Abstract
In human gene therapy applications, lentiviral vectors may have advantages over γ-retroviral vectors in several areas, including the ability to transduce nondividing cells, resistance to gene silencing and a potentially safer integration site profile. However, unlike γ-retroviral vectors it has been problematic to drive the expression of multiple genes efficiently and coordinately with approaches such as internal ribosome entry sites or dual promoters. Using different 2A peptides, lentiviral vectors expressing two-gene T-cell receptors directed against the melanoma differentiation antigens gp100 and MART-1 were constructed. We demonstrated that addition of amino-acid spacer sequences (GSG or SGSG) before the 2A sequence is a prerequisite for efficient synthesis of biologically active T-cell receptors and that addition of a furin cleavage site followed by a V5 peptide tag yielded optimal T-cell receptor gene expression. Furthermore, we determined that the furin cleavage site was recognized in lymphocytes and accounted for removal of residual 2A peptides at the post-translational level with an efficiency of 20–30%, which could not be increased by addition of multiple furin cleavage sites. The novel bicistronic lentiviral vector developed herein afforded robust anti-melanoma activities to engineered peripheral blood lymphocytes, including cytokine secretion, cell proliferation and lytic activity. Such optimal vectors may have immediate applications in cancer gene therapy.
Keywords: T-cell receptor, adoptive immunotherapy, tumor immunity, lentivirus, 2A peptide
Introduction
The genetic engineering of human lymphocytes as a potential therapy for inherited, acquired or infectious disease requires efficient transfer and expression of the transgene. In the case of adoptive immunotherapy for cancer, naturally occurring antitumor T-cell receptors (TCRs) have been used to endow normal T cells with antitumor reactivity.1 In a recent clinical trial, we demonstrated the successful conferral of antitumor function through ex vivo γ-retroviral transduction of a MART-1-reactive TCR gene into the peripheral blood lymphocytes (PBLs) in patients with metastatic melanoma resulted in clinical tumor responses.2 Although successful in demonstrating the potential of this cancer gene therapy approach, there are several limitations to current γ-retroviral vector-based gene transfer systems.
In T-cells, the level of transgene expression is modulated by the activation state of the cell,3 and γ-retroviral based vectors have been shown to be subject to gene silencing in certain cell types,4,5 and they have a preference for integration near transcription start sites,6 which may increase the potential for insertional mutagenesis. Furthermore, the current γ-retroviral vector based protocols require T cells to be fully activated for efficient transduction and this may be deleterious to their function.7,8 We previously reported that in a murine model, prolonged ex vivo stimulation yielded fully differentiated effector T cells which were capable of in vitro tumor killing and high-level INF-γ release but, exhibited inferior activity for in vivo tumor treatment compared to naive and less differentiated effectors.9 Finally, there is a growing need for gene delivery systems to carry multiple genes in one construct, and γ-retroviral vectors have a limited coding capacity (<7 kb).
Lentiviral vectors with their larger coding capacity and ability to more easily express complex expression cassettes may be advantageous in this regard compared to γ-retroviral vectors.10,11 An example of a clinically important multi-gene construct is a tumor-associated antigen TCR, which is a heterodimer of TCR-α and -β chains.12 The TCRs for a variety of tumor-associated antigens (TAA) have been identified including the MART-1 and gp100 melanoma differentiation antigens, the NY-ESO-1 cancer-testis antigen and the p53 tumor suppressor.13–18 An important aspect of TCR expression vector design is that, the TCR-α- and -β chains must be coordinately expressed for proper biologically activity.12
The most common strategy for the expression of multiple genes has been based on internal ribosome entry site (IRES) elements.19 However, bicistronic lentiviral vectors containing IRES elements have consistently demonstrated a biased expression of two transgenes with the second gene being under expressed.20–22 We confirmed these reports (data not shown) and thus assembled a series of dual promoter-containing lentiviral vectors to express the α- and β-TCR chains, but consistently failed to achieve a high percentage of TCR expression in PBL (S Jones, data not shown). Naldini and co-workers recently reported that lentiviral vectors coordinately expressing two genes could be assembled using a synthetic bidirectional promoter,21 but this synthetic promoter exhibited poor activity in PBL (approximately 5–10% of transduced cells expressed both genes). An alternative to these approaches is the use of ribosomal skipping via 2A peptides.23,24 Several viruses use 2A peptides to mediate protein cleavage, including foot-and-mouth disease virus (F2A), equine Rhinitis A virus, porcine teschovirus-1 (P2A) and Thosea asigna virus (T2A). The 2A peptide consensus motif (DVEXNPGP) is extremely rare and is associated with cleavage-like activity between 2A and 2B genes through a ribosomal skip mechanism; the 2A peptide impairs normal peptide bond formation between 2A and 2B without affecting the translation of 2B. 2A peptides have been shown in γ-retroviral vectors to initiate the production of up to four proteins both in vitro and in vivo.24,25 When exogenous genes are linked using this approach, about 18 amino acids are left on the C terminus of the first gene and a single proline residue remains on the N terminus of the second gene. Although linking of the TCR-α and -β chains by 2A peptide has been reported,25 the effect of the residual amino acids on the function of expressed TCR has not been fully explored. In this study, we inserted a furin protease recognition site ahead of the 2A peptide to obtain a more native TCR chain by post-translational processing. Furin is a ubiquitous subtilisin-like proprotein convertase, whose natural substrates include certain serum proteins and growth factor receptors, such as the insulin-like growth factor receptor. The consensus sequence for furin cleavage is RXXR but the potential for actual cleavage is dependent on substrate tertiary structure and the amino acids immediately surrounding the recognition site.26
Herein, we developed a novel bicistronic lentiviral vector that combines a furin cleavage site, and an aminoacid spacer followed by a 2A ribosomal skip peptide. When the spacer sequence was augmented by the addition of a synthetic V5 peptide tag sequence, this specifically boosted protein processing and resulted in a lentiviral vector capable of mediating high-level TCR expression in transduced lymphocytes.
Results
2A peptides along with furin and spacer facilitate transgene expression
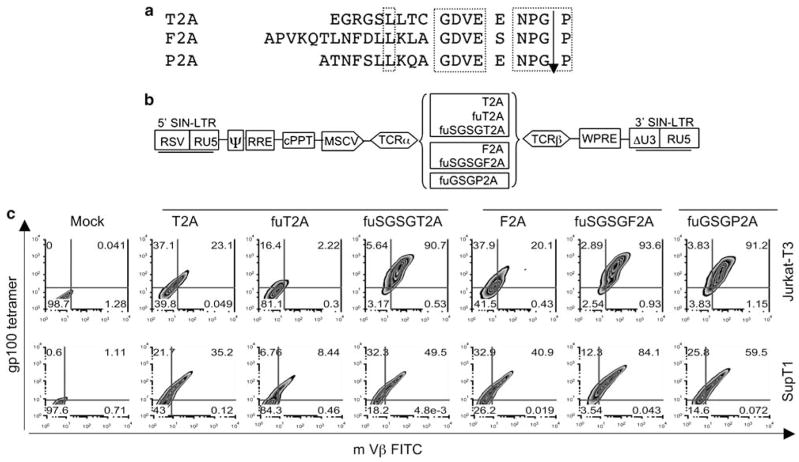
In preliminary experimentation, we investigated several strategies such as dual promoters or IRES, to drive a two-gene TCR expression cassette, but none of these lentiviral vectors yielded significant levels of TCR expression (data not shown). Encouraged by the successful use of 2A peptides in γ-retroviral vectors to express multiple genes in one construct,24,25,27 we attempted to develop lentiviral vectors expressing antitumor antigen-specific TCRs using different 2A peptides. In Figure 1a, the amino acids from different 2A peptides are illustrated and the conservative domains highlighted. We utilized T2A, F2A and P2A to link the α- and β-chains of a murine TCR that specifically recognized the human melanoma differentiation antigen gp100 (154–162) (Figure 1b). It has been reported that in γ-retroviral constructs, expression of multiple genes linked with 2A peptides can be facilitated by a spacer sequence (GSG) ahead of the 2A peptides.25,27 We designed several constructs combining a spacer (SGSG or GSG) and furin (R-A-K-R)28 cleavage site with different 2A peptides in bicistronic lentiviral vectors (Figure 1b).
Figure 1.
Schematic illustration of lentiviral constructs harboring T-cell receptor (TCR) expression cassettes linked with different 2A peptides. (a) 2A peptides derived from different viruses. P2A, porcine teschovirus-1; F2A, foot-and-mouth disease virus; T2A, thosea asigna virus. The conserved regions of 2A peptides are highlighted in dotted boxes. (b) 2A peptide TCR vector designs. α- and β-chains of TCR targeted against the melanoma differentiation antigen gp100 (154–162) linked with different 2A peptides (T2A, F2A and P2A) in conjunction of spacer sequences (GSG or SGSG) and furin were assembled as shown. The promoter used derived from the murine stem cell virus (MSCV). (c) TCR expression in T-cell lines. The lentiviral constructs harboring gp100 TCR linked with different 2A peptides combined with spacers and furin were pseudotyped with VSV-G envelope by transiently transfection in 293FT cells and the lentiviral particles were concentrated by ultracentrifugation. The Jurkat-T3 and SupT1 cells were transduced with equal amount of lentiviral particles in the presence of 10 μg ml−1 protamine sulfate. Four days post-transduction, the expression of the TCR was determined by fluorescence-activated cell sorting (FACS) analysis using gp100 tetramer and murine Vβ antibody, with the percent marker expression shown in each quadrant.
To compare the ability of different 2A peptide linkers to express TCRs, lentiviral vectors were used to transduce human T-cell lines Jurkat-T3 and SupT1. All the constructs, irrelevant to the origin of 2A peptides (T2A, F2A, P2A), functioned to produce the TCR in these two cell lines as demonstrated by fluorescence-activated cell sorting (FACS) analysis following gp100 tetramer staining (Figure 1c). In contrast to a previous report,29 the addition of furin to the 2A peptide inhibited TCR expression, whereas furin plus the addition of spacer sequences (GSG or SGSG) enabled highly efficient gene expression (up to 95%). While active in T-cell lines, these constructs produced significantly less TCR in primary PBL (<34% tetramer+; Supplementary Figure 1).
Addition of peptide tags increases TCR expression
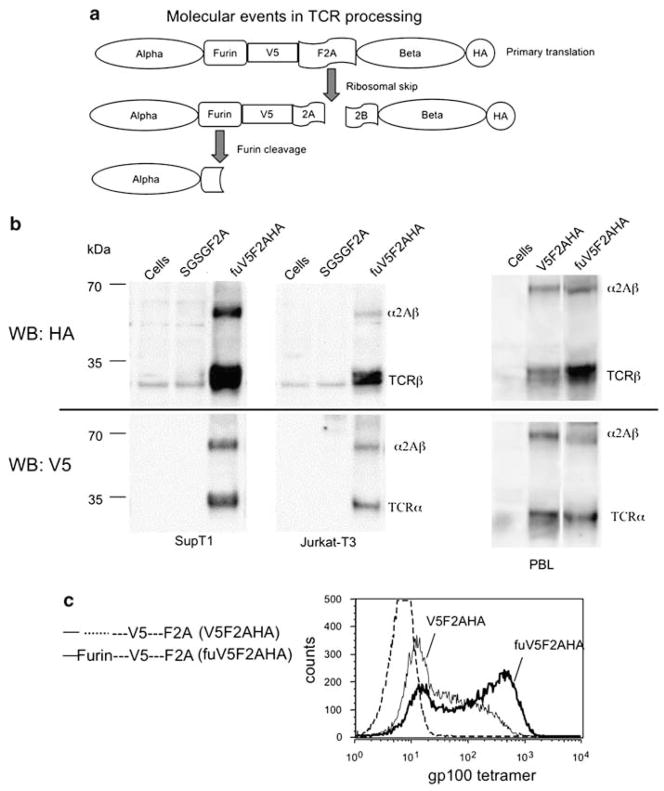
In an attempt to optimize TCR expression in primary human T cells, we determined the maturation events of the expressed precursors proteins. The lack of available α- and β-chain antibodies that functioned in western blot analysis necessitated the addition of peptide tags. The V5 synthetic peptide tag was added between the furin cleavage site and the amino-acid spacer-F2A sequence (at the C terminus of α-chain) and a hemagglutinin (HA) tag was added to the C terminus of the β-chain (Figure 2a). As depicted in Figure 2a, when 2A peptides initiate a ribosomal skip during translation, two molecules can result: the TCR-α chain with an F2A residual peptide (including the V5 tag), and the TCR-β chain with an HA tag. If the furin site is additionally recognized, this leads to the production of the TCR-α chain without the V5 tag. Western blotting with anti-HA antibody demonstrated approximately 90% efficiency of processing of the β-chain in the T-cell lines (Figure 2b, quantitation not shown). When the anti-V5 antibody was used for western blot analysis, a relatively lower percentage of the α-chain was detected, which was likely caused by the loss of the V5 tag following furin cleavage (Figure 2a). On the basis of the difference in processing detected by the two antibodies, we estimated that cleavage at the furin site occurred at a post-translational level of 28% in SupT1 and 19% in Juarkt-T3 cells (Figure 2b, compare HA and V5 blots, quantitation not shown). Interestingly, this ‘tag-modified’ vector also showed enhanced tetramer staining in the two transduced T-cell lines (data not shown).
Figure 2.
2A peptide and furin contribute to α- and β-chain maturation. (a) Proposed molecular events of expressed T-cell receptor (TCR). Diagrammatic illustration of gp100 TCR fused with V5 (between furin and foot-and-mouth disease virus (F2A) peptide) and hemagglutinin (HA) (in C terminus of TCR-β chain) tags. Translation continues up to the F2A peptide, which can initiate a ribosomal skip to generate two proteins; the TCR-α and -β chains as illustrated. The α-chain with furin site can be cleaved at the post-translational level as shown. (b) TCR products detected by western blotting. In upper panel, the cell lysates were prepared (3 days post-transduction) and 30 μg of lysates was subjected to SDS–polyacrylamide gel electrophoresis (PAGE) gels, the level of TCR expression was measured by immunoblotting with HA antibody. In lower panel, the level of TCR-α chain was detected by V5 antibody. Cells analyzed were SupT1 (left) Jurkat-T3 (middle) and primary human peripheral blood lymphocytes (PBLs) (right). The unprocessed TCR-α and -β chains are indicated in the image as α2Aβ. The bands of TCR-α or -β and α2Aβ were quantified using a CCD camera and image analysis software. (c) Furin accounts for the enhanced TCR expression in lentiviral construct. Lentiviral constructs harboring gp100 TCR modified with tags with or without furin are illustrated (left panel). PBL from melanoma patient was transduced with the same amount of lentiviral particles derived from above constructs, and expression of the TCR analyzed by tetramer staining. The images were overlaid, and histograms reflecting specific vector are as denoted.
We next tested whether the superior performance of the tag-modified vector also applied to primary human PBL. PBLs were transduced with peptide tag vectors with or without a furin cleavage site (Figure 2c). By western blot analysis we observed a similar pattern of TCR chain maturation (Figure 2b, right panel) as described in T-cell lines, where furin contributed to 28% cleavage at the post-translational level in primary PBL (quantitation not shown). The unexpectedly high protein processing of the F2A fusion protein with the V5 and HA peptide tags led us to further investigate the biological properties of this tag-modified vector. When primary PBLs were transduced with the tag-modified vectors, we observed a significant enhancement in TCR expression measured by tetramer staining using the vector with a furin site and peptide tags (Figure 2c). The lower tetramer staining observed without the furin site suggested that post-translational cleavage at the furin site may be important in the maturation of the TCR heterodimer in transduced human PBL.
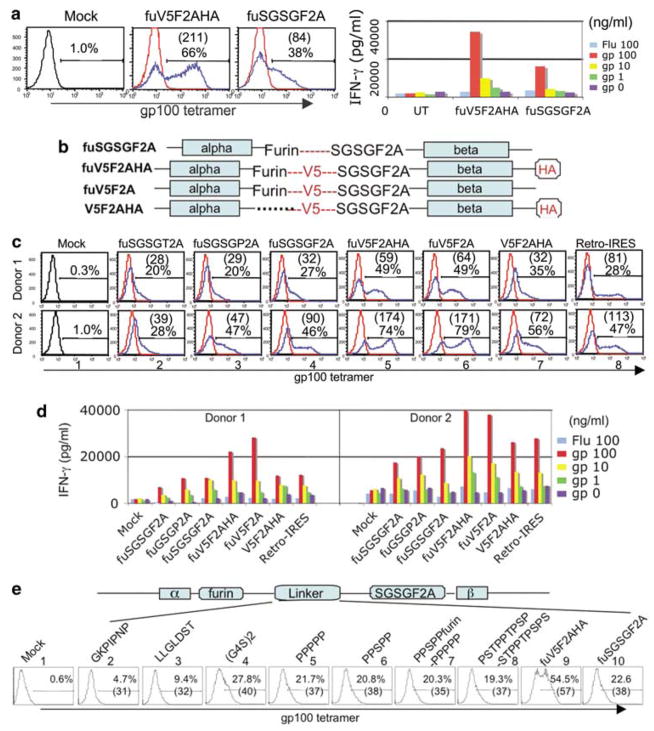
Furin plus V5 is critical for optimal TCR expression in PBL
We next systematically tested the biological activities of several 2A linker modifications following transduction of PBL from melanoma patients. Analysis of PBL transduced with the vectors containing the furin site and peptide tags demonstrated a significant and reproducible improvement in TCR production (Figure 3a) as measured by both an increased transduction rate (66% versus 38%) and enhanced expression per cell mean fluorescence intensity ((MFI) 211 versus 84). To test the antigen reactivity of the expressed TCR, transduced cells were co-cultured with peptide-pulsed cells and specific dose-dependent induction of interferon (IFN)-γ was demonstrated (Figure 3a, right panel). The level of IFN-γ induction was correlated with the transduction rate and MFI, that is, the TCR with the peptide tags was more active than TCR without the peptide tags. To further define the role of the tag sequences, the V5 and HA tags were removed and the various combinations tested in one experiment (Figure 3b). As depicted in Figure 3c, the combination of a furin cleavage site followed by the V5 peptide tag sequence showed the highest amount of TCR expression in transduced PBL (image 5). Removal of the HA tag did not affect the performance of the vector (compare images 5 versus 6). Removal of the furin site significantly decreased TCR expression from the vector (compare images 5 and 7). The tag-modified vectors also demonstrated superior biological activity when transduced PBLs were co-cultured with peptide-pulsed T2 cells (Figure 3d). To address whether the few residual amino acids from 2A peptide affect the functionality of expressed TCR, we monitored TCR activity using a γ-retroviral construct where TCR-α and -β chains were linked by an IRES (this arrangement produced α- and β-chains without residual amino acids). When compared to PBL expressing similar amounts of TCR by tetramer staining (images 4 versus 8) the level of IFN-γ induction was similar in both vectors (Figure 3d), indicating that the extra amino acids added to the TCR-α and -β chains using the 2A linker approach did not significantly affect the functionality of this TCR.
Figure 3.
2A fusion peptides containing the V5 peptide correlated with enhanced activities. (a) Lentiviral vector modified with peptide tags demonstrates improved performance. Peripheral blood lymphocyte (PBL) from a melanoma patient was transduced with gp100 T-cell receptor (TCR) lentiviral vectors containing either foot-and-mouth disease virus (F2A) peptide or F2A containing the V5 peptide. Transduction efficiency was evaluated by percentage of positive cells and MFI (in parenthesis) (left panel). In right panel, the transduced lymphocytes were co-cultured with peptide-pulsed T2 cells (using specific gp100 (154–162) or control, Flu, peptides). The level of interferon (IFN)-γ was measured by enzyme-linked immunosorbent assay (ELISA). (b) Vector designs and role of peptide tags. Using the existing vector constructs, new gp100 TCR vectors were designed, with and without the V5 and hemagglutinin (HA) tags, and with both tags but without the furin cleavage site. (c) Measuring the transduction efficiency of lentiviral vectors in PBL. Lentiviral particles derived from the above constructs and the previous vectors with furin and spacer sequence were titered and equal amounts of lentiviral particles used to transduce PBL from two melanoma patients. Four days after transduction, the expression of TCR was analyzed by fluorescence-activated cell sorting (FACS). The FACS image-labeled Retro-IRES is a control in which gp100 TCR chains are linked with IRES in a γ-retroviral vectors. Percentage of tetramer staining and MFI (in parenthesis) were calculated as shown. (d) Activity of TCR vectors. Transduced PBLs were co-cultured with specific peptide-pulsed T2 cells, the induction of IFN-γ over 16 h period was measured by ELISA. (e) Comparison of peptide linkers. Linker peptides from the following sources were inserted between furin and F2A and compared to the V5 peptide. First half of V5 peptide, GKPIPNP (image 2); second half of V5 peptide, LLGLDST(image 3); hinge region of a chimeric TCR, (G4S)2 (image 4); immunoglobulin A (IgA) hinge region of human Cα2, PPPPP (image 5); hinge region of Gorilla Cα2, PPSPP (image 6); chimeric hinge region and furin, PPSPPfurinPPPPP (image 7); IgA hinge region of human Cα1, PSTPPTPSPSTPPTPSPS (image 8); V5 synthetic peptide, GKPIPNPLLGLDST (fuV5F2A, image 9) and a construct without additional amino acid between furin and the F2A sequences (fuSGSGF2A, image 10). PBL from a melanoma patient was transduced with equal amount of lentiviral vector following activation by CD3/CD28 beads. Twelve days after transduction, the expression of TCR was analyzed by FACS, the percentage of tetramer-positive cells and MFI (in parenthesis) were individually plotted.
We next screened a series of linker peptides inserted between furin and F2A sequences to determine if the specific amino acids in the V5 peptide influenced the enhanced biological activity of this construct (Figure 3e). In two constructs we dissected the V5 into two parts and accordingly made the two constructs as depicted in Figure 3e (second and third images). Next, we replaced the V5 peptide with a synthetic hinge region commonly used in chimeric TCR constructs30 ((G4S)2, construct 4) or naturally occurring hinge regions from primate immunoglobulins31 (constructs 5–8). The original vector with the furin site plus the complete V5 peptide (construct 9) consistently displayed the highest level of TCR expression, whereas other vectors showed a performance similar to the vector where furin was linked directly to F2A (construct 10).
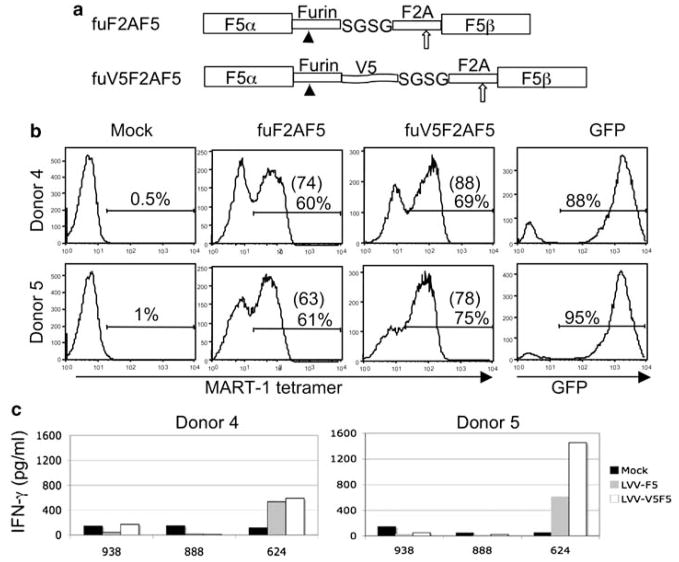
To verify the applicability of this novel molecular strategy to other TCR, we constructed a human anti-MART-1 TCR linked by furin-V5-SGSGF2A in the same lentiviral vector backbone (Figure 4a). This TCR, DMF5 was previously shown to be highly reactive against MART-1 expressing melanoma tumor cell lines.32 Similar to the mouse anti-gp100 TCR vector, superior performance was observed in two patient PBL transduced with a vector where the TCR chains were linked with a furin site plus the V5 peptide (Figure 4b). We observed enhancement not only in the number of tetramer-positive cells (60–69 and 61–75%) but also in expression measured by MFI (74–88 and 63–78). When transduced PBLs were co-cultured with antigen expressing human lymphocyte antigen (HLA)-A2-matched tumor line 624, we observed a significant increase in IFN-γ production in one of the donors tested, while a modest increase in cytokine production was observed with the other donor (Figure 4c). As the magnitude of increased biological activity was less pronounced than noted for the gp100 TCR, thus it is possible that the improved function of V5 peptide linked TCR constructs may vary depending on the genes involved.
Figure 4.
The V5 tag-modified MART-1 T-cell receptor (TCR) lentiviral vector leads to robust expression in peripheral blood lymphocytes (PBLs). (a) Schematic illustration of lentiviral constructs harboring human anti-MART-1 TCR (called DMF5). Upper panel, the human DMF5 TCR receptor-α and -β chain were linked with furin, SGSG and foot-and-mouth disease virus (F2A) peptide; lower panel, the vector with furin site, V5 tag and SGSGF2A peptide were extended with the V5 peptide. (b) MART-1 TCR transduction of PBL. Lentiviral vectors were prepared and equal amount of virus used to transduce PBL from two donors. The expression of TCR was confirmed by MART-1 tetramer staining at 11 days post-transduction. A lentiviral vector containing green fluorescent protein (GFP) was transduced in parallel to monitor transduction. (c) Induction of interferon (IFN)-γ by melanoma cells. Transduced PBLs were co-cultured with equal ratio of tumor cells for 16 h, and the level of IFN-γ was measured by enzyme-linked immunosorbent assay (ELISA). Melanoma cells included MART-1-positive human lymphocyte antigen (HLA)-A2+ 624, and two MART-1-positive HLA-A2− cells 888 and 938. Data shown represent the mean of triplicate cultures.
One furin site is sufficient for TCR maturation
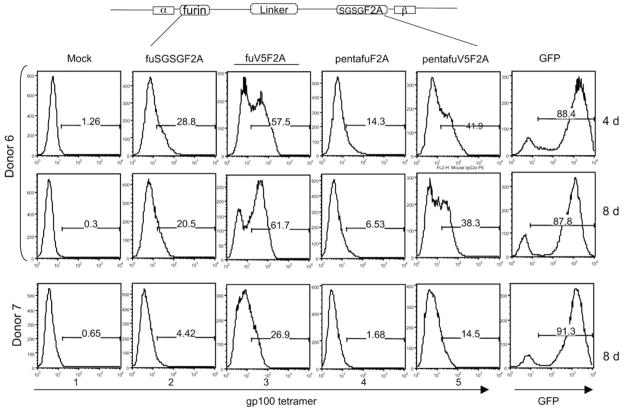
In a last series of vectors, we tested alternatives to the single furin cleavage site. Some naturally occurring growth factors like insulin-like growth factor I are synthesized as a proprotein that is converted to the mature form by cleavage within a unique pentabasic-processing motif (KXXKXXRXXRXXR) containing four furin recognition sites. We tested if these tetra-furin sites when engineered into the vector could further improve the performance of the vector. In Figure 5, we assembled two constructs where tetra-furin sites were inserted either with the V5 tag or directly fused to the F2A peptide. These vectors were used to transduce PBL, and TCR expression assayed by tetramer staining. FACS data demonstrated that the vectors with tetra-furin sites did not improve TCR expression compared to the vector with a single furin site (compare images 3–5 and images 2–4). When tetra-furin was combined with the V5 peptide, we again observed an improvement in TCR expression (compare images 4 and 5), indicating that the V5 peptide tag facilitated the cleavage by endoproteolysis at the tetra-furin site.
Figure 5.
Alternative furin site vectors. Pentabasic furin-processing motif (K-X-X-K-X-X-R-X-X-R-X-X-R), including tetra-furin sites was engineered into two bicistronic-lentiviral vectors. In one construct tetra-furin was fused directly following α-chain and the other tetra-furin was extended with V5 peptide tag sequence. Along with previous fuSGSGF2A and furin extended with V5 (underlined) constructs, the lentiviral vectors were prepared and equal amount of virus was used to transduce peripheral blood lymphocytes (PBLs) from two donors. The expression of T-cell receptor (TCR) was confirmed by tetramer staining starting 4 and 8 days post-transduction with donor 6, and at 8 days for donor 7. A lentiviral vector containing green fluorescent protein (GFP) was used in parallel to monitor the transduction system.
Both TCR-engineered CD4 and CD8 cells recognize and kill tumor cells
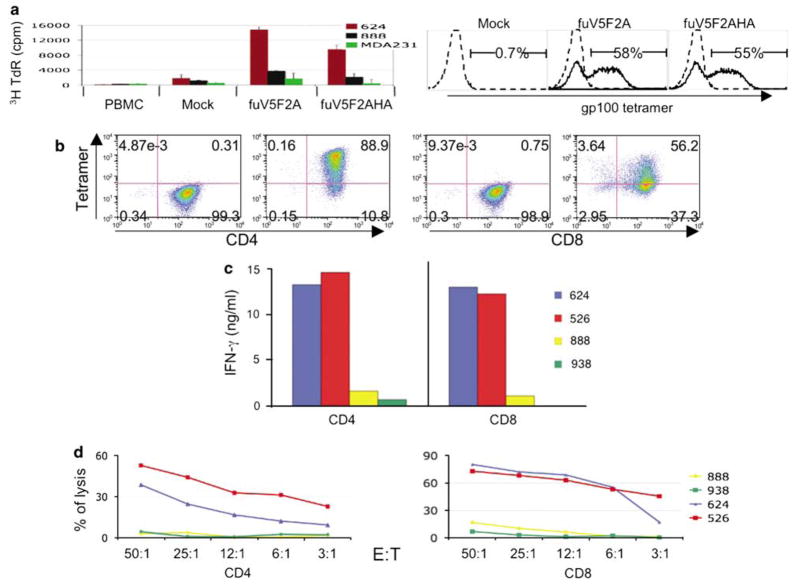
As a final test of the optimized vector design, we transduced melanoma patient PBL with the anti-gp100 TCR vectors and analyzed these engineered cells for anti-melanoma activity. The ability to expand following encounter with tumor antigen in vivo may be essential to successful TCR gene therapy. To test the proliferative capacity the V5 peptide-containing vectors, we transduced PBL from a melanoma patient with the V5 tag-containing F2A vectors and determined antigen-specific cell proliferation (Figure 6a, left panel). When the TCR-engineered PBLs were co-cultured with melanoma tumor cell lines, the gp100 TCR-engineered PBL underwent proliferation with HLA-A2+-matched gp100+ melanoma cell line 624, but not with control tumor lines (Figure 6a). The specific TCR used in these studies, gp100 (154–162), has been determined to be CD8-independent (L Cassard, manuscript in preparation). To test TCR activities in potential helper versus killer T cells, we separated CD4 and CD8T cell subsets from PBL by negative selection, and the purified lymphocytes were transduced with the V5 peptide tag-containing TCR lentiviral vector. The expression of TCR and purity of CD4 or CD8 was analyzed by FACS analysis (Figure 6b). The CD4 cells were 89% tetramer-positive, whereas the CD8T cells were 56% tetramer-positive (the difference in transduction efficiency may relate to the growth of the cells during the time of transduction, as the growth rate of CD4 was greater than CD8 cells, data not shown). The biological activity of the TCR-engineered CD4 and CD8T cells were tested by co-culture with melanoma tumor lines. Both T-cell subsets exhibited significant release of IFN-γ to HLA A2-matched tumor cells (Figure 6c). Lastly, tumor cell lysis by the TCR-engineered CD4/CD8 lymphocytes was determined by 51Cr release assay following co-culture with two HLA A2+ and two HLA A2− melanoma lines (Figure 6d). Both CD4 and CD8 TCR-transduced T cells demonstrated specific lytic activity and as expected, CD8T cells lysed tumors to a greater extent than CD4T cells.
Figure 6.
V5 peptide containing T-cell receptor (TCR) vectors demonstrate superior biological activity. (a) Antigen-specific proliferation. Peripheral blood lymphocytes (PBLs) from melanoma patient were transduced with lentiviral vectors harboring the V5 tag-modified gp100 (154) TCR and TCR expression confirmed by fluorescence-activated cell sorting (FACS) analysis (right panel). Four days post-transduction, PBLs were co-cultured with different tumor cell lines (ratio 1:1) in media without interleukin IL-2 for 72 h, and16 h before harvest 1 μCi of (3H) thymidine was added. Radionucleotide incorporation was determined by liquid scintillation counter. Results representing mean c.p.m. of triplicate cultures were plotted as shown (left panel). (b) Robust expression of TCR in CD4/CD8 subsets. CD4/CD8 cells were negatively selected from PBL, and following anti-CD3/CD28 bead activation, cells were transduced with lentiviral vector fuV5F2A. Seven days post-transduction, TCR expression in CD4 and CD8 cells was determined by FACS analysis. (c) Induction of interferon (IFN)-γ by melanoma cells. Transduced CD4/CD8 cells were co-cultured with equal ratio of tumor cells for 16 h, and the concentration of IFN-γ determined by enzyme-linked immunosorbent assay (ELISA) (data are the mean of triplicate cultures). (d) Lytic activities of transduced PBL. Transduced CD4/CD8 cells were co-cultured with 51Cr-labeled tumor cells at the indicated ratio and the release of 51Cr was measured. The percentage of cell lysis was calculated using the formula ((specific release−spontaneous release)/(total release−spontaneous release)) × 100. Results representing mean of triplicate cultures were plotted. Melanoma cells included MART-1-positive human lymphocyte antigen (HLA)-A2+ 624, and 526, plus two MART-1-positive HLA-A2− cells 888 and 938. Data shown represent the mean of triplicate cultures.
Discussion
Lentiviral vectors were developed in the mid-1990s by adopting strategies defined over the past decade to optimize γ-retroviral vectors for safety and effectiveness.33,34 Lentiviral vectors can integrate in nondividing cells, making them more effective than γ-retroviral vectors for gene transfer to postmitotic or slowly dividing cells, which may include hematopoietic stem cells and naive T cells.35 The lentiviral vector used in this report contain several elements previously shown to enhance vector function, including a central polypurine tract (cPPT) for improved replication and nuclear import,36 a promoter from the murine stem cell virus (MSCV), which has been shown to lessen vector silencing in some cell types,37,38 a woodchuck hepatitis virus post-transcriptional responsive element (WPRE) for improved transcriptional termination,39 and the backbone was a deleted 3′-LTR self-inactivating (SIN) vector design that may have improved safety, sustained gene expression and antisilencing properties.5,40–42
The ability of lentiviral vector to transduce minimally stimulated T cells43 may have significant advantages. In vitro analysis of human T cells transduced with γ-retroviral vectors following T-cell activation indicated that both TCR Vβ usage and global gene expression patterns were altered44,45 and such changes may affect the in vivo survival and function of gene-modified T cells. The use of a transgenic mouse model of adoptive cell transfer (ACT)46 also indicated that ex vivo stimulated and fully differentiated effector T cells, where inferior for in vivo tumor treatment compared to naive or less differentiated T cells (this was despite the fact that they demonstrated superior in vitro tumor killing and high-level IFN-γ release).9 We recently confirmed and extended these observations in this murine model of ACT by demonstrating that TCR gene transfer into stimulated murine splenocytes was less efficacious than T cells containing the same TCR isolated from transgenic mice.47 These reports suggested that the ability to use lentiviral vectors to genetically modify T lymphocytes at a less differentiated stage may have important applications in cancer immunotherapy.
The individual α- and β-chains of the TCR heterodimer must be expressed at equal levels for full biologically activity.12 In the case of TCR gene transfer into mature T cells, there is an additional complication that the introduced genes must compete with the endogenous TCR chains for TCR complex assembly. If mixed heterodimers were formed, they would not recognize their appropriate target antigens, and while mixed heterodimer formation can be lessened by protein modifications,48,49 the individual α- and β-chains must still be optimally expressed. A common strategy for multiple gene expression has relied on IRES element, but the use of these elements in bicistronic lentiviral vectors was demonstrated to lead to biased expression of the transgenes.20–22,50 Although it has been reported that lentiviral vectors containing two independent internal promoters transferred high-level expression of multiple transgenes,20 we consistently failed to achieve a high percentage of TCR expression using this approach in PBL (data not shown). Naldini and co-workers recently developed lentiviral vectors coordinately expressing dual-genes driven by synthetic bidirectional promoters.21 While they observed coordinated gene expression in various cells and tissues, these synthetic promoters exhibited lower activities in activated (10%) and naive PBL (5%).
Encouraged by the successful use of 2A peptides in γ-retroviral vectors to express multiple genes in one construct,24,25,27,51 we attempted to develop lentiviral vectors expressing antitumor antigen TCR using different 2A peptides for adoptive immunotherapy. The main advantage of using 2A peptides in the construction of bicistronic vectors is the potential for co-expression of both genes at equal levels. Our western blot analysis indicated that cleavage of the polyprotein occurred approximately 90% of the time (Figure 2), thus assuring near stoichiometric synthesis of the TCR-α- and -β chains. A potential disadvantage in the use of 2A peptides is the residual amino acids left on the C terminus of the first protein with the potential for inappropriate targeting of proteins to different subcellular organelles.52 The residual 2A peptide left on the α-chain did not appear to significantly affect TCR function in comparison to an α-chain without these residues (Figure 3), but this observation is likely gene specific as we have observed the loss of biological activity of cytokine genes produced using 2A peptides (data not shown).
The concern for optimizing TCR gene expression led us to compare three 2A peptides-T2A, F2A and P2A,25,29 and consistent with published observations,21,50 2A peptides alone failed to achieve high level of TCR expression (Figure 1). Interestingly, when these 2A peptides were combined with spacer sequences (SGSG or GSG) and a furin cleavage site, there was significant increase in TCR expression (>95%) in T-cell lines, but expression in transduced PBL was significantly lower (generally 20%; Supplementary data). This result suggested a failure of 2A peptide-mediated ribosomal skipping in PBL, and led us to perform protein-processing studies using TCRs modified with peptide tags compatible with western blot analysis. This analysis led to the surprising observation that a vector in which the furin cleavage site was followed by a V5 peptide tag significantly improved TCR expression compared to TCR vectors without the tag. Although the precise mechanism that accounts for enhanced processing is unknown, we speculate that the specific V5 peptide sequence may permit enhanced ribosomal skipping in this context. Attempts to substitute other sequences for the V5 peptide were unsuccessful (Figure 3e), suggesting that the specific amino-acid sequence of the V5 peptide may be involved in the enhanced activity. This effect was observed with both murine and human TCRs.
By western blotting (Figure 2), we saw a robust processing of the TCR 2A fusion protein into the individual α- and β-chains (more than 90% of the initial polyprotein as processed). By analyzing TCR-α chain fused with V5 tag we observed that post-translational cleavage at furin site occurred approximately 30% of the time. Cleavage could not be enhanced using a naturally occurring tetra-furin sequence and this sequence may have inhibited the ribosomal skip, as TCR expression was lower in tetra-furin sequence-containing vectors (Figure 5). Taken together, the combination of a single furin cleavage site followed by a V5 peptide tag plus a 2A ribosomal skip peptide represents a unique molecular strategy, which may overcome technical difficulties using 2A peptides in lentiviral vectors to express multiple genes in one construct.
This report also points out the importance of testing transgene expression in the ultimate target cell. On the basis of the data from human T-cell lines (where >95% of the simple 2A constructs were processed and expressed), we might have concluded TCR vector design to be optimized. The fact that TCR expression in PBL was generally less than one quarter of the activity in T-cell lines lead to the continued refinement in vector design such that all current TCR vectors now produce robust expression in transduced primary human lymphocytes. Recently, Levine et al.53 reported on a phase 1 clinical trial using lentiviral vectors that demonstrated efficient and safe gene delivery to patients’ T cells with good persistence in vivo. The novel bicistronic lentiviral vectors harboring specific antitumor TCR described herein provide the opportunity not only for sustained transgene expression but also new methodologies to modify T lymphocytes at a less differentiated stage, and may have important applications in cancer immunotherapy.
Materials and methods
Cell culture
PBLs used in this study were from metastatic melanoma patients seeking treatments at Surgery Branch, National Cancer Institute. Briefly, PBLs were collected by leukapheresis, and lymphocytes were separated by Ficoll/Hypaque cushion centrifugation, washed and resuspended at a concentration of 1 × 106 per ml in AIM-V medium (Invitrogen, Carlsbad, CA, USA) supplemented with 300 IU ml−1 interleukin IL-2 and 5% heat-inactivated human AB serum (Valley Biomedical, Winchester, VA, USA). CD4+- and CD8+-enriched populations were separated using a magnetic beads-based method for both negative and positive selection of these subsets (Invitrogen). The other cell lines used in this study, including a human lymphoid cell line SupT1, (ATCC, CRL-1942), a T-cell acute leukemia, Jurkat (ATCC, TIB-152) and a lymphoblastoid cell line deficient in TAP function, T2 (Starter, 1985) were maintained in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with heat-inactivated 10% fetal calf serum (FCS), 100 U ml−1 penicillin/streptomycin, 2 mM L-glutamine and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer solution. Melanoma cells included MART-1-positive HLA-A2+ 624, 526 and two MART-1-positive HLA-A2− cells 888 and 938. Other lines included breast cancer line MDA231 (HLA-A2+ MART-1 negative), 293T and 293FT (Invitrogen) that were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS, 100 U ml−1 penicillin/streptomycin, 2 mM L-glutamine, 20 μM 2-mercaptoenthanol and 25 mM HEPES buffer solution. All cell lines were cultured at 37 °C in a 5% CO2-humidified incubator.
Peptide synthesis
Peptides in this study were synthesized using a solid-phase method on a peptide synthesizer (Gilson, Middleton, WI USA) at the Surgery Branch (NCI). The quality of peptide was evaluated by the mass spectrometry (Biosynthesis, Lewisville, TX, USA). The peptides used in this study are as follows: gp100 (154–162) (KTWGQYWQV) and control influenza peptide (GILGFVFTL).
Vector construction
The lentiviral constructs utilized are from pLenti6/V5-D-TOPO vector (Invitrogen) and pRRLSIN.cPPT. MSCV/GFP.WPRE harboring a green fluorescent protein (GFP) gene driven by MSCV U3 promoter (S Jones, unpublished data). Mouse TCR (sp0.01 A-α and -β, DQ452619 and DQ452620) targeting gp100 (154–162) melanoma differentiation antigen was screened and established in Surgery Branch (L Cassard, unpublished data). MSGV1-154-AIB (γ-retroviral construct with gp100 (154) TCR gene linked with IRES) and MSGV-154-A2AB (γ-retroviral construct with gp100 (154) TCR gene linked with T2A peptide) were used as template and PCR products were cloned into lentiviral-related constructs, namely pLV.CMV.154AIB and pLV.CMV.154AT2AB, respectively. Using clones sp0.01A-α and -β as templates, four primers for each construct containing different combination of 2A peptides, spacers (GSG, SGSG) and furin (RAKR) were synthesized and resulting two PCR products for each gene with complement ends were annealed and ligated into the pRRLSIN.cPPT.MSCV/GFP.WPRE vector to the restriction enzyme sites (AscI and SalI) replacing GFP. The following 2A peptides were utilized in the lentiviral vectors, insect virus T2A encodes EGRGSLLTCGD VEENPGP, F2A encodes APVKQTLNFDLLKLAGDVES NPGP and P2A encodes ATNFSLLKQAGDVEE NPGP.25,29,54 Supplementary Table 1 contains all primer sequences. The primer flanking 5′ of gp100 (154) TCR-α chain (154A5′) and primer flanking 3′ of gp100 (154) β-chain (154B3′) containing AscI and SalI restrictive enzyme sites were designed for all the following constructs, including fuT2A, fuSGSGT2A, F2A, fuSGSG F2A and fuSGSGP2A and so on. For furinT2A the primers with complementing ends were alphaFurin-T2AR and betafurinT2AF; for fuSGSGT2A, the primers are alphafurinSGSGT2AR and betafurinSGSGT2AF that used the same primer as betafurinT2AF; for F2A, the primers were alphaF2AR and betaF2AF; for fuSGSGF2A, the primers were alphafurinSGSGF2AR and betafur-inSGSGF2AF remained the same as betaF2AF; for fuGSGP2A, the primers were alphafurinGSGP2AR and betafurinGSGP2AF. Using lentiviral construct fuSGSG-F2A as a template, we synthesized a pair of primers DV5F and DV5R flanking furin region and by site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) removed the furin to yield SGSGF2A. To produce the construct with peptide tags, primers containing V5 and HA sequences with the lentiviral construct fuSGSGF2A were used as template for PCR. The following primers were used, gp154xpressF and gp154HAR; alphagp154V5R and betagp154V5F, to produce construct fuV5SGSGF2AHA. The α- and β-chains from human TCR targeting melanoma-Ag MART-132 were subcloned into lentiviral vector with the following primers. The primer flanking 5′ of DMF5 TCR-α chain (F5A5′) and primer flanking 3′ of DMF5 β-chain (F5B3′) containing AscI and SalI restrictive enzyme sites were designed for constructs fuV5F2AF5 and fuF2AF5. The primers for fuV5F2AF5 with complementing ends were F5P3alphaR and F5P4betaF for fuF2AF5 the primers with complementing ends were fuSGSGF2AalphaR and fuSGSGF2A-betaF. All the constructs are confirmed by restrictive enzyme digestion and sequencing.
Lentivirus preparation and transduction
293FT cells (Invitrogen) were cultured in complete culture medium (DMEM containing 10% fetal bovine serum) supplemented with Geneticin (500 μg ml−1). The day before transfection, 25 × 106 293FT cells were plated onto 150 mm2 culture dishes with 15 ml of culture medium. On the day of transfection, the culture medium was replaced with 15 ml of normal growth medium containing serum without antibiotics. Pseudotyped lentiviral vector was produced by transfection using Lipofectamine 2000 (Invitrogen) of 293T cells using a three plasmid (with the helper packaging construct pCMVΔR8.91) or a four plasmid system of pMDLg/pRRE plus pRSV-Rev, the gp100(154) TCR or MART-1 TCR vectors and the plasmid pMD-G encoding VSV-G envelope protein. At 6 h after transfection, the plates were washed with phosphate-buffered saline (PBS) for thrice and 20 ml fresh medium was added to each plate for 24 h before collection of virus. The supernatant was collected 30–48 h post-transfection and cell debris was removed by centrifugation at 6000 g for 10 min, then passed through a 0.45 μm polyvinylidene fluoride filter. Virus was concentrated using ultracentrifugation at 50 000 g for 2 h in Beckman centrifuge tube (25 × 89 mm) using rotor SW-28. Virus was resuspended in DMEM without serum, aliquoted and stored at −80 °C. The lentiviral titer was determined by p24 enzyme-linked immunosorbent assay (ELISA) assay as described.55 For transduction of Jurkat-T3 and SupT1, 5 × 105 cells were plated per well in 24-well plate, equal amount of virus was applied to the cells in the presence of 8 μg ml−1 polybrene or 10 μg ml−1 protamine sulfate. PBLs from donors were cultured in AIM-V supplemented with IL-2 as described above. For transduction of activated PBLs, T cells were activated for 1–3 days with addition of OKT3 (50 ng ml−1). For transduction of minimally stimulated cells, PBLs were transduced by concurrent addition of vector plus anti-CD3/CD28 beads (Invitrogen) using three beads per cell. In all transductions, 5 × 105 cells were plated per well in 24-well plate and equal amount of virus particles was applied in the presence of 10 μg ml−1 protamine sulfate.
FACS analysis
Cell-surface expression of gp100 (154) TCR, MART-1 TCR, CD3, CD4, CD8 and murine Vβ was measured using fluorescein isothiocyanate- or phycoerythrin-conjugated antibody or tetramers. For the gp100 TCR, a customer-designed anti-gp100(154–162) tetramer was used (iTAg MHC Tetramer, Beckman Coulter, Fullerton, CA, USA); all other reagents were commercially available. Immunofluorescence staining was analyzed as the relative log fluorescence of live cells, determined using a FACScan flow cytometer (Beckton Dickinson, San Jose, CA, USA). A combination of forward angle light scatter and propidium iodide staining was used to gate out the dead cells. About 1 × 105 cells were analyzed. Cells were washed twice with PBS (Invitrogen) and stained in FACS buffer composed of PBS supplemented with 2% FCS. The incubation of antibodies or tetramers with the cells was about 1 h at 4 °C. FACS data were analyzed using FlowJo 8.1.1 software (FlowJo, Ashland, OR, USA).
Measurement of lymphocyte reactivity to antigen
T2 cells were pulsed with peptides 1–100 ng ml−1 in cell culture medium for 2 h at 37 °C. Transduced PBL effector cells (1 × 105) were co-cultured with peptide-pulsed T2 cells (1 × 105) in a final volume of 0.2 ml in each well of a round-bottom 96-well plate. Cell culture supernatants were harvested and assayed 16 h later for IFN-γ by ELISA (Endogen, Rockford, IL, USA). The culture supernatants were diluted to be in the linear range of the assay. Transduced lymphocyte proliferation assays were conducted in triplicate wells of round-bottom 96-well plates (Costar, Lowel, MA, USA). About 1 × 105 transduced PBLs were co-cultured with different melanoma cells in a ratio of 1:1 for 3 days. The melanoma cells were irradiated using gamma irradiator at a dose of 200 Gy. The co-cultured lymphocytes were radiolabeled for the past 16 h of culture with 1 μCi of (3 H) thymidine (DuPont, New England Nuclear, Shelton, CT, USA) per well of cells and harvested onto glass filters, radio-nucleotide incorporation was determined using a Beta-plate 1205 liquid scintillation counter (Wallac, Ramsey, MN, USA). Results representing mean (±s.d.) c.p.m. of triplicate cultures are plotted.
Western blotting
Whole-cell lysates were prepared 3 days post-transduction using radioimmuno precipitation assay buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and freshly added 1/10 volume of protease inhibitor cocktail) (Sigma, St Louis, MO, USA), incubated on ice for 20 min and centrifuged at 12 000 g for 10 min at 4 °C. Protein concentrations were quantitated with a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Aliquots (30 μg) were mixed with 4 × SDS loading buffer containing 0.35 M 2-mercaptoethanol, subjected to 10% NuPAGE BT gel (Invitrogen) and then transferred onto nitrocellulose membranes (Invitrogen). The primary antibodies, mouse anti-HA-HRP (Roche, Basel, Switzerland), mouse anti-V5-HRP (Invitrogen) antibodies were used. The ECL Detection Kit (Amersham Pharmacia, Piscataway, NJ, USA) was used to visualize each protein. The amount of protein was quantitated by CCD camera driven by LAS-1000 Pro software and quantitated with ImageGauge software (Fuji medical systems).
51Cr release assay
The ability of transduced PBL to lyse HLA-A2+/gp100 (154) melanoma cells was evaluated using a 51Cr assay as described.56 Briefly, 106 tumor cells were labeled for 1 h at 37 °C with 100 μCi of 51Cr (Amersham Biosciences). Labeled target cells (2 × 103) were co-cultured with effector cells at the ratios indicated in the figure for 4 h at 37 °C in 0.15 ml of complete medium. Harvested supernatants were counted using a Wallac 1470 Wizard automatic gamma counter. Total and spontaneous 51Cr release was determined by incubating 2 × 103 labeled target cells in either 2% SDS or medium alone for above condition, respectively. Each data point was conducted as a mean of quadruplicate wells. The percentage of specific lysis was calculated as indicated in the figure legend.
Supplementary Material
Acknowledgments
We thank FACS laboratory and TIL laboratory in Surgery Branch for providing technical support and maintenance of tumor cells from patients. This work is supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
References
- 1.Thomas S, Hart DP, Xue SA, Cesco-Gaspere M, Stauss HJ. T-cell receptor gene therapy for cancer: the progress to date and future objectives. Expert Opin Biol Ther. 2007;7:1207–1218. doi: 10.1517/14712598.7.8.1207. [DOI] [PubMed] [Google Scholar]
- 2.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper LJ, Topp MS, Pinzon C, Plavec I, Jensen MC, Riddell SR, et al. Enhanced transgene expression in quiescent and activated human CD8+ T cells. Hum Gene Ther. 2004;15:648–658. doi: 10.1089/1043034041361217. [DOI] [PubMed] [Google Scholar]
- 4.Ikawa M, Tanaka N, Kao WW, Verma IM. Generation of transgenic mice using lentiviral vectors: a novel preclinical assessment of lentiviral vectors for gene therapy. Mol Ther. 2003;8:666–673. doi: 10.1016/s1525-0016(03)00240-5. [DOI] [PubMed] [Google Scholar]
- 5.Pfeifer A, Ikawa M, Dayn Y, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci USA. 2002;99:2140–2145. doi: 10.1073/pnas.251682798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 7.Sauce D, Bodinier M, Garin M, Petracca B, Tonnelier N, Duperrier A, et al. Retrovirus-mediated gene transfer in primary T lymphocytes impairs their anti-Epstein–Barr virus potential through both culture-dependent and selection process-dependent mechanisms. Blood. 2002;99:1165–1173. doi: 10.1182/blood.v99.4.1165. [DOI] [PubMed] [Google Scholar]
- 8.Sauce D, Tonnelier N, Duperrier A, Petracca B, de Carvalho Bittencourt M, Saadi M, et al. Influence of ex vivo expansion and retrovirus-mediated gene transfer on primary T lymphocyte phenotype and functions. J Hematother Stem Cell Res. 2002;11:929–940. doi: 10.1089/152581602321080592. [DOI] [PubMed] [Google Scholar]
- 9.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang AH, Sadelain M. The genetic engineering of hematopoietic stem cells: the rise of lentiviral vectors, the conundrum of the LTR, and the promise of lineage-restricted vectors. Mol Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- 11.Wiznerowicz M, Trono D. Harnessing HIV for therapy, basic research and biotechnology. Trends Biotechnol. 2005;23:42–47. doi: 10.1016/j.tibtech.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Krogsgaard M, Davis MM. How T cells ‘see’ antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002;2:512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 14.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan RA, Couture L, Elroy-Stein O, Ragheb J, Moss B, Anderson WF. Retroviral vectors containing putative internal ribosome entry sites: development of a polycistronic gene transfer system and applications to human gene therapy. Nucleic Acids Res. 1992;20:1293–1299. doi: 10.1093/nar/20.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Zhan X, D’Costa J, Tanavde VM, Ye Z, Peng T, et al. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther. 2003;7:827–838. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 21.Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol. 2005;23:108–116. doi: 10.1038/nbt1049. [DOI] [PubMed] [Google Scholar]
- 22.Osti D, Marras E, Ceriani I, Grassini G, Rubino T, Vigano D, et al. Comparative analysis of molecular strategies attenuating positional effects in lentiviral vectors carrying multiple genes. J Virol Methods. 2006;136:93–101. doi: 10.1016/j.jviromet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther. 2005;5:627–638. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- 24.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 26.van de Ven WJ, Voorberg J, Fontijn R, Pannekoek H, van den Ouweland AM, van Duijnhoven HL, et al. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990;14:265–275. doi: 10.1007/BF00429896. [DOI] [PubMed] [Google Scholar]
- 27.Holst J, Vignali KM, Burton AR, Vignali DA. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat Methods. 2006;3:191–197. doi: 10.1038/nmeth858. [DOI] [PubMed] [Google Scholar]
- 28.Krysan DJ, Rockwell NC, Fuller RS. Quantitative characterization of furin specificity. Energetics of substrate discrimination using an internally consistent set of hexapeptidyl methylcoumarinamides. J Biol Chem. 1999;274:23229–23234. doi: 10.1074/jbc.274.33.23229. [DOI] [PubMed] [Google Scholar]
- 29.Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 30.Simmons A, Whitehead RP, Kolokoltsov AA, Davey RA. Use of recombinant lentivirus pseudotyped with vesicular stomatitis virus glycoprotein G for efficient generation of human anti-cancer chimeric T cells by transduction of human peripheral blood lymphocytes in vitro. Virol J. 2006;3:8. doi: 10.1186/1743-422X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumiyama K, Saitou N, Ueda S. Adaptive evolution of the IgA hinge region in primates. Mol Biol Evol. 2002;19:1093–1099. doi: 10.1093/oxfordjournals.molbev.a004167. [DOI] [PubMed] [Google Scholar]
- 32.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 34.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 35.Kohn DB. Lentiviral vectors ready for prime-time. Nat Biotechnol. 2007;25:65–66. doi: 10.1038/nbt0107-65. [DOI] [PubMed] [Google Scholar]
- 36.Dardalhon V, Herpers B, Noraz N, Pflumio F, Guetard D, Leveau C, et al. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Therapy. 2001;8:190–198. doi: 10.1038/sj.gt.3301378. [DOI] [PubMed] [Google Scholar]
- 37.Barklis E, Mulligan RC, Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986;47:391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- 38.Colicelli J, Goff SP. Isolation of a recombinant murine leukemia virus utilizing a new primer tRNA. J Virol. 1986;57:37–45. doi: 10.1128/jvi.57.1.37-45.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schambach A, Galla M, Maetzig T, Loew R, Baum C. Improving transcriptional termination of self-inactivating gamma-retroviral and lentiviral vectors. Mol Ther. 2007;15:1167–1173. doi: 10.1038/sj.mt.6300152. [DOI] [PubMed] [Google Scholar]
- 40.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- 41.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 2003;21:111–117. doi: 10.1634/stemcells.21-1-111. [DOI] [PubMed] [Google Scholar]
- 43.Cavalieri S, Cazzaniga S, Geuna M, Magnani Z, Bordignon C, Naldini L, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 44.Deschamps M, Robinet E, Certoux JM, Mercier P, Sauce D, De Vos J, et al. Transcriptome of retrovirally transduced CD8(+) lymphocytes: influence of cell activation, transgene integration, and selection process. Mol Immunol. 2008;45:1112–1125. doi: 10.1016/j.molimm.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Coito S, Sauce D, Duperrier A, Certoux JM, Bonyhadi M, Collette A, et al. Retrovirus-mediated gene transfer in human primary T lymphocytes induces an activation- and transduction/selection-dependent TCR-B variable chain repertoire skewing of gene-modified cells. Stem Cells Dev. 2004;13:71–81. doi: 10.1089/154732804773099272. [DOI] [PubMed] [Google Scholar]
- 46.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abad JD, Wrzensinski C, Overwijk W, De Witte MA, Jorritsma A, Hsu C, et al. T-cell receptor gene therapy of established tumors in a murine melanoma model. J Immunother. 2008;31:1–6. doi: 10.1097/CJI.0b013e31815c193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of Tcells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chinnasamy D, Milsom MD, Shaffer J, Neuenfeldt J, Shaaban AF, Margison GP, et al. Multicistronic lentiviral vectors containing the FMDV 2A cleavage factor demonstrate robust expression of encoded genes at limiting MOI. Virol J. 2006;3:14. doi: 10.1186/1743-422X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA. Generation of T-cell receptor retrogenic mice. Nat Protoc. 2006;1:406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- 52.de Felipe P, Ryan MD. Targeting of proteins derived from self-processing polyproteins containing multiple signal sequences. Traffic (Copenhagen, Denmark) 2004;5:616–626. doi: 10.1111/j.1398-9219.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 53.Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan MD, King AM, Thomas GP. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol. 1991;72(Part 11):2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- 55.Geraerts M, Willems S, Baekelandt V, Debyser Z, Gijsbers R. Comparison of lentiviral vector titration methods. BMC Biotechnol. 2006;6:34. doi: 10.1186/1472-6750-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.