Abstract
Context: Cortisol has a distinct circadian rhythm regulated by the brain’s central pacemaker. Loss of this rhythm is associated with metabolic abnormalities, fatigue, and poor quality of life. Conventional glucocorticoid replacement cannot replicate this rhythm.
Objectives: Our objectives were to define key variables of physiological cortisol rhythm, and by pharmacokinetic modeling test whether modified-release hydrocortisone (MR-HC) can provide circadian cortisol profiles.
Setting: The study was performed at a Clinical Research Facility.
Design and Methods: Using data from a cross-sectional study in healthy reference subjects (n = 33), we defined parameters for the cortisol rhythm. We then tested MR-HC against immediate-release hydrocortisone in healthy volunteers (n = 28) in an open-label, randomized, single-dose, cross-over study. We compared profiles with physiological cortisol levels, and modeled an optimal treatment regimen.
Results: The key variables in the physiological cortisol profile included: peak 15.5 μg/dl (95% reference range 11.7–20.6), acrophase 0832 h (95% confidence interval 0759–0905), nadir less than 2 μg/dl (95% reference range 1.5–2.5), time of nadir 0018 h (95% confidence interval 2339–0058), and quiescent phase (below the mesor) 1943–0531 h. MR-HC 15 mg demonstrated delayed and sustained release with a mean (sem) maximum observed concentration of 16.6 (1.4) μg/dl at 7.41 (0.57) h after drug. Bioavailability of MR-HC 5, 10, and 15 mg was 100, 79, and 86% that of immediate-release hydrocortisone. Modeling suggested that MR-HC 15–20 mg at 2300 h and 10 mg at 0700 h could reproduce physiological cortisol levels.
Conclusion: By defining circadian rhythms and using modern formulation technology, it is possible to allow a more physiological circadian replacement of cortisol.
A modified release formulation of hydrocortisone that allows for delayed and sustained release of hydrocortisone can potentially replicate normal un-stressed physiological cortisol levels.
Cortisol secretion follows a distinct circadian rhythm, with circulating levels low at sleep onset, beginning to increase between 0200 and 0400 h, peaking within 1 h waking, and then declining through the day (1). This circadian rhythm is determined by the central endogenous clock (pacemaker) of the hypothalamic-pituitary-adrenal (HPA) axis, located in the hypothalamic suprachiasmatic nucleus, which drives release of CRH, in turn leading to secretion of ACTH from the pituitary and, thus, cortisol from the adrenal.
The central suprachiasmatic nucleus clock has an approximate period length of 24.2 h, and requires daily adjustment by photoperiod to synchronize to the 24-h day/night cycle. In addition to the central clock, there are molecular oscillators (peripheral clocks) in most mammalian cells (2). The phase of these peripheral clocks is reset by signals from the central pacemaker. The specific signals from the central to peripheral clocks have not been fully established. Circadian gene expression can be induced by serum shock (3), and glucocorticoids are able to phase delay or advance peripheral oscillators (4). Thus, cortisol appears to act as one secondary messenger from central to peripheral clocks.
The HPA axis plays an important role in maintaining alertness and modulating sleep (5). Conditions associated with insomnia, including depression, sleep apnea, and chronic fatigue, disrupt the circadian rhythm of cortisol, leading to metabolic abnormalities and increased cardiovascular risk (6,7). Patients with adrenal insufficiency have a loss of the normal circadian rhythm of cortisol and excess mortality, mainly from cardiovascular events and infections (8,9,10). This may be explained in part by the fact that current replacement regimens cannot replace the normal physiological rhythm of cortisol (11). Moreover, with current replacement regimes, the majority of patients with adrenal insufficiency report impaired health-related quality of life (12,13), and early morning fatigue (14,15) with subsequent socioeconomic health problems. Therefore, physiological cortisol replacement in patients with adrenal insufficiency may be advantageous when compared with conventional treatments.
Infusions of hydrocortisone (cortisol) can mimic the normal circadian rhythm of cortisol, and improve biochemical control and quality of life in patients with adrenal insufficiency (16,17). Because infusions are not a practical solution, we designed a modified-release hydrocortisone (MR-HC). A small proof of concept study confirmed the potential utility of the formulation to reproduce the normal overnight increase in cortisol levels (18).
We have now analyzed circadian cortisol levels from a group of healthy subjects to define the parameters of physiological cortisol secretion. These can then be used to judge any new therapy that aims for physiological circadian replacement of cortisol. We have then performed a detailed phase 1 study on new modified-release formulations of hydrocortisone and compared them with the kinetics of 10 mg immediate-release hydrocortisone (IR-HC), the conventional hydrocortisone preparation currently used in the routine clinical care for adrenal insufficiency and congenital adrenal hyperplasia. These data were then used to define optimal treatment regimens with MR-HC for mimicking physiological cortisol levels, a novel concept in the delivery of glucocorticoid replacement.
Subjects and Methods
Healthy reference group
A total of 33 normal individuals who had undergone detailed, 24-h, 20-min, cortisol profiling provided data for definition of the physiological cortisol circadian rhythm (Table 1) (19). This data set was used because all individual measurements were available to us, and the data were generated with the same modern cortisol assay. To validate further that this data set is representative of the general population, all mean [±95% confidence intervals (CIs)] cortisol concentrations, area under the curve (AUC) from 0–24 h [AUC(0–24)], and time variables obtained from the healthy reference group were compared with similar variables derived from the previously published literature (20,21,22,23,24,25,26,27,28,29).
Table 1.
Characteristics of cortisol circadian rhythm: comparison of mean (±95% CIs) cortisol concentrations, AUC, and time variables in our healthy reference group (19) with similar variables in previously published data
| Author | Sample mean age (range) | AUC (h*μg/dl) | Peak (μg/dl) | Trough (μg/dl) | 24-hr mean cortisol (μg/dl) | Time of peak or acrophase (h:min) | Time of nadir (h:min) | Quiescent phase start (h:min) | Quiescent phase end (h:min) |
|---|---|---|---|---|---|---|---|---|---|
| Darzy and Shalet (19) (24 males; 9 Females)a | 27 (17–57) | 160.2 (148.6–171.6) | 16.0 (14.6–17.4) | 2.5 (1.8–3.2) | 6.7 (5.9–7.5) | 08:32 (07:59–09:05) | 00:18 (23:39–0:58) | 19:43 (18:36–20:49)b | 05:31 (04:45–06:18)c |
| Other authors (20,21,22,23,24,25,26,27,28,29) (127 males; 38 females)d | 32 (19–59) | 169.0 (138.5−207.0) | 15.0 (11.6−19.0) | 2.04 (1.0–3.2) | 7.7 (5.7–15.8) | 07:49 (06:28−09:01) | 00:30 (22:00–02:00) | 19:40 (16:30−22:00)e | 04:11 (03:00−05:30)f |
Sample subgroups used in analysis were: 20- to 29-yr subgroup (21); 19- to 25-yr subgroup (22); S1 session (24); control group (25); and Chinese, 30-yr subgroup (26). The AUC (23) represents the average of harmonic regressions. Not used in analysis of: Trough (22), 24-h mean cortisol (22,28), Time of Nadir (26,27,29), Quiescent phase start (26,27,29), and Quiescent phase end (27,29).
Variable mean (95% CIs).
Period starts when the cortisol level is approximately 5.2 μg/dl or less for at least 1 h.
Period starts when the cortisol levels are approximately 5.2 μg/dl or more for at least 1 h.
Values have been estimated from published data: mean (range).
Period starts when the cortisol level is approximately 5.0 μg/dl or less for at least 1 h.
Period starts when the cortisol levels are approximately 5.0 μg/dl or more for at least 1 h.
Defining physiological ranges
Cortisol levels generally show a skewed distribution. Therefore, in defining physiological ranges, cortisol levels were transformed to the natural logarithm, enabling the cortisol geometrical mean to be calculated at each time point. The 95% CIs were calculated for: the AUC(0–24), peak cortisol, trough cortisol, and 24-h mean cortisol. In addition, we report 95% reference ranges (±2 sd values) for the peak and trough cortisol.
Cosinor analysis
For each individual cortisol profile, a cosinor model with a second harmonic was fitted to the data (30). A group cosinor model was computed by averaging the coefficients from the individual fits. Circadian timing estimates were obtained for each individual cortisol profile. This allowed us to calculate the mesor (rhythm adjusted mean), acrophase (time of peak in rhythm), nadir (lowest point of the rhythm), and quiescent phase (start, taken as the time when the cortisol level was equal to or less than the mesor for more than 1 h, and end, when the cortisol level was equal to or more than the mesor for more than 1 h). In a previous study reporting the quiescent phase, the cutoff for the start and end was taken as 5 μg/dl (138 nmol/liter) (21), a very similar value to the mesor 5.2 μg/dl (143.6 nmol/liter) that we obtained for use in this study.
Pharmacokinetic (PK) analysis for MR-HC
A total of 32 healthy male subjects was recruited. Entry criteria were: 18–50 yr old; no illness, operation, or steroid use in the previous 3 months; and no regular medication. The study was approved by the Plymouth Independent Ethics Committee, United Kingdom, and all subjects gave informed written consent. The sampling was performed in the Chiltern Clinical Research Unit, United Kingdom.
Dose-response study
There were 20 subjects randomized to receive three of the following four single-dose regimes: 5 mg MR-HC (1 × 5 mg), 15 mg MR-HC (1 × 15 mg), 30 mg MR-HC (2 × 15 mg), or 10 mg IR-HC, with a 1-wk washout between treatments. Twelve other subjects were randomized to receive either 10 mg (2 × 5 mg) MR-HC or 10 mg IR-HC, with a 1-wk washout period.
The MR-HC or IR-HC dose was taken at 2200 h. Participants had their HPA axis suppressed with 1 mg oral dexamethasone at 1800 and 2200 h on d 1, and then at 0600, 1200, and 1800 h on d 2 during each treatment period. Plasma ACTH levels were taken at 2155 h on d 1 and at 0600 h on d 2, whereas serum cortisol levels were taken before ingestion of the drug and were repeated every 30 min for the first 4 h, then at 0300, 0400, 0500, 0600, 0800, 1000, 1300, 1600, and 2200 h (Table 2).
Table 2.
Study scheme
| Study assessment | d 1
|
d 2
|
||||
|---|---|---|---|---|---|---|
| 1800 h | 2200 h | 0600 h | 1200 h | 1800 h | 2200 h | |
| ACTH measurement | Ö | Ö | ||||
| Dexamethasone 1 mg orally | Ö | Ö | Ö | Ö | Ö | |
| 20a Subjects dosed with MR-HC 5, 15, or 30 mg, or IR-HC 10 mg | ||||||
| 12 Subjects dosed with MR-HC 10 mg or IR-HC 10 mg | Ö | |||||
Subjects were admitted to the Clinical Research Facility on d 1, and were tested on up to three occasions with a 1-wk washout between treatments. Cortisol measurement, pre-dose at 2200 h and post-dose sampling every 30 minutes for the first 4 h, then at 5, 6, 7, 8, 10, 12, 15, 18, and 24 h after dose.
Four subjects withdrawn.
MR-HC
The tablet has an insoluble barrier coat protecting all but the upper face of the tablet. The unprotected face exposes a delaying layer that slowly erodes in the small intestine to present the sustained release drug-containing layer. Two different dose units of MR-HC were available, 5 and 15 mg. MR-HC was supplied by Phoqus Pharmaceuticals, plc (Kent, UK).
IR-HC
Ten-milligram tablets were from Merck Sharp & Dohme Ltd. (Hertfordshire, UK). A 10-mg dose was used to avoid unphysiological peak values that exceed the binding capacity of cortisol binding globulin, which in our view would complicate the analysis when comparing PK values with a modified-release formulation of hydrocortisone (31).
Assays
Serum cortisol was measured using the Bayer Advia Centaur Automated Immunoassay System (Bayer Diagnostics, a division of Bayer HealthCare, Bayer, Fernwald, Germany). The interassay coefficient variation was 7% at 7.2 μg/dl (200 nmol/liter), and 8% at 38 μg/dl (1050 nmol/liter). Plasma ACTH was measured using the Immulite 2000 assay (Diagnostic Products Corp., Los Angeles, CA). The interassay coefficient variation was 4% at 28 ng/liter (6.2 pmol/liter). The same assay methodology was used in the healthy reference group.
Statistical analysis
PK parameters were computed by noncompartmental analysis. Because only a 10-mg dose of IR-HC was used, to assess relative bioavailability, MR-HC was dose adjusted as follows: AUC (MR-HC) × dose (IR-HC)/AUC (IR-HC) × dose (MR-HC). An independent sample t test was used to compare PK data of MR-HC and IR-HC. A significant difference was taken as P < 0.05. Dose proportionality was determined by comparing AUC from zero to infinity [AUC(0-inf)] and maximum observed concentration (Cmax) of the 5, 15, and 30 mg MR-HC doses using a linear model approach on the log-transformed PK parameters vs. log-transformed doses. The slope of the response against dose estimated, and dose proportionality was accepted if the 90% CI for the slope included unity.
In modeling, we simulated giving MR-HC doses at different times and once or twice daily to establish whether MR-HC could produce a 24-h physiological profile. To examine the best fit, we calculated the ratio between the MR-HC AUC vs. the physiological AUC at each of the pharmacokinetically relevant time intervals, e.g. the trapezoidal segment between each sampling interval. A ratio between 0.8 and 1.2 (within 20% of identity) was considered to indicate an acceptable MR-HC fit for that time interval. We could then determine the proportion of cortisol levels after MR-HC that were between the upper and lower 95% reference ranges for physiological cortisol levels.
Results
Defining the physiological cortisol circadian rhythm
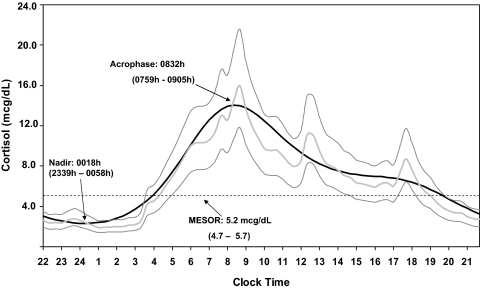
A circadian rhythm is clearly demonstrated in the data from the healthy reference group (Fig. 1). A cosinor model with second harmonic gave an excellent fit to the mean cortisol data (r2 = 0.97; P < 0.001). All individuals had a significant (P < 0.001) sinusoidal rhythm. Cortisol levels reached a peak at around 0832 h (95% CI 0759–0905), then levels gradually decreased until reaching a nadir at 0018 h (95% CI 2339–0058). Two smaller peaks occurred at meal times. The means for all variables of the healthy reference group were similar to the previously published literature with overlap of all 95% CIs (Table 1) (20,21,22,23,24,25,26,27,28,29). In this cohort there was no difference in the level or phase of the circadian rhythm between men and women.
Figure 1.
Physiological cortisol circadian rhythm. The figure shows the geometrical mean (−) ± 2 sd values (−) of serum cortisol concentration calculated from 20 min sampling over a 24-h period in 33 healthy subjects. The fitted cosinor (−) is the average of harmonic regressions that were a fit for the individual subject data. Cortisol has a distinct circadian rhythm with a peak of 15.5 μg/dl (95% reference range 11.7–20.6) occurring at 0832 h and a nadir (time of trough cortisol level) less than 2.0 μg/dl (95% reference range 1.5–2.5) at 0018 h. The mean and 95% CI are shown for the mesor (midline estimating statistic of rhythm), acrophase (time of peak using a 24 h clock with midnight taken as origin), and nadir. mcg, μg.
PK analysis of MR-HC and comparison with IR-HC
A total of 32 subjects was recruited. Four participants were withdrawn: two for violation of the protocol, one withdrew consent, and one had an adverse event while on IR-HC (hiccups). The 28 other subjects completed the study (Table 3). Of all ACTH values, 98% were below the lower limit of the assay, confirming dexamethasone-induced suppression of endogenous HPA axis, a mandatory precondition for correct PK analysis of cortisol bioavailability.
Table 3.
Demographics for study subjects
| Variable | Healthy reference group | Main study group |
|---|---|---|
| No. of subjects | 33 | 28 |
| Sex | 24 males/9 females | 28 males |
| Mean age (range) | 27 (17–57) | 33 (21–46) |
| Mean weight (range) | 67.1 (54–102) | 80.0 (58–103) |
| Mean BMI (range) | 22.9 (17–29) | 25.6 (20–31) |
BMI, Body mass index.
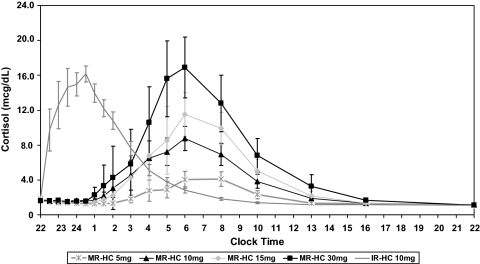
The cortisol concentration-time profiles after administration of MR-HC and IR-HC are shown in Fig. 2. The summary statistics for the PK parameters are represented in Table 4 for comparison. The geometric mean (meangeo) (90% CI) relative bioavailability of MR-HC formulations compared with that of IR-HC, as measured by dose-normalized AUC values, based on 12 subjects who went through both arms of the study that were subjects of this comparison, were: 100% (90–112) for 5 mg, 79% (66–95) for 10 mg, 86% (77–96) for 15 mg, and 69% (62–77) for 30 mg, respectively. The corresponding analysis for the maximum exposure, as measured by Cmax (without dose normalization), indicated equivalence of the MR-HC 15-mg dose to IR-HC 10 mg [100% (87–116)]. As expected from a delayed and sustained release formulation, the MR-HC 15-mg dose showed a marked delay in mean time to reach Cmax (Tmax), prolongation in time delay between drug administration and cortisol concentration more than 3.5 μg/dl (Tlag), and lower dose-normalized Cmax compared with IR-HC 10 mg dose [Tmax (95% CI of mean difference), 7.41 vs. 1.8 h (4.6–6.6; P ≤ 0.001); Tlag, 5.19 vs. 0.5 h (4.1–5.4; P ≤ 0.001); and dose-adjusted Cmax, 11.04 vs. 18.4 μg/dl (4.75–9.8; P ≤ 0.001)]. All other formulations also showed characteristics of sustained and delayed release (Table 4). The peak concentrations and cortisol exposure increased predictably with increasing MR-HC dose. Dose proportionality was seen for MR-HC 5 and 15 mg: slopes (90% CIs) were 0.82 (0.62–1.02) and 0.9 (0.69–1.1) for AUC(0-inf) and Cmax, respectively. Dose proportionality was not shown between 15 and 30 mg MR-HC formulation: slopes (90% CI) for AUC(0-inf) and Cmax were 0.58 (0.30–0.85) and 0.64 (0.40–0.87), respectively. There was no correlation between weight and any variable of MR-HC PKs.
Figure 2.
Concentration-time profiles for MR-HC and IR-HC. Concentration-time profiles for different doses of MR-HC given at 2200 h compared with 10 mg IR-HC using geometrical means (±sem) of serum cortisol concentrations at 18 different time points over 24 h. Profiles of MR-HC show a prolonged Tmax, Tlag, and a lower dose-adjusted Cmax (data not shown) when compared with IR-HC, all typical of a formulation with delayed and sustained release characteristics. mcg, μg.
Table 4.
PK data for MR-HC and IR-HC
| Variable mean (sem) | MR-HC 5 mg (12 subjects) | MR-HC 10 mg (12 subjects) | MR-HC 15 mg (12 subjects) | MR-HC 30 mg (12 subjects) | 10 mg IR-HC (24 subjects) |
|---|---|---|---|---|---|
| AUC(0–24) (h*μg/dl) | 35.2 (4.0) | 69.7 (6.2) | 90.4 (9.0) | 137.7 (10.5) | 80.5 (4.2) |
| AUC (0-inf)(h*μg/dl) | 41.5 (4.8) | 77.3 (6.8) | 98.5 (9.2) | 143.2 (10.8) | 88.2 (5.3) |
| Cmax (μg/dl) | 6.4 (0.7) | 10.9 (0.9) | 16.6 (1.4) | 24.9 (1.1) | 18.4 (0.7) |
| Tmax (h) | 8.25 (0.49) | 7.83 (0.5) | 7.41 (0.57) | 7.17 (0.66) | 1.8 (0.2) |
| Tlag (h) | 6.6 (0.47) | 4.78 (0.45) | 5.19 (0.43) | 4.6 (0.44) | 0.5 (0.08) |
| CL/F (liter/h) | 13.8 (1.3) | 14.08 (1.1) | 16.56 (1.1) | 22.08 (1.7) | 11.04 (0.5) |
CL/F, Apparent clearance of drug after oral administration [= dose/AUC(0-inf)].
Comparison of MR-HC with the physiological cortisol profile
Administration of 30 mg MR-HC provided the best cortisol exposure over 24 h based on AUC compared with the physiological profile: mean (90% CI) = 88% (77–99). However, the 30-mg MR-HC Cmax was above that seen for the physiological peak (mean ± sem = 24.9 ± 1.1 vs. 15.5 ± 0.8 μg/dl), and the peak occurred earlier than the physiological peak (mean: 0600 vs. 0832 h). The cortisol profile after MR-HC suggests that it only provides approximately 12 h exposure to hydrocortisone (Fig. 2), and when looking at the AUC for 12 h after 2200 h for 15 mg MR-HC cortisol exposure was 84% (71–97) of physiological, the Cmax similar to physiological peak (16.6 ± 1.4 vs. 15.5 ± 0.8 μg/dl) but occurred earlier (0600 vs. 0832 h) when given at 2200 h.
Modeling MR-HC data to provide physiological dosing regimen
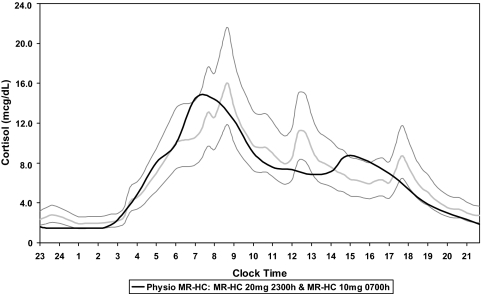
Based on the phase 1 MR-HC data obtained from the dose-response study using single-dose regimes, we simulated giving MR-HC doses at different times and once or twice daily. For MR-HC 30 mg given at 2200 h, only two cortisol AUCs of the 16 (12.5%) were within the 0.8–1.2 ratio. However, 12 (75%) of 16 cortisol AUCs were within the 0.8–1.2 ratio for MR-HC given as 20 mg at 2300 h and 10 mg at 0700 h. Using this analysis we defined the best dose combination to provide physiological cortisol levels as either 15 or 20 mg MR-HC at 2300 h and 10 mg MR-HC at 0700 h. An example profile is shown in Fig. 3 against physiological cortisol levels.
Figure 3.
Simulation of physiological (Physio) cortisol rhythm using MR-HC. Phase 1 PK data were used to simulate giving MR-HC doses at different times and once or twice daily. PK modeling included comparison of MR-HC with the physiological cortisol rhythm over 12 and 24 h, and at different time intervals (trapezoidal segments), using AUCs. The graph shows modeled concentration-time profile (−) obtained when giving 20 mg (15 plus 5 mg) MR-HC at 2300 h and 10 mg MR-HC at 0700 h superimposed on the physiological cortisol rhythm [geometrical mean (−) ± 2 sd values (−) of serum cortisol concentration calculated from 20 min sampling over a 24-h period in 33 healthy subjects].mcg, μg.
Discussion
Physiological cortisol replacement therapy for adrenal insufficiency is not possible with current oral hydrocortisone formulations (32). To address this we have developed a novel modified-release formulation of hydrocortisone. We have undertaken detailed PKs of MR-HC and compared these with the physiological profile of cortisol in normal individuals. Based on PK modeling, we suggest that a twice-daily regimen of MR-HC given at 2300 and 0700 h can provide levels of cortisol similar to normal physiological cortisol: AUC, Cmax, and Tmax.
Defining the normal physiological profile of cortisol was critical to our study. We had access to a data set of 33 healthy individuals who had undergone 20 min blood sampling over a 24-h period (19). The generalizability of these data is reflected by the fact that the peak and nadir cortisol, timing of the peak and nadir, duration of the quiescent phase, and onset of cortisol secretion and cosinor analysis were all very similar to that in the literature (20,21,22,23,24,25,26,27,28,29). The key variables in the cortisol profile that were defined were a peak cortisol of 15.5 μg/dl (95% reference range 11.7–20.6), with the acrophase at 0832 h (95% CI 0759–0905), a nadir cortisol of less than 2 μg/dl (95% reference range 1.5–2.5) occurring at 0018 h (95% CI 2339–0058), and the quiescent phase (below the mesor) occurring from 1943–0531 h.
We found no difference in physiological cortisol profiles according to weight or gender. Gender has previously had a minor effect on physiological cortisol levels (21), although not found in all studies (33). Body mass index does not have any significant effect on parameters quantifying the 24-h cortisol profile in lean or modestly overweight individuals (21). In the literature there is an age effect on the cortisol profile, and older subjects have an earlier onset of the cortisol rhythm (22). Most of our subjects were young and Caucasian. However, different populations and ethnic groups may exhibit differences in circadian cortisol rhythm parameters. For example, trough and peak cortisol levels occur earlier in Chinese men compared with Caucasians, possibly due to genetic differences and/or environmental influences, including social activity and everyday habits on entrainment of circadian rhythms, such as an average earlier bedtime (26).
The PKs of MR-HC showed similar bioavailability to IR-HC with dose proportionality for MR-HC between 5 and 15 mg, but not between 15 and 30 mg. This is likely to be explained by the binding characteristics of cortisol-binding globulin (CBG). Under basal conditions about 5–10% of circulating cortisol is free, about 75% is bound to CBG, and the remainder is bound to albumin. Increases in total plasma cortisol concentrations above 20 μg/dl (550 nmol/liter) exceed the binding capacity of CBG and result in rapid increases in levels of free cortisol concentration, which, thus, exhibits more rapid clearance from the circulation (31). Because the peak cortisol after 30 mg MR-HC exceeded 20 μg/dl, the lack of dose proportionality is probably explained by this mechanism.
The profile after MR-HC was compared with physiological cortisol levels. The PK studies were all undertaken with subjects taking a single dose at 2200 h. Based on this the onset of cortisol release preceded the end of the physiological quiescent phase, and the peak preceded the timing of physiological peak cortisol. In addition, a single dose of MR-HC could only replace the 24-h AUC if the peak cortisol exceeded the physiological peak. Therefore, we simulated giving MR-HC at different time points and once or twice daily. In the simulations, AUC at time intervals were compared with the normal circadian rhythm. This analysis shows that MR-HC 20 and 10 mg, given at 2300 and 0700 h, respectively, could provide a cortisol profile with the least difference from normal physiological cortisol levels. However, this conclusion requires validation by an appropriate clinical study, and it should be recognized that all simulations have been done on data derived from adults, and a different regimen both in timing and dose will be required for children.
The future of endocrine replacement lies in using modern pharmaceutical formulations to provide hormone replacement that replicates physiological hormone levels. We recognize that it is unlikely that any future drug regimen will be able to replicate completely the rapid adaptation of physiological cortisol secretion to different conditions of stress. However, we have shown that a modified release formulation of hydrocortisone that allows for delayed and sustained release of hydrocortisone can potentially replicate normal unstressed physiological cortisol levels. Currently, in patients with adrenal insufficiency, health-related quality of life is significantly compromised, affecting their ability to work and cope with activities of daily life, and even more significant, mortality is increased. Future studies will determine the beneficial effects of physiological cortisol replacement, but we have demonstrated here that it is possible to generate hydrocortisone formulations that provide cortisol profiles closer to baseline cortisol physiology.
Footnotes
The study was funded by Diurnal Ltd. and Phoqus Pharmaceuticals, plc. W.A. is a Medical Research Council Senior Clinical Fellow (G116/172). This research was supported (in part) by the Intramural Research Program of the National Institutes of Health.
Disclosure Summary: M.D., C.G., M.J.C., J.N.-P., K.D., and W.A. have nothing to declare. D.P.M. received research funds from Phoqus Pharmaceuticals, plc. R.J.R. and A.R.-H. have equity interests in Diurnal Ltd. H.H. is employed as a consultant for Diurnal Ltd.
First Published Online February 17, 2009
Abbreviations: AUC, Area under the curve; AUC(0-inf), area under the curve from zero to infinity; AUC(0–24), area under the curve from 0–24 h; CBG, corticosteroid-binding globulin; CI, confidence interval; Cmax, maximum observed concentration; HPA, hypothalamic-pituitary-adrenal; IR-HC, immediate-release hydrocortisone; MR-HC, modified-release hydrocortisone; PK, pharmacokinetic; Tlag, time delay between drug administration and cortisol concentration more than 3.5 μg/dl; Tmax, time to reach maximum observed concentration.
References
- Krieger DT, Allen W, Rizzo F, Krieger HP 1971 Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab 32:266–284 [DOI] [PubMed] [Google Scholar]
- Dunlap JC 1999 Molecular bases for circadian clocks. Cell 96:271–290 [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U 1998 A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93:929–937 [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U 2000 Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289:2344–2347 [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF 2005 On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 90:3106–3114 [DOI] [PubMed] [Google Scholar]
- Plat L, Leproult R, L’Hermite-Baleriaux M, Fery F, Mockel J, Polonsky KS, Van Cauter E 1999 Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab 84:3082–3092 [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E 1999 Impact of sleep debt on metabolic and endocrine function. Lancet 354:1435–1439 [DOI] [PubMed] [Google Scholar]
- Bergthorsdottir R, Leonsson-Zachrisson M, Oden A, Johannsson G 2006 Premature mortality in patients with Addison’s disease: a population-based study. J Clin Endocrinol Metab 91:4849–4853 [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, Sheppard MC, Stewart PM 2001 Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet 357:425–431 [DOI] [PubMed] [Google Scholar]
- Bensing S, Brandt L, Tabaroj F, Sjoberg O, Nilsson B, Ekbom A, Blomqvist P, Kampe O 2008 Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf) 69:697–704 [DOI] [PubMed] [Google Scholar]
- Mah PM, Jenkins RC, Rostami-Hodjegan A, Newell-Price J, Doane A, Ibbotson V, Tucker GT, Ross RJ 2004 Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 61:367–375 [DOI] [PubMed] [Google Scholar]
- Arlt W, Allolio B 2003 Adrenal insufficiency. Lancet 361:1881–1893 [DOI] [PubMed] [Google Scholar]
- Hahner S, Loeffler M, Fassnacht M, Weismann D, Koschker AC, Quinkler M, Decker O, Arlt W, Allolio B 2007 Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab 92:3912–3922 [DOI] [PubMed] [Google Scholar]
- Lovas K, Loge JH, Husebye ES 2002 Subjective health status in Norwegian patients with Addison’s disease. Clin Endocrinol (Oxf) 56:581–588 [DOI] [PubMed] [Google Scholar]
- Lovas K, Husebye ES, Holsten F, Bjorvatn B 2003 Sleep disturbances in patients with Addison’s disease. Eur J Endocrinol 148:449–456 [DOI] [PubMed] [Google Scholar]
- Merza Z, Rostami-Hodjegan A, Memmott A, Doane A, Ibbotson V, Newell-Price J, Tucker GT, Ross RJ 2006 Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 65:45–50 [DOI] [PubMed] [Google Scholar]
- Lovas K, Husebye ES 2007 Continuous subcutaneous hydrocortisone infusion in Addison’s disease. Eur J Endocrinol [Erratum (2008) 158:939] 157:109–112 [DOI] [PubMed] [Google Scholar]
- Newell-Price J, Whiteman M, Rostami-Hodjegan A, Darzy K, Shalet S, Tucker GT, Ross RJ 2008 Modified-release hydrocortisone for circadian therapy: a proof-of-principle study in dexamethasone-suppressed normal volunteers. Clin Endocrinol (Oxf) 68:130–135 [DOI] [PubMed] [Google Scholar]
- Darzy KH, Shalet SM 2005 Absence of adrenocorticotropin (ACTH) neurosecretory dysfunction but increased cortisol concentrations and production rates in ACTH-replete adult cancer survivors after cranial irradiation for nonpituitary brain tumors. J Clin Endocrinol Metab 90:5217–5225 [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L 1971 Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab 33:14–22 [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ 1996 Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81:2468–2473 [DOI] [PubMed] [Google Scholar]
- Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair NP 1989 Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry 25:305–319 [DOI] [PubMed] [Google Scholar]
- Hurwitz S, Cohen RJ, Williams GH 2004 Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol 96:1406–1414 [DOI] [PubMed] [Google Scholar]
- Selmaoui B, Touitou Y 2003 Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: a study of three different 24-h cycles over six weeks. Life Sci 73:3339–3349 [DOI] [PubMed] [Google Scholar]
- Selmaoui B, Lambrozo J, Touitou Y 1997 Endocrine functions in young men exposed for one night to a 50-Hz magnetic field. A circadian study of pituitary, thyroid and adrenocortical hormones. Life Sci 61:473–486 [DOI] [PubMed] [Google Scholar]
- Zhao ZY, Xie Y, Fu YR, Li YY, Bogdan A, Touitou Y 2003 Circadian rhythm characteristics of serum cortisol and dehydroepiandrosterone sulfate in healthy Chinese men aged 30 to 60 years. A cross-sectional study. Steroids 68:133–138 [DOI] [PubMed] [Google Scholar]
- Kanabrocki EL, Sothern RB, Scheving LE, Vesely DL, Tsai TH, Shelstad J, Cournoyer C, Greco J, Mermall H, Ferlin H, Nemchansky BM, Bushnell DL, Kaplan E, Kahn S, Augustine G, Holmes E, Rumbyrt J, Sturtevant RP, Sturtevant F, Bremmer F, Third JLHC, McCormick JB, Mudd CA, Dawson S, Sackett-Lundeen L, Haus E, Halberg F, Pauly JE, Olwin JH 1990 Reference values for circadian rhythms of 98 variables in clinically healthy men in the fifth decade of life. Chronobiol Int 7:445–461 [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Nair M, Zhang Q, Hill EE, Brown MB 1997 Circadian immune measures in healthy volunteers: relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med 59:42–50 [DOI] [PubMed] [Google Scholar]
- Reinberg A, Lagoguey M, Cesselin F, Touitou Y, Legrand JC, Delassalle A, Antreassian J, Lagoguey A 1978 Circadian and circannual rhythms in plasma hormones and other variables of five healthy young human males. Acta Endocrinol (Copenh) 88:417–427 [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Krzyzanski W, Jusko WJ 1999 Mathematical modeling of circadian cortisol concentrations using indirect response models: comparison of several methods. J Pharmacokinet Biopharm 27:23–43 [DOI] [PubMed] [Google Scholar]
- Tunn S, Mollmann H, Barth J, Derendorf H, Krieg M 1992 Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clin Chem 38(8 Pt 1):1491–1494 [PubMed] [Google Scholar]
- Crown A, Lightman S 2005 Why is the management of glucocorticoid deficiency still controversial: a review of the literature. Clin Endocrinol (Oxf) 63:483–492 [DOI] [PubMed] [Google Scholar]
- Halbreich U, Asnis GM, Zumoff B, Nathan RS, Shindledecker R 1984 Effect of age and sex on cortisol secretion in depressives and normals. Psychiatry Res 13:221–229 [DOI] [PubMed] [Google Scholar]