Abstract
A recent report demonstrated that JCV employs serotonin receptor 2A (5HT2AR) to infect the glial cells. To assess the ability of a potent 5HT2AR blocker, risperidone, to inhibit JCV infection, we treated primary human fetal glial (PHFG) cells in-vitro with risperidone for 24 hr and inoculated with JCV(Mad1). There was no significant difference in JCV genome copies or mRNA transcripts and protein expression in treatment-naive and risperidone-treated PHFG cells. This data indicate that risperidone does not inhibit JCV(Mad1) attachment, internalization and replication in PHFG cells, and 5HT2AR blockers may not be effective in treating progressive multifocal leukoencephalopathy (PML).
Keywords: JCV, human polyomavirus, PML, serotonin receptor, risperidone, mirtazapine
Progressive multifocal leukoencephalopathy (PML), a fatal, subacute demyelinating disease of the central nervous system (CNS), is caused by human polyomavirus JC (JCV) (Padgett et al, 1971). Although, some AIDS-associated PML patients had survival benefit when treated with highly active antiretroviral therapy (HAART) (Antinori et al, 2003; Lima et al, 2007), in the post-HAART era incidence of PML has not significantly changed (Antinori et al, 2001), and PML currently is the second most frequently diagnosed neurological disorder among AIDS patients (Antinori et al, 2001). Few reports have demonstrated beneficial effect of cytosine arabinoside (Aksamit, 2001; De Luca et al, 1999; Elphick et al, 2004) and cidofovir (De Luca et al, 2001) in treating PML patients, however studies have concluded that cytosine arabinoside (Enting and Portegies, 2000) and cidofovir (Marra et al, 2002; Wyen et al, 2004) therapy have no significant therapeutic benefit. Although, clinical outcome among PML patients treated with interferon is controversial (Colosimo et al, 1992; Geschwind et al, 2001; Huang et al, 1998; Nath et al, 2006), IFN is effective in in-vitro inhibition of JCV replication and intrathecal infusion of IFN may be beneficial as an adjunct therapy for PML (Co et al, 2007). It is important to note that there is currently no proven therapy or vaccine available for treatment and prevention of the fatal disease, PML.
A recent study demonstrated that the chimera polyomavirus JC (Mad-1/SVEΔ) consisting of JCV-SV40 promoter enhancer sequences in the backbone of JCV coding region sequences employs the 5HT2AR to infect SVG-A cells, a subclone of transformed human fetal glial cells by an origin-defective SV40 mutant (Elphick et al, 2004). Altschuler and Kast (2005), have suggested that newer safer antipsychotic drugs such as risperidone, ziprasidone and olanzapine with significantly more potent 5HT2AR receptor antagonist activity, may be useful in treating or preventing PML. Since, half of the healthy adult population shed large amounts of JCV in the urine (Agostini et al, 2001; Agostini et al, 1997; Agostini et al, 1996; Shah et al, 1997), this provides an unique opportunity to test safe and easy-to-tolerate drugs such as, risperidone and mirtazapine for anti-JCV activity, using urinary tract clearance of JCV as an additional surrogate marker for potential in treating PML (Focosi et al, 2007b). Sure enough, these drugs were tested and some recent case reports have suggested survival benefit among HIV naive PML patients treated with risperidone (Focosi et al, 2007) or mitrazepam (Owczarczyk et al, 2007; Verma et al, 2007; Vulliemoz et al, 2006) and demonstrated clearance of JCV from blood (Focosi et al, 2007). It is possible that 5HT2AR blockers may be effective in controlling JCV replication in vivo. However, it was unclear whether the beneficial effect observed in these patients was the result of modification of immunosuppressive treatment leading to immune reconstitution or was contributed by the 5HT2AR blockers risperidone or mirtazapine. We recently demonstrated that human brain microvascular endothelial cells can be infected with JCV, independent of 5HT2AR (Chapagain et al, 2007). Although, PHFG cells in- vitro express 5HT2AR mRNA transcripts and protein (Chapagain et al, 2007), it remains yet unclear, whether oligodendrocytes, the primary target cells in-vivo express 5HT2AR (Santagata and Kinney, 2005) and if 5HT2AR blockers inhibit JCV replication in oligodendrocytes and are useful in preventing or treating PML.
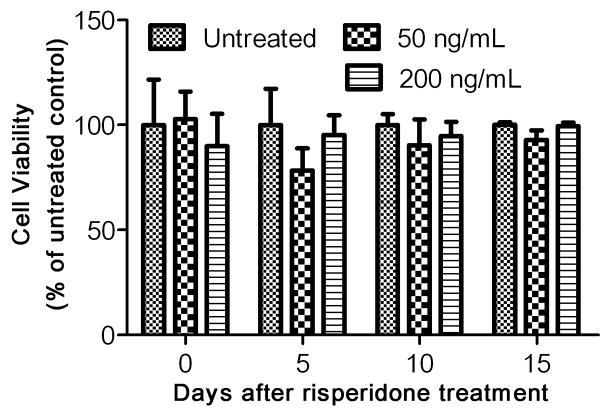
To assess the effect of risperidone on JCV infection and replication in PHFG cells, we first tested the toxicity of risperidone to the PHFG cells. PHFG cells were isolated and cultured from therapeutically aborted 10- to 14-weeks-old fetal brains, as described previously, after obtaining approval from the institutional review board of the Kapi‘olani Medical Center for Women and Children, Honolulu, Hawai‘i (Chapagain et al, 2006; Padgett et al, 1977). Approximately 5,000 PHFG cells were grown per well in a 96-well plate either with media alone or in the continuous presence of risperidone (RISPERDAL® oral solution, Cat#: NDC 50458–305–03, Janssen Pharmaceutica N.V., Beerse, Belgium). Risperidone a monoaminergic antagonist for the serotonin Type 2, dopamine Type 2, α-1 and α-2 adrenergic, and H1 histaminergic receptors, is widely used to treat psychotic disorders and has high affinity for 5HT2AR (Morisset et al, 1999). The medium, with or without risperidone was changed every fifth day and cell proliferation and viability were determined by the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) on days 0, 5, 10, 15 after-incubation with risperidone. The absorbance in each well was measured according to the manufacturer’s protocol at 490 nm using a multiplate reader (Victor3, Perkin Elmer, MA), and was expressed as percentage of untreated cells. Risperidone in concentration up to 200 ng/mL was non-toxic to PHFG cells in-vitro and PHFG cells untreated or treated with risperidone for up to 15 days did not show any change in the cell viability based on the cell proliferation assay (Fig. 1).
Fig. 1. Risperidone is non-toxic to PHFG cells.
The cell proliferation and viability of PHFG cells grown in a 96-well plate either with media alone (untreated) or in the continuous presence of risperidone 50 or 200 ng/mL was determined by the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit on days 0, 5, 10, 15 after-incubation with risperidone. The absorbance was measured at 490 nm and expressed as percentage of untreated-treated cells. Data are representative of mean of six independent samples in each group at each time point and error bars represent standard deviations.
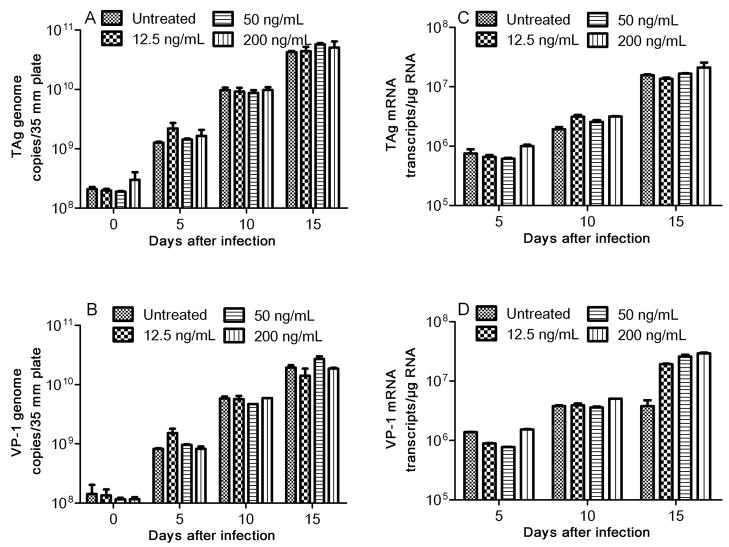
We next examined the effect of risperidone in PHFG cells infected with JCV (Mad1). PHFG cells grown to 70–80% confluency in each 35-mm culture plate, were incubated for 24 hr either with medium alone or with medium containing 12.5, 50 or 200 ng/mL of risperidone and inoculated with JCV containing 1.96 (± 0.53) × 109 and 1.36 (± 0.42) × 109 TAg and VP-1 genome copies, respectively, for 2 hr in the absence or continuous presence of risperidone. Cells were harvested on days 0, 5, 10 and 15 after-inoculation, and DNA (Qiagen QIAprep® Spin Miniprep Kit; Cat #27104) and RNA (Qiagen RNeasy® Plus kit; Cat #74134), extracted from 35-mm plates, were eluted in 100 μL and 50 μL of elution buffer, respectively. cDNA was synthesized from 0.5 μg of total RNA using Bio-Rad’s iScript® cDNA synthesis kit (Bio-Rad Inc., Hercules, CA) in a 20 μL reaction mixture. Two μL of template DNA or cDNA were amplified and quantitated in the Bio-Rad’s iCycler iQ™ Multicolor Real-Time PCR Detection System using Bio-Rad 2X iQ™ Taqman® supermix, 12.5 pmol each of forward and reverse primers and 5 pmole of probes specific for JCV TAg (forward: 5′-AGA GTG TTG GGA TCC TGT GTT TT-3′, reverse: 5′-GAG AAG TGG GAT GAA GAC CTG TTT-3′ and probe 5′-/FAM/TCA TCA CTG GCA AAC ATT TCT TCA/BHQ-1/-3′) or VP-1 (forward: 5′-ACT GTC CAT ATT TGT CAA CGT ATC-3′, reverse: 5′-AAG GTC CAG CTA AGG AAA AGG-3′ and probe 5′-/FAM/TCT GGG TCC CCT GGA AGC TCC TCT/BHQ-1/-3′) in a final reaction volume of 20 μL. Thermal cycling was initiated with the first denaturation step of 4 min at 95°C, followed by 40 cycles of 95°C (30 sec for TAg or 10 sec for VP-1) and 60°C (45 sec for TAg) or 58°C (10 sec for VP-1). Copies of JCV TAg or VP-1 genomes or mRNA transcripts in experimental samples were calculated from the standard curve and expressed as copies of viral genome per 35-mm plate or mRNA transcripts per microgram of total RNA. Based on the TAg and VP-1 genome copies, approximately 11.2% (SD±2.6%) and 9.3% (SD±0.98%), respectively, of JCV was attached or internalized into PHFG cells at 2 hr after-inoculation with JCV. However, there was no significant (p>0.05) difference in the JCV TAg (Fig. 2A) or VP-1 (Fig. 2B) genome copies recovered at 2 hr after-inoculation from PHFG cells untreated or treated with risperidone at concentrations of 12.5, 50 or 200 ng/mL suggesting that there was no effect of risperidone on JCV attachment or internalization into PHFG cells. Similar numbers of JCV TAg and VP-1 genome copies were recovered from drug naive and risperidone treated PHFG cells harvested at different time points after-inoculation (Fig 2A and 2B) suggesting that risperidone has no effect in JCV genome replication. There was no significant difference (p >0.05) in the JCV early (TAg) or late (VP-1) gene mRNA transcripts (Fig 2C and 2D) copies recovered from naive or risperidone-treated PHFG cells. Moreover, immunoprecipitation/immunoblotting of JCV T antigen protein (Chapagain et al, 2007), demonstrated similar amount of JCV T-antigen protein in naive or risperidone-treated PHFG cells harvested on days 10 and 15 after inoculation (Fig. 3) suggesting that risperidone does not have any significant effect on JCV early protein expression in PHFG cells.
Fig. 2. Risperidone has no effect in JCV infection of PHFG cells.
PHFG cells were treated with risperidone at a concentration of 12.5, 50, or 200 ng/mL for 24 hr and inoculated with JCV for 2 hr and harvested at the indicated time points for DNA or RNA extraction. JCV TAg (Fig 2A) and VP-1 (Fig. 2B) genome or TAg (Fig. 2C) and VP-1 (Fig. 2D) transcripts were amplified and quantitated by real-time PCR or real-time RT-PCR. Data are representative of duplicate samples assayed in three independent experiments. Error bars represent standard deviations.
Fig. 3. Risperidone does not inhibit JCV T antigen protein expression in PHFG cells.
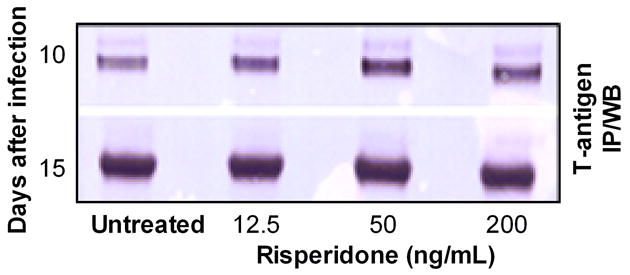
JCV-infected and naive PHFG cells were harvested at the indicated time points and JCV T antigen protein was detected in JCV-infected HBMVE cells by immunoprecipitation and Western blotting.
Since, risperidone did not have significant effect on JCV replication, it was important to demonstrate that the risperidone we employed was functional. We therefore conducted receptor binding assays on membrane preparation of HEK-293 cells transfected with 5-HT2AR pDNA. HEK-293 cells were transfected with the open reading frame (ORF) of human 5- hydroxytryptamine receptor 2A (GB accession no. NM_000621, University of Missouri cDNA resource center, Rolla, MO) using Lipofectamine™ 2000 transfection reagent (Invitrogen, cat# 11668–019), according to the manufacturer’s instructions. Cells were either untreated or treated with 12.5, 50, 200 or 1000 ng/mL of risperidone for 24 hr, harvested at 72 hr after transfection, RNA was extracted, and 0.5 μg of RNA was employed for cDNA synthesis. 5-HT2AR gene was amplified using the 5-HT2AR primer pair [forward: GGC ACA CGG GCC AAA TTA GC and reverse: TTG CTC ATT GCT GAT GGA CTG C] in a GeneAmp Thermal Cycler 9700 (Perkin- Elmer, Wellesley, MA) with 5 μL of cDNA in a 50-μL reaction mixture containing 1.0 U AmpliTaq Gold (Applied Biosystems, Foster City, CA), PCR buffer with 1.5 mM MgCl2, 0.2 mM dNTPs and 0.4 μM of each primer. An initial pre-PCR denaturation step (4 min at 95°C) was followed by 35 three-step PCR cycles of denaturation (95°C for 10 sec), annealing (58°C for 20 sec) and elongation (72°C for 30s) followed by a final elongation step (72°C for 10 min). Simultaneously, PCR was conducted using primers for house keeping gene GAPDH [forward: AGT TAG CCG CAT CTT CTT TTG C and reverse: CAA TAC GAC CAA ATC CGT TGA CT] as an internal control (Chapagain et al, 2007). The amplicons were electrophoresed on a 2% agarose gel, and the ethidium bromide fluorescence was visualized after scanning with a Bio-Rad Molecular Phosphorimager. Our data suggest that transfected HEK-293 cells expressed 5-HT2AR transcripts, and risperidone treatment at all concentrations does not inhibit 5-HT2AR transcripts levels (fig. 4A). 5-HT2AR transfection was further confirmed by immunostaining. At 72 hr after transfection, cells were fixed with 4% paraformaldehyde for 10 min, lightly permeabilized on ice (0.2% Triton X-100 in PBS) for 20 min, and incubated with blocking buffer (5% fetal bovine serum) for 1 hr. Cells were then incubated overnight with rabbit polyclonal anti 5-HT2AR antibody (Cat# sc-50396, Santa Cruz Biotech, Inc) at 1:150 dilution, washed and incubated with FITC-conjugated goat anti-rabbit IgG secondary antibody (Cat# N1034, Amersham Biosciences, 1:150) for 1 hr. The cells were washed and mounted in vectashield mounting medium with DAPI (VECTOR Laboratories; Burlingame, California). The slides were examined by the Carl Zeiss Inverted microscope and images were captured with Zeiss AxioCam MRm camera and processed with the Axio Vision Rel. 4.5 software. Our data suggest that transfected HEK-293 cells express 5-HT2AR on the cell surface (fig 4B), and risperidone treatment has no significant effect on 5-HT2AR immunoreactivity (data not shown). Further, ketanserin binding assay was conducted to analyze the effect of risperidone treatment on the binding of [Ethylene-3H]-labelled ketanserin hydrochloride, (Cat# R41 468, 25 μCi, 925 kBq) (PerkinElmer Life and Analytical Sciences, Inc, Waltham, MA) as described elsewhere (Decker et al, 2004). Briefly, HEK-293 cells were seeded on 100-mm plates and transfected with 12 μg 5-HT2AR pDNA and 24 μL of LipofectamineTM 2000 transfection reagent (Invitrogen) according to the manufacturer’s instructions. At 72 hr after transfection, cells were washed with 1X PBS, scraped and centrifuged at 1,000 rpm for 10 min. The pellet was homogenized in 50 mM Tris-HCl (pH = 7.7) with a polytron homogenizer, centrifuged at 20,000 rpm, resuspended with 25 mM Tris-HCl (pH = 7.7), and aliquoted and stored at −80°C after measuring protein concentration by the Bradford assay. On the day of assay, the protein preparation was thawed and adjusted to 5 mg protein/80 mL with 25 mM Tris-HCl (pH 7.7) and 0.8 mL of cell homogenate (0.05 mg/protein/well) was added to wells containing 100 μL of the risperidone at the final concentration of 0.125, 1.25, 12.5, 50 or 200 ng/mL or buffer, and 100 μL of [3H] ketanserin (2.0 nM final concentration). The mixture was incubated at 37°C for 30 min, reaction was stopped by rapidly filtering through the glass fiber filter paper, washed four times with ice-cold 50 mM Tris-HCl (pH = 7.7) using FilterMate™ Cell Harvester (Packard Instrument Company, Meriden, CT), dried for 4 hr, and the radioactivity was measured using a Tri-Carb 2900TR Scintillation Counter (Perkin-Elmer). Nonspecific binding was determined by incubating with 10.0 μM ritanserin. Risperidone at concentrations of 0.125, 1.25, 12.5, 50 or 200 ng/mL reduced [3H] ketanserin binding to 67.58 (±4.28), 13.48 (±2.35), 0.21 (±1.1), 0.48 (±3.28) and 0.16 (±0.26) percent of the binding of untreated control respectively (fig. 4D). These data indicate that risperidone significantly reduces ketanserin binding in a dose dependent manner (fig 4D), and confirms that the risperidone we employed is functional.
Fig. 4. Transfection of HEK-293 cells with 5-HT2AR pDNA construct.
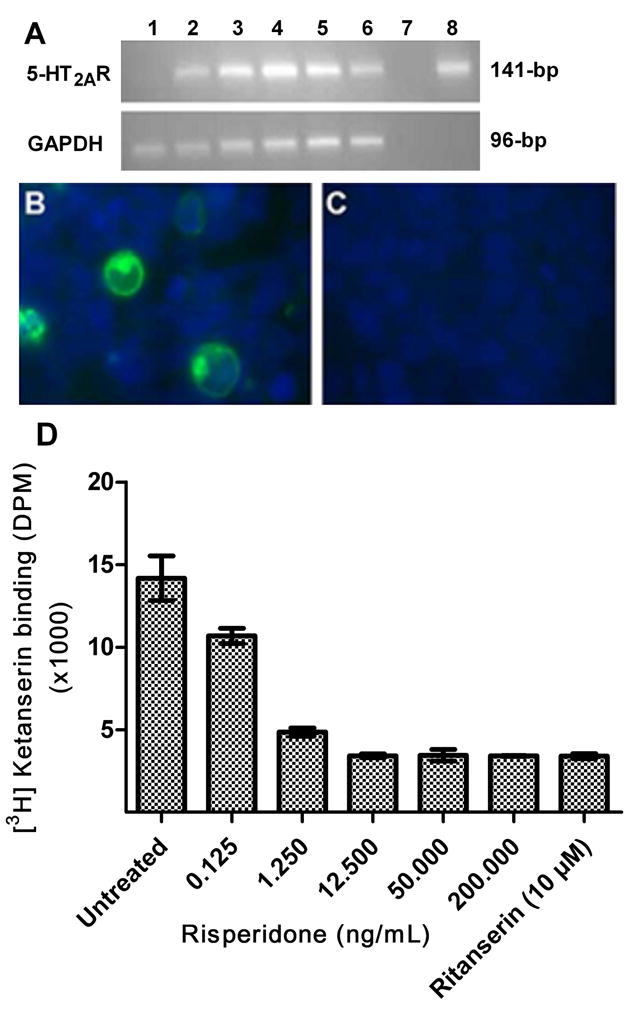
(A) HEK-293 cells at 72 hr after transfection expressed 5-HT2AR transcripts but risperidone treatment had no negative effect on mRNA expression. HEK-293 cells untransfected (lane 1); transfected and untreated (lane 2); transfected and treated with 12.5 (lane 3); 50 (lane 4), 200 (lane 5); and 1,000 (lane 6) ng/mL of risperidone; transfected and untreated HEK-293 cells without reverse transcriptase (negative control) (lane 7); and 5-HT2AR pDNA (positive control) (lane 8). (B) Transfected HEK-293 cells demonstrated 5-HT2AR immunoreactivity (green), and nuclei were stained with bisbenzidine (blue). (C) Control empty vector transfected cells. (D) Ketanserin binding is inhibited by risperidone in a dose dependent manner. [Ethylene-3H]-labeled ketanserin hydrochloride with or without risperidone was co-incubated with HEK293 membrane protein preparation and the radioactivity was measured. Data represents the mean DPM with standard deviation.
Our data suggest that risperidone, a potent 5HT2AR blocker (Altschuler and Kast, 2005; Morisset et al, 1999), does not inhibit JCV entry and replication in PHFG cells in in-vitro. This result is consistent with our previous study demonstrating 5HT2AR independent JCV infection of HBMVE cells (Chapagain et al, 2007), however, it contradicts with the previous study that suggested JCV uses 5HT2AR to infect glial cells (Elphick et al, 2004). This inconsistency may be attributed to the use of different JCV strains or cells. While Elphick and colleagues employed chimeric JCV that has SV40 promoter, in this study, we used the JCV(Mad1), which is predominantly found among brains of PML patients (Dubois et al, 2001). It is possible that chimeric JCV (Mad-1/SVEΔ) behaves differently than JCV(Mad1) found in the PML brains. Moreover, we used PHFG cells, that most closely resemble the JCV target cells population in-vivo whereas Elphick et al used SVG-A cells, a subclone of SV-40 transformed human fetal glial cells (Elphick et al, 2004). It is also possible that JCV may use different receptors to infect different target cells. Although, PHFG cells in-vitro express 5HT2AR mRNA transcripts and protein (Chapagain et al, 2007), 5HT2AR immunoreactivity was not detected in oligodendrocytes of rat spinal cord (Maxishima et al, 2001), and there is as yet no clear report demonstrating that human oligodendrocytes express 5HT2AR (Santagata and Kinney, 2005). Interestingly, a recent publication also suggests that there was only a modest effect if any of risperidone on JCV infection of SVGA cells and more importantly, there was no significant difference in JC viral load between 5-HT2AR blockers-treated and -untreated SVGA cells, which concur with our findings (O’Hara and Atwood, 2008). It is possible that human oligodendrocytes may not express 5HT2AR, JCV may employ receptors other than 5HT2AR to infect oligodendrocytes, and 5HT2AR blockers may not be effective in treating PML. Further studies are essential to demonstrate expression of 5HT2AR in oligodendrocytes, the primary target cells of JCV in the human brain, and to document the effectiveness of 5HT2AR blockers in reducing JCV viral load in clinical trials before widespread use of 5HT2AR blockers for treatment or prevention of PML.
Acknowledgments
This work was supported by U.S. Public Health Service grants from the Collaborative Neurological Sciences Program (S11 NS041833) and the Specialized Neurosciences Research Program (U54 NS039406), National Institute of Neurological Disorders and Stroke, as well as from the Research Centers in Minority Institutions Program (G12 RR003061) and Centers of Biomedical Research Excellence (P20 RR018727), National Center for Research Resources, National Institutes of Health. We thank Dr. Pratibha V. Nerurkar for assistance with the ketanserin binding assay, and the staff and students of the Retrovirology Research Laboratory for their technical assistance.
References
- Agostini HT, Deckhut A, Jobes DV, Girones R, Schlunck G, Prost MG, Frias C, Perez-Trallero E, Ryschkewitsch CF, Stoner GL. Genotypes of JC virus in East, Central and Southwest Europe. J Gen Virol. 2001;82:1221–331. doi: 10.1099/0022-1317-82-5-1221. [DOI] [PubMed] [Google Scholar]
- Agostini HT, Ryschkewitsch CF, Singer EJ, Stoner GL. JC virus regulatory region rearrangements and genotypes in progressive multifocal leukoencephalopathy: two independent aspects of virus variation. J Gen Virol. 1997;78 ( Pt 3):659–64. doi: 10.1099/0022-1317-78-3-659. [DOI] [PubMed] [Google Scholar]
- Agostini HT, Ryschkewitsch CF, Stoner GL. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996;34:159–64. doi: 10.1128/jcm.34.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksamit AJ. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirol. 2001;7:386–90. doi: 10.1080/13550280152537292. [DOI] [PubMed] [Google Scholar]
- Altschuler EL, Kast RE. The atypical antipsychotic agents ziprasidone [correction of zisprasidone], risperdone and olanzapine as treatment for and prophylaxis against progressive multifocal leukoencephalopathy. Med Hypotheses. 2005;65:585–6. doi: 10.1016/j.mehy.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Antinori A, Ammassari A, Giancola ML, Cingolani A, Grisetti S, Murri R, Alba L, Ciancio B, Soldani F, Larussa D, Ippolito G, De Luca A. Epidemiology and prognosis of AIDS-associated progressive multifocal leukoencephalopathy in the HAART era. J Neurovirol. 2001;7:323–8. doi: 10.1080/13550280152537184. [DOI] [PubMed] [Google Scholar]
- Antinori A, Cingolani A, Lorenzini P, Giancola ML, Uccella I, Bossolasco S, Grisetti S, Moretti F, Vigo B, Bongiovanni M, Del Grosso B, Arcidiacono MI, Fibbia GC, Mena M, Finazzi MG, Guaraldi G, Ammassari A, d’Arminio Monforte A, Cinque P, De Luca A. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA) J Neurovirol. 2003;9(Suppl 1):47–53. doi: 10.1080/13550280390195388. [DOI] [PubMed] [Google Scholar]
- Chapagain ML, Nguyen T, Bui T, Verma S, Nerurkar VR. Comparison of real-time PCR and hemagglutination assay for quantitation of human polyomavirus JC. Virol J. 2006;3:3. doi: 10.1186/1743-422X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapagain ML, Verma S, Mercier F, Yanagihara R, Nerurkar VR. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology. 2007;364:55–63. doi: 10.1016/j.virol.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co JK, Verma S, Gurjav U, Sumibcay L, Nerurkar VR. Interferon- alpha and - beta restrict polyomavirus JC replication in primary human fetal glial cells: implications for progressive multifocal leukoencephalopathy therapy. J Infect Dis. 2007;196:712–8. doi: 10.1086/520518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo C, Lebon P, Martelli M, Tumminelli F, Mandelli F. Alpha-interferon therapy in a case of probable progressive multifocal leukoencephalopathy. Acta Neurol Belg. 1992;92:24–9. [PubMed] [Google Scholar]
- De Luca A, Giancola ML, Ammassari A, Grisetti S, Cingolani A, Larussa D, Alba L, Murri R, Ippolito G, Cauda R, Monforte A, Antinori A. Potent anti-retroviral therapy with or without cidofovir for AIDS-associated progressive multifocal leukoencephalopathy: extended follow-up of an observational study. J Neurovirol. 2001;7:364–8. doi: 10.1080/13550280152537256. [DOI] [PubMed] [Google Scholar]
- De Luca A, Giancola ML, Cingolani A, Ammassari A, Gillini L, Murri R, Antinori A. Clinical and virological monitoring during treatment with intrathecal cytarabine in patients with AIDS-associated progressive multifocal leukoencephalopathy. Clin Infect Dis. 1999;28:624–8. doi: 10.1086/515153. [DOI] [PubMed] [Google Scholar]
- Decker M, Schleifer KJ, Nieger M, Lehmann J. Dopamine/serotonin receptor ligands. Part VIII: the dopamine receptor antagonist LE300 - modelled and X-ray structure plus further pharmacological characterization, including serotonin receptor binding, biogenic amine transporter testing and in vivo testings. Eur J Med Chem. 2004;39:481–9. doi: 10.1016/j.ejmech.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Dubois V, Moret H, Lafon ME, Brodard V, Icart J, Ruffault A, Guist’hau O, Buffet-Janvresse C, Abbed K, Dussaix E, Ingrand D. JC virus genotypes in France: molecular epidemiology and potential significance for progressive multifocal leukoencephalopathy. J Infect Dis. 2001;183:213–217. doi: 10.1086/317927. [DOI] [PubMed] [Google Scholar]
- Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, Dugan A, Stanifer M, Bhatnagar A, Kroeze WK, Roth BL, Atwood WJ. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–3. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- Enting RH, Portegies P. Cytarabine and highly active antiretroviral therapy in HIV-related progressive multifocal leukoencephalopathy. J Neurol. 2000;247:134–8. doi: 10.1007/pl00007794. [DOI] [PubMed] [Google Scholar]
- Focosi D, Fazzi R, Montanaro D, Emdin M, Petrini M. Progressive multifocal leukoencephalopathy in a haploidentical stem cell transplant recipient: a clinical, neuroradiological and virological response after treatment with risperidone. Antiviral Res. 2007;74:156–8. doi: 10.1016/j.antiviral.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Focosi D, Kast RE, Maggi F, Ceccherini-Nelli L, Petrini M. Risperidone-induced reduction in JC viruria as a surrogate marker for efficacy against progressive multifocal leukoencephalopathy and hemorrhagic cystitis. J Clin Virol. 2007b;39:63–4. doi: 10.1016/j.jcv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Geschwind MD, Skolasky RI, Royal WS, McArthur JC. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J Neurovirol. 2001;7:353–7. doi: 10.1080/13550280152537238. [DOI] [PubMed] [Google Scholar]
- Huang SS, Skolasky RL, Dal Pan GJ, Royal W, 3rd, McArthur JC. Survival prolongation in HIV-associated progressive multifocal leukoencephalopathy treated with alpha-interferon: an observational study. J Neurovirol. 1998;4:324–32. doi: 10.3109/13550289809114533. [DOI] [PubMed] [Google Scholar]
- Lima MA, Katz-Brull R, Lenkinski RE, Nunez R, Feinrider D, Koralnik IJ. Remission of progressive multifocal leukoencephalopathy and primary central nervous system lymphoma in an HIV-infected patient. Eur J Neurol. 2007;14:598–602. doi: 10.1111/j.1468-1331.2007.01820.x. [DOI] [PubMed] [Google Scholar]
- Marra CM, Rajicic N, Barker DE, Cohen BA, Clifford D, Donovan Post MJ, Ruiz A, Bowen BC, Huang ML, Queen-Baker J, Andersen J, Kelly S, Shriver S. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. Aids. 2002;16:1791–7. doi: 10.1097/00002030-200209060-00012. [DOI] [PubMed] [Google Scholar]
- Maxishima M, Shiga T, Shutoh F, Hamada S, Maeshima T, Okado N. Serotonin 2A receptor-like immunoreactivity is detected in astrocytes but not in oligodendrocytes of rat spinal cord. Brain Res. 2001;889:270–3. doi: 10.1016/s0006-8993(00)03150-4. [DOI] [PubMed] [Google Scholar]
- Morisset S, Sahm UG, Traiffort E, Tardivel-Lacombe J, Arrang JM, Schwartz JC. Atypical neuroleptics enhance histamine turnover in brain via 5-Hydroxytryptamine2A receptor blockade. J Pharmacol Exp Ther. 1999;288:590–6. [PubMed] [Google Scholar]
- Nath A, Venkataramana A, Reich DS, Cortese I, Major EO. Progression of progressive multifocal leukoencephalopathy despite treatment with beta-interferon. Neurology. 2006;66:149–50. doi: 10.1212/01.wnl.0000191322.93310.a1. [DOI] [PubMed] [Google Scholar]
- O’Hara BA, Atwood WJ. Interferon beta1-a and selective anti-5HT(2a) receptor antagonists inhibit infection of human glial cells by JC virus. Virus Res. 2008;132:97–103. doi: 10.1016/j.virusres.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owczarczyk K, Hilker R, Brunn A, Hallek M, Rubbert A. Progressive multifocal leucoencephalopathy in a patient with sarcoidosis--successful treatment with cidofovir and mirtazapine. Rheumatology (Oxford) 2007;46:888–90. doi: 10.1093/rheumatology/kem049. [DOI] [PubMed] [Google Scholar]
- Padgett BL, Rogers CM, Walker DL. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977;15:656–62. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1:1257–60. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Santagata S, Kinney HC. Mechanism of JCV entry into oligodendrocytes. Science. 2005;309:381–2. doi: 10.1126/science.309.5733.381b. [DOI] [PubMed] [Google Scholar]
- Shah KV, Daniel RW, Strickler HD, Goedert JJ. Investigation of human urine for genomic sequences of the primate polyomaviruses simian virus 40, BK virus, and JC virus. J Infect Dis. 1997;176:1618–21. doi: 10.1086/517340. [DOI] [PubMed] [Google Scholar]
- Verma S, Cikurel K, Koralnik IJ, Morgello S, Cunningham-Rundles C, Weinstein ZR, Bergmann C, Simpson DM. Mirtazapine in progressive multifocal leukoencephalopathy associated with polycythemia vera. J Infect Dis. 2007;196:709–11. doi: 10.1086/520514. [DOI] [PubMed] [Google Scholar]
- Vulliemoz S, Lurati-Ruiz F, Borruat FX, Delavelle J, Koralnik IJ, Kuntzer T, Bogousslavsky J, Picard F, Landis T, Du Pasquier RA. Favourable outcome of progressive multifocal leucoencephalopathy in two patients with dermatomyositis. J Neurol Neurosurg Psychiatry. 2006;77:1079–82. doi: 10.1136/jnnp.2006.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyen C, Hoffmann C, Schmeisser N, Wohrmann A, Qurishi N, Rockstroh J, Esser S, Rieke A, Ross B, Lorenzen T, Schmitz K, Stenzel W, Salzberger B, Fatkenheuer G. Progressive multifocal leukencephalopathy in patients on highly active antiretroviral therapy: survival and risk factors of death. J Acquir Immune Defic Syndr. 2004;37:1263–8. doi: 10.1097/01.qai.0000136093.47316.f3. [DOI] [PubMed] [Google Scholar]