Abstract
Context: Graves’ disease (GD) is an autoimmune process of the thyroid and orbital connective tissues. The fraction of T and B cells expressing IGF-I receptor (IGF-IR) is increased in GD. It is a potentially important autoantigen in GD. Susceptibility to GD arises from both genetic and acquired factors.
Objective: The aim of the study was to determine whether the increased frequency of IGF-IR-expressing T and B cells in GD results from genetic or nongenetic factors.
Design/Setting/Participants: Display of IGF-IR was assessed on blood lymphocytes from 18 pairs of monozygotic twins in the Danish Twin Registry, including seven discordant pairs, four pairs concordant for GD, and seven healthy pairs.
Main Outcome Measures: Subjects underwent physical examination and laboratory analysis. Surface display of IGF-IR on T and B cells was analyzed by flow cytometry.
Results: Twins with GD display increased IGF-IR-expressing CD3+ T cells and T cell subsets including total CD4+, CD4+ naive, CD4+ memory, and CD8+ cells (P < 0.0001, P = 0.0001, P = 0.0003, P = 0.01, and P = 0.02, respectively) compared to healthy twins. The frequency of IGF-IR-expressing B cells from affected twins was increased relative to healthy controls (P = 0.009). In pairs discordant for GD, affected twins exhibited increased frequency of IGF-IR+ CD3+, CD4+, and CD4+ naive T cells (P < 0.05, P = 0.03, and P = 0.03, respectively) compared to their healthy twin.
Conclusion: Our findings suggest that more frequent IGF-IR+ T cells in GD cannot be attributed to genetic determinants. Rather, this skew appears to be acquired. These results underscore the potential role of nongenetic, acquired factors in genetically susceptible individuals.
Monozygotic twins manifesting Graves’ disease exhibit an increased frequency of lymphocytes expressing IGF-1 receptor while their unaffected siblings do not.
Graves’ disease (GD), an autoimmune syndrome, comprises disordered thyroid function and remodeling of connective tissues. Orbital tissue inflammation and expansion are associated with excessive deposition of the glycosaminoglycan, hyaluronan, in a process known as thyroid-associated ophthalmopathy (TAO) (1). Activating autoantibodies directed against the TSH receptor, termed thyroid-stimulating antibodies (TSI), drive thyroid overactivity and enlargement (2,3). Recently, the IGF-I receptor (IGF-IR) has been identified as another potentially important autoantigen in GD (4). Activating IgGs directed against the IGF-IR (GD-IgG) are detectable in nearly all patients with GD but are usually absent in unaffected, healthy individuals (4,5). Overexpression of IGF-IR appears to represent a phenotypic hallmark of orbital fibroblasts from donors with TAO (4). Exposure of these cells to GD-IgG results in the accelerated production of hyaluronan (6) and T cell chemoattractants, including IL-16 and regulated upon activation, normal T cell-expressed, and secreted (4,5). Overrepresentation of IGF-IR-expressing lymphocytes has recently been described. T cells isolated from orbital connective tissue and those circulating in peripheral blood of patients with GD demonstrate increased IGF-IR display (7). The IGF-IR+ phenotype is found in a disproportionate fraction of CD45RO+ (memory) CD4+, and memory CD8+ T cells. Its expression is associated with enhanced T cell proliferation and resistance to Fas-mediated apoptosis (7). B cells are also skewed in GD toward the IGF-IR+ phenotype, and IGF-I enhances IgG production and promotes B cell expansion (8). Thus, substantial evidence points to widespread involvement of IGF-IR in the pathogenesis of multiple hallmarks of GD, including aberrant IgG production, T cell infiltration, and hyaluronan production.
Genetic, environmental, and epigenetic factors contribute to GD susceptibility (9,10,11,12). But the relative importance of each to the overexpression of IGF-IR in GD has remained unexplored, and thus important questions remain unanswered. For instance, is the skew toward IGF-IR+ T cells and B cells genetically or environmentally determined? Is the increased frequency of the IGF-IR+ lymphocyte phenotype durable, or is it diminished after disease treatment and with the passage of time? For the first time, we report that the phenotypic skew toward IGF-IR+ T lymphocytes in monozygotic twin pairs appears to derive, at least in part, from nongenetic factors, and its appearance may therefore serve as a marker for evolving clinical disease.
Subjects and Methods
Twin pairs were recruited from the population-based Danish Twin Registry. Selection criteria for the registry are detailed elsewhere (13). For this study, only monozygotic twin pairs were eligible, and one or both siblings needed to exhibit clinical and biochemical hyperthyroidism, a diffuse uptake on scinti-scan, and/or presence of TSI without or with TAO. Monozygotic twin pairs without any evidence of autoimmune or thyroid dysfunction served as controls. Eleven of 18 eligible twin pairs in which at least one twin carried the diagnosis of GD identified in the registry agreed to participate. In seven of these pairs, only one twin was affected with GD. Seven additional control monozygotic unaffected twin pairs were randomly selected from registry pairs that were age- and sex-matched to the seven GD-discordant pairs. Both twins in each pair were examined simultaneously. All but two twin pairs lived in the same geographical region of Denmark. A total of 36 individuals (18 twin pairs) were examined. Blood samples from all 18 twin pairs were assayed for serum TSH, serum free T4, free T3, and TSI. At the time of participation, all subjects were euthyroid. The mean interval between diagnosis of GD and entrance into the study was 18.1 ± 7.3 yr, with a range of 8–34 yr. DNA analysis confirmed that all participants were monozygotic, and all underwent clinical examination and completed health-related questionnaires. Written informed consent was obtained from all participants, and the study was approved by all regional Danish Scientific-Ethical Committees.
Flow cytometry
Peripheral blood (∼5 ml) was obtained and stored in tubes containing EDTA. Staining buffer was prepared in PBS containing 4% fetal bovine serum with 0.1% sodium azide (Sigma Aldrich, St. Louis, MO). Staining for flow cytometry was performed within 24 h of blood collection and according to the manufacturer’s instructions (BD Biosciences, San Jose, CA). Briefly, 100 μl whole blood was placed in 12 × 75 mm polypropylene tubes, and fluorochrome-conjugated monoclonal antibodies were added, including anti-CD3, CD4, CD8, CD19, CD20, CD23, CD45RO, CD45RA, CD25, CD69, and IGF-IR at a concentration of 1 μg/106 cells. These were then incubated in the dark for 20 min at room temperature. FACSlyse solution (2 ml) was added for 10 min at room temperature to promote red blood cell lysis. Cells were washed twice with staining buffer, resuspended in Cytofix (BD Biosciences), and kept in the dark at 4 C until cytometric analysis (within 24 h). Analysis was performed on a FACS Calibur flow cytometer (BD Biosciences). Mean fluorescent intensity was calculated as a ratio of mean fluorescence sample/isotype fluorescence. Percentage of positive expression was determined as the population of cells with increased fluorescent intensity compared with isotype.
Statistics
Subject demographic and clinical characteristics are summarized in Table 1 and are expressed as mean ± sd. The mean percentage of IGF-IR+ cells in GD subjects was compared with the percentage for control subjects using two-sample t tests for each T and B cell type. To assess the role of genotype, twin pairs discordant for GD were separately examined. In that analysis, the within-pair affected to unaffected ratio of the percentage of IGF-IR+ cells was summarized with a median for each cell type due to skewness in the distribution of ratios, and significance for ratios shifted away from 1 was determined by the nonparametric Wilcoxon signed rank test. The mean ratio over cell types for each twin pair is used as an overall measure of increased IGF-IR+ phenotype in subjects with GD and analyzed identically, as were the individual cell types. P < 0.05 was considered statistically significant.
Table 1.
Clinical characteristics of the study subjects
| Normal range | 7 Discordant pairs
|
4 Concordant GD pairs | 7 Concordant healthy pairs | ||
|---|---|---|---|---|---|
| GD | Non-GD | ||||
| No. of individuals | 7 | 7 | 8 | 14 | |
| Age (yr) | 48.9 ± 6.2 | 48.9 ± 6.2 | 45.0 ± 7.1 | 49.3 ± 3.6 | |
| Males, n (%) | 0 (0) | 0 (0) | 4 (50) | 0 (0) | |
| Ever smoker, n (%) | 7 (100) | 7 (100) | 6 (75) | 10 (71) | |
| GD duration (yr) | 17.7 ± 4.5 | 18.7 ± 9.6 | |||
| TSH (mIU/liter) | 0.3–4.0 | 2.8 ± 2.8 | 4.4 ± 4.5 | 2.7 ± 1.3 | 1.8 ± 1.5 |
| T4 (nmol/liter) | 70–140 | 139 ± 16 | 122 ± 28 | 124 ± 14 | 125 ± 20 |
| Free T4 (pmol/liter) | 9.9–17.7 | 14.6 ± 4.0 | 11.2 ± 0.8 | 14.1 ± 2.3 | 12.8 ± 1.5 |
| T3 (nmol/liter) | 1.45–2.5 | 2.0 ± 0.3 | 2.2 ± 0.3 | 1.9 ± 0.2 | 2.1 ± 0.3 |
| Free T3 (pmol/liter) | 4.3–7.4 | 6.0 ± 1.9 | 5.6 ± 0.7 | 6.0 ± 1.5 | 5.5 ± 0.8 |
| Anti-TPO Ab >10 (%) | 2–10 κIU/liter | 3 (43) | 4 (57) | 5 (63) | 2 (14) |
| Anti-TR Ab >0.7 (%) | <0.7 IU/liter | 2 (29) | 0 (0) | 2 (25) | 0 (0) |
Data are expressed as mean ± sd, unless described otherwise. Ab, Antibody.
Results
A total of 36 individuals (18 twin pairs) were studied. In seven pairs, one twin manifested GD, whereas the other was unaffected (discordant GD). In another four twin pairs, both twins manifested the disease (concordant GD). In seven control twin pairs, both subjects were healthy and exhibited no evidence of GD or any other autoimmune disease. Demographic and laboratory data are provided in Table 1. Overall, patient groups were of similar age, smoking history, and current thyroid function status.
Case-control study with external controls
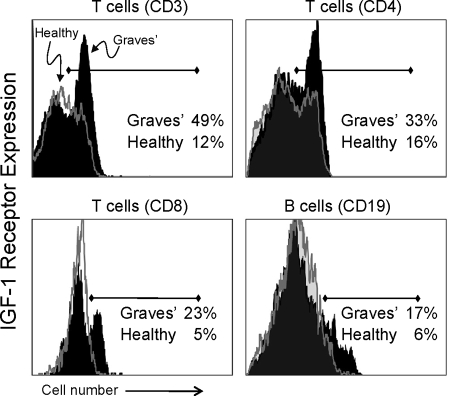
An increased fraction of T and B lymphocytes from patients with GD in the Danish twin registry display IGF-IR, as was found previously in a patient cohort in the United States (7,8). As demonstrated in Fig. 1 and congruent with those previously reported findings, donors with GD exhibit a larger fraction of peripheral blood T cells (CD3+CD4+ and CD3+CD8+ subsets) and B cells (CD19+) expressing IGF-IR compared with controls. Cumulative data, shown in Table 2, demonstrate that 30 ± 2% (mean ± sd) of CD3+ T cells from patients with GD (n = 15) express IGF-IR, whereas the receptor was detected in 20 ± 1% cells from control donors (n = 21; P < 0.0001, GD vs. controls). Furthermore, CD3+CD4+ T cell subsets from these patients with GD also demonstrate a substantially greater fraction of IGF-IR+ cells; 34 ± 2% of CD4+ T cells from these patients express IGF-IR compared with 20 ± 1% cells from control donors (P = 0.0001; GD vs. controls). CD4+ naive (CD45RA+) and memory (CD45RO+) T cells from patients with GD also demonstrate a substantial skew toward the IGF-IR+ phenotype [CD4+CD45RA+: GD, 69 ± 2%; control, 52 ± 3%; (P = 0.0003); and CD4+CD45RO+: GD, 13 ± 1%; control, 9 ± 1% (P = 0.01)]. CD8+ T cell subsets from patients with GD also exhibit a substantially greater fraction of IGF-IR+ cells (Fig. 1 and Table 2). A total of 17 ± 2% of CD8+ T cells from patients express IGF-IR compared with 11 ± 1% cells from control donors (P = 0.02, GD vs. controls). In contrast, CD8+CD45RA+ and CD8+CD45RO+ T cells from GD and control patients demonstrated similar fractions of IGF-IR+ cells (Table 2).
Figure 1.
Increased frequency of IGF-IR-expressing T and B lymphocytes from monozygotic twins with GD. Expression of IGF-IR by CD3+ T cells (upper left panel), CD4+ T cell subset (upper right panel), CD8+ T cell subset (lower left panel), and CD19+ B cells (lower right panel) from twins with GD (solid black histograms) and those not manifesting the disease (open gray histograms). Representative histograms (GD, n = 15; healthy, n = 21).
Table 2.
Comparison between frequency of IGF-IR+ lymphocytes from healthy subjects and those with GD
| GD (n = 15) | Healthy (n = 21) | P value | |
|---|---|---|---|
| T cell | |||
| CD3 | 30 ± 2 | 20 ± 1 | 0.0001 |
| CD4 | 34 ± 2 | 20 ± 1 | 0.0001 |
| CD45RA+ | 69 ± 2 | 52 ± 3 | 0.0003 |
| CD45RO+ | 13 ± 1 | 9 ± 1 | 0.01 |
| CD8 | 17 ± 2 | 11 ± 1 | 0.02 |
| CD45RA+ | 33 ± 4 | 25 ± 3 | 0.10 |
| CD45RO+ | 4 ± 1 | 4 ± 1 | 0.97 |
| B cell | |||
| CD19 | 18 ± 2 | 10 ± 1 | 0.009 |
Data are expressed as percentage IGF-IR+ (mean ± sd).
Analogous to their T cells, CD19+IGF-IR+ B cells from these patients are more frequent (Fig. 1). Cumulative data (Table 2) demonstrate that 18 ± 2% of B cells express IGF-IR, whereas the receptor was detected on 10 ± 1% cells from control donors (P = 0.009, GD vs. controls). The mean fluorescent intensity of IGF-IR staining on T and B cells was indistinguishable for GD and control cells, suggesting similar receptor densities. Expression of surface antigens, including CD69, CD25, CD23, CD80, and CD86 was not significantly different in T and B cells from the two cohorts.
Case-control study with discordant co-twin controls
We next investigated whether a genetic basis could be established for the disproportionately large fraction of IGF-IR+ T and IGF-IR+ B lymphocytes found circulating in blood from patients with GD by examining discordant twin pairs. In six of seven pairs, the affected twin exhibited a larger fraction of IGF-IR+ peripheral blood CD3+ T cells as well as CD4+ and CD8+ subsets when compared with the healthy twin (Tables 3 and 4). CD3+IGF-IR+ T cells from the twin with GD were 1.4-fold more frequent than those from the healthy sibling (n = 7; P < 0.05; Table 3). Similarly, the proportions of CD4+IGF-IR+ T cells and CD4+CD45RA+IGF-IR+ naive T cells from affected twins were 1.6- and 1.3-fold more frequent, respectively (n = 7; both P = 0.03). IGF-IR-expressing CD4+ memory T cells and CD8+T cells were also more abundant in six of seven twin pairs but failed to reach significance (Tables 3 and 4; P = 0.08). The frequencies of CD8+ naive and CD8+ memory T cells expressing IGF-IR were similar in affected and normal twins. CD19+IGF-IR+ B cells were more frequent in five of seven affected twins (Tables 3 and 4; P = 0.16). Averaged over all cell types, the affected twin exhibited the IGF-IR+ phenotype among lymphocytes 1.55-fold more frequently than did the unaffected twin (P < 0.03). In seven of seven twin pairs discordant for GD, an increased frequency of IGF-IR+ lymphocytes in either the CD3+ T cell or CD19+ B cell population was found in the affected twin.
Table 3.
Comparison between frequency of IGF-IR+ lymphocytes from discordant twins without and with GD
| GD twin | Unaffected twin | GD/unaffected ratio | P value | |
|---|---|---|---|---|
| T cell | ||||
| CD3 | 32 ± 10 | 20 ± 7 | 1.41 | 0.047 |
| CD4 | 37 ± 8 | 23 ± 7 | 1.61 | 0.03 |
| CD45RA+ | 72 ± 9 | 50 ± 13 | 1.31 | 0.03 |
| CD45RO+ | 15 ± 6 | 9 ± 5 | 1.83 | 0.08 |
| CD8 | 16 ± 7 | 9 ± 5 | 2.48 | 0.08 |
| CD45RA+ | 28 ± 12 | 20 ± 10 | 1.30 | 0.22 |
| CD45RO+ | 5 ± 3 | 3 ± 2 | 2.00 | 0.20 |
| B cell | ||||
| CD19 | 18 ± 12 | 9 ± 5 | 1.91 | 0.16 |
Data are expressed as percentage IGF-IR+ (mean ± sd) (n = 7).
Table 4.
Percentage IGF-IR+ lymphocytes by subset
| Twin pairs discordant for GD
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD3+ T cells
|
CD4+ T cells
|
CD8+ T cells
|
CD4+CD45RA+ T cells
|
CD4+CD45RO+ T cells
|
CD8+CD45RA+ T cells
|
CD8+CD45RO+ T cells
|
CD19+ B cells
|
||||||||
| Healthy | GD | Healthy | GD | Healthy | GD | Healthy | GD | Healthy | GD | Healthy | GD | Healthy | GD | Healthy | GD |
| 21 | 30 | 29 | 43 | 5 | 11 | 61 | 80 | 13 | 15 | 14 | 24 | 2 | 4 | 13 | 11 |
| 34 | 36 | 24 | 39 | 9 | 11 | 40 | 63 | 6 | 11 | 20 | 26 | 1 | 1 | 10 | 11 |
| 20 | 15 | 24 | 23 | 9 | 6 | 68 | 68 | 16 | 21 | 23 | 12 | 6 | 3 | 12 | 40 |
| 13 | 29 | 16 | 33 | 6 | 22 | 60 | 74 | 9 | 19 | 17 | 51 | 1 | 9 | 4 | 12 |
| 23 | 30 | 31 | 33 | 20 | 25 | 45 | 58 | 12 | 6 | 40 | 33 | 5 | 3 | 17 | 15 |
| 19 | 35 | 23 | 42 | 8 | 20 | 46 | 82 | 5 | 12 | 22 | 24 | 6 | 3 | 5 | 10 |
| 12 | 49 | 13 | 48 | 5 | 20 | 31 | 77 | 4 | 24 | 7 | 23 | 1 | 9 | 2 | 29 |
| Twin pairs concordant for GD
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD3+ T cells | CD4+ T cells | CD8+ T cells | CD4+CD45RA+ T cells | CD4+CD45RO+ T cells | CD8+CD45RA+ T cells | CD8+CD45RO+ T cells | CD19+ B cells | ||||||||
| 32 | 33 | 31 | 37 | 11 | 21 | 60 | 68 | 11 | 13 | 27 | 37 | 6 | 2 | 14 | 16 |
| 29a | 32 | 27a | 32 | 14a | 45 | 62a | 74 | 15a | 9 | 65a | 56 | 3a | 2 | 32a | 30 |
| 26a | 26 | 27a | 32 | 15a | 17 | 60a | 80 | 10a | 12 | 15a | 14 | 2a | 2 | 12a | 11 |
| 19 | 32a | 11 | 54a | 5 | 23a | 58 | 77a | 8 | 14a | 47 | 47a | 2 | 3a | 6 | 17a |
| Control twin pairs | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD3+ T cells | CD4+ T cells | CD8+ T cells | CD4+CD45RA+ T cells | CD4+CD45RO+ T cells | CD8+CD45RA+ T cells | CD8+CD45RO+ T cells | CD19+ B cells | ||||||||
| 21 | 30 | 20 | 22 | 17 | 14 | 54 | 33 | 9 | 15 | 35 | 30 | 1.3 | 12 | 10 | 12 |
| 18 | 13 | 16 | 10 | 11 | 12 | 50 | 57 | 6 | 9 | 34 | 32 | 7 | 6 | 12 | 5 |
| 25 | 18 | 25 | 12 | 12 | 9 | 75 | 60 | 6 | 9 | 26 | 26 | 1 | 1 | 9 | 11 |
| 15 | 10 | 15 | 14 | 3 | 10 | 49 | 35 | 13 | 12 | 28 | 13 | 1 | 1 | 12 | 13 |
| 25 | 23 | 26 | 23 | 16 | 14 | − | − | − | − | − | − | − | − | − | − |
| 15 | 19 | 13 | 20 | 11 | 9 | 21 | 60 | 5 | 13 | 12 | 26 | 3 | 5 | 8 | 9 |
| 17 | 28 | 20 | 22 | 7 | 20 | 71 | 68 | 9 | 8 | 21 | 56 | 1 | 9 | 14 | 6 |
Peripheral blood mononuclear cells were analyzed by flow cytometry as described. Data from each subject are presented. −, Not determined.
TAO.
Analysis of twins concordant for GD was limited because of the small sample size (four twin pairs). However, the IGF-IR+ phenotype was consistently more frequent in both T and B cell populations compared with controls (Table 4). TAO was manifested in three of the eight GD twins from the four concordant pairs, but frequency of IGF-IR+ lymphocyte phenotype was not significantly different compared with the matched twin without orbital disease.
Discussion
Numerous studies have attempted to dissect the genetic and environmental causes of GD and other autoimmune thyroid diseases (9,10,11,12). These have used family and twin-based cohorts to estimate the magnitude of contributions from each in the pathogenesis of disease (14). A substantial component of susceptibility emanates from genetic makeup, and a number of candidate genes have been identified (15,16,17,18,19,20,21). However, factors acquired after conception also play a major role (22,23). Exposure to cigarette smoke (24,25,26), stress (27), high dietary iodine content (28), and several infectious agents (29,30,31) has been implicated in provoking the development of GD. Female predisposition has been linked to skewed X-chromosome inactivation (9,32) where the resulting tissue chimerism could underlie inadequate tolerance to potential self-antigens. This mechanism appears to play an important role in the development of scleroderma (33,34).
The physiological consequences of IGF-IR displayed on circulating lymphocytes have been examined previously. IGF-I supports the development and normal function of the thymus and can participate in its diseases (35,36,37). Precursor thymocytes (CD4−CD8−) express substantially higher IGF-IR levels than do immature CD3−/lowCD4+CD8+, mature CD4+CD8−, or CD4−CD8+ lymphocytes (38). Furthermore, IGF-I plays an essential role in pro B cell development from bone marrow CD34+ cells and regulates B cell function (39,40). IGF-I selectively increases expression of CD23 (type II IgE receptor, FcεRII) by human primary immune cells and established B cell lines (41). Its administration also enhances IgG production by human tonsillar and peripheral B cells and increases circulating antibody levels in mice (42,43,44,45). Thus, a role for IGF-I and its pathway has been established in both normal and abnormal lymphocyte function.
Increased frequency of IGF-IR-expressing T cells and B cells in the peripheral circulation appears characteristic of a majority of recently diagnosed patients with GD and is a durable finding (7,8). Skewed representation of this phenotype does not appear to discriminate between individuals with TAO and those without it. Our current findings suggest that, at least in part, a factor acquired as a feature of clinical disease development might prompt the emergence of increasingly numerous IGF-IR+ lymphocytes. They demonstrate that the skew toward a IGF-IR+ phenotype includes CD8+ T cells but fails to recapitulate the strong bias among CD8+ memory T cells found earlier (7). This disparity may arise from potentially important differences in the participant profiles of the two studies. Patients in the earlier series included only those individuals who manifested clinically recognized TAO. At the time of their participation, they were considerably closer to their initial diagnosis of GD (all within 1 yr). In contrast, the current study involved a population diagnosed approximately 18 yr before their participation, the vast majority without TAO. Both differences may be critical determinants of the CD8+IGF-IR+ T cell skew. Expansion of that IGF-IR+ population of T cells after an encounter with a relevant antigen might abate as a function of disease duration, a situation analogous to well-recognized declining TSI levels occurring with time.
Earlier studies have suggested that the IGF-I pathway might participate in the pathogenesis of GD. Weightman et al. (46) found that an IgG constituent of serum from patients with GD inhibits [125I]-labeled IGF-I binding to the surface of fibroblasts. This interaction between GD-IgG and the cell-surface was subsequently identified as mediated through IGF-IR, a protein the expression of which is increased in GD (4). Moreover, activating GD-IgG against IGF-IR has been detected in most patients with GD thus far examined (4,5). Activating GD-IgG promotes downstream effector functions in fibroblasts and lymphocytes peculiar to those from patients with GD. Thus, IGF-IR may represent an autoantigen relevant to disease pathogenesis. In the current study, we examined whether a genetic association could link the overrepresentation of the IGF-IR+ phenotype among lymphocytes by using monozygotic twin pairs. Our findings suggest that this skew cannot be attributed to genetic determinants but rather appears to be acquired as susceptible individuals manifest the disease. It may represent a clinically useful marker on lymphocytes that heralds the onset of disease. Furthermore, the results highlight the need to identify environmental triggers of autoimmunity in genetically susceptible individuals.
Acknowledgments
The authors are indebted to Dr. Peter D. Christenson for his help with the statistical analysis of this data and to Ms. Debbie Hanaya for her help in preparing the manuscript.
Footnotes
Funding for the study was provided in part by National Institutes of Health Grants EY008976, EY011708, DK063121, EY016339, and RR00425; an unrestricted grant and a career development award from the Research to Prevent Blindness Foundation; the Bell Charitable Foundation; the Novo Nordisk Foundation; and the Agnes and Knut Mørk Foundation.
Disclosure Summary: All authors have nothing to declare.
First Published Online February 24, 2009
Abbreviations: GD, Graves’ disease; GD-IgG, IgGs directed against the IGF-IR; IGF-IR, IGF-I receptor; TAO, thyroid-associated ophthalmopathy; TSI, thyroid-stimulating antibodies.
References
- Prabhakar BS, Bahn RS, Smith TJ 2003 Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev 24:802–835 [DOI] [PubMed] [Google Scholar]
- Akamizu T 2003 Monoclonal antibodies to thyroid specific autoantigens. Autoimmunity 36:361–366 [DOI] [PubMed] [Google Scholar]
- Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM 2003 The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim. J Clin Invest 111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ 2003 Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol 170:6348–6354 [DOI] [PubMed] [Google Scholar]
- Pritchard J, Horst N, Cruikshank W, Smith TJ 2002 Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol 168:942–950 [DOI] [PubMed] [Google Scholar]
- Smith TJ, Hoa N 2004 Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab 89:5076–5080 [DOI] [PubMed] [Google Scholar]
- Douglas RS, Gianoukakis AG, Kamat S, Smith TJ 2007 Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol 178:3281–3287 [DOI] [PubMed] [Google Scholar]
- Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, Smith TJ 2008 B Cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol 181:5768–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix TH, Knudsen GP, Kristiansen M, Kyvik KO, Orstavik KH, Hegedus L 2005 High frequency of skewed X-chromosome inactivation in females with autoimmune thyroid disease: a possible explanation for the female predisposition to thyroid autoimmunity. J Clin Endocrinol Metab 90:5949–5953 [DOI] [PubMed] [Google Scholar]
- Brix TH, Kyvik KO, Christensen K, Hegedus L 2001 Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab 86:930–934 [DOI] [PubMed] [Google Scholar]
- Prummel MF, Strieder T, Wiersinga WM 2004 The environment and autoimmune thyroid diseases. Eur J Endocrinol 150:605–618 [DOI] [PubMed] [Google Scholar]
- Holm IA, Manson JE, Michels KB, Alexander EK, Willett WC, Utiger RD 2005 Smoking and other lifestyle factors and the risk of Graves’ hyperthyroidism. Arch Intern Med 165:1606–1611 [DOI] [PubMed] [Google Scholar]
- Skytthe A, Kyvik K, Bathum L, Holm N, Vaupel JW, Christensen K 2006 The Danish Twin Registry in the new millennium. Twin Res Hum Genet 9:763–771 [DOI] [PubMed] [Google Scholar]
- Villanueva R, Greenberg DA, Davies TF, Tomer Y 2003 Sibling recurrence risk in autoimmune thyroid disease. Thyroid 13:761–764 [DOI] [PubMed] [Google Scholar]
- Taylor JC, Gough SC, Hunt PJ, Brix TH, Chatterjee K, Connell JM, Franklyn JA, Hegedus L, Robinson BG, Wiersinga WM, Wass JA, Zabaneh D, Mackay I, Weetman AP 2006 A genome-wide screen in 1119 relative pairs with autoimmune thyroid disease. J Clin Endocrinol Metab 91:646–653 [DOI] [PubMed] [Google Scholar]
- Tomer Y, Davies TF 2003 Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocr Rev 24:694–717 [DOI] [PubMed] [Google Scholar]
- Jacobson EM, Tomer Y 2007 The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun 28:85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TY, Park YJ, Hwang JK, Song JY, Park KS, Cho BY, Park DJ 2003 A C/T polymorphism in the 5′-untranslated region of the CD40 gene is associated with Graves’ disease in Koreans. Thyroid 13:919–925 [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Amemiya S, Kobayashi K, Shimura Y, Ishihara T, Nakagomi Y, Onigata K, Tamai S, Kasuga A, Nanazawa S 2003 Association of the CTLA-4 gene 49 A/G polymorphism with type 1 diabetes and autoimmune thyroid disease in Japanese children. Diabetes Care 26:843–847 [DOI] [PubMed] [Google Scholar]
- Lo FS, Lee YJ, Huang CY, Lin CH, Chang SC, Dang CW, Liu HF 2003 Polymorphism in the transmembrane region of the major histocompatibility complex class I chain-related gene A: association of five GCT repetitions with Graves’ disease in children. Thyroid 13:839–843 [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Turakulov RI 2003 CTLA-4 and its role in autoimmune thyroid disease. J Mol Endocrinol 31:21–36 [DOI] [PubMed] [Google Scholar]
- Villanueva R, Inzerillo AM, Tomer Y, Barbesino G, Meltzer M, Concepcion ES, Greenberg DA, MacLaren N, Sun ZS, Zhang DM, Tucci S, Davies TF 2000 Limited genetic susceptibility to severe Graves’ ophthalmopathy: no role for CTLA-4 but evidence for an environmental etiology. Thyroid 10:791–798 [DOI] [PubMed] [Google Scholar]
- Brix TH, Kyvik KO, Hegedus L 1998 What is the evidence of genetic factors in the etiology of Graves’ disease? A brief review. Thyroid 8:727–734 [DOI] [PubMed] [Google Scholar]
- Lois N, Abdelkader E, Reglitz K, Garden C, Ayres JG 2008 Environmental tobacco smoke exposure and eye disease. Br J Ophthalmol 92:1304–1310 [DOI] [PubMed] [Google Scholar]
- Bufalo NE, Santos RB, Cury AN, Andrade RA, Morari J, Morari EC, Leite JL, Monte O, Romaldini JH, Ward LS 2008 Genetic polymorphisms associated with cigarette smoking and the risk of Graves’ disease. Clin Endocrinol (Oxf) 68:982–987 [DOI] [PubMed] [Google Scholar]
- Brix TH, Hansen PS, Kyvik KO, Hegedus L 2000 Cigarette smoking and risk of clinically overt thyroid disease: a population-based twin case-control study. Arch Intern Med 160:661–666 [DOI] [PubMed] [Google Scholar]
- Tsai CC, Cheng CY, Liu CY, Kao SC, Kau HC, Hsu WM, Wei YH 10 October 2008 Oxidative stress in patients with Graves’ ophthalmopathy: relationship between oxidative DNA damage and clinical evolution. Eye 10.1038/eye.2008.310 [DOI] [PubMed] [Google Scholar]
- Laurberg P, Jorgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, Rasmussen LB, Carle A, Vejbjerg P 2006 The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol 155:219–228 [DOI] [PubMed] [Google Scholar]
- Levy BS 2008 Graves’ disease. N Engl J Med 359:1408; author reply, 1409 [PubMed] [Google Scholar]
- Kraemer MH, Donadi EA, Tambascia MA, Magna LA, Prigenzi LS 1998 Relationship between HLA antigens and infectious agents in contributing towards the development of Graves’ disease. Immunol Invest 27:17–29 [DOI] [PubMed] [Google Scholar]
- Brix TH, Hansen PS, Hegedus L, Wenzel BE 2008 Too early to dismiss Yersinia enterocolitica infection in the aetiology of Graves’ disease: evidence from a twin case-control study. Clin Endocrinol (Oxf) 69:491–496 [DOI] [PubMed] [Google Scholar]
- Yin X, Latif R, Tomer Y, Davies TF 2007 Thyroid epigenetics: X chromosome inactivation in patients with autoimmune thyroid disease. Ann NY Acad Sci 1110:193–200 [DOI] [PubMed] [Google Scholar]
- Ozbalkan Z, Bagislar S, Kiraz S, Akyerli CB, Ozer HT, Yavuz S, Birlik AM, Calguneri M, Ozcelik T 2005 Skewed X chromosome inactivation in blood cells of women with scleroderma. Arthritis Rheum 52:1564–1570 [DOI] [PubMed] [Google Scholar]
- Uz E, Loubiere LS, Gadi VK, Ozbalkan Z, Stewart J, Nelson JL, Ozcelik T 2008 Skewed X-chromosome inactivation in scleroderma. Clin Rev Allergy Immunol 34:352–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerot I, Fabien N, Thivolet C 1996 Effects of insulin like growth factor-1 and insulin on effector T cells generating autoimmune diabetes. Diabetes Metab 22:235–239 [PubMed] [Google Scholar]
- de Mello Coelho V, Villa-Verde DM, Farias-de-Oliveira DA, de Brito JM, Dardenne M, Savino W 2002 Functional insulin-like growth factor-1/insulin-like growth factor-1 receptor-mediated circuit in human and murine thymic epithelial cells. Neuroendocrinology 75:139–150 [DOI] [PubMed] [Google Scholar]
- Beschorner WE, Divic J, Pulido H, Yao X, Kenworthy P, Bruce G 1991 Enhancement of thymic recovery after cyclosporine by recombinant human growth hormone and insulin-like growth factor I. Transplantation 52:879–884 [DOI] [PubMed] [Google Scholar]
- Kooijman R, Scholtens LE, Rijkers GT, Zegers BJ 1995 Type I insulin-like growth factor receptor expression in different developmental stages of human thymocytes. J Endocrinol 147:203–209 [DOI] [PubMed] [Google Scholar]
- Kim JH, Park HH, Lee CE 2003 IGF-1 potentiation of IL-4-induced CD23/Fc(ε)RII expression in human B cells. Mol Cells 15:307–312 [PubMed] [Google Scholar]
- Landreth KS, Narayanan R, Dorshkind K 1992 Insulin-like growth factor-I regulates pro-B cell differentiation. Blood 80:1207–1212 [PubMed] [Google Scholar]
- Stuart CA, Meehan RT, Neale LS, Cintron NM, Furlanetto RW 1991 Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphocytes. J Clin Endocrinol Metab 72:1117–1122 [DOI] [PubMed] [Google Scholar]
- Kimata H, Fujimoto M 1994 Growth hormone and insulin-like growth factor I induce immunoglobulin (Ig)E and IgG4 production by human B cells. J Exp Med 180:727–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata H, Yoshida A 1994 Differential effect of growth hormone and insulin-like growth factor-I, insulin-like growth factor-II, and insulin on Ig production and growth in human plasma cells. Blood 83:1569–1574 [PubMed] [Google Scholar]
- Robbins K, McCabe S, Scheiner T, Strasser J, Clark R, Jardieu P 1994 Immunological effects of insulin-like growth factor-I—enhancement of immunoglobulin synthesis. Clin Exp Immunol 95:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardieu P, Clark R, Mortensen D, Dorshkind K 1994 In vivo administration of insulin-like growth factor-I stimulates primary B lymphopoiesis and enhances lymphocyte recovery after bone marrow transplantation. J Immunol 152:4320–4327 [PubMed] [Google Scholar]
- Weightman DR, Perros P, Sherif IH, Kendall-Taylor P 1993 Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity 16:251–257 [DOI] [PubMed] [Google Scholar]