Abstract
Background
Endoscopic cyclophotocoagulation (ECP) is a relatively new method of cyclodestruction which can be used in the management of refractory glaucomas.
Aim
To evaluate the safety and efficacy of ECP in the management of refractory glaucomas.
Settings and Design
Prospective interventional non-comparative study.
Materials and Methods
Fifty eyes of 50 patients with refractory glaucoma, whose intraocular pressures (IOP) were not under control with maximal medical therapy underwent ECP, by the anterior, or pars plana route. IOP, best corrected visual acuity (BCVA), and the number of anti-glaucoma medications, were compared postoperatively to preoperative values. Success was defined as IOP ≤ 22 mmhg, with or without use of medications.
Statistical analysis used
Student's t test and repeated measures ANOVA were used to evaluate change in IOP and Student's t test, for comparison of BCVA. Kaplan Meier survival curve was plotted. Wilcoxon signed rank test was used to evaluate reduction in medications.
Results
Patients were followed for an average of 12.27 months (3-21months). IOP decreased significantly from 32.58 ± 9.16 mmHg to 13.96 ± 7.71 mmHg at last follow-up (P<0.001, student's t test). BCVA was significantly improved in the postoperative period (P<0.001, student's t test). The average number of antiglaucoma medications decreased from 2.51 ± 0.97 to 1.09 ± 1.16 (P<0.001, Wilcoxon signed rank test). ECP had a success rate of 82.2%.
Conclusion
Endoscopic cyclophotocoagulation is an effective procedure in this subset of refractory glaucomas.
Keywords: Cyclophotocoagulation, endoscopic cyclophotocoagulation, refractory glaucoma
Cyclodestruction is one of the surgical modalities of management of glaucoma refractory to other means of surgical and medical treatment. Cyclodestruction has been practiced by either cyclocryotherapy, or by non-contact or contact trans-scleral cyclophotocoagulation by diode, or YAG lasers. Traditionally, this method of reduction of intraocular pressure (IOP) is used as a last resort, and is complicated by loss of best-corrected visual acuity (BCVA), and risk of phthisis bulbi.[1] In addition, the anatomical localization of the ciliary processes through the sclera can be faulty[2] by the trans-scleral methods.
Endoscopic cyclophotocoagulation (ECP) is a method of cyclodestruction which enables accurate anatomical localization, and photocoagulation of the ciliary processes.[3] It enables precise titration of laser delivery to the ciliary processes. The instrument combines a light source, endolaser, and videoendoscope in a 20-guage single probe. Reports of the efficacy of this procedure are encouraging and it is associated with less postoperative complications when compared to the traditional trans-scleral methods.[4–6] Comparison with glaucoma drainage implants has also been encouraging, with less long-term complications with ECP.[7]
To our knowledge, there are no studies of ECP on Indian eyes with refractory glaucoma. The aim of the study was to investigate the safety and efficacy of this procedure in a subset of patients with refractory glaucoma.
Materials and Methods
This was a prospective, interventional, non-comparative study. All patients who underwent ECP by the first three authors in the period between June 2005 to February 2007, were included in this study. Any patient with refractory glaucoma (neovascular glaucoma, post-traumatic, post-vitrectomy glaucomas, post penetrating keratoplasty glaucoma, congenital and primary glaucoma previously having undergone either trabeculectomy, or other intraocular surgery, where trabeculectomy has a high rate of failure due to extensive conjunctival scarring), whose IOP was not under control with maximal medical therapy was included in the study. Anterior or posterior uveitis, and uveitic secondary glaucomas were exclusion criteria.
Fifty eyes of 50 patients were included in the study. Patient demographics, preoperative diagnoses, previous surgeries, were noted. Patients were informed about the invasive nature of the procedure, the risks and benefits of the procedure, and informed consent was obtained. Institutional ethics committee approval was obtained for the study. Endoscopic cyclophotocoagulation was preformed using the Uram E2 endoscopic photocoagulation machine (Medtronic Inc). The procedure was performed either by the anterior (limbal), or pars plana route.
Anterior ECP: A paracentesis was fashioned using a side-opening knife enough to admit the 20-guage probe, and enable movement of the probe. A highly retentive viscoelastic (Sodium hyaluronate 2.3%, Healon 5, AMO Inc, Uppsala, Sweden) was placed under the iris to inflate the ciliary sulcus, and push the lens back, and create a space between the iris and lens back. The endoscope probe was introduced, and advanced until the ciliary processes were in adequate view. Diode laser 810 nm incorporated in the endoscope probe was used to photocoagulate the ciliary processes. The laser settings used were 25-30 mJ of power, continuous delivery mode. The end point was whitening and shrinkage of the ciliary processes. A second paracentesis was done wherever necessary, to ensure adequate coverage of the ciliary processes. At the end of the procedure, removal of the viscoelastic was ensured, and the paracentesis were sutured.
Pars plana ECP: A standard three-port vitrectomy was performed. One of the superior ports was temporarily closed by a scleral plug, and the endoscope was introduced in the other superior port. Ciliary photocoagulation was done as in the anterior route.
At least 270 degrees of cyclophotocoagulation was aimed for in all cases. In eyes which had highly elevated preoperative IOPs (>30 mmHg) and were on maximum anti-glaucoma medications including maximum doses (>750 mg/day) of systemic acetazolamide, 360 degrees of cyclophotocoagulation was aimed for. Also, in eyes with no visual potential to start with, where the procedure was being done for pain relief, 360 degrees of photocoagulation was aimed for. However, if visualization of the ciliary body processes was difficult due to causes like retained cortical material, or fibrous/ fibro vascular tissue over the ciliary body, lesser degrees of cyclophotocoagulation was done, and the extent treated was recorded.
Supplemental procedures like endolaser to the retina, and phacoemulsification with intraocular lens implantation were done where necessary along with the ECP procedure.
The patients were not randomized between anterior and pars plana routes of ECP. The criteria for performing pars plana ECP were: any eye with coexistent retinal pathology which would require pars plana vitrectomy, and/or endolaser, and post penetrating keratoplasty glaucomas, to minimize damage to the Comeal endothelium. The remaining eyes underwent anterior ECP. Phakic eyes underwent anterior ECP.
BCVA was recorded by Snellen charts, and was converted to a number on a continuous scale of 1 to 16, for ease of analysis, with one indicating no perception of light (PL) vision, and 16 indicating 20/20 vision. This was done as large number of eyes had less than 20/200 vision preoperatively
Preoperative and postoperative IOP was measured by Goldmann Applanation tonometry, and where the cornea was irregular, by Tono Pen (Reichert, NY, USA). Preoperative and postoperative BCVA, and number of anti-glaucoma medications were compared.
Topical anti-glaucoma medications were enumerated according to the generic drug used, and were numbered separately, even if combination drugs were used. Oral acetazolamide, when given, was also counted as a separate anti-glaucoma medication.
Complications during the procedure and postoperative complications were noted. Patients were followed up at postoperative day one, day three, two weeks, four weeks, six weeks, three months, six months, nine months, 12 months, 15 months, and three-monthly thereafter. All patients were put on topical steroid antibiotic drops and cycloplegics following the procedure, which were tapered and stopped by the seventh or eighth week. All preoperative anti-glaucoma medications were continued, except prostaglandin analogues, and pilocarpine. Based on the postoperative recording of IOP, the anti-glaucoma medications were reduced in a phased manner, wherever possible. Success was defined as IOP ≤ 22 mmHg with or without use of anti-glaucoma medications in the postoperative period. Hypotony was defined as IOP less than 6 mmHg.
Statistical methods
Statistical software SYSTAT and SPSS were used to analyze the results. Student's t-test, and multiple measures ANOVA were used to evaluate a change in IOP following the procedure. Kaplan Meier survival curve was plotted for success of IOP control over time. Wilcoxon signed rank test was used to evaluate reduction in anti-glaucoma medications, pre and post ECP. Relation of success to route (anterior or pars plana), was analyzed by the Fischer's exact test applied on a 2x2 table. Multivariate analysis has been used to study the relation between preoperative diagnosis, and extent of cyclophotocoagulation to success. Change in BCVA post operative compared to pre operative was analysed using the student's t-test.
Results
The results of the treatment of 45 eyes were subjected to analysis because five eyes were excluded due to inadequate follow-up (<three months). The mean age of the patients was 48.82 years ± 21.76 and there were 22 males and 23 females. Thirteen eyes underwent ECP through the limbal route, and 32 eyes underwent ECP through the pars plana route. The preoperative diagnoses were as in Table 1. Twenty-seven eyes had undergone one previous intraocular surgery, nine eyes had undergone two previous surgeries, four eyes had undergone three previous surgeries, and four eyes had undergone four previous surgeries. Only one eye with neovascular glaucoma (NVG) had undergone no previous intraocular surgery. Twenty-seven eyes were pseudophakic, nine were aphakic, and nine were phakic. Supplemental procedures were performed, as required, apart from the ECP procedure [Table 2].
Table 1.
Preoperative diagnoses
| Diagnosis | Number of eyes |
|---|---|
| Post penetrating keratoplasty glaucoma | 8 |
| Neovascular glaucoma | 16 |
| Chronic glaucoma | 7 |
| Congenital glaucoma | 5 |
| Postvitrectomy glaucoma | 6 |
| Posttraumatic glaucoma | 3 |
| Total | 45 |
Table 2.
Details of surgical procedures done in study eyes
| Procedures | Number | % |
|---|---|---|
| ECP | 12 | 26.7 |
| ECP + Pars Plana Vitrectomy | 12 | 26.7 |
| ECP+PPV+EL | 6 | 13.3 |
| ECP+SOR | 3 | 6.7 |
| ECP+PCIOL | 2 | 4.4 |
| ECP+ PPV + ACIOL Explantation | 2 | 4.4 |
| ECP + PPV + MP + PCIOL | 3 | 6.7 |
| ECP + Vitreous Lavage +ACIOL | ||
| Explantation+PCIOL+C3F8 gas | 3 | 6.7 |
| ECP+ PPL+PPV | 1 | 2.2 |
| ECP+ Pupilloplasty +PPV+EL | 1 | 2.2 |
Endoscopic cyclophotocoagulation (ECP), Pars PlanaVitrectomy (PPV), Retinal Endolaser (EL), Silicone oil removal (SOR), Posterior chamber intraocular lens (PCIOL), Anterior chamber Intraocular lens (ACIOL), Membrane peeling (MP), Pars plana lensectomy (PPL)
The extent of cyclophotocoagulation done was 360 degrees in 10 eyes, 270 degrees in 28 eyes, and 180 degrees in six eyes, and 90 degrees in one eye. One eye underwent repeat ECP because of an increase in IOP at seven months following ECP.
All patients were followed up for a minimum of at least three months, and maximum follow-up was up to 21 months, with an average follow-up of 12.27 months.
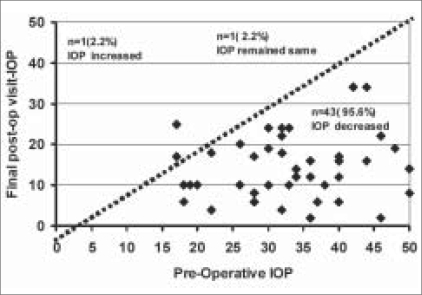
Mean preoperative IOP was 32.58 ± 9.16 mmHg. This showed a sustained decrease in the postoperative period at Day 1(P< 0.001) and at three months (P<0.001) and was maintained at 21 months of follow-up (P< 0.001). Mean IOP at final post operative visit was 13.96 ± 7.71 mmHg. Mean IOP was significantly reduced from preoperative to final postoperative visit with a very large effect of treatment of 2.03 (t=11.332, P<0.001). Fig. 1 shows a scatter plot depicting final postoperative versus preoperative IOP. Mean pattern of IOP during the study period is shown in Table 3. The change was found to be significant, P<0.001** (F=7.803), by repeated measures ANOVA. At last follow-up 82.2% (n=37) of the eyes had success outcome with 95% CI (68.67-90.71%). Eight eyes (17.8%), had IOP > 22 mmHg, with supplemental medications and were considered as failures.
Figure 1.
Scatter plot depicting pre-op vs. final postoperative intraocular pressure
Table 3.
Mean pattern of IOP during the study period
| Study period | IOP mm Hg | |||
|---|---|---|---|---|
| Number of eyes | Range | Mean ± SD | ||
| Preoperative | 45 | 17-50 | 32.58 ± 9.16 | |
| Post-op Day1 | 45 | 4-48 | 20.69 ± 10.29 | |
| Post-op Day3 | 45 | 4-37 | 16.27 ± 7.94 | |
| Post-op Week1 | 45 | 0-34 | 14.36 ± 8.66 | |
| Post-op Week2 | 45 | 0-36 | 14.42 ± 9.29 | |
| Post-op Week4 | 45 | 0-42 | 15.49 ± 9.36 | |
| Post-op Week6 | 45 | 0-42 | 15.98 ± 9.37 | |
| Post-op Month3 | 45 | 0-42 | 15.60 ± 8.59 | |
| Post-op Month6 | 40 | 2-40 | 15.13 ± 7.98 | |
| Post-op Month9 | 31 | 2-34 | 14.45 ± 8.12 | |
| Post-op Month12 | 27 | 2-34 | 14.85 ± 8.37 | |
| Post-op Month15 | 21 | 2-34 | 13.29 ± 8.94 | |
| Post-op Month18 | 14 | 2-34 | 12.00 ± 6.87 | |
| Post-op Month21 | 5 | 6-22 | 12.80 ± 7.71 | |
Significance F=7.803, P<0.001**, Repeated Measures ANOVA is used to find the significance
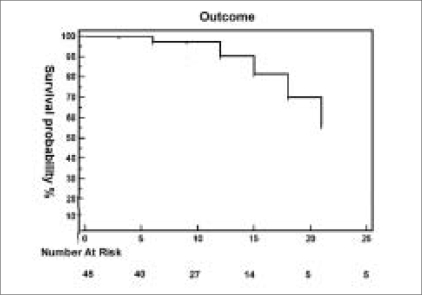
Kaplan Meier survival analysis was done for success of IOP control over time, which showed a cumulative success of 81.7% at 15 months (standard error 7.58%), 70% at 18 months (std error 10.0%), and 56.0% at 21 months (std error 14.9%) follow-up [Fig. 2].
Figure 2.
Kaplan Meier Survival curve
Table 4 gives details of eyes with hypotony. Of the four eyes with hypotony, only one eye had a decrease in the postoperative BCVA from hand movement close to face (HMCF) to PL +; all other eyes maintained or had improved acuities after ECP and supplemental procedures. All the eyes had a preoperative diagnosis of neovascular glaucoma.
Table 4.
Details of eyes with hypotony at last follow-up
| Diagnosis | Pre-op | IOP IOP 6 week | IOP last follow-up (duration of follow-up) | Pre-ECP BCVA | Post- ECP BCVA | Degrees of ECP done |
|---|---|---|---|---|---|---|
| NVG | 36 | 0 | 2 (18 months) | PL+ PR acc | CFCF | 360 |
| NVG | 22 | 4 | 4(18 months) | CFCF | CFCF | 270 |
| NVG | 32 | 4 | 4(18 months) | 20/63 | 20/32 | 270 |
| NVG | 46 | 0 | 2(6 months) | HM CF | PL+ | 270 |
IOP- Intraocular pressure, ECP- Endoscopic cyclophotocoagulation, BCVA- Best corrected visual acuity, NVG- Neovascular glaucoma. PL- Perception of light, PR-projection of light, Acc- Accurate, CFCF- Counting Fingers close to face, HMCF- hand movements close to face
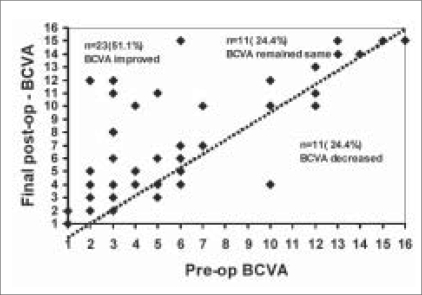
Table 5 shows the distribution of BCVA preoperative versus postoperative. There is a significant improvement in the BCVA at last postoperative follow-up compared to the preoperative BCVA (P =0.005) [Fig. 3]. Twenty-three patients (51.1%) had an improvement in BCVA, while 11 patients (24.4%) maintained their preoperative BCVA.
Table 5.
Best corrected visual acuity distribution in study eyes
| BCVA (N=45) | Pre-op | Post -op | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| 1. PL negative | 3 | 6.7 | 2 | 4.4 |
| 2. PL+ | 6 | 13.3 | 5 | 11.1 |
| 3. HM | 10 | 22.2 | 4 | 8.9 |
| 4. CFCF | 4 | 8.9 | 7 | 15.6 |
| 5. 20/1000 | 4 | 8.9 | 3 | 6.7 |
| 6. 20/600 | 5 | 11.1 | 3 | 6.7 |
| 7. 20/400 | 2 | 4.4 | 2 | 4.4 |
| 8. 20/350 | - | - | 1 | 2.2 |
| 9. 20/300 | - | - | - | - |
| 10. 20/200 | 3 | 6.7 | 4 | 8.9 |
| 11. 20/125 | - | - | 4 | 8.9 |
| 12. 20/80 | 3 | 6.7 | 3 | 6.7 |
| 13. 20/63 | 2 | 4.4 | 1 | 2.2 |
| 14. 20/40 | 1 | 2.2 | 2 | 4.4 |
| 15. 20/32 | 1 | 2.2 | 4 | 8.9 |
| 16. 20/20 | 1 | 2.2 | - | - |
| Mean ± SD | 5.82±4.21 | 7.29±4.53 | ||
| Min-Max) | (1-16) | (1-15) | ||
Significance Student t=2.938, P=0.005**, PL- Perception of light, HM- Hand movement close to face, CFCF- Counting fingers close to face
Figure 3.
Scatter plot of preoperative vs. postoperative best corrected visual acuity
In 11 patients (24.4%), a decrease in BCVA was observed [Table 6]. In seven of the 11 eyes, the decrease could probably be related to the procedure. Of these, four eyes had <20/200 BCVA to start with and were eyes with very advanced glaucoma with complex retinal problems due to diabetic retinopathy as well. In two eyes, the cause for decrease in BCVA was indeterminate, as the preoperative diagnosis (proliferative diabetic retinopathy with clinically significant macular edema in one eye, epithelial ingrowth in one eye) influenced the subsequent outcome in terms of visual acuity.
Table 6.
Details of patients with decreased Best Corrected Visual Acuity post Endoscopic cyclophotocoagulation
| Diagnosis | Pre-op BCVA | Final Post-op BCVA | IOP pre op | IOP final post op | Cause for BCVA reduction | Related to ECP/unrelated cause |
|---|---|---|---|---|---|---|
| Post PK | 20/600 | CFCF | 37 | 6 | Graft rejection at 12 months post ECP | Unrelated cause |
| Post PK | 20/600 | 20/1000 | 50 | 8 | Comeal ulcer at 13 months post ECP | Unrelated cause |
| Congenital Glaucoma | 20/1000 | CFCF | 28 | 8 | Comeal decompensation | Probably related to procedure |
| ACG | 20/20 | 20/32 | 32 | 6 | Advanced Glaucoma | Probably related to procedure |
| Post traumatic Glaucoma | HM CF | PL+ | 32 | 18 | Uncontrolled IOP | Probably related to procedure |
| NVG | HMCF | PL+ | 28 | 6 | Cataract progression | Probably related to procedure |
| Post Vit Glaucoma | 20/1000 | HMCF | 48 | 19 | Coexistent Diabetic retinopathy | Indeterminate |
| Post Vit Glaucoma | 20/80 | 20/125 | 18 | 6 | Coexistent retinal pathology | Probably related |
| Post PK | 20/200 | CFCF | 30 | 19 | Comeal edema, Graft failure | Probably related |
| NVG | HMCF | PL+ | 46 | 2 | Coexistent PDR | Probably related |
| Post PK, epithelial ingrowth with sec glaucoma | 20/80 | 20/200 | 42 | 34 | Comeal epithelial ingrowth progression | Indeterminate |
IOP-Intraocular pressure, PK- Penetrating Keratoplasty, ACG- Angle closure Glaucoma, NVG-Neovascular Glaucoma, HMCF- Hand movements close to face, PL+- Perception of light, CFCF- Counting fingers close to face, PDR-Proliferative diabetic retinopathy, Vit- Vitrectomy
There was a significant decrease in the number of anti-glaucoma medications required to control IOP in the postoperative period compared to the preoperative period (P<0.001), as depicted in Table 7. No statistically significant association was noted for preoperative diagnosis, and extent of ECP or route of ECP with success or failure of the procedure. Complications noted in the postoperative period were fibrin three patients, hyphema- two patients, cystoid macular edema one patient. All these resolved without any final effect on either IOP or BCVA.
Table 7.
Anti-glaucoma medications required postoperatively compared to preoperatively
| Anti-glaucoma medications required | Pre-op (n=45) | Final Post-op (n=45) | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| 0 | 2 | 4.4 | 19 | 42.2 |
| 1 | 3 | 6.7 | 11 | 24.4 |
| 2 | 15 | 33.3 | 8 | 17.8 |
| 3 | 21 | 46.7 | 6 | 13.3 |
| 4 | 3 | 6.7 | 1 | 2.2 |
| 5 | 1 | 2.2 | - | - |
| Mean ± SD | 2.51 ± 0.97 | 1.09 ± 1.16 | ||
Inference Number of medications are significantly reduced postoperatively with (Wilcoxon Signed Rank test) Z=4.453 P<0.001**.
Discussion
The purpose of this study was to evaluate the effect of ECP on the three outcome variables, IOP, anti-glaucoma medications and BCVA, in the subset of patients with refractory glaucoma. IOP was significantly reduced (P<0.001), and 82.2% of the eyes were in the success category. Mean number of antiglaucoma medications needed postoperatively compared to preoperatively was also significantly reduced (P<0.001). The study eyes comprised refractory glaucomas with poor vision preoperatively and also had diagnoses traditionally associated with poor prognoses for postoperative vision (neovascular glaucomas, post vitrectomy glaucoma, post penetrating keratoplasty glaucoma).
In 75.5% of the study eyes, BCVA either improved or remained the same, with 24.5% showing reduction in BCVA compared to preoperative values. When the 11 eyes showing decrease in BCVA were analyzed, only in seven of the 11 eyes (15.5%) could the procedure have any relation to decrease in BCVA. In our study, we have divided patients with BCVA < 20/200, into multiple categories, and used this to analyze the BCVA change post procedure. For example, a patient having drop of vision from HM to PL+, is recorded as having a drop in BCVA. In contrast most studies of BCVA in cyclophotocoagulation, group all these homogenously into the less than 20/200 category.[5,11] Only a drop of vision of greater than two Snellen lines is considered as decrease in BCVA in some other studies.[5] Therefore, in our series, the reduction in BCVA may be slightly overestimated, however, it is a more accurate and true estimate.
Review of published literature shows higher rates of BCVA loss in trans-scleral methods of cyclophotocoagulation. Bloom et al.,[8] reported that 28% of their patients experienced vision decrease after trans-scleral photocoagulation (TSCPC), which was more common in neovascular and silicone oil-induced glaucoma. In the diode laser ciliary ablation study group,[9] in a prospective study of 27 eyes of 27 patients, 30% of patients lost vision, and 3.7% had hypotony.
Brancato et al.,[10] in a prospective series of TSCPC in 68 Caucasian patients reported 53% vision loss, and two of 68 eyes experienced phthisis. Goldberg et al.,[11] in a comparative series between TSCPC and cyclocryotherapy reported deterioration in vision in 31.5% of the cylcocryo group, and deterioration of vision in 37.5% of the TSCPC group were noted. Severe visual loss to no light perception in six eyes (cryo) and two eyes (TSCPC), and phthisis in two eyes - cryo, one eye- TSCPC was also noted.
In contrast, similar to the findings in our series, studies of ECP show lesser rates of BCVA decrease. Chen et al.,[4] in a study of ECP in refractory glaucomas, reported a success rate of 90% at 12.9 months, with respect to IOP control. They had a reduction in anti-glaucoma medications from a preoperative mean of 3.0 ± 1.3, to 2.0 ± 1.3 postoperatively. BCVA was stable or improved in 94%, with 6% losing two or more lines of Snellen acuity. No cases of hypotony or phthisis were observed.
In most studies involving ECP, including ours, the extent of IOP reduction is similar or slightly superior to TSCPC. However, the maintenance, or improvement of BCVA, is significantly better in the ECP studies compared to other methods of cyclodestruction.[4–11] In addition, the rates of reported hypotony, or phthisis are lower in the ECP studies.[4–6]
One of the differences in our series when compared to other studies of ECP is that in majority of the eyes (71.1%), pars plana route has been used to perform ECP. This is due to the fact that in majority of the eyes in our series the preoperative diagnoses involved coexistent retinal pathologies which could be addressed by the pars plana route. In addition, in eyes status post keratoplasty, the pars plana route was preferred to minimize Comeal endothelial damage.
One of the limitations of this study is that it is a non-comparative study. A randomized comparison between TSCPC and ECP, as well as ECP and drainage implants could clarify these differences in outcome better. As endoscopy offers excellent anatomic localization of cyclophotocoagulation and excellent titration in terms of endpoint of photocoagulation, this stands out as a more attractive option compared to the other blind techniques. However, the only drawback of this procedure is its invasive nature when compared to the other methods. As illustrated in our case series, a significant number of patients who require cyclophotocoagulation can also benefit from supplemental procedures like pars plana vitrectomy, and membranectomy, cataract extraction with intraocular lens implantation, and in these cases, combining the procedures with the ECP procedure does not carry any extra risk. Also to date, there are no reported incidences of endophthalmitis following the ECP procedure.
In conclusion, ECP is an efficacious procedure in the management of refractory glaucoma. It may offer better preservation of BCVA compared to the other trans-scleral methods of cyclodestruction, as reported in the literature.
Acknowledgments
Mr. Suresh KP, statistical assistance. The authors have no financial interest in any of the products used in the study, the manuscript has been read and approved by all authors and they all meet the authorship criteria as specified.
Footnotes
Nil
None declared.
References
- 1.Pastor SA, Singh K, Lee DA, Juzych MS, Lin SC, Netland PA, et al. Cyclophotocoagulation: A report by the American Academy of Ophthalmology. Ophthalmology. 2001;108:2130–8. doi: 10.1016/s0161-6420(01)00889-2. [DOI] [PubMed] [Google Scholar]
- 2.Walland MJ, McKelvie PA. Diode laser cyclophotocoagulation: Histopathology of two cases of clinical failure. Ophthalmic Surg Lasers. 1998;29:852–6. [PubMed] [Google Scholar]
- 3.Uram M. Endoscopic cyclophotocoagulation in glaucoma management. Curr Opin Ophthalmol. 1995;6:19–29. doi: 10.1097/00055735-199504000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of refractory Glaucomas. Am J Ophthalmol. 1997;124:787–96. doi: 10.1016/s0002-9394(14)71696-4. [DOI] [PubMed] [Google Scholar]
- 5.Lin S. Endoscopic cyclophotocoagulation. Br J Ophthalmol. 2002;86:1434–8. doi: 10.1136/bjo.86.12.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS. 2001;5:221–9. doi: 10.1067/mpa.2001.116868. [DOI] [PubMed] [Google Scholar]
- 7.Lima FE, Magacho L, Carvalho DM, Susanna R, Jr, Avila MP. A prospective comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory Glaucoma. J Glaucoma. 2004;13:233–7. doi: 10.1097/00061198-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NS, Hitchings RA, et al. Cyclodiode Trans scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology. 1997;104:1508–19. doi: 10.1016/s0161-6420(97)30109-2. [DOI] [PubMed] [Google Scholar]
- 9.Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long term outcome of initial ciliary ablation with contact trans scleral diode laser cyclophotocoagulation: The Diode laser ciliary ablation study group. Ophthalmology. 1996;103:1294–302. doi: 10.1016/s0161-6420(96)30508-3. [DOI] [PubMed] [Google Scholar]
- 10.Brancato R, Carassa RG, Bettin P, Fiori M, Trabucchi G. Contact Trans scleral cyclophotocoagulation in refractory glaucoma. Eur J Ophthalmol. 1995;5:32–9. doi: 10.1177/112067219500500106. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg-Cohen N, Bahar I, Ostashinski M, Lusky M, Weinberger D, Gaton DD. Cyclocryotherapy versus Trans scleral Diode laser Cyclophotocoagulation for uncontrolled Intraocular pressure. Ophthalmic Surg Lasers Imaging. 2005;36:272–9. [PubMed] [Google Scholar]