Abstract
Context: Resistance to TSH (RTSH) is a condition of impaired responsiveness of the thyroid gland to TSH, characterized by elevated serum TSH, low or normal thyroid hormone levels, and hypoplastic or normal-sized thyroid gland.
Objectives: The aim of the study was to evaluate the clinical course and the genotype-phenotype relationship of RTSH caused by two different TSH receptor (TSHR) gene mutations in a consanguineous population.
Patients and Methods: We conducted a clinical and genetic investigation of 46 members of an extended family and 163 individuals living in the same town. In vitro functional studies of the mutant TSHRs were also performed.
Results: Two TSHR gene mutations (P68S and L653V) were identified in 33 subjects occurring as homozygous L653V (five subjects), heterozygous L653V (20 subjects), heterozygous P68S (four subjects), and compound heterozygous L653V/P68S (four subjects). With the exception of one individual with concomitant autoimmune thyroid disease, all homozygotes and compound heterozygotes presented with compensated RTSH (high TSH with free T4 and T3 in the normal range). Only nine of 24 heterozygotes had mild hyperthyrotropinemia. The L653V mutation resulted in a higher serum TSH concentration and showed a more severe in vitro abnormality than P68S. Haplotype analysis predicted a founder of the L653V six to seven generations earlier, whereas the P68S is older. Cross-sectional and prospective longitudinal studies indicate that TSH and T4 concentrations remain stable over time.
Conclusions: High frequency hyperthyrotropinemia in an Israeli Arab-Muslim consanguineous community is attributed to two inactivating TSHR gene mutations. Concordant genotype-phenotype was demonstrated clinically and by in vitro functional analysis. Retrospective and prospective studies indicate that in the absence of concomitant autoimmune thyroid disease, elevated TSH levels reflect stable compensated RTSH.
Subjects with TSHR mutations producing partial loss-of-function show no significant change of thyroid function over time.
Resistance to TSH (RTSH) is a syndrome of reduced sensitivity to TSH. It is characterized by elevated serum TSH concentrations, absence of goiter (normal or hypoplastic thyroid gland), and normal to very low levels of thyroid hormones (1). The G protein-coupled TSH receptor (TSHR) gene, located on chromosome 14q31, encodes a protein with a large N-terminal ligand-binding extracellular domain, a heptahelical transmembrane, and an intracellular domain. Mutations in the TSHR gene result in either gain or loss of the receptor function. Loss-of-function mutations are mainly recessively inherited and lead to a spectrum of phenotypes, ranging from euthyroid hyperthyrotropinemia to severe congenital hypothyroidism (1,2). The degree of insensitivity to TSH depends on the type and location of the TSHR mutations. Since the first report of familial euthyroid hyperthyrotropinemia caused by a TSHR gene mutation (3), several cases of loss-of-function mutations of the TSHR have been reported; the majority are missense mutations, but deletions and insertions have been identified as well (4) (see http://gris.ulb.ac.be/ and OMIM no. 275200).
Loss-of-function mutations have been reported in 40 families and 24 individuals without family studies. Affected individuals of 17 families were homozygous, 16 compound heterozygotes, and in seven families all affected members were heterozygous. Approximately half of the mutant TSHRs have partial impairment of function, and in one third of these cases affected subjects could maintain a euthyroid state by an appropriate increase in serum TSH levels (fully compensated RTSH) (3,5,6). More severe loss of TSHR function manifests as mild or borderline hypothyroidism (partially compensated RTSH) (7,8). When both alleles carry mutant receptors with complete lack of function, the result is severe hypothyroidism (uncompensated RTSH), usually presenting as congenital hypothyroidism (9,10,11,12,13). Despite several reports of patients affected with TSHR gene mutations, there are limited data on the outcome of this condition (14). Whether compensated hyperthyrotropinemia progresses to clinical hypothyroidism, remains stable, or normalizes over time has yet to be elucidated. Controversy exists about the need for l-T4 therapy in these patients (15).
We previously reported a family of five siblings, three of whom had RTSH due to a loss-of-function TSHR gene mutation (L653V) with strong impairment of the inositol phosphate signaling cascade (16). This family was subsequently found to belong to an extended kindred of a highly consanguineous community living in northern Israel. In addition to L653V, we identified another TSHR gene mutation (P68S). These mutations were identified in 33 individuals that were homozygous, heterozygous, or compound heterozygous. Screening 163 random individuals also assessed the frequencies of the two mutant alleles in the town’s population. Our finding of TSHR mutations in a large cohort of patients with carefully kept medical records allowed us to evaluate the clinical course over time and the genotype-phenotype association of the RTSH syndrome resulting from the two different TSHR gene mutations.
Subjects and Methods
Studies were approved by the review board of Ha’Emek Medical Center, the Israeli Ministry of Health, and the University of Chicago.
Original (index) family and extended kindred
The proposita of Arab-Muslim descent presented at the age of 10 yr with sinus tachycardia. Laboratory tests revealed compensated hyperthyrotropinemia [TSH, 53 (normal range, 0.4–3.6) mU/liter; free T4 (FT4), 13.3 (normal range, 11–18.8) pmol/liter; and total T3, 2.49 (normal range, 1.34–3.35) nmol/liter]. Two of her sisters had compensated hyperthyrotropinemia as well. A missense mutation (c.1957C >G) in the TSHR gene, resulting in the replacement of the normal leucine with a valine (L653V), was identified in both alleles of the three sisters (subjects IV-6, IV-9, and IV-10 in Fig. 1) (16). These findings prompted the study of individuals with compensated hyperthyrotropinemia in the community and ultimately the discovery that some belonged to the extended kindred of the index family.
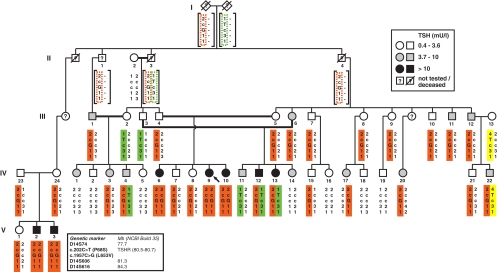
Figure 1.
Pedigree of the extended family with RTSH. The shadings of symbols indicate ranges of serum TSH concentrations as explained in the legend on the upper right corner. Subjects were tested for the indicated polymorphic markers. The haplotypes are shown below each symbol, and different colors help to trace the inheritance of the different alleles harboring the mutations, as indicated in the legend on the bottom left. Predicted haplotypes are in squared brackets.
Forty-six subjects belonging to 11 nuclear families linked to the original family were identified and agreed to participate in the study. The extended family belongs to a hamulah (clan) that lives in the same town of 45,000 inhabitants, located in northern Israel. The clan consists of approximately 15,000 members who share a common ancestor six generations back. The founder presumably belonged to a tribe originating from the southern Arabian peninsula. The combination of recent common ancestry and intense cultural consanguinity accounts for a high inbreeding rate in this population. Members of each family underwent clinical, hormonal, and genetic evaluation. During the course of this study, another novel mutation, a C to T transition in codon 68 (P68S), was identified.
Control subjects
To determine the overall prevalence of the two TSHR gene mutations, blood samples were obtained from 163 unrelated individuals from the same town presenting at the local clinic for non-thyroid-related medical reasons. Thyroid function tests were obtained from all carriers of P68S and L653V TSHR gene mutations. Twenty-two age- and gender-matched individuals, negative for these two mutations, were selected to serve as controls for thyroid function tests. Four were excluded because of detectable thyroperoxidase and/or thyroglobulin (TG) antibodies.
Thyroid function tests
Total T4, total T3, and TSH were measured by chemiluminescence immunometric assays using the Elecsys Automated System (Roche Molecular Biochemicals GmbH and Hitachi, Ltd., both located in Indianapolis, IN). rT3 was measured by a commercial RIA (Adaltis Italia S.p.A, Bologna, Italy) and TG by an in-house RIA (17). The serum free T4 and free T3 indexes (FT4I and FT3I, respectively) were calculated as the product of their total serum concentrations and the normalized resin T4 uptake ratio. Antibodies against TG and thyroperoxidase were measured by passive hemagglutination (Fujirebio, Inc., Tokyo, Japan). FT4 and FT3 concentrations were measured by a direct automated chemiluminescence assay using ADVIA Centaur (Bayer Corporation, Tarrytown, NY). Data are expressed as mean ± sd and analyzed by ANOVA.
Linkage analysis and mutation screening of the TSHR gene
Three microsatellite markers (D14S74, D14S606, and D14S616) spanning the TSHR locus were used for haplotyping as detailed previously (18). For sequencing, all coding regions of the TSHR gene were amplified using primers and thermocycler settings similar to those previously described (19). Presence of the TSHR gene mutations was confirmed by electrophoretic analysis of PCR products after restriction endonuclease digestion with XmnI (for P68S mutation) or MnlI (for L653V mutation).
In vitro analyses
An expression vector for the P68S mutant TSHR was constructed by site-directed mutagenesis (Quikchange II; Stratagene, La Jolla, CA) of the wild-type (WT) TSHR sequence cloned in the plasmid pSVL (20), and the sequence was confirmed. The expression vector for the L653V mutant TSHR was prepared as described previously (16). The reporter construct pCRE-Luc (Clontech Laboratory Inc., Palo Alto, CA) contains two copies of the cAMP-responsive element (CRE; TGACGTCA) directing expression of firefly luciferase.
For functional analysis, HEK-293 cells were maintained in DMEM (Life Technologies Inc., Gaithersburg, MD) supplemented with 2 mm l-glutamine, 4.5 g/liter d-glucose, 50 U/ml penicillin, 50 mg/ml streptomycin, and 5% fetal bovine serum under 5% CO2/95% air at 37 C. Cells grown in 12-well plates to 70–80% confluence were cotransfected with 0.5 μg of reporter plasmid, 3 ng of pRL-Tk internal control vector (Promega Corp., Madison, WI), and 125–250 ng of effector plasmid/empty vector per well using FuGENE 6 reagent (Roche Applied Science, Indianapolis, IN). The medium was replaced 12 h after transfection by fresh medium with or without various doses of bovine TSH (Sigma, St. Louis, MO). Cells were harvested 18 h later and analyzed sequentially for firefly and Renilla luciferase activities (Dual-Luciferase Reporter Assay System; Promega). The ratios between the measured firefly and Renilla luciferase activities were expressed relative to the ratios obtained in cells transfected with reporter and empty pSVL vector only.
Cell-surface expression of WT and mutant TSHR proteins expressed in COS-7 cells was quantified by flow immunocytofluorometry (FACS) 48 h after transfection (21).
Results
Genetic and clinical results
The TSHR loss-of-function missense mutation (L653V) found in three homozygous siblings of the index family (subjects IV-6, IV-9, and IV-10; Fig. 1) was previously reported (16). Both parents and two other siblings were heterozygous for this mutation and had normal thyroid function tests. Detailed family history identified 10 additional nuclear families (Fig. 1), some of which had family members with hyperthyrotropinemia and have been followed on and off l-T4 treatment. Sequencing of the entire TSHR gene of subject IV-12 revealed a second mutation, a C to T transition in codon 68. This mutation results in the substitution of the normal phenylalanine with a serine (P68S) in the ectodomain of the receptor.
Of a total of 46 family members studied, 33 were found to harbor TSHR gene mutations. Five were homozygous for L653V, 20 heterozygous for L653V, four heterozygous for P68S, and four compound heterozygous for L653V and P68S (Fig. 1 and Supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). None were homozygous for the P68S mutation. The five subjects homozygous for the L653V mutation included the three sisters from the original nuclear family and two brothers from another nuclear family (V-2 and V-3; Fig. 1). With the exception of IV-6 with autoimmune thyroid disease (AITD) (Supplemental Table 1), all family members with TSHR gene mutations had normal FT4I and FT3I and had no clinical stigmata of hypothyroidism. No thyroid cancer, nodular goiter, or deafness was found in subjects included in this study or reported in the extended kindred.
Newborn screening for hypothyroidism in Israel uses dried blood T4 measurement. A value less than 10 μg/dl (formerly 7 μg/dl) prompts a TSH determination in the same blood spot. This explains why, despite markedly elevated TSH levels when tested later in life, only one of five homozygous subjects (V-3; total T4, 8.8 μg/dl; TSH, 61 mU/liter) was identified by the national neonatal screening. At the age of 3 wk, TSH was 90 mU/liter, FT4 was 15.6 pmol/liter (normal range, 9.9–22.7), and FT3 was 5.97 pmol/liter (normal range, 3.5–6.5). His x-ray showed normal ossification of the distal femur. On d 21, l-T4 therapy was started at a dose of 50 μg/d. At the age of 2 yr, l-T4 therapy was withdrawn, and thyroid function tests revealed compensated hyperthyrotropinemia.
Genotype frequency in the local population
The prevalence of the two TSHR mutations in the town’s population was determined by genotyping of 163 consecutive patients with no known thyroid disease. Four were heterozygous for L653V, and three were heterozygous for P68S. Three of the four who harbored one L653V allele and one who had the P68S allele belonged to the same clan as the index family.
Despite substantial frequency of consanguineous marriages, the observed genotype frequencies in the randomly obtained control samples were compatible with Hardy-Weinberg equilibrium (by χ2 test). Under this assumption, the estimated allelic frequencies are P ∼ 0.012 for L653V and P ∼ 0.009 for P68S. The combined frequency of L653V homozygosity and L653V/P68S compound heterozygosity can be estimated as 1/2650; 2.4% of the local population of 45,000 are predicted to be heterozygous carriers of the L653V mutation. Based on the finding that one third of these heterozygotes (eight of 24; Fig. 2) have serum TSH levels above the upper limit of normal, 360 individuals in the local population are expected to have elevated serum TSH levels secondary to the mutation.
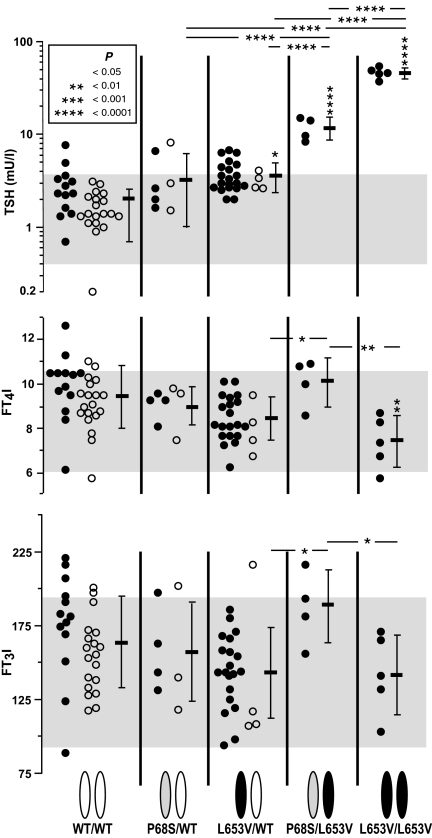
Figure 2.
Serum TSH (upper graph), FT4I (middle graph), and FT3I (lower graph) concentrations in individual subjects grouped according to the genotype indicated on the bottom of the figure. Closed symbols represent members of the extended family. Open symbols represent mutation carriers identified by screening the population and the corresponding normal WT controls matched for age and gender. Mean values ± sd for each genotype group are shown on the left of data points. Asterisks above the sd bar indicate P values for difference with the group expressing only the WT alleles.
Haplotype analysis
In all cases, the L653V allele was found on the same three-marker haplotype spanning 5.3 cM. Based on the segregation of the mutant allele in the extended kindred, we assume a minimum of five generations between extant mutation carriers and the most recent common ancestor segregating the mutant allele. The P68S mutation was observed on two distinct haplotypes, differing at D14S74 (Fig. 1). The sequence context of the mutated site does not lend support for recurrent mutational events, suggesting that recombinations between the markers and the TSHR locus and/or marker mutations had occurred since the mutation of the original haplotype. The P68S mutation was also more frequently detected in the random controls not considered to be members of the clan. Together, these findings support the notion that P68S is an even older variant that may well be found in other populations of Arab ethnicity.
Phenotype-genotype correlation (Fig. 2)
Individuals homozygous for L653V had significantly higher TSH levels than any of the other genotypes. The mean TSH level in compound heterozygotes was significantly higher than that of heterozygotes and carriers of the WT allele only. Heterozygotes for the L653V allele, but not for the P68S allele, also had significantly higher mean TSH level than family members with WT allele only.
Compared with individuals homozygous for the WT allele, L653V homozygotes had significantly lower mean FT4I but not FT3I. However, the P value increased to less than 0.05 when subject IV-6 with AITD was excluded from the calculation. For unknown reasons, both FT4I and FT3I were significantly higher in compound heterozygotes compared with heterozygous and homozygous carriers of the L653V allele. No significant differences in the mean T3/T4 ratios were found between the different genotype groups (data not shown).
Effect of age and passage of time on thyroid function tests
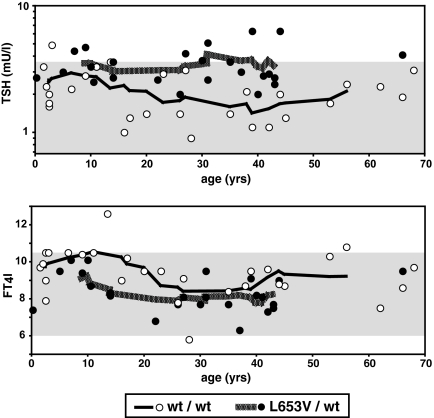
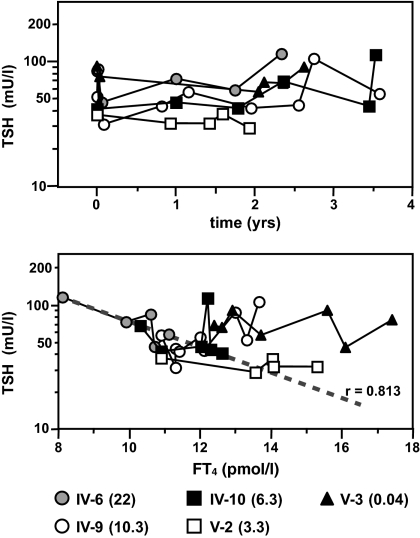
Compared with subjects with the WT allele only, heterozygotes for L653V had increased mean TSH levels in individuals older than 20 yr (mean TSH, 3.5 ± 1.4 vs. 1.9 ± 0.7 mU/liter; P < 0.005) and younger (1–20 yr of age; mean TSH, 3.4 ± 0.8 vs. 2.5 ± 1.1 mU/liter; P < 0.04; one-tailed t test) (Fig. 3). In a prospective study, we examined the evolution of TSH and FT4 in the five homozygotes for the L653V mutation over the period of 2 to 3.5 yr during which five to nine samples were obtained from each subject. Linear regressions generated by the method of least mean square were analyzed for the significance of the slopes. TSH values fluctuated without a significant change with time (Fig. 4). With the exception of one subject (IV-6), there was no correlation between the TSH and FT4 levels within the same individual (Fig. 4). Subject IV-6 with AITD was the only individual with a borderline low FT4I (Supplemental Table 1) and a significant inverse correlation between TSH and FT4 (Fig. 4).
Figure 3.
Serum TSH (upper graph) and FT4I (lower graph) concentrations in individual subjects graphed according to age. Shaded areas are the respective normal ranges. Open symbols are homozygotes for the WT allele, and closed symbols are heterozygotes for L653V. Note that there is no trend of change with age for both parameters and in both genotypes.
Figure 4.
Prospective longitudinal measurement of TSH and FT4 in the five subjects homozygous for the L653V mutation. The evolution of TSH over time is displayed in the upper graph. Individuals are identified by different symbols, and age at the time of the first measurement is given in parentheses in the key. Note that regression analysis showed no significant upward or downward trend of TSH with time (slope) for any of the individuals. In the bottom graph, TSH values are correlated to those of FT4. Note that only subject IV-6, with AITD, showed a significant (P < 0.02) reciprocal correlation between TSH and FT4. This is indicated by the regression line (dashed) plotted by the least mean square method for the five pairs of TSH and FT4 measurements in subject IV-6 over the 2.5 yr of observation.
In vitro analysis confirms the functional significance of P68S
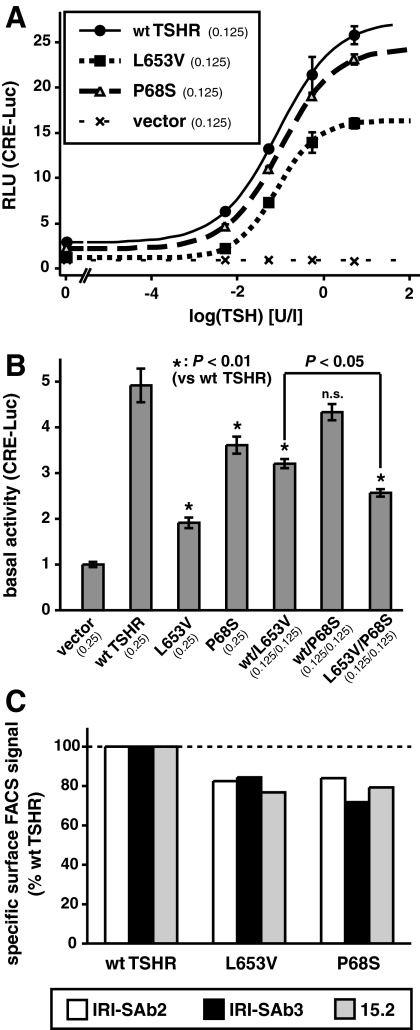
To determine the functional properties of the P68S mutation compared with that of the WT and L653V mutation in the same assay, we measured the expression of a cAMP-responsive reporter gene (pCRE-Luc) in HEK-293 cells. Results showed approximately 20% loss in maximum stimulated luciferase expression (Fig. 5A).
Figure 5.
In vitro functional analysis of the mutant compared with the WT TSHR. A, TSH dose response curves of a cotransfected CRE-driven reporter. B, Basal activity in transfections simulating the different genotypes observed in humans. C, Cell surface expression of the mutant receptors detected by FACS using three different antibodies. Results are expressed relative to those obtained with WT-TSHR.
Simulation of compound heterozygosity by cotransfection of L653V with P68S produced consistently less CRE-Luc activation than WT-TSHR/L653V cotransfection, indicating the functional significance of P68S in the heterozygous state (Fig. 5B). The results of the coexpression experiments under basal conditions did not suggest activation in trans- or dominant-negative effects between the mutant receptors, and the results are most compatible with a simple additive effect of the two mutations in the compound heterozygous state. The basal activities of the single transfections of either P68S or L653V alone (simulating homozygosity) and of the cotransfection of WT-TSHR/L653V (simulating L653V heterozygosity), but not that of WT-TSHR/P68S (simulating P68S heterozygosity), were lower than that of the WT-TSHR (Fig. 5B).
In FACS analysis with three different monoclonal antibodies directed against distinct portions of the TSHR molecule, both mutant TSHRs showed consistent and similar reduction of cell surface expression to approximately 80% that of the WT (Fig. 5C). This completely accounted for the functional defect of P68S but not L653V. For P68S, this finding is consistent with the location of the mutated site on the concave (outer) surface of the extracellular domain (22), neither being part of the TSH binding surface nor expected to be directly involved in the signal-transduction mechanism. In contrast, the impairment caused by L653V also comprises a defect in intramolecular signal transduction (16).
Discussion
In the present study, we identified 40 subjects (33 of whom belong to the same extended family and seven from the control subjects) segregating at least one of two distinct TSHR mutations. One mutation (L653V) is located in the third extracellular loop of the TSHR, and the other (P68S) is located within the ectodomain of the TSHR.
This communication reports the largest kindred studied with loss-of-function TSHR mutations, allowing genotype-phenotype correlations in a similar genetic background. The most severely affected patients were homozygous for L653V (mean TSH, 46.2 mU/liter), followed by compound heterozygous for L653V/P68S (mean TSH, 11.7 mU/liter). Heterozygotes for L653V and P68S had only slightly elevated TSH (mean values, 3.6 and 3.2 mU/liter, respectively). To simulate the various TSHR genotypes on TSHR activity in vitro, we cotransfected both mutations and the WT-TSHR in various combination. A significant reduction in CRE reporter activity was shown in cells transfected with L653V and P68S alone, L653V/WT and L653/P68S, but not with P68S/WT. Thus, functional studies confirm that both mutations result in loss of function of the TSHR and that the correlation of genotype to phenotype observed in vivo holds also in vitro. In fact, the degree of functional impairment L653V/L653V > L653V/P68S > L653V/WT > P68S/WT ∼ WT/WT (Fig. 5B) correlates with the magnitude of mean TSH values (Fig. 2).
Based on cross-sectional observations in small families, it has been previously suggested that mild RTSH due to heterozygous TSHR gene mutations shows euthyroidism in individuals of various ages (3,7) or spontaneous regression over time (14) without the need for l-T4 therapy. However, there are no longitudinal studies to confirm this hypothesis. Our identification of a large cohort of individuals heterozygous for the same TSHR gene mutation allowed a more reliable estimation of an age effect on degree of hyperthyrotropinemia. In addition, a prospective longitudinal study of five homozygotes for the L653V allele over the period of 3.5 yr allowed us to determine whether serum TSH levels changed over time. In the cross-sectional analysis of L653V heterozygotes, we found no change in TSH levels with age, suggesting a stable compensated RTSH with an appropriately adjusted set point of pituitary-thyroid feedback regulation. FT4I levels were not significantly different from those of subjects homozygous for the WT allele (relatives or members of the same clan) (Fig. 3). Similarly, no significant changes in the TSH concentration were observed in the longitudinal study in the L653V homozygotes. In the absence of concomitant AITD, serum FT4 values remained normal and did not show an inverse correlation with the TSH concentration (Fig. 4). In contrast to subclinical hypothyroidism in the context of AITD (23), the thyroidal compensation in mild to moderate RTSH should be clinically stable with no progression toward true hypothyroidism or spontaneous regression toward normal TSH levels. In fact, the single patient with TSHR gene mutation that showed a significant reduction of FT4 (subject IV-6) had concomitant AITD (Fig. 4). In a previous study (16), we have shown by in vitro analysis that the L653V mutation is associated with a specific TSHR defect, preferentially affecting the inositol phosphate pathway, with a phenotypical presentation distinct from previously reported TSHR loss-of-function mutations.
Thus, this report demonstrates that hyperthyrotropinemia caused by TSHR mutations with mild to moderate loss-of-function maintains a stable compensated RTSH and may not necessitate thyroid hormone replacement. Our results provide, for the first time, actual data in response to the controversy whether l-T4 therapy is required in RTSH caused by partial loss of TSHR function (15). The presence of normal FT4 levels argues against the need for l-T4 treatment, especially when inadvertent overtreatment, producing subclinical hyperthyroidism, can have untoward effects (24). On the other hand, the significantly lower mean FT4I level in homozygotes for the L653V mutation compared with individuals with the WT allele (even when subject IV-6 with AITD is deleted), indicates only partially compensated RTSH. In such individuals, careful follow-up and judicious administration of l-T4 is recommended.
The frequencies of the L653V and P68S mutations among the control subjects were 1.2% and 0.9%, respectively. The 2.4% heterozygosity for the L653V allele may be a major cause of hyperthyrotropinemia in this isolated population. Screening for the common TSHR gene mutations should therefore be considered in individuals with apparent nonautoimmune subclinical hypothyroidism to avoid unnecessary treatment.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants DK15070, DK20595, DK07011, and RR04999.
Current addresses: for S.M., Department of Medicine, Ratchaburi Hospital, Ratchaburi, 70000 Thailand; for U.R., Department of Medicine, Bhumibol Adulyadej Hospital, Bangken, Bangkok 10220, Thailand; and for L.M., Department of Endocrinology and Metabolism, University of Pisa, 56124 Pisa, Italy.
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 24, 2009
Abbreviations: AITD, Autoimmune thyroid disease; CRE, cAMP response element; FACS, flow immunocytofluorometry; FT3, free T3; FT4, free T4; FT3I, FT3 index; FT4I, FT4 index; RTSH, resistance to TSH; TG, thyroglobulin; TSHR, TSH receptor; WT, wild-type.
References
- Refetoff S 2003 Resistance to thyrotropin. J Endocrinol Invest 26:770–779 [DOI] [PubMed] [Google Scholar]
- Beck-Peccoz P, Persani L, Calebiro D, Bonomi M, Mannavola D, Campi I 2006 Syndromes of hormone resistance in the hypothalamic-pituitary-thyroid axis. Best Pract Res Clin Endocrinol Metab 20:529–546 [DOI] [PubMed] [Google Scholar]
- Sunthornthepvarakul T, Gottschalk ME, Hayashi Y, Refetoff S 1995 Resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. N Engl J Med 332:155–160 [DOI] [PubMed] [Google Scholar]
- Van Durme J, Horn F, Costagliola S, Vriend G, Vassart G 2006 GRIS: glycoprotein-hormone receptor information system. Mol Endocrinol 20:2247–2255 [DOI] [PubMed] [Google Scholar]
- de Roux N, Misrahi M, Brouner R, Houang M, Carel JC, Granier M, Le Bouc Y, Ghinea N, Boumedienne A, Toublanc JE, Milgrom E 1996 Four families with loss of function mutations of the thyrotropin receptor. J Clin Endocrinol Metab 81:4229–4235 [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Perri A, De Marco G, Agretti P, Banco ME, Di Cosmo C, Grasso L, Vitti P, Chiovato L, Pinchera A 2004 Low prevalence of thyrotropin receptor mutations in a large series of subjects with sporadic and familial nonautoimmune subclinical hypothyroidism. J Clin Endocrinol Metab 89:5787–5793 [DOI] [PubMed] [Google Scholar]
- Alberti L, Proverbio MC, Costagliola S, Romoli R, Boldrighini B, Vigone MC, Weber G, Chiumello G, Beck-Peccoz P, Persani L 2002 Germline mutations of TSH receptor gene as cause of nonautoimmune subclinical hypothyroidism. J Clin Endocrinol Metab 87:2549–2555 [DOI] [PubMed] [Google Scholar]
- Clifton-Bligh RJ, Gregory JW, Ludgate M, John R, Persani L, Asteria C, Beck-Peccoz P, Chatterjee VKK 1997 Two novel mutations in the thyrotropin (TSH) receptor gene in a child with resistance to TSH. J Clin Endocrinol Metab 82:1094–1100 [DOI] [PubMed] [Google Scholar]
- Biebermann H, Schöneberg T, Krude H, Schultz G, Gudermann T, Grüters A 1997 Mutations of the human thyrotropin receptor gene causing thyroid hypoplasia and persistent congenital hypothyroidism. J Clin Endocrinol Metab 82:3471–3480 [DOI] [PubMed] [Google Scholar]
- Gagné N, Parma J, Deal C, Vassart G, van Vliet G 1998 Apparent congenital athyreosis contrasting with normal plasma thyroglobulin levels and associated with inactivating mutations in the thyrotropin receptor gene: are athyreosis and ectopic thyroid distinct entities? J Clin Endocrinol Metab 83:1771–1775 [DOI] [PubMed] [Google Scholar]
- Tiosano D, Pannain S, Vassart G, Parma J, Gershoni-Baruch R, Mandel H, Lotan R, Zaharan Y, Pery M, Weiss RE, Refetoff S, Hochberg Z 1999 The hypothyroidism in an inbred kindred with congenital thyroid hormone and glucocorticoid deficiency is due to a mutation producing a truncated thyrotropin receptor. Thyroid 9:887–894 [DOI] [PubMed] [Google Scholar]
- Park SM, Clifton-Bligh RJ, Betts P, Chatterjee VK 2004 Congenital hypothyroidism and apparent athyreosis with compound heterozygosity or compensated hypothyroidism with probable hemizygosity for inactivating mutations of the TSH receptor. Clin Endocrinol (Oxf) 60:220–227 [DOI] [PubMed] [Google Scholar]
- Abramowicz MJ, Duprez L, Parma J, Vassart G, Heinrichs C 1997 Familial congenital hypothyroidism due to inactivating mutation of the thyrotropin receptor causing profound hypoplasia of the thyroid gland. J Clin Invest 99:3018–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilot M, Teofoli F, Gandini A, Franceschi R, Rapa A, Corrias A, Bona G, Radetti G, Tato L 2005 Thyrotropin receptor gene mutations and TSH resistance: variable expressivity in the heterozygotes. Clin Endocrinol (Oxf) 63:146–151 [DOI] [PubMed] [Google Scholar]
- Utiger RD 1995 Thyrotropin-receptor mutations and thyroid dysfunction. N Engl J Med 332:183–185 [DOI] [PubMed] [Google Scholar]
- Grasberger H, Van Sande J, Hag-Dahood Mahameed A, Tenenbaum-Rakover Y, Refetoff S 2007 A familial TSH receptor mutation provides in vivo evidence that the inositol phosphates/Ca2+ cascade mediates TSH action on thyroid hormone synthesis. J Clin Endocrinol Metab 92:2816–2820 [DOI] [PubMed] [Google Scholar]
- Barsano CP, Skosey C, DeGroot LJ, Refetoff S 1982 Serum thyroglobulin in the management of patients with thyroid cancer. Arch Int Med 142:763–767 [PubMed] [Google Scholar]
- Grasberger H, Mimouni-Bloch A, Vantyghem M-C, van Vliet G, Abramowicz M, Metzger DL, Abdullatif H, Rydlewski C, Macchia PE, Scherberg NH, van Sande J, Mimouni M, Weiss RE, Vassart G, Refetoff S 2005 Autosomal dominant resistance to thyrotropin as a distinct entity in five multigenerational kindreds: clinical characterization and exclusion of candidate-loci. J Clin Endocrinol Metab 90:4025–4034 [DOI] [PubMed] [Google Scholar]
- de Roux N, Misrahi M, Chatelain N, Gross B, Milgrom E 1996 Microsatellites and PCR primers for genetic studies and genomic sequencing of the human TSH receptor gene. Mol Cell Endocrinol 117:253–256 [DOI] [PubMed] [Google Scholar]
- Libert F, Lefort A, Gerald C, Parmentier M, Perret J, Ludgate M, Dumont JE, Vassart G 1989 Cloning, sequencing and expression of the human thyrotropin (TSH) receptor: evidence for binding of autoantibodies. Biochem Biophys Res Commun 165:1250–1255 [DOI] [PubMed] [Google Scholar]
- Govaerts C, Lefort A, Costagliola S, Wodak SJ, Ballesteros JA, Van Sande J, Pardo L, Vassart G 2001 A conserved Asn in transmembrane helix 7 is an on/off switch in the activation of the thyrotropin receptor. J Biol Chem 276:22991–22999 [DOI] [PubMed] [Google Scholar]
- Nunez Miguel R, Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Blundell TL, Rees Smith B, Furmaniak J 2004 Analysis of the thyrotropin receptor-thyrotropin interaction by comparative modeling. Thyroid 14:991–1011 [DOI] [PubMed] [Google Scholar]
- Ross DS2000 Subclinical hypothyroidism. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s the thyroid a fundamental and clinical text. 8th ed. Philadelphia: Lippincott, Williams, Wilkins; 467–473 [Google Scholar]
- Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS 2008 Health status, mood, and cognition in experimentally induced subclinical thyrotoxicosis. J Clin Endocrinol Metab 93:1730–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.