Abstract
Aims
To evaluate the outcomes and complications of 23-gauge transconjunctival sutureless vitrectomy (TSV) with Silicone oil (SO) tamponade in complex vitreoretinal diseases.
Settings and Design
Ege university hospital ophthalmology department. Retrospective case series.
Materials and Methods
Forty eyes of 40 patients with diabetic tractional retinal detachment (DTRD) and proliferative vitreoretinopathy (PVR) were included in the study. Vitrectomy using 23-gauge system with SO endotamponade was performed. Peroperative and postoperative complications, anatomical and visual results were evaluated.
Statistical analysis used
Paired Student's t-test.
Results
Simultaneous cataract surgery was performed in 17 eyes. Peroperative complications were posterior capsule rupture during phacoemulsification in one patient, vitreous and retinal incarceration in one patient. One eye required suture placement at the end of surgery due to SO leakage. Postoperatively, a small subconjunctival SO bubble in three patients, and hypotony in one patient (6 mmHg) were observed. Recurrent retinal detachment under SO occurred in one patient. Mean follow-up was 6.5 months (±2.7). Pre- and postoperative mean visual acuity was 2.22±0.91 logMAR and 1.11±0.8 logMAR, respectively (P<0.001). Mean intraocular pressure (IOP) on the first postoperative day was lower than preoperative IOP (11.3 ±3.2 versus 14.0 ±2.4 mmHg) (P<0.001).
Conclusions
Twenty-three gauge instrumentation seems to be feasible, effective and safe for vitrectomy with SO injection in DTRD and PVR, and can be considered in the surgical management of these complex vitreoretinal diseases.
Keywords: Silicone oil, transconjunctival sutureless vitrectomy
One of the innovative vitreoretinal surgery techniques introduced in recent years is the 25-gauge transconjunctival sutureless vitrectomy (TSV) developed by Fujii et al.[1,2] Compared to standard 20-gauge vitrectomy, the smaller 25-gauge system and accompanying instrumentation allow for self-sealing transconjunctival pars plana sclerotomies. This leads to less postoperative inflammation, and faster postoperative recovery.
Initially, 25-gauge vitrectomy was utilized in limited cases such as core vitrectomy, epiretinal membrane peeling and macular hole surgery.[3,4] The development of second generation 25-gauge instruments and brighter xenon light sources enabled more peripheral vitreous dissection and facilitated the usage of 25-gauge pars plana vitrectomy (PPV) in more complex surgical cases, such as retinal detachment, proliferative vitreoretinopathy (PVR), diabetic tractional retinal detachment (DTRD), and pathologic myopia with vitreomacular traction.[5,6] However, inferior fluidics, and flexible instruments are inherent to the 25-gauge system. Besides, infusion of silicone oil (SO) in the 25-gauge vitrectomy setting is more time-consuming due to its reduced microcannula lumen when compared to the 20-gauge system. Slightly larger instruments may be more feasible to solve some of these problems.
Recently, Eckardt improved the transconjunctival sutureless technique introducing the 23-gauge TSV system.[7] Twenty-three-gauge system seems to have similar design and utility as that of the standard 20-gauge instruments. Additionally, it also has the advantages of 25-gauge TSV, such as decreased postoperative inflammation, less patient discomfort, and shorter recovery time.[7]
In this study, the feasibility of our technique for SO infusion with 23-gauge instrumentation in complex vitreoretinal cases was evaluated. The indications for surgery, clinical courses, and complications were analyzed.
Materials and Methods
In this study, 23-gauge TSV with SO injection was performed in 40 eyes of 40 consecutive patients by the same surgeon (TE) from April 2006 to February 2007. All patients provided informed consent before surgery. The data collected included age, sex, ophthalmic history, pre- and postoperative Snellen visual acuities, pre- and postoperative intraocular pressures (IOP), results of anterior segment and fundus examinations, and intra- and postoperative complications. Surgical indications were DTRD (n=21), and PVR Grade C1-3 (n=19) (classification of Machemer).[8] No patient was excluded from the study.
Local anesthesia with systemic monitorization was achieved with peribulbar anesthetic injection in all patients. Simultaneous clear corneal cataract surgery using standard phacoemulsification was performed by the same surgeon on all patients in whom a visually significant cataract precluded adequate visualization for the operative procedure and postoperative care. In these combined cases, insertion of microcannulae for the instruments and infusion line was performed before the creation of clear corneal wound for cataract surgery.
The surgical approach consisted of pushing the conjunctiva 1 mm to 2 mm laterally in the infero-temporal, superotemporal, and superonasal quadrants using a special pressure plate to hold it firmly to the sclera. A 23-gauge stiletto blade (45° angle; DORC, Zuidland, The Netherlands) was then inserted 3.5 mm from the limbus at a 20-30° angle through the conjuntiva, sclera, and pars plana. The scleral incisions were made radial to the corneoscleral limbus to obtain scleral tunnels parallel to the corneoscleral limbus. The microcannula was then inserted through the conjunctival incision and into the scleral tunnel using a specially designed blunt inserter. The microcannula has a length of 4 mm, an internal diameter of 0.65 mm, and an external diameter of 0.75 mm. The infusion catheter was connected to the inferotemporal cannula.
The 23-gauge instruments used were pneumatic vitreous cutter, endoillumination probe, straight forceps, scissors, back-flush needle, endodiatermy probe and endolaser probe. The twinlight chandelier illumination system (DORC, Zuidland, The Netherlands) was used in all cases for vitreous base removal with scleral self-indentation.
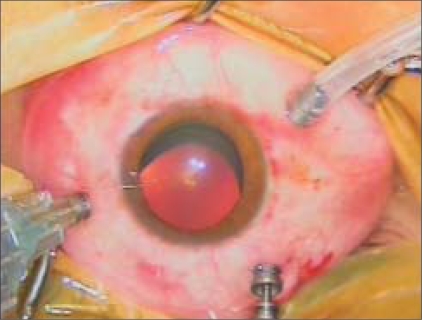
The surgical procedure varied depending upon the diagnosis. Core vitrectomy and peripheral vitreous dissection was performed in all cases. Perfluorocarbon liquid was utilized to stabilize the retina in PVR cases and in some severe DTRD cases. After complete fluid-air exchange, 1000 centistoke SO was injected through the superotemporal cannula using a 23-gauge polyamide cannula (DORC, Zuidland, The Netherlands) and a viscous fluid injector with an injection pressure of 5 Barr until the desired fill was achieved [Fig. 1]. In aphakic patients, an inferior iridotomy was performed to prevent SO migration to the anterior chamber. At the end of surgery, endoillumination with scleral indentation was performed in all cases to identify any other peripheral retinal pathology. The plugs were inserted to the superior cannulae, the infusion line was clamped, and the superotemporal and superonasal cannulae were removed with repositioning and inspection of the conjunctiva to cover the sclerotomy. Finally, the inferotemporal cannula with infusion line was removed followed by repositioning and inspection of the conjunctiva. The eye was then patched and shielded.
Figure 1.
Intraoperative photograph of the silicone injection with 23-gauge vitrectomy system
For data analysis, Snellen visual acuity was converted into a logarithm of the minimal angle of resolution (logMAR) score. Hypotony was defined as IOP equal to or less than 6 mmHg. A paired Student's t-test was used to make statistical comparisons between preoperative and postoperative visual acuity (logMAR) and IOP. A value of P<0.05 was taken to indicate statistical significance.
Results
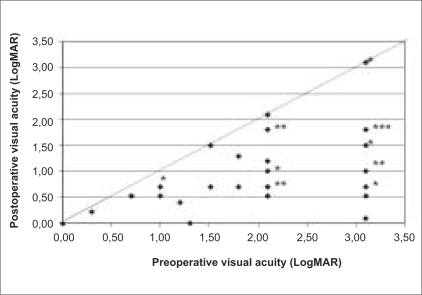
Prior to surgery, 24 patients were phakic, 11 pseudophakic, and five aphakic. Simultaneous phacoemulsification followed by intraocular lens implantation was performed in 17 of 24 eyes (71%). Seventeen women and 23 men, with a mean age of 58.9 (±11.8) years, were observed for a mean of 6.5 months (±2.7), ranging from 4 to 12 months. Mean overall best corrected visual acuity improved from logMAR 2.22 ±0.9 preoperatively to logMAR 1.11±0.8 at final visit (P<0.001) [Fig. 2]. Postoperative visual acuity improved in 33 patients (83%), remained unchanged in seven patients (17%). The mean overall preoperative IOP was 14.0±2.4 mmHg, ranging from 8-18 mmHg. The mean overall postoperative IOP on Day 1, Week 1 and at final visit was 11.4±3.2 (range, 6 to 19), 14.5±4.1 (range, 8 to 30), 16.2±4.5 (range, 8 to 30) mmHg, respectively. The decrease in IOP was statistically significant on postoperative Day 1 (P<0.001), but the difference was not significant in postoperative Week 1 (P=0.407). The increase in IOP was statistically significant at final visit (P=0.003).
Figure 2.
Preoperative and postoperative visual acuities in 40 patients undergoing 23-gauge pars plana vitrectomy and silicone oil tamponade. *2 patients. **3 patients. ***6 patients
In all patients (40/40), surgery was successfully completed using the 23-gauge system alone. All surgical manipulations including rotating the eye and vitreous base vitrectomy could be performed. The cannulae remained stable in place in all surgeries. No slippage or loss of the cannulae and damage to the crystalline lens or retina was observed. Withdrawal of cannulae resulted in minor subconjunctival bleeding which was arising from the episcleral vessels in most of the cases. This hemorrhage was stopped by a short tamponade with gentle pressure without any extra intervention. Suture placement to the sclerotomy sites was required in a 24-year-old patient (3%) with PVR associated to congenital glaucoma due to excess leakage of SO.
Posterior capsular rupture during phacoemulsification occurred in one patient (6%), however, implantation of a foldable intraocular lens through the 3.0 mm clear corneal incision was successfully conducted in all eyes. Perfluorocarbon liquid was utilized in all patients with PVR, and in three patients with DTRD. No significant complication was observed during perfluorocarbon liquid injection. Vitreous and retinal incarceration to the sclerotomy site which was freed without retinotomy occurred in one patient (3%).
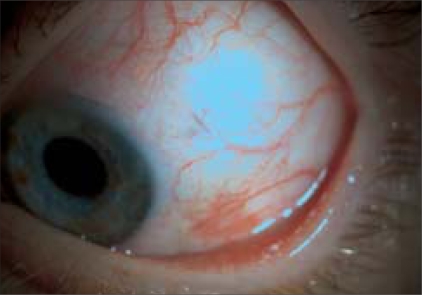
On the first postoperative day, minimal trauma was seen in the sclerotomy sites in most of the patients [Fig. 3]. Hypotony with an IOP of 6 mmHg was seen in one patient with DTRD (3%). IOP rose to 8 mmHg at Week 1 and remained stable at this level in the follow-up visits without suture requirement. No patient developed choroidal effusion. Postoperatively, a small subconjunctival SO bubble which was well tolerated without complication was observed in three patients (8%). Elevated IOP (≥21 mmHg) which was controlled by topical antiglaucoma medication (dorzolamid hydrochloride + timolol maleate twice daily) in SO-filled eyes occurred in five patients (13%).
Figure 3.
Postoperative first day photograph of one patient. Minimal trauma was seen in the sclerotomy sites
Inferior retinal redetachment developed in one patient with PVR (3%) under SO in the first postoperative month. The retina was successfully reattached with subsequent surgery using 20-gauge vitrectomy with 5000-centistoke SO endotamponade. Intraoperative and postoperative complications are shown in Table 1.
Table 1.
Intraoperative and postoperative complications of 23-gauge vitrectomy
| Complications | Number of eyes | % |
|---|---|---|
| Intraoperative | ||
| Scleral sulture | 1/40 | 3 |
| Posterior capsular rupture | 1/17 | 6 |
| Retinal incarceration | 1/40 | 3 |
| Postoperative | ||
| Hypotony(≤6 mmHg) | 1/40 | 3 |
| Elevated IOP (≥21 mmHg) | 5/40 | 13 |
| Subconjunctival SO | 3/40 | 8 |
| Redetachment (under SO) | 1/40 | 3 |
IOP: Intraocluar pressure, SO: Silicone oil
Discussion
The utility of SO endotamponade in selected patients with complex vitreoretinal pathologies such as retinal detachment associated with PVR, giant retinal tears, DTRD, or ocular trauma is well established.[9–11 Placement of SO tamponade in the setting of 25-gauge pars plana vitrectomy without the need to create a 20-gauge sclerotomy was reported by Riemann et al.[6] Recently, the 1000 centistokes SO endotamponade use in 23-gauge TSV was described for retinal detachments of different etiologies by Oliveira et al.[12] In this series, 23-gauge TSV associated with SO endotamponade was applied to 40 patients. Preoperative diagnosis was DTRD or PVR in all cases.
SO of 1000 centistoke was injected without any difficulty through the superotemporal cannula after the air-fluid, or air-perfluorocarbon exchange, using a 23-gauge polyamide cannula and a viscous fluid injector with an injection pressure of 5 Barr. No complication was encountered during injection.
Due to its smaller port size and inner diameter, the 25-gauge vitrector has a lower aspiration rate compared with the 20-gauge cutter; the infusion rate in the 25-gauge system is similarly decreased.[1] Hypotony during aspiration is therefore rarely encountered, but maximum cut settings and increased aspiration power must be employed to achieve reasonable cutting and aspiration rates. This will minimize occlusion of the vitreous cutter with large or dense fragments of fibrous or other intraocular tissue. Optimal cases for 25-gauge system are therefore those that do not require extensive membrane dissection or manipulation of dense intraocular tissues. In 23-gauge surgery both the cutter and the infusion cannula have improved designs affording higher flow rates which bring an advantage compared with 25-gauge vitrectomy. In this series, removal of dense intraocular hemorrhage and membraneous tissues was performed successfully, and the surgery was completed with the 23-gauge vitrectomy system in all eyes.
Stiff 23-gauge rather than flexible 25-gauge instruments behave like traditional 20-gauge instruments in the eye offering improved surgical capability. Compared with the increased flexibility of 25-gauge instruments which do not allow as much torsion of the eye itself, firmer and larger 23-gauge instruments seem to support easier use by the vitreoretinal surgeon to perform all the steps of traditional 20-gauge surgery. This advantage may also help the surgeon to convert from 20-gauge vitrectomy to TSV. In this series, surgical indications were DTRD (n=21), and PVR Grade C1-3 (n=19) which required a complete peripheral vitrectomy. Using the instruments to affect rotation of the eye to expose the periphery for visualization and instrument access, and to trim peripheral vitreous was comparable to 20-gauge system in all cases. No intra- or postoperative retinal break was seen in any eye.
Inserting the microcannulae at an oblique angle to the scleral surface prevents hypotony in 23-gauge as well as in 25-gauge TSV. Rates of hypotony in early reports on 25-gauge TSV in which the initial incision was perpendicular to the sclera ranged from 3.8-30%,[2,3,13–15] however, this complication dramatically reduced by bevelling the trochar entry.[16] The incision used for 23-gauge surgery is made at an angle to the wall of the eye, like the angled incisions used in cataract surgery. This angled incision does not present a direct perpendicular opening from the vitreous to outside of the eye. Instead a tunnel incision is created that has the potential to self-seal because the IOP helps to close the tunnel. Fine et al. reported a postoperative hypotony in two of 77 eyes in their series of 23-gauge vitrectomy.[17] In our series, suture placement to the sclerotomy sites at the end of the surgery was required in one 24-year-old patient with PVR associated to congenital glaucoma (3%). The scleral tunnel may have been relatively short and insufficient to self-seal the wound in this patient with thin sclera. On the first postoperative day, hypotony with an IOP of 6 mmHg was seen in one patient with DTRD (3%). IOP rose to 8 mmHg at Week 1 and remained stable at this level in the follow-up visits without suture requirement. In the study eyes, the decrease in IOP was statistically significant on postoperative Day 1 (P<0.001), but the difference was not significant in postoperative Week 1 (P=0.407). No choroidal effusion or choroidal hemorrhage was seen in any eyes.
In our series, a small subconjunctival SO bubble which was well tolerated during the follow-up period was observed in three patients (8%) on the first postoperative day. Recently, a SO leakage rate of 9.7% to necessitate its removal was reported in a series of 23-gauge TSV.[18] SO leakage into the subconjunctival space may be associated with some problems such as reduced SO endotamponade effect, postoperative discomfort, and interference with subsequent glaucoma filtering surgery. Some aspects have to be considered to avoid this complication. Having a tight self-sealing incision at the end of the surgery is mandatory. For this purpose, an incision of about 20°-30° through the eye wall has to be created. During surgical maneuvers, minimizing the stretching of the sclera may help to have a tighter incision at the end of the surgery. Trying to torque the eye using the instruments as we used to do in 20-gauge surgery will enhance the scleral stretching. Rotating the shaft of the instruments around the microcannulae (pivotal point) during the maneuvers will minimize scleral stretching. Another aspect which is very important to avoid SO leakage is having a normal IOP before removing the microcannulae. In our series, in three patients in whom the SO migrated to the subconjunctival space, we speculated that a slightly higher IOP while the microcannulae were being removed played a role in this leakage.
Another important complication in TSV is endophthalmitis. One study reported the rate of endophthalmitis following 5498 cases of 20-gauge vitrectomy as 0.018%, and 0.23% following 3103 cases of 25-gauge vitrectomy.[19] In this study population, 25-gauge vitrectomy had a statistically significant 12-fold higher incidence of endophthalmitis compared to 20-gauge vitrectomy. In 25-gauge vitrectomy, open sclerotomies and wound leaks may allow for increased influx of bacteria, and decreased infusion during vitrectomy itself may decrease the amount of fluid that dilutes or flushes out organisms within the eye. Oblique cannula insertion to facilitate scleral wound closure, and suturing any wound leaks seen following cannula removal may decrease the endophthalmitis risk. Although no endophthalmitis was seen during the follow-up period in our series, a small number of patients is not enough to have an idea about the endophthalmitis incidence of 23-gauge vitrectomy system.
In this study in which the feasibility and reproducibility of the technique of 23-gauge TSV with SO injection in complex cases was evaluated, small sample size, and lack of comparison with a control group are some of the limitations. Furthermore, only 1000-centistoke SO was used as an internal tamponade in our series. The usage of SO with higher viscosity was not evaluated in the setting of 23-gauge PPV. In one patient in whom inferior retinal redetachment was seen under SO, 5000-centistoke SO endotamponade was used in 20-gauge vitrectomy setting.
The 23-gauge TSV system has some advantages such as increased instrument flexibility and higher fluidics than the 25-gauge system to perform the steps of a standard 20-gauge vitrectomy. In the setting of 23-gauge TSV, the technique described in this study allows for the feasible, efficient and safe placement of SO tamponade.
In conclusion, SO injection in 23-gauge TS may have a role in cases of complex vitreoretinal diseases such as DTRD, and PVR
Footnotes
Nil
None.
References
- 1.Fujii GY, De Juan E, Jr, Humayun MS, Pieramici DJ, Chang TS, Awh C, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109:1807–12. doi: 10.1016/s0161-6420(02)01179-x. discussion 1813. [DOI] [PubMed] [Google Scholar]
- 2.Fujii GY, De Juan E, Jr, Humayun MS, Chang TS, Pieramici DJ, Barnes A, et al. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology. 2002;109:1814–20. doi: 10.1016/s0161-6420(02)01119-3. [DOI] [PubMed] [Google Scholar]
- 3.Lakhanpal RR, Humayun MS, deJuan E, Lim JI, Chong LP, Chang TS, et al. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology. 2005;112:817–24. doi: 10.1016/j.ophtha.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Ibarra MS, Hermel M, Prenner JL, Hassan TS. Long-term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am J Ophthalmol. 2005;139:831–6. doi: 10.1016/j.ajo.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Lesnoni G, Rossi T, Gelso A. 25-Gauge vitrectomy instrumentation: A different approach. Semin Ophthalmol. 2004;19:49–54. doi: 10.1080/08820530490520077. [DOI] [PubMed] [Google Scholar]
- 6.Riemann CD, Miller DM, Foster RE, Petersen MR. Outcomes of transconjunctival sutureless 25-gauge vitrectomy with silicone oil infusion. Retina. 2007;27:296–303. doi: 10.1097/01.iae.0000242761.74813.20. [DOI] [PubMed] [Google Scholar]
- 7.Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25:208–11. doi: 10.1097/00006982-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Machemer R, Aaberg TM, Freeman HM, Irvine AR, Leam JS, Michels RM. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991;112:159–65. doi: 10.1016/s0002-9394(14)76695-4. [DOI] [PubMed] [Google Scholar]
- 9.The Silicone Study Group. Vitrectomy with silicone oil or sulfur hexafluoride gas in eyes with severe proliferative vitreoretinopathy: Results of a randomized clinical trial, Silicone Study Report 1. Arch Ophthalmol. 1992;110:770–9. doi: 10.1001/archopht.1992.01080180042027. [DOI] [PubMed] [Google Scholar]
- 10.The Silicone Study Group. Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe proliferative vitreoretinopathy: Results of a randomized clinical trial, Silicone Study Report 2. Arch Ophthalmol. 1992;110:780–92. doi: 10.1001/archopht.1992.01080180052028. [DOI] [PubMed] [Google Scholar]
- 11.Abrams GW, Azen SP, McCuen BW, 2nd, Flynn HW, Jr, Lai MY, Ryan SJ. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: Results of additional and long-term follow-up, Silicone Study report 11. Arch Ophthalmol. 1997;115:335–44. doi: 10.1001/archopht.1997.01100150337005. 2nd. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira LB, Reis PA. Silicone oil tamponade in 23-gauge transconjunctival sutureless vitrectomy. Retina. 2007;27:1054–8. doi: 10.1097/IAE.0b013e318113235e. [DOI] [PubMed] [Google Scholar]
- 13.Yanyali A, Celik E, Horozoglu F, Oner S, Nohutcu AF. 25-gauge transconjunctival sutureless pars plana vitrectomy. Eur J Ophthalmol. 2006;16:141–7. doi: 10.1177/112067210601600123. [DOI] [PubMed] [Google Scholar]
- 14.Shimada H, Nakashizuka H, Mori R, Mizutani Y. Expanded indications for 25-gauge transconjunctival vitrectomy. Jpn J Ophthalmol. 2005;49:397–401. doi: 10.1007/s10384-004-0214-4. [DOI] [PubMed] [Google Scholar]
- 15.Byeon SH, Chu YK, Lee SC, Koh HJ, Kim SS, Kwon OW. Problems associated with the 25-gauge transconjunctival sutureless vitrectomy system during and after surgery. Ophthalmologica. 2006;220:259–65. doi: 10.1159/000093081. [DOI] [PubMed] [Google Scholar]
- 16.Shimada H, Nakashizuka H, Mori R, Mizutani Y, Hattori T. 25-gauge scleral tunnel transconjunctival vitrectomy. Am J Ophthalmol. 2006;142:871–3. doi: 10.1016/j.ajo.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 17.Fine HF, Iranmanesh R, Iturralde D, Spaide RF. Outcome of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment disease. Ophthalmol. 2007;114:1197–200. doi: 10.1016/j.ophtha.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Siqueira RC, Gil AD, Jorge R. Retinal detachment surgery with silicone oil injection in transconjunctival sutureless 23-gauge vitrectomy. Arq Bras Oftalmol. 2007;70:905–9. doi: 10.1590/s0004-27492007000600004. [DOI] [PubMed] [Google Scholar]
- 19.Kunimoto DY, Kaiser RS, Wills Eye Retina Service Incidence of endophthalmitis after 20- and 25-gauge vitrectomy. Ophthalmology. 2007;114:2133–7. doi: 10.1016/j.ophtha.2007.08.009. [DOI] [PubMed] [Google Scholar]