants receiving allopurinol would likely have significant decreases in uric acid levels that would cross this threshold and that would not be expected by children administered placebo.
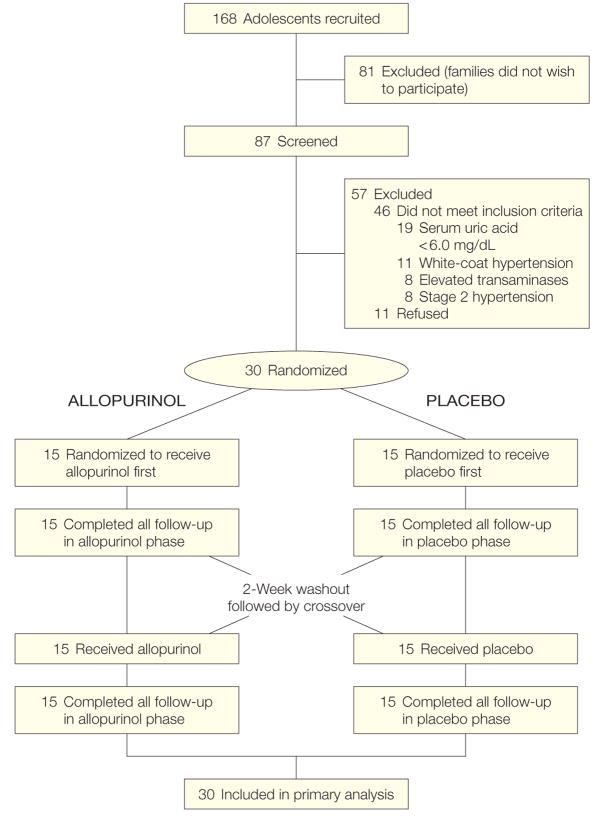
Exclusion criteria included pre-hypertension or stage 2 hypertension (BP >99th percentile + 5 mm Hg for sex, age, and height percentile), serum uric acid levels lower than 6 mg/dL, prior or current treatment with an antihypertensive agent, serum transaminase levels higher than the laboratory normal range or any abnormalities on screening complete blood cell count. Race assessment was made by the principal investigator and included only as a demonstration of the diversity of the recruited population. Of 168 invited patients, the parents or guardians of 81 adolescents (48%) did not wish to be screened and 46 children (27%) were screened but did not enroll because they did not meet inclusion criteria or had 1 or more exclusion criteria (Figure 1). Eleven children met enrollment criteria but withdrew prior to enrollment. All of the 30 adolescents randomized completed the protocol and data collection. A low ratio of children screened to children enrolled is common among pediatric trials. Our rate of enrollment is consistent with published observations.29 All participants had consultation with a trained nutritionist and received counseling in how to establish a healthful diet, reduce sodium, and, when appropriate, reduce weight.
Figure 1.
Study Design
To convert uric acid to μmol/L, multiply values by 59.485.
Study Design
The study was a randomized, double-blind, placebo-controlled, crossover trial. Medication preparation of allopurinol and placebo in identical, unmarked capsules was performed in the Investigational Pharmacy at Texas Children’s Hospital. Informed consent was obtained by the principal investigator (D.I.F.), study coordinator (B.S.), or both in a face-to-face interview that included the participant with at least 1 of his/her parents. The discussion included risks of hypertension, a discussion of standard treatments for hypertensive children, the study hypothesis, and the risks and benefits of study participation, including adverse effects of allopurinol. Informed consent by a parent and informed assent by the participant were both required before study enrollment or any screening procedures. Both consent and assent forms were approved by the Baylor College of Medicine institutional review board. After screening and enrollment, participants were assigned by random number table in the investigational pharmacy and treated with either allopurinol, 200 mg twice daily, or placebo capsule twice daily for 4 weeks, followed by a 2-week washout period then a 4-week crossover phase (Figure 1). The principal investigator and study staff responsible for patient contact and end point measurement were blinded to medication assignment and serum uric acid values until after enrollment and data collection were completed.
All participants had clinic visits 3 to 7 days before initiation of any medication, on the day of medication initiation for each medication phase, at 5 to 9 days after initiation of each medication phase, and at 26 to 30 days after initiation of each medication. Pill counts were performed at the end of each treatment phase. Adherence was assessed as the number of pills taken divided by the total number of pills prescribed. End point testing included casual BP monitoring (primary end point), 24-hour ambulatory BP monitoring (secondary end point), clinical laboratory testing, and noninvasive bioimpedance performed 3 to 7 days before initiation of any medication and on the last day of each of the medication phases. The study design was approved by the Baylor College of Medicine institutional review board.
Adverse Event Screening
After 1 and 4 weeks of each medication phase, participants had a review of systems that included skin, urinary, gastrointestinal, and neurological symptoms; a physical examination; and laboratory tests, including complete blood cell count and differential, electrolytes, blood urea nitrogen, creatinine, and transaminases to screen for skin, hepatic, hematological, and renal adverse events.
BP Measurements
Blood pressure measurements were made by trained personnel using aneroid BP monitors. Cuff size was selected in accordance with task force recommendations29 and once selected, the same cuff and monitor were used subsequently for each patient. Each BP data point was the mean of 4 upper extremity measurements, performed on seated children who had been relaxing in a quiet examination room for more than 10 minutes. Standard, aneroid (Mabis Medic Kit-5; Mabis Healthcare Inc, Waukegan, Illinois) auscultatory monitors were used and were calibrated with T-valve connector and mercury sphygmomanometer each month to ensure consistency and accuracy in the equipment. We did not use mercury sphygmomanometers because they are prohibited from use in patient areas at our institution for environmental safety concerns. Twenty-four-hour ambulatory BP monitoring was performed using SpaceLabs 90217 monitors (SpaceLabs Medical, Issaquah, Washington) at the time of study screening, within a week prior to starting study medication, and at the end of each of the 4-week treatment phases (while the participant was still receiving medication). The same cuff size was used for all 3 ambulatory BP monitoring studies for each patient. Monitors measure BP every 20 minutes from 6 AM to 10 PM and every 30 minutes from 10 PM to 6 AM.
The definition of casual (in office) hypertension used in this study follows the Fourth Report of the Task Force on the Diagnosis, Evaluation and Treatment of Hypertension in Children and Adolescents.28 This definition, which represents the current consensus guideline, used greater than 95th percentile of systolic or diastolic BP stratified for age, sex, and height and does not include any modification for body weight or body mass index. Hypertension by ambulatory BP monitoring criteria was defined using sex- and height-based normative data30 as the 24-hour systolic or diastolic mean BP greater than the 95th percentile or systolic or diastolic BP load (percentage of readings exceeding the 95th percentile) greater than 30%. Dipping is the percentage decrease in systolic and diastolic BP between sleep and awake periods. The normal pattern is for a decrease of more than 10%. Participants whose BP increased or did not decrease by at least 10% were considered nocturnal nondippers.
Laboratory Analyses
At each visit, patient samples were tested for uric acid, complete blood cell count, electrolytes, blood urea nitrogen, creatinine, alanine transaminase levels, and plasma renin activity, and urine pregnancy for girls. All clinical laboratory testing was performed in the Texas Children’s Hospital Clinic Laboratory. Plasma renin activity was measured using a continuous fluorescence assay as developed by Wang et al31 and with reagents purchased from Cayman Chemicals (Ann Arbor, Michigan).
Bioimpedance
Cardiac bioimpedance studies were performed with a Bio-Z device (Cardio-Dynamics, San Diego, California) using manufacturer’s specifications. The device measures heart rate, cardiac output, total body water, and systemic vascular resistance of patients in a supine position using noninvasive impedance skin electrodes on the neck and chest. This device has been used for monitoring BP treatment in patients with hypertension and has been found to correlate well with invasive BP in volume status measurements.32–35 Impedance cardiography has also been used to monitor systemic vascular resistance and cardiac output in healthy and hypertensive children.36,37
Statistical Analysis
Sample size calculations were made for detection of the difference in change in systolic BP of 6 mm Hg and diastolic BP of 5 mm Hg with a power of 90% for each BP end point. For the purposes of these calculations, individual BP parameters were not considered independent; a 50% covariance of the BP end points was assumed. Because this was a clinical trial with multiple prospectively defined end points, nominal assessment of significance tests would be likely to yield at least 1 α error. For this reason, the family-wise error rate was conserved by prospective α allocation (.03 for change in office measures of systolic and diastolic BP, .01 for systolic BP load, and .01 for 24-hour mean systolic BP). Using these assumptions in the model, sample size was calculated with Statistica 8.0 software (StatSoft Inc, Tulsa, Oklahoma). To preserve a family-wise error rate lower than 0.05, a minimum of 27 participants were required.
The analysis was on an intent-to-treat basis so that only the treatment phase, not medication adherence or actual change in serum uric acid, were considered in the analysis of data. The mean of each patient’s change in BP between pretreatment and placebo and pretreatment and allopurinol was analyzed by paired t test and Hotelling T2 test for repeated measurements, after confirmation of the absence of treatment order effect. The dichotomous variable, presence or absence of hypertension, was analyzed by the McNemar test. The change in mean systemic vascular resistance and plasma renin activity values were analyzed by analysis of variance for repeated measurements. All analyses were performed using Statistica 8.0 (Statsoft Inc).
RESULTS
We recruited 30 adolescents with newly diagnosed stage 1 essential hypertension and serum uric acid levels of 6.0 mg/dL or higher. The population was mixed in terms of race/ethnicity and sex (Table 1). Seventy-three percent (22/30) of the participants were overweight or obese (>90th percentile body mass index for sex and age), and 30% (9/30) met diagnostic criteria for metabolic syndrome,38 which is representative of patients referred to our clinic. The 24-hour mean ambulatory BP readings were significantly lower than the casual BP readings because sleep-period BP contributed to the mean. At the time of screening, all 30 participants had hypertension by at least 1 ambulatory BP monitoring criterion. There was no difference in casual BP readings at the beginning of the placebo and medication phases, indicating that carryover from the previous treatment phase, particularly when allopurinol was first, did not contribute to the BP results (Table 1).
Table 1.
Patient Population Throughout Study Participation
| Characteristic | Value |
|---|---|
| Sex, No. (%) Male |
18 (60) |
| Female | 12 (40) |
| Age, mean (95% CI), y | 15.1 (13.5–17.8) |
| Height, mean (95% CI), cm | 170 (165–175) |
| Weight, mean (95% CI), kg At enrollment |
97 (82–108) |
| End of placebo phase | 98 (84–106) |
| End of allopurinol phase | 96 (83–108) |
| BMI at enrollment, mean (95% CI) | 33 (28–36) |
| BMI percentile at enrollment, mean (95% CI) | 94.3 (91.1–99.5) |
| Race/ethnicity, No. (%) White |
14 (47) |
| Black | 9 (30) |
| Hispanic | 7 (23) |
| Serum uric acid, mean (95% CI), mg/dL At enrollment |
6.9 (6.5–7.4) |
| Beginning of placebo phase | 6.2 (5.5–6.9) |
| End of placebo phase | 6.4 (5.8–7.0) |
| Beginning of allopurinol phase | 7.0 (6.5–7.5) |
| End of allopurinol phase | 4.2 (3.7–4.6) |
| Casual systolic BP, mean (95% CI), mm Hg At enrollment |
139 (137–141) |
| Beginning of placebo phase | 139 (135–141) |
| End of placebo phase | 137 (135–140) |
| Beginning of allopurinol phase | 137 (134–142) |
| End of allopurinol phase | 132 (129–134) |
| Casual diastolic BP, mean (95% CI), mm Hg At enrollment |
83 (80–85) |
| Beginning of placebo phase | 82 (79–85) |
| End of placebo phase | 81 (78–83) |
| Beginning of allopurinol phase | 83 (80–86) |
| End of allopurinol phase | 78 (74–80) |
| 24-Hour ambulatory systolic BP, mean (95% CI), mm Hg At enrollment |
127 (124–130) |
| End of placebo phase | 128 (124–132) |
| End of allopurinol phase | 120 (117–123) |
| 24-Hour ambulatory diastolic BP, mean (95% CI), mm Hg At enrollment |
74 (69–83) |
| End of placebo phase | 74 (70–76) |
| End of allopurinol phase | 68 (65–70) |
Abbreviations: BMI, body mass index (measured as weight in kilograms divided by height in meters squared); BP, blood pressure; CI, confidence interval.
SI conversion factor: To convert uric acid to μmol/L, multiply values by 59.485.
The mean adherence rate was 76% (range, 27%–100%) when both placebo and allopurinol groups were included, suggesting that, on average, approximately 11 of 14 weekly doses were taken. There was a tendency toward more missed doses during the allopurinol treatment phase (73% adherence) than the placebo phase (79% adherence), but the difference was not statistically significant (P=.09). None of the participants were withdrawn for deviation from the expected pill counts, and the degree of adherence was not accounted for in the data analysis. There were no observed adverse reactions among the participants by review of symptoms, physical examination, or laboratory tests.
From the beginning to the end of the medication phase, treatment with placebo resulted in no statistically significant change in uric acid levels, whereas allopurinol resulted in a marked decrease (Table 1). Two of 30 patients had no change in serum uric acid levels while taking allopurinol. Twenty-two of 30 patients achieved serum uric acid levels lower than 5.0 mg/dL by the end of the allopurinol phase, whereas only 2 of 30 patients had serum uric acid levels lower than 5.0 mg/dL at the end of the placebo phase.
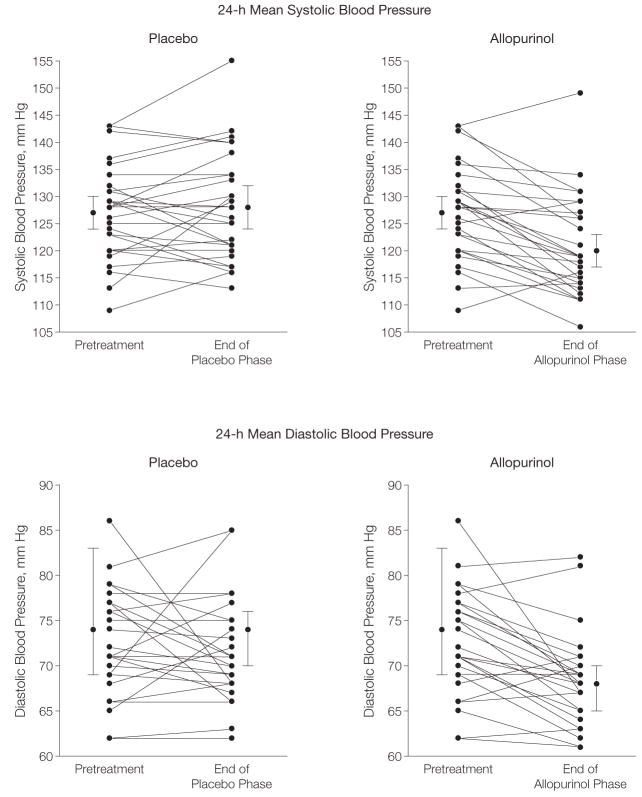
Allopurinol treatment was associated with a significant decrease in casual and ambulatory systolic and diastolic BP (Table 2). The mean decrease in casual BP during allopurinol treatment was −6.9 mm Hg for systolic and −5.1 mm Hg for diastolic BP; for placebo the respective changes were −2.0 and −2.4 mm Hg. The mean changes in 24-hour ambulatory BP during allopurinol were −6.3 mm Hg systolic and −4.6 diastolic BP. Systolic BP increased slightly during the placebo phase by 0.8 mm Hg and diastolic BP slightly decreased by 0.3. The decrease in ambulatory BP directly correlated with allopurinol treatment (Figure 2).
Table 2.
Blood Pressure (BP) Response to Placebo and Allopurinol (Posttreatment Values)
| Mean (95% Confidence Interval) | |||
|---|---|---|---|
| Parameter | Placebo | Allopurinol | P Value |
| Change in casual systolic BP, mm Hg | −2.0 (0.3 to −4.3) | −6.9 (−4.5 to −9.3) | .009a |
| Change in casual diastolic BP, mm Hg | −2.4 (0.2 to −4.1) | −5.1 (−2.5 to −7.8) | .05 |
| Change in 24-h ambulatory systolic BP, mm Hg | 0.8 (3.4 to −2.9) | −6.3 (−3.8 to −8.9) | .001a |
| Change in 24-h ambulatory diastolic BP, mm Hg | −0.3 (2.3 to −2.1) | −4.6 (−2.4 to −6.8) | .004b |
| Systolic BP load, %c | 48.6 (34.0 to 50.2) | 23.3 (15.8 to 30.9) | .01a |
| Diastolic BP load, %c | 29.2 (25.6 to 37.1) | 18.1 (12.3 to 23.8) | .01b |
| Hypertensive, No./total (%)d | 29/30 (97) | 10/30 (33) | .001b |
Calculated with the paired t test.
Exploratory end points.
Load (as measured by ambulatory BP) is the percentage of time during the study that BP exceeds the 95th percentile.
BP above 95th percentile, casual systolic BP, casual diastolic BP, ambulatory mean systolic BP, ambulatory mean diastolic BP, systolic BP load, or diastolic BP load, as described in the “Methods.”
Figure 2.
Blood Pressure Response of Adolescents to Allopurinol and Placebo
Fifteen individuals received allopurinol first and 15 received placebo first but the x-axis is defined by treatment arm rather than time for clarity. Each panel shows the data for all 30 participants. Because of overlap in the blood pressure values and change in blood pressure, 30 distinct points and lines are not visible on each diagram. Data points with error bars are overall mean (95% confidence interval) values.
Ambulatory systolic BP load decreased from 44% (95% CI, 37%–49%) before allopurinol medication to 23% (95% CI, 16%–31%) after and the diastolic load decreased from 31% (95% CI, 25%–37%) before to 18% (95% CI, 12%–24%) after treatment. But those readings remained unchanged during the placebo phase.
The degree of nocturnal dipping did not significantly change between treatment phases. At baseline, the mean systolic BP dipped by 12.1% (95% CI, 7.5%–19.9%) and the diastolic BP dipped by 19.2% (95% CI, 13.0%–29.7%) between wake and sleep periods. During the placebo phase, systolic BP dipped 10.7% (95% CI, 5.2%–17.7%, P=.24) and diastolic BP dipped 16.6% (95% CI, 9.7%–28.6%, P=.13) between wake and sleep periods. During the allopurinol phase, systolic BP dipped 11.8% (95% CI, 8.0%–18.4%, P=.51) and diastolic BP dipped 18.5% (95% CI, 12.8%–28.0%, P=.48).
Twenty of the 30 participants achieved normal BP by casual and ambulatory criteria during the allopurinol phase, whereas only 1 of 30 achieved normal BP during the placebo phase. Of the 10 participants who remained hypertensive while taking allopurinol, 7 had a serum uric acid level of 5.0 mg/dL or higher at the end of the allopurinol phase.
A potential weakness of the crossover study design is the possibility of differential effect secondary to the order of treatments received. For this reason, we examined the BP of patients based on the whether they received placebo or allopurinol first. For 15 who received placebo first, the mean casual baseline BP was 139/81 mm Hg (95% CI, 135–141/78–84 mm Hg). During the placebo phase, it was 137/80 mm Hg (95% CI, 134–140/77–84 mm Hg) and 132/77 (95% CI, 127–134/75–80 mm Hg) during the allopurinol phase. For patients who received allopurinol first, the mean casual baseline BP was 139/81 mm Hg (95% CI, 135–141/78–83 mm Hg). During the placebo phase it was 138/82 mm Hg (95% CI, 135–140/79–85 mm Hg) and 132/78 mm Hg (95% CI, 126–134/73–80 mm Hg) during the allopurinol phase. With respect to the 24-hour ambulatory BP monitoring, the 15 individuals who received placebo first had a mean baseline BP of 126/73 mm Hg (95% CI, 123–130/69–75 mm Hg). During the placebo phase, it was 128/73 mm Hg (95% CI, 125–132/70–77 mm Hg) and 120/67 (95% CI, 115–123/61–69 mm Hg) during the allopurinol phase. The 15 participants who received allopurinol first had a mean baseline 24-hour BP of 128/75 mm Hg (95% CI, 124–131/72–78 mm Hg). During the placebo phase, it was 128/74 mm Hg (95% CI, 125–131/72–77 mm Hg) and 119/68 mm Hg (95% CI, 116–122/64–70 mm Hg) during the allopurinol phase. In short, there was no treatment order effect on either casual or ambulatory BP.
Because the early uric acid–induced hypertension in the animal model was, at least in part, mediated by the renin angiotensin system,17,18 we assessed both plasma renin activity and systemic vascular resistance of children during the study. The mean plasma renin activity decreased from 1.9 ng/mL per hour (95% CI, 1.7–2.2 ng/mL per hour) to 1.4 ng/mL per hour (95% CI, 0.8–2.1 ng/mL per hour) during the allopurinol phase, whereas there was no significant change during the placebo phase: 2.1 ng/mL per hour (95% CI, 1.8–2.4 ng/mL per hour). Bioimpedance measurement of heart rate, cardiac output, and total body water revealed no differences between pretreatment and treatment with allopurinol or placebo. The systemic vascular resistance index, however, decreased an average of 14% in response to allopurinol with no change in response to placebo (Table 3).
Table 3.
Effect of Placebo and Allopurinol on Non–Blood Pressure End Points
| Mean (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Parameter | Pretreatmenta | Placebo | Allopurinol | P Value |
| Heart rate, beats/min | 72 (67–78) | 74 (69–80) | 75 (69–80) | .87 |
| Cardiac output, L/min | 6.4 (5.6–7.1) | 6.2 (5.4–7.0) | 6.6 (5.9–7.2) | .56 |
| Systemic vascular resistance index, (dyne s/cm5)/m2 | 2478 (2223–2731) | 2473 (2232–2615) | 2136 (2056–2228) | .03b |
| Total body water, L | 27.8 (26.0–29.7) | 28.0 (26.1–30.1) | 28.1 (26.0–29.9) | .86 |
| Plasma renin activity, ng/mL/h | 1.9 (1.7–2.2) | 2.1 (1.8–2.4) | 1.4 (0.8–2.1) | .02b |
Pretreatment values were measured prior to first treatment phase.
Exploratory end points.
COMMENT
We performed a small, carefully controlled, double-blind study to determine if lowering uric acid with a xanthine oxidase inhibitor can lower BP in asymptomatic adolescents with high serum uric acid levels (≥6.0 mg/dL) and newly diagnosed mild essential hypertension. The study was intended as a proof of physiological mechanisms and not to establish new therapy. However, hypertension is a very common disease, affecting 30% to 35% of adults and is especially common in groups at high risk of cardiovascular disease. Despite a large number of safe and effective antihypertensive agents and useful lifestyle modification measures, optimal BP control is attained in less than 40% of patients receiving therapy.39 The results of this study represent a potentially new therapeutic approach, that of control of a biochemical cause of hypertension, rather than nonspecifically lowering elevated BP. Although not representing a fully developed therapeutic strategy, this study raises an alternative strategy that may prove to be more effective than currently available options.
One hesitation in considering allopurinol as a therapy for hypertension is its potential for adverse effects. Allopurinol is approved for use in children to treat gout and the dose used in this study is generally considered safe. Although no adverse effects were seen in our small study, about 1 in 75 children typically develop nausea, vomiting, or diarrhea. More serious rare adverse effects include liver damage, neutropenia, and Stevens-Johnson syndrome, which are generally reversible but can be life-threatening. The risks vs benefits of allopurinol remain to be studied.
Allopurinol was administered as 200 mg twice daily according to pediatric dosing guidelines.40 Uric acid levels decreased from a mean of 6.9 mg/dL to 4.2 mg/dL (P<.001), although 8 of 30 patients continued to have serum uric acid levels 5 mg/dL or higher with allopurinol. The variability in response to allopurinol is most likely associated with differences in adherence, but individuals could vary in their biological response to the medication as well. Because of the small size of this study, we were unable to definitively determine the etiology of the variable response. Participants in the placebo phase also showed a mild nonstatistically significant decrease in serum uric acid level (6.2 mg/dL, P=.04), which likely reflects either dietary changes made during the course of the study or that the serum uric acid was still increasing after 2 weeks of washout in the patients who received allopurinol first.
The major finding was that allopurinol treatment resulted in normal BP, by both casual and ambulatory criteria, in 20 of 30 participants, including 19 of the 22 (86%) whose uric acid levels were lowered to less than 5.0 mg/dL. In contrast, only 1 of 30 participants became normotensive while receiving placebo during the study. The primary end point (clinic BP) showed a greater mean decrease of 5 mm Hg in systolic BP and a 2.5-mm Hg decrease in diastolic BP over placebo. By ambulatory BP the differences were even greater, with a 7-mm Hg greater decline in systolic BP and a 4-mm Hg greater decline in diastolic BP.
The relative reduction in BP we observed with allopurinol was similar to what is observed with conventional antihypertensive agents in the treatment of mild hypertension. For example, in the Treatment of Mild Hypertension Study, the effect of β-blocker, calcium channel blocker,α-blocker, and angiotensin-converting enzyme inhibitors were evaluated in adults with mild hypertension.41 Over 48 months only 70% of patients responded to any given agent and the mean change in systolic BP ranged from 2.7 to 6.0 mm Hg and the change in diastolic BP from 1.1 to 3.6 mm Hg. In a meta-analysis to assess the efficacy of individual classes of antihypertensive medications in children, Simonetti et al42 found an average total BP decrease of 10 mm Hg systolic and 7 mm Hg diastolic in populations that included patients with moderate and severe hypertension. While the observed degree of reduction may appear modest, a reduction of 5 to 7 mm Hg in systolic BP can translate to as much as a 25% decrease in long-term cardiovascular mortality.43
A clue to the mechanism by which allopurinol lowered BP was the observation that systemic vascular resistance and plasma renin activity both decreased significantly with treatment. In experimental animals intrarenal renin expression has been shown to be mediated by uric acid.17 More recently, Toma et al44 reported that uric acid stimulates renin release via a macula densa dependent mechanism using an in vitro microperfused afferent arteriole-glomerular preparation. These studies suggest that lowering uric acid may act, at least in part, by reducing plasma renin activity.
There are a number of limitations to the study. First, the number of participants was small and the population limited to adolescents with mild, newly diagnosed hypertension and hyperuricemia. We do not know if the findings will extend to populations that include lower serum uric acid levels, more severe or long-standing hypertension, older patients, or different ethnic or geographic mixtures. Indeed, we have previously reported that once microvascular disease develops in the kidney, hypertension is largely driven by renal-and sodium-dependent mechanisms,45 suggesting that individuals with long-established hypertension might be expected to be resistant to hypouricemic therapy.
Second, the population was predominantly obese. Because approximately 2% to 3% of lean children and 18% to 20% of obese children have hypertension,29,46 the 70% rate of obesity in our study is representative of the adolescent hypertensive population, although it may be less representative of the general hypertensive population in the United States or worldwide.
Third, because the purpose of the study was to investigate a causal principle, the duration of treatment was short and we have no data as to whether the observed effect would be sustained over time.
Fourth, since allopurinol reduces both uric acid and xanthine oxidase–induced oxidants, it is possible that the effect of allopurinol to lower BP may not be due to the lowering of uric acid. George et al47 reported that allopurinol but not the uricosuric probenecid could improve endothelial function in patients with heart failure. However, xanthine oxidase inhibitors may be more effective at lowering uric acid within the cell where it is believed most of the effects are mediated.48,49
Fifth, we also do not know how dietary, exercise, or weight loss interventions might modify the effect of xanthine oxidase inhibition because all participants received similar counseling.
Sixth, ambulatory BP monitoring was performed only 3 times during the study: at enrollment and at the end of each of the 2 medication phases. This was done because the number of ambulatory BP monitoring procedures was the most common reason for individuals to refuse to enroll. Since both posttreatment ambulatory BPs were compared with the enrollment BP reading, there is potential concern for a carryover effect from the first to second medication phase. This is unlikely because the participants were randomized and such effects should impact both treatment groups equally. There was also no difference between casual BP measurements at the beginning of each medication phase, suggesting no carryover. Furthermore, because this was a placebo-controlled study, persistence of medication effect from those who received allopurinol first would be expected to increase the apparent placebo effect, while no placebo effect would be expected to carry over to the allopurinol treatment. Consequently, medication carryover would be expected to favor the null hypothesis, which was rejected by the data analysis.
Finally, the lack of adverse events for hypertensive participants receiving allopurinol in a small and short-term study should not be construed to suggest that allopurinol is without adverse effects or even comparable to conventional antihypertensive medications because the study was not designed to make such an evaluation.
In conclusion, we found that allopurinol treatment can reduce BP in hyperuricemic adolescents with newly diagnosed hypertension. Despite these findings, this clinical trial is a small one and allopurinol is not indicated for the treatment of hypertension in adolescents or other populations. The potential adverse effects of allopurinol, including gastrointestinal complaints and especially Stevens-Johnson syndrome, make allopurinol an unattractive alternative to available antihypertensive medications. More clinical trials are needed to determine the reproducibility of the data and whether it can be generalized to the larger hypertensive population. Nevertheless, the observation that lowering uric acid can reduce BP in adolescents with newly diagnosed hypertension raises intriguing questions about its role in the pathogenesis of hypertension.
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health grants 5 K23 DK064587 (Dr Feig) and HL-68607 (Dr Johnson).
Role of the Sponsors: The National Institutes of Health had no direct involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Feig had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Feig, Johnson.
Acquisition of data: Feig, Soletsky.
Analysis and interpretation of data: Feig, Johnson.
Drafting of the manuscript: Feig, Johnson.
Critical revision of the manuscript for important intellectual content: Feig, Soletsky.
Statistical analysis: Feig.
Obtained funding: Feig.
Administrative, technical, or material support: Soletsky.
Study supervision: Feig, Johnson.
Financial Disclosures: Dr Johnson reported being listed as an inventor on several patent applications related to the lowering of uric acid as a means of lowering BP, reducing the complications of metabolic syndrome, or slowing diabetic renal disease. These include a patent application from the University of Washington and Merck Inc (lowering uric acid with allopurinol to reduce hypertension); from the University of Florida (lowering uric acid to improve metabolic syndrome and slow diabetic renal disease); from TAP Inc (lowering uric acid with febuxostate to reduce BP); and from Human Cell Systems Inc (blocking uric acid uptake into cells as a means to reduce cardiovascular disease). None of these patent applications has been patented or licensed. Dr Johnson reports not receiving any remuneration related to patent applications. He reports not having any current stocks, consultantships, or any other sources of financial reimbursements related to these studies or the involved companies. Dr Johnson reports that he consulted for TAP Pharmaceuticals but this ended in 2006; he also occasionally lectures for Merck but total reimbursement per year is less than $10 000. He reports never consulting for Human Cell Systems and has no financial relationship with this group. In terms of the study, Dr Johnson reports no involvement in the recruitment nor in the analysis of the data. His primary role was to aid Dr Feig in the study design and interpretation of the results. Dr Feig and Ms Soletsky report no financial or other potential conflicts of interest relevant to the article.
Additional Contributions: We express our sincere gratitude to the children and their families who participated in this study.
References
- 1.Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275(9):457–464. doi: 10.1056/NEJM196609012750902. [DOI] [PubMed] [Google Scholar]
- 2.Williams J. The total nonprotein nitrogen constituents of the blood in arterial hypertension. Arch Intern Med. 1922;27:748–754. [Google Scholar]
- 3.Mohamed FA. On chronic Bright’s disease, and its essential symptoms. Lancet. 1879;1:399–401. [Google Scholar]
- 4.Haig A. On uric acid and arterial tension. BMJ. 1889;1:288–291. doi: 10.1136/bmj.1.1467.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis N. The cardiovascular and renal relations and manifestations of gout. JAMA. 1897;29:267–262. [Google Scholar]
- 6.Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG. Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Ann Intern Med. 1980;93(6):817–821. doi: 10.7326/0003-4819-93-6-817. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Vaccarino V, Krumholz HM. Risk factors for cardiovascular disease: one down, many more to evaluate. Ann Intern Med. 1999;131(1):62–63. doi: 10.7326/0003-4819-131-1-199907060-00012. [DOI] [PubMed] [Google Scholar]
- 9.Sundström J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45(1):28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 10.Alper AB, Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure:the Bogalusa Heart Study. Hypertension. 2005;45(1):34–38. doi: 10.1161/01.HYP.0000150783.79172.bb. [DOI] [PubMed] [Google Scholar]
- 11.Selby JV, Friedman GD, Quesenberry CP., Jr Precursors of essential hypertension: pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. Am J Epidemiol. 1990;131(6):1017–1027. doi: 10.1093/oxfordjournals.aje.a115593. [DOI] [PubMed] [Google Scholar]
- 12.Mellen PB, Bleyer AJ, Erlinger TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the Atherosclerosis Risk in Communities study. Hypertension. 2006;48(6):1037–1042. doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 13.Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031–1036. doi: 10.1161/01.HYP.0000248752.08807.4c. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49(2):298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 15.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RJ, Rideout BA. Uric acid and diet–insights into the epidemic of cardiovascular disease. N Engl J Med. 2004;350(11):1071–1073. doi: 10.1056/NEJMp048015. [DOI] [PubMed] [Google Scholar]
- 17.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 18.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282(6):F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RJ, Segal MS, Srinivas T, et al. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16(7):1909–1919. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Lozada LG, Tapia E, Lopez-Molina R, et al. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol. 2007;292(4):F1238–F1244. doi: 10.1152/ajprenal.00164.2006. [DOI] [PubMed] [Google Scholar]
- 21.Erdogan D, Gullu H, Caliskan M, et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 2005;59(11):1276–1282. doi: 10.1111/j.1742-1241.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 22.Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F. Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol. 2006;17(5):1466–1471. doi: 10.1681/ASN.2005090949. [DOI] [PubMed] [Google Scholar]
- 23.Gruskin AB. The adolescent with essential hypertension. Am J Kidney Dis. 1985;6(2):86–90. doi: 10.1016/s0272-6386(85)80146-3. [DOI] [PubMed] [Google Scholar]
- 24.Saito I, Saruta T, Kondo K, et al. Serum uric acid and the renin-angiotensin system in hypertension. J Am Geriatr Soc. 1978;26(6):241–247. doi: 10.1111/j.1532-5415.1978.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 25.Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105(22):2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 26.Mercuro G, Vitale C, Cerquetani E, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004;94(7):932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 27.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106(2):221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 28.The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114(2 suppl 4th report):555–576. [PubMed] [Google Scholar]
- 29.Isaacman DJ, Reynolds EA. Effect of a research nurse on patient enrollment in a clinical study. Pediatr Emerg Care. 1996;12(5):340–342. doi: 10.1097/00006565-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Soergel M, Kirschstein M, Busch C, et al. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130(2):178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang GT, Chung CC, Holzman TF, Krafft GA. A continuous fluorescence assay of renin activity. Anal Biochem. 1993;210(2):351–359. doi: 10.1006/abio.1993.1207. [DOI] [PubMed] [Google Scholar]
- 32.Lasater M. The view within: the emerging technology of thoracic electrical bioimpedance. Crit Care Nurs Q. 1998;21(3):97–101. doi: 10.1097/00002727-199821030-00010. [DOI] [PubMed] [Google Scholar]
- 33.Ventura HO, Pranulis MF, Young C, Smart FW. Impedance cardiography: a bridge between research and clinical practice in the treatment of heart failure. Congest Heart Fail. 2000;6(2):94–102. doi: 10.1111/j.1527-5299.2000.80147.x. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg BH, Hermann DD, Pranulis MF, Lazio L, Cloutier D. Reproducibility of impedance cardiography hemodynamic measures in clinically stable heart failure patients. Congest Heart Fail. 2000;6(2):74–80. doi: 10.1111/j.1527-5299.2000.80140.x. [DOI] [PubMed] [Google Scholar]
- 35.Pranulis MF. Impedance cardiography (ICG) non-invasive hemodynamic monitoring provides an opportunity to deliver cost effective, quality care for patients with cardiovascular disorders. J Cardiovasc Manag. 2000;11(3):13–17. [PubMed] [Google Scholar]
- 36.Smith RD, Levy P, Ferrario CM. Value of noninvasive hemodynamics to achieve blood pressure control in hypertensive subjects. Hypertension. 2006;47(4):771–777. doi: 10.1161/01.HYP.0000209642.11448.e0. [DOI] [PubMed] [Google Scholar]
- 37.Pianosi PT. Measurement of exercise cardiac output by thoracic impedance in healthy children. Eur J Appl Physiol. 2004;92(4–5):425–430. doi: 10.1007/s00421-004-1167-5. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira AP, Oliveira CER, Franca NM. Metabolic syndrome and risk factors for cardiovascular disease in obese children: the relationship with insulin resistance (HOMA-IR) J Pediatr. 2007;83(1):21–26. doi: 10.2223/JPED.1562. [DOI] [PubMed] [Google Scholar]
- 39.Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008;10(2):130–139. doi: 10.1111/j.1751-7176.2008.07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crom WR, Webster SL, Bobo L, Teresi ME, Relling MV, Evans WE. Simultaneous administration of multiple model substrates to assess hepatic drug clearance. Clin Pharmacol Ther. 1987;41(6):645–650. doi: 10.1038/clpt.1987.90. [DOI] [PubMed] [Google Scholar]
- 41.Neaton JD, Grimm RH, Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study: final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270(6):713–724. [PubMed] [Google Scholar]
- 42.Simonetti GD, Rizzi M, Donadini R, Bianchetti MG. Effects of antihypertensive drugs on blood pressure and proteinuria in childhood. J Hypertens. 2007;25(12):2370–2376. doi: 10.1097/HJH.0b013e3282efeb7e. [DOI] [PubMed] [Google Scholar]
- 43.Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet. 2001;358(9290):1305–1315. doi: 10.1016/S0140-6736(01)06411-X. [DOI] [PubMed] [Google Scholar]
- 44.Toma I, Kang J, Meer E, Pet-Peterdi J. Uric acid triggers renin release via a macula densa-dependent pathway; Presented at: American Society of Nephrology Annual Meeting; November 2007; San Francisco, CA. p. F-P0240. [Google Scholar]
- 45.Watanabe S, Kang DH, Feng L, et al. Uric acid hominoid evolution and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 46.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113(3 pt 1):475–482. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 47.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114 (23):2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 48.Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41(6):1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 49.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]