Abstract
Context: Progesterone has been associated with promoting growth of uterine leiomyomas. The mechanisms involved remain unclear.
Objective: In this study we investigated the activation of the AKT pathway and its downstream effectors, glycogen synthase kinase-3b and Forkhead box O (FOXO)-1 by progesterone as a mechanism of proliferation and survival of leiomyoma cells. Inhibitors of the AKT pathway were used to demonstrate the role of phosphatidylinositol 3-kinase, AKT, and FOXO1 in contributing to cell proliferation and apoptosis.
Results: Treatment of leiomyoma cells with R5020 over a period of 72 h resulted in higher cell numbers compared with untreated cells. When cells were treated with 100 nm R5020 for 1 and 24 h, the levels of phospho(Ser 473)-AKT increased. This increase was inhibited when cells were cotreated with RU486. Treatment of leiomyoma cells with a phosphatidylinositol 3-kinase inhibitor, LY294 dramatically decreased levels of phospho(Ser 473)-AKT, despite R5020 treatment. In addition to increased phospho(Ser 473)-AKT levels, R5020 treatment resulted in an increase in phospho(Ser 256)-FOXO1 and phosphoglycogen synthase kinase-3b. Inhibition of AKT using API-59 decreased proliferation and cell viability even in the presence of R5020. Higher concentrations of API-59-induced apoptosis of leiomyoma cells, even in the presence of R5020. Psammaplysene A increased nuclear FOXO1 levels and did not affect cell proliferation but induced apoptosis of leiomyoma cells.
Conclusions: The progestin, R5020, can rapidly activate the AKT pathway. Inhibition of the AKT pathway inhibits cell proliferation and promotes apoptosis of leiomyoma cells.
The rapid activation of the AKT pathway by progesterone in leiomyoma cells is demonstrated, providing a mechanism by which progesterone promotes growth of uterine leiomyomas.
Uterine fibroids, also known as leiomyomas, are benign tumors of the myometrium. They are composed of smooth muscle cells and large amounts of extracellular matrix. Uterine leiomyomas are common, estimated to be present in 77% of women (1). The incidence in African-American women is 60% at age 35 yr and more than 80% by age 50 yr, whereas Caucasian women have an incidence of 40% by age 35 yr and almost 70% by age 50 yr (2). Although the tumors are considered benign, they cause significant morbidity, such as pain, discomfort, and excessive menstrual bleeding. Leiomyomas are the primary indication for hysterectomy in the United States, which is performed 200,000 times per year (3). To date, medical treatments for leiomyomas are limited due to the fact that the mechanisms regulating the development and growth of these tumors remain unclear. A better understanding of leiomyomas at the molecular level would be beneficial toward the development of more effective treatments of this disease.
Studies have identified possible factors responsible for the development of leiomyomas, including chromosome rearrangements, congenitally elevated estrogen receptors, and hormonal changes and injury due to potential hypoxic conditions (4). Whereas it is unlikely that steroid hormones are responsible for the initiation of leiomyomas, it has been well documented that leiomyoma growth depends on these hormones. Traditionally estrogen has been considered the major promoter of leiomyoma growth and increased levels of enzymes that favor estradiol production, such as aromatase and 17β-hydroxysteroid dehydrogenase in leiomyoma cells have been reported (5,6,7). This could lead to the up-regulation of estrogen and progesterone receptors, hyperresponsiveness to steroid hormones, and leiomyoma growth. There is also sufficient biochemical, clinical, and pharmacological evidence that suggests progesterone is important in the pathogenesis of leiomyomas. First, leiomyomas have increased levels of progesterone receptors A and B compared with normal myometrium (8,9). Higher mitotic rates are found in leiomyomas during the secretory phase of the menstrual cycle when progesterone levels peak (10). Women treated with medroxyprogesterone acetate have higher mitotic counts in the leiomyomas than in untreated controls (10). When progestin is given concurrently with GnRH, it prevents a decrease in size normally observed with GnRH only treatment (11,12). RU486, a progesterone antagonist, is used to treat fibroids (13). Despite the strong implications that progesterone promotes leiomyoma growth or survival, the mechanisms by which this occurs is unknown.
Progesterone’s effects are mediated through interaction with its receptor (PR), a transcription factor and member of a large family of structurally related gene products known as the nuclear receptor superfamily (14). PR is recruited to the DNA and thereby regulates gene transcription. Pioneering work by the group of Edwards and colleagues (15) demonstrated that progesterone can also activate nongenomic signaling molecules by directly binding to the Src homology (SH)-3 domain of Src kinase and thereby activating the kinase. It has also been shown that the phosphatidylinositol 3-kinase (PI3K)/AKT pathway can be rapidly activated by estrogen and progesterone in rat uterine cells and human breast cancer cells (15,16,17,18,19). PR can interact with the SH3 domain of the p85 regulatory subunit of PI3K in a cell-free system using glutathione-S-transferase pulldown (15).
Once activated, AKT plays a central role in modulating cell survival, proliferation, migration, differentiation and apoptosis (20). AKT directly phosphorylates the Forkhead box O (FOXO) transcription factors as well as other proteins such as glycogen synthase kinase (GSK)-3 and tuberous sclerosis-2 (TSC2) to promote cell proliferation and survival. Hyperactivation of the PI3K/AKT pathway is common in many cancers such as breast, endometrium, prostate, lung, pancreatic, liver, ovarian, and colorectal cancers (21) underlying its importance in promoting cell survival and in inhibiting apoptosis.
In this study, we demonstrate for the first time the rapid increase in phospho(Ser 473)-AKT levels in response to progestins in leiomyoma cells. We show that R5020 acts potentially through PR, which then activates AKT, leading to phosphorylation and cytoplasmic translocalization of FOXO1. Inhibiting molecules of the AKT pathway results in decreased proliferation and increased apoptosis of leiomyoma cells, supporting the importance of activated AKT in progesterone-mediated cell proliferation and survival of leiomyoma cells.
Subjects and Methods
Tissue collection and cell culture
Human uterine leiomyoma were collected from premenopausal women undergoing hysterectomy or abdominal myomectomy. The subjects had not received any hormonal treatment 6 months before surgery. The tissues were collected from the Prentice Women’s Hospital at Northwestern University, and permission for using these human specimens was approved by the Northwestern University Institutional Review Board. Tissue pieces were taken from the peripheral portions (∼1 cm to the capsule) of the leiomyoma. Cells were isolated and cultured as previously described (22). Cells used in the experiments were passaged up to three times before they were discarded. Leiomyoma cells were maintained in DMEM/F12 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, sodium pyruvate, and antibiotics (5000 U/ml penicillin G, 5000 μg/ml streptomycin) at 37 C. All cell culture reagents were purchased from Invitrogen. Cells were grown to 80% confluence and serum starved overnight before treatments with vehicle, R5020, or R5020+RU486 for 1 and 24 h.
Western blot analysis
Leiomyoma cells were lysed with radioimmunoprecipitation assay buffer [(pH 8.0), 150 mm NaCl, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1% tert-Octylphenoxy poly (oxyethylene)ethanol, 50 mm Tris] with protease and phosphatase inhibitors to collect whole-cell lysates. The lysates were stored at −20 C pending analysis. Nuclear and cytoplasmic proteins were isolated using the NE-PER nuclear and cytoplasmic extraction kit (Pierce, Rockford, IL). Protein content was determined with the microbicinchoninic assay protein assay kit. Protein extracts were heated at 95 C for 3 min, run on a precast 10% acrylamide gel, and transferred onto polyvinyl difluoride membrane. Membranes were blocked with 5% milk or 1.5% BSA in Tris-buffered saline with 0.1% Tween 20 and then incubated with primary antibody followed by incubation with secondary peroxidase-conjugated IgG. Protein complexes were detected with the ECL Plus Western blotting detection system (Amersham Biosciences, Buckinghamshire, UK). Antibodies used included total AKT, phospho(Ser473)-AKT, phospho(Ser256)-FOXO1, cleaved poly(ADP-ribose) polymerase (PARP; Cell Signaling, Danvers, MA), and total FOXO1 (Bethyl, Montgomery, TX). All Western blots are representative of experiments performed on at least four patient tissues.
Cell viability and proliferation
Leiomyoma cells were plated in 96-well plates at 1 × 105 cells/well and allowed to attach overnight. Cells were then washed, and fresh media without serum were added for 4–6 h. Media were again replaced with or without API-59CJ-OMe (API-59; EMD Biosciences, San Diego, CA) or psammaplysene A (PsA; donated by J. Clardy, Harvard University) (23) in the presence or absence of 100 nm promegestrone (R5020; PerkinElmer Life Sciences, Waltham, MA). Cells were incubated at 37 C for 24–72 h. The Quick Cell proliferation assay kit (Biovision, Mountain View, CA) was used to measure cell viability according to the manufacturer’s protocol. The absorbance of the wells was measured using a microtiter plate reader (Synergy HT model no. SIAFRID; Bio-Tek, Winooski, VT) at wavelengths of 440 and 650 nm. Data were presented as the fold increase or decrease from the untreated controls. To measure cell proliferation, 5-bromo-2′-deoxyuridine (BrdU) incorporation immunoassay was used. Cells were treated as above and BrdU was added during the 24 h of inhibitor or R5020 treatment. During this time, BrdU is incorporated in place of thymidine into the DNA of cycling cells. BrdU was then detected by an immunoassay according to the manufacturer’s protocol (Roche Applied Sciences, Indianapolis, IN) and the reaction product quantified by measuring the absorbance using a scanning multiwell spectrophotometer at 370 nm wavelength.
Apoptosis
Leiomyoma cells were grown in 100-mm plates until 80% confluency. Cells were serum starved overnight followed by treatment with inhibitors with or without R5020 for 24 h. Cells were trypsinized and resuspended in annexin-binding buffer [10 mm HEPES, 140 mm NaCl, 2.5 mm CaCl2 (pH 7.4)] to a concentration of approximately 1 × 105 cells/ml. Annexin V, Alexa Fluor 647 conjugate (Invitrogen), and 4′,6′-diamino-2-phenylindole (Invitrogen) were added to each cell solution, and samples were analyzed using the CyAn flow cytometer (Dako, Fort Collins, CO) for early and late apoptosis. Immunoblotting for cleaved PARP was done as described above for monitoring apoptosis.
Immunofluorescent staining
Leiomyoma cells were grown on glass coverslips and treated with 100 nm R5020 or 100 nm PsA for 24 h. Cells were fixed with 4% paraformaldehyde (Sigma, St. Louis, MO). Cells were blocked with 5% BSA (Sigma) made in PBS. Subsequently, the FOXO1 primary antibody made in filtered 5% BSA was added to each sample and incubated for 2 h at room temperature. A fluorescein secondary peroxidase-conjugated goat antirabbit IgG (Vector Laboratories Inc., Burlingame, CA) was used. Cells were then mounted with Vectashield Hard Set mounting medium for fluorescence (Vector Laboratories) and visualized using a fluorescent inverted microscope, Axiovert 200 (Zeiss, Thornwood, NY).
Statistical analysis
The data from cell viability assay after treatment with R5020 and the annexin V binding assay were statistically analyzed using a pairwise t test, comparing treated samples with the corresponding untreated samples. Differences are denoted with the letter a.
Results
Effect of progestin on the AKT pathway
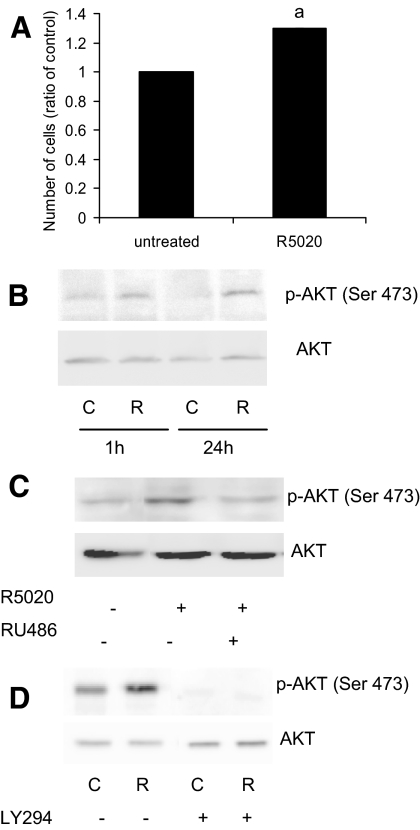
Progesterone promotes leiomyoma growth by mechanisms that remain unclear. In culture, leiomyoma cells continue to express PRA and PRB [supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org; (22)]. When leiomyoma cells are treated with the progestin, R5020, the number of viable cells increases in a period of 72 h compared with the untreated cells (Fig. 1A), suggesting an increase in proliferation and/or cell survival. We hypothesized that one mechanism by which progesterone elicits this response in leiomyoma cells is through the activation of the AKT pathway. When leiomyoma cells were treated with R5020 for 1 h, the levels of phospho(Ser437)-AKT increased compared with the untreated cells, as determined by Western blot (Fig 1B). The higher levels of phospho(Ser437)-AKT were evident, even after 24 h of R5020 treatment, suggesting a prolonged activation of AKT. Treatment of leiomyoma cells with progesterone also similarly increased phospho(Ser437)-AKT (supplemental Fig. 1C). Phospho(Ser437)-AKT levels in matched myometrial cells with or without treatment with R5020 or progesterone was lower than that of leiomyoma cells, and no consistent pattern of increased phosphorylation was observed with the progestins (supplemental Fig. 1, B and C).
Figure 1.
Effect of progestin on AKT. A, Leiomyoma cells were treated with 100 nm R5020 for 72 h. Cell number was assessed using the cell viability assay. B, Leiomyoma cells were treated with 100 nm R5020 for 1 and 24 h. Levels of phospho(Ser473)-AKT and total AKT proteins were detected by Western blot. C, Leiomyoma cells were pretreated with 1 μm RU486 for 30 min before R5020 treatment for 1 h. Levels of phospho(Ser473)-AKT and total AKT proteins were detected by Western blot. D, Leiomyoma cells were pretreated with 10 μm LY294 for 30 min before R5020 treatment for 15 min. Levels of phospho(Ser473)-AKT and total AKT proteins were detected by Western blot. C, Control; R, R5020; a, Statistical significance.
Addition of the antiprogestin RU486 along with R5020 inhibited the increase of phospho(Ser437)-AKT levels induced by R5020 in leiomyoma cells, suggestive of the involvement of the progesterone receptor in activation of AKT (Fig. 1C). Interestingly, phospho(Ser437)-AKT protein was also detected in the untreated cells, albeit weakly, indicating an activation of AKT at the basal level (Fig. 1, B and C). Because it has been demonstrated that the p85 subunit of PI3K can bind to PR in a cell-free system (15), we next determined whether PI3K was involved in the rapid phosphorylation of AKT using the specific PI3K inhibitor, LY294. Leiomyoma cells were pretreated for 30 min with LY294 and then treated with R5020 for 15 min. Treatment with LY294 not only prevented the increase of phosphorylated (p)-AKT observed with R5020 but also decreased p-AKT levels beyond that of the basal level (Fig. 1D). These data demonstrate that PI3K is involved in phosphorylation of AKT by R5020.
To determine whether activation of AKT by R5020 also influences phosphorylation of direct downstream targets, levels of phospho-GSK3β and phospho(Ser256)-FOXO1 after 1 h of R5020 treatment was measured by Western blot. Levels of both phospho-GSK3β and phospho(Ser256)-FOXO1 increased after R5020 treatment, whereas total GSK3β and FOXO1 levels remained the same (Fig. 2A). In addition, R5020 treatment resulted in increased levels of FOXO1 protein in the cytoplasm (Fig. 2B), suggesting that increased phosphorylation of FOXO1 promoted translocation of this protein to the cytoplasm.
Figure 2.
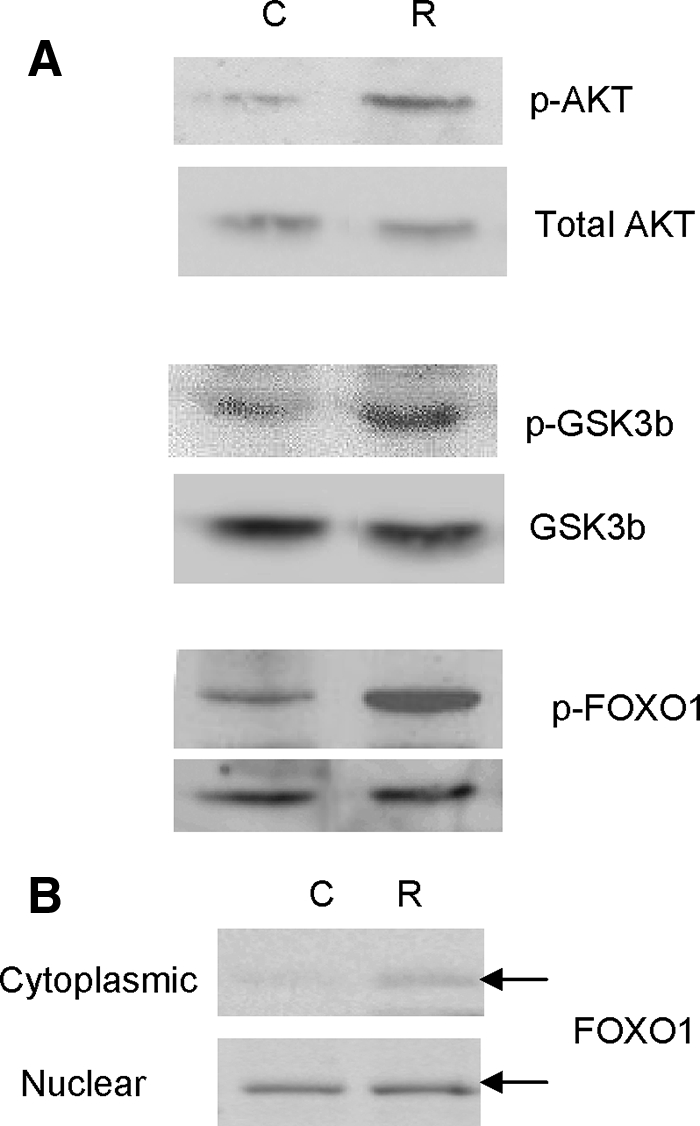
Effect of progestin on AKT pathway. A, Leiomyoma cells were treated with 100 nm R5020 for 1 h. Levels of phospho(Ser473)-AKT, total AKT, phospho-GSKβ, GSKβ, phospho(Ser256)-FOXO1, and FOXO1 proteins were detected by Western blot. B, Leiomyoma cells were treated with 100 nm R5020 for 1 h. Nuclear and cytoplasmic fractions were isolated and levels of total FOXO1 were detected in each fraction by Western blot. C, Control; R, R5020.
Effect of inhibiting AKT activity on cell proliferation
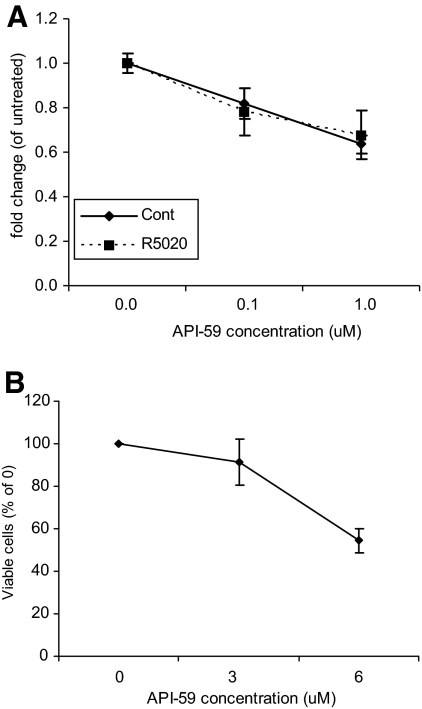
Thus far, we have demonstrated that AKT is activated in leiomyoma cells both at the basal level and more so in response to R5020. In an effort to determine whether activation of this pathway played a role in cell proliferation and survival, cells were treated with an AKT inhibitor, API-59, that has been shown to inhibit AKT kinase activity (24), and cell proliferation or apoptosis was measured. These studies were done in the presence or absence of R5020 to determine whether API-59 was able to inhibit the effects of R5020 on leiomyoma cells. When cells were treated with 100 nm or 1 μm of API-59 with or without R5020 for 24 h, a decrease in proliferation was observed as determined by BrdU incorporation (Fig. 3A). There was no difference in this effect whether R5020 was present, suggesting that the AKT inhibitor acts downstream of the effects of R5020. At higher concentrations of API-59 (6 μm), cell death was evident as observed under the light microscope and as quantitatively measured using a cell viability assay (Fig. 3B).
Figure 3.
Effect of API-59 on cell proliferation and viability. A, Leiomyoma cells were treated with 100 nm or 1 μm of API-59 with or without 100 nm R5020 for 24 h. Cell proliferation was measured using BrdU incorporation assay. B, A Leiomyoma cells were treated with 3 or 6 μm of API-59. The number of viable cells was measured using the cell viability assay. Data are the mean ± sem of four experiments.
Effect of increased nuclear FOXO1 on cell proliferation
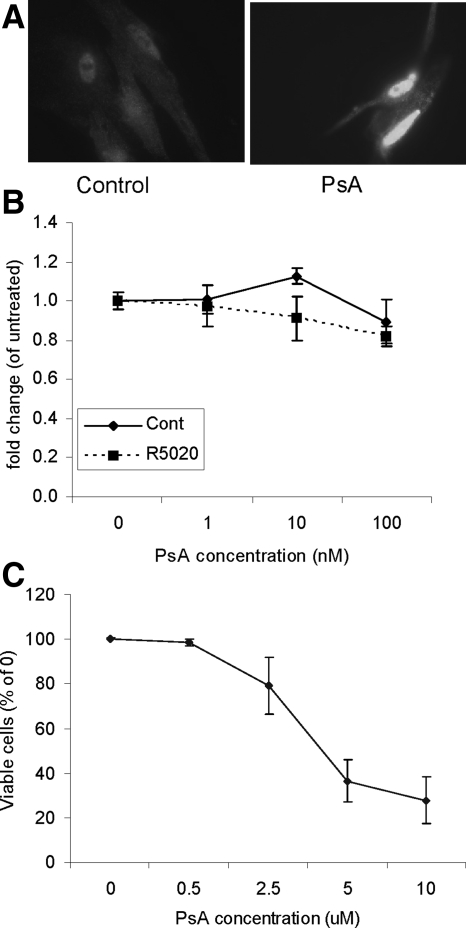
One of the direct targets of AKT activity is FOXO1, which regulates cell proliferation and apoptosis (25). As observed in Fig. 2, levels of phospho(Ser256)-FOXO1 increase in response to R5020 and more FOXO1 is found in the cytoplasm. This is indicative that on phosphorylation of FOXO1 by R5020, translocation to the cytoplasm occurs. A chemical compound derived from a marine sponge, PsA, has been shown to enforce nuclear relocalization of FOXO1 in cells with a phosphatase and tensin homolog deleted from chromosome 10 (PTEN) mutation (26). Treatment of leiomyoma cells with PsA for 24 h did indeed increase FOXO1 protein in the nucleus (Fig. 4A). Next, leiomyoma cells were treated with 1–100 nm PsA, and cell proliferation was not significantly affected (Fig 4B). Again, no significant difference was observed between the control and R5020-treated cells, suggesting that PsA acts downstream of the effects of R5020. Higher concentrations of PsA significantly reduced cell viability (Fig. 4C).
Figure 4.
Effect of PsA on cell proliferation and viability. A, Leiomyoma cells were treated with 100 nm PsA for 24 h. Immunofluorescent staining for FOXO1 was done. B, Leiomyoma cells were treated with 1–100 nm of PsA with or without 100 nm R5020. Cell proliferation was measured using BrdU incorporation assay. C, Leiomyoma cells were treated with 0.5–10 μm of PsA. The number of viable cells was measured using the cell viability assay. Data are the mean ± sem of four experiments.
Effect of API-59 and PsA on apoptosis
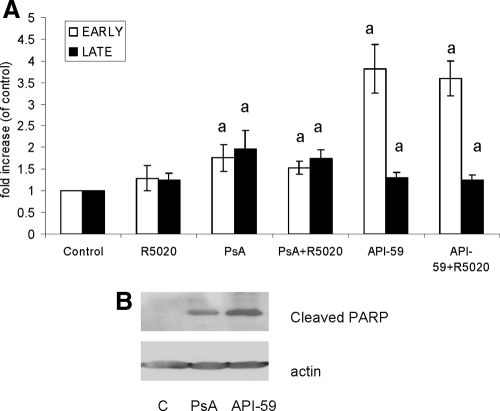
The decrease in cell viability observed in leiomyoma cells treated with API-59 or PsA was further investigated using apoptotic assays. Leiomyoma cells were treated with 6 μm API-59 or 100 nm PsA with or without R5020, and apoptosis was measured using the annexin V binding assay. As shown in Fig. 5A, both API-59 and PsA significantly increased the percentage of early and late apoptotic cells compared with untreated cells. Treatment of leiomyoma cells with R5020 alone did not induce apoptosis, and its presence with API-59 or PsA did not affect the percentage of cells undergoing apoptosis. These observations were confirmed by measuring levels of cleaved PARP, which is indicative of cells undergoing apoptosis. Both API-59 and PsA increased levels of cleaved PARP compared with untreated cells (Fig. 5B). Treatment of R5020 did not affect levels of cleaved PARP (data not shown). These data demonstrate that API-59, which inhibits AKT kinase activity, and PsA, which increases levels of nuclear FOXO1 protein, are able to induce apoptosis in leiomyoma cells.
Figure 5.
Effect of API-59 and PsA on apoptosis. A, Leiomyoma cells were treated with 6 μm API-59 or 100 nm PsA with or without 100 nm R5020 for 24 h. Annexin V binding assay was done and the percentage of cells in early and late apoptosis was measured. B, Leiomyoma cells were treated as above and levels of cleaved PARP was measured by Western blot. Data are the mean ± sem of four experiments. a, Statistical significance.
Discussion
It has been well documented by biochemical, histological, clinical, and pharmacological studies that PR plays a key role in uterine leiomyoma growth (27). PRs are highly expressed in leiomyomas compared with adjacent myometrium [supplemental Fig. 1A (28)] and PR expression increases during the secretory phase of the menstrual cycle (29). This observation has not always been reported to be consistent, and one study (30) reported that expression of PR in leiomyoma and matched myometrium varied, depending on tumor size and patient age. Thus, the variability and heterogeneity of the disease may contribute to conflicting observations. The PR modulators, RU486 and asoprisnil, can cause fibroids to regress, providing additional support that progesterone promotes uterine leiomyoma growth through its receptor, PR (12,31,32). We have shown that progestins increased leiomyoma cell viability over a period of 72 h, possibly through enhanced cell survival or cell proliferation (Fig. 1A). This supports previous studies showing an increase in leiomyoma cell thymidine incorporation or proliferating cell nuclear antigen expression with progesterone treatments (33,34). The mechanism by which progesterone promotes leiomyoma growth remains to be elucidated. Studies have demonstrated that progesterone increases epithelial growth factor (29), thereby promoting proliferation. Progesterone can also increase levels of the antiapoptotic molecule, Bcl2, which would inhibit apoptosis and promote survival (22,29). Recently it was shown that asoprisnil decreased Bcl-2 expression and increased apoptosis in leiomyoma cells (35). Similarly, asoprisnil decreased the mRNA and protein expressions of epithelial growth factor, IGF-I, and TGFβ as well as their corresponding receptors in leiomyoma cells. We have demonstrated for the first time that progesterone can rapidly phosphorylate AKT in human leiomyoma cells, providing yet another mechanism by which progesterone can promote cell proliferation and survival.
There has been much progress in elucidating the nongenomic role of progesterone in the activation of signaling molecules, specifically Src kinase (15). This has been shown to occur through physical interaction of the polyproline sequence motif in the N-terminal domain of PR to the SH3 domain of Src. This interaction causes a physical displacement of the SH3, creating an open conformation of Src, activating the kinase domain. This in turn activates the Src/Ras-Raf/MAPK kinase-1/MAPK signaling pathway. Interestingly, the p85 regulatory subunit of PI3K also contains two SH2 domains and an SH3 domain. In fact, it has been shown in a cell-free glutathione-S-transferase pulldown system that liganded PR interacts with the SH3 domain of p85 (15). We have demonstrated that inhibiting PI3K using LY294 inhibits the increase in p-AKT by R5020, strongly implicating the involvement of PI3K. We are currently investigating the involvement of PI3K in more detail in the rapid progestin-mediated activation of AKT. We are in the process of determining whether PR physically associates with PI3K in leiomyoma cells and whether this mediates increased p-AKT.
Once activated, AKT plays a central role in modulating cell survival, proliferation, migration, differentiation, and apoptosis (20). AKT directly phosphorylates multiple proteins, including FOXO transcription factors, GSK3, TSC2, and AKT1 substrate 1 (proline-rich) which leads to activation of Mammalian target of rapamycin complex 1. In doing so, AKT activation promotes cell survival by regulating glycolysis and glucose uptake and inactivating molecules associated with apoptosis, i.e. FOXO and caspases (36). In this study, we have shown that both FOXO1 and GSK3b are phosphorylated on R5020 treatment. In addition, we observed an increase in cytoplasmic FOXO1 levels with R5020. We performed this experiment in at least six different patients and consistently found similar observations of increased cytoplasmic FOXO1 without a visible difference in nuclear FOXO1 levels. We speculate that this may be due to the fact that levels of FOXO1 translocating to the cytoplasm after 1 h is modest and that the expected decrease in nuclear levels of FOXO1 cannot be visualized by Western blot. It is also possible that levels of total FOXO1 increases after R5020 treatment due to effects that are independent of AKT. Nevertheless, although effects of progesterone on FOXO1 appear modest after an hour, the consequential downstream effect on FOXO1 after prolonged exposure to progesterone may be significant.
Hyperactivation of the PI3K/AKT pathway is common in many cancers such as breast, endometrium, prostate, lung, pancreatic, liver, ovarian, and colorectal cancers (21), underlying its importance in promoting cell survival and inhibition of apoptosis. Kovacs et al. (37) reported that phospho-Akt levels were abundant in leiomyoma, and its expression was higher during menstrual cycle than in myometrium. Furthermore, at menopause, no differences were observed in the expression of p-AKT between the two types of tissues, strongly suggesting the involvement of steroid hormones in the activation of the AKT pathway in leiomyomas. We consistently observed higher p-AKT levels in leiomyoma cells compared with matched myometrial cells at the basal level, suggesting that the AKT pathway is more highly activated in leiomyoma cells. The reasons for this increased activation remains unclear and could involve modifications in receptor tyrosine kinases, PI3K, PTEN, and AKT itself, which is currently under investigation. The importance of the AKT pathway in the pathogenesis of leiomyoma is underscored by the studies performed in the transgenic Eker rat, which carry a germ-line defect in the TSC-2, a direct target of AKT. Approximately 65% of female Tsc2Ek/+carriers develop leiomyomas (38). Similar to human leiomyomas, Eker rat leiomyomas express estrogen receptor and PR, respond to steroid hormones, have aberrant high mobility group AT-hook 2 expression, and overexpress IGF-I. Thus, the relevance in the AKT activation in leiomyomas is underscored by these studies.
Targeting AKT in the cell that has an overactive AKT may provide to be a potentially useful biological target for pathologies that are associated with cell proliferation and survival, leiomyomas included. API-59CJ-Ome, an AKT inhibitor, was identified using a bioinformatics approach to screen 35,000 compounds in the anticancer database of the National Cancer Institute (NCI) and correlated that with the p-AKT levels in 60 human cancer cell lines from NCI (24). Compounds whose in vitro anticancer activities significantly correlated with p-AKT levels in the 60 cancer cell lines were considered as candidate inhibitors for the AKT pathway. Additional studies demonstrated that API-59CJ-Ome inhibited AKT kinase activity and induced apoptosis in the endometrial cancer cell lines that had high AKT activity and had little effect on cells without AKT activity (24). Preliminary data suggest that leiomyoma cells are more sensitive to API-59CJ-Ome-induced apoptosis than myometrial cells (data not shown). In Ishikawa cells, we demonstrated that this inhibitor can localize FOXO1 to the nucleus and rescue it from ubiquitination as demonstrated by increased protein expression (39). Thus, whereas inhibition of AKT can influence numerous downstream molecules, FOXO1 may be actively involved in inhibiting proliferation and promoting apoptosis. This is supported by the response we saw to PsA in this study. PsA was first identified using a cell-based chemical genetic screen using FOXO1 subcellular localization as the readout. More than 18,000 compounds from the NCI Structural Diversity Set, Chemridge DiverSetE, and a small collection of NCI marine extracts were tested for their ability to relocalize FOXO1 to the nucleus in PTEN null cells (26). The most active fraction from reversed-phase HPLC of the psammaplysenes was named PsA. This compound has now been chemically synthesized by Georgiades and Clardy (40). Thus far, it has been demonstrated that PsA neither inhibited exportin 1 (nuclear export receptor) nor reduced AKT phosphorylation and thus must have a target, yet unidentified downstream of AKT that alters cellular localization of FOXO1 (26). Identifying this downstream target will be important in describing the mechanism of PsA in leiomyoma cells and also give insight to other players in FOXO1 regulation of apoptosis. In our hands, this compound is very effective in inducing cell death in leiomyoma cells which appear more sensitive to this compound compared with myometrial cells (data not shown). Additionally, the ability of API-59 and PsA to decrease cell proliferation or induce apoptosis, irrespective of the presence of R5020, suggest that inhibiting AKT kinase activity and FOXO1 cytoplasmic translocation is sufficient to override the increase in cell viability in response to R5020. Thus, whereas inhibition of AKT can influence numerous downstream molecules, FOXO1 may be actively involved in inhibiting proliferation and promoting apoptosis. Further investigation of PsA action in leiomyoma cells is warranted.
In conclusion, we provide here a novel mechanism of progesterone action to promote leiomyoma cell proliferation and survival. Because the choices for nonsurgical alternatives for treatment of leiomyomas are limited, the use of biological agents, such as inhibitors of the AKT pathway, should be further explored.
Supplementary Material
Footnotes
This work was supported by a grant from the Friends of Prentice (to J.J.K.) and National Institutes of Health Grant CA24487 (to J.C.).
Disclosure Summary: A.V.H., E.C.S., E.B., Z.L., J.H., E.M., P.Y., J.C., D.C., S.B., and J.J.K. have nothing to declare.
First Published Online February 24, 2009
Abbreviations: BrdU, 5-Bromo-2′-deoxyuridine; FOXO, Forkhead box O; GSK, glycogen synthase kinase; NCI, National Cancer Institute; p, phosphorylated; PARP, poly(ADP-ribose) polymerase; PI3K, phosphatidylinositol 3-kinase; PR, progesterone receptor; PsA, psammaplysene A; PTEN, phosphatase and tensin homolog deleted from chromosome 10; SH, Src homology; TSC2, tuberous sclerosis-2.
References
- Cramer SF, Patel A 1990 The frequency of uterine leiomyomas. Am J Clin Pathol 94:435–438 [DOI] [PubMed] [Google Scholar]
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM 2003 High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 188:100–107 [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Steiner CA 2002 Hysterectomy rates in the United States 1990–1997. Obstet Gynecol 99:229–234 [DOI] [PubMed] [Google Scholar]
- Parker WH 2007 Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 87:725–736 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Imir G, Utsunomiya H, Thung S, Gurates B, Tamura M, Lin Z 2005 Aromatase in endometriosis and uterine leiomyomata. J Steroid Biochem Mol Biol 95:57–62 [DOI] [PubMed] [Google Scholar]
- Imir AG, Lin Z, Yin P, Deb S, Yilmaz B, Cetin M, Cetin A, Bulun SE 2007 Aromatase expression in uterine leiomyomata is regulated primarily by proximal promoters I.3/II. J Clin Endocrinol Metab 92:1979–1982 [DOI] [PubMed] [Google Scholar]
- Kasai T, Shozu M, Murakami K, Segawa T, Shinohara K, Nomura K, Inoue M 2004 Increased expression of type I 17β-hydroxysteroid dehydrogenase enhances in situ production of estradiol in uterine leiomyoma. J Clin Endocrinol Metab 89:5661–5668 [DOI] [PubMed] [Google Scholar]
- Englund K, Blanck A, Gustavsson I, Lundkvist U, Sjoblom P, Norgren A, Lindblom B 1998 Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab 83:4092–4096 [DOI] [PubMed] [Google Scholar]
- Nisolle M, Gillerot S, Casanas-Roux F, Squifflet J, Berliere M, Donnez J 1999 Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum Reprod 14:2844–2850 [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T 1989 Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol 160:637–641 [DOI] [PubMed] [Google Scholar]
- Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, Roark M, Steinkampf MP 1993 An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab 76:1217–1223 [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Daly M, Juneau-Norcross M, Rein MS, Fine C, Gleason R, Leboff M 1993 A prospective, randomized trial of gonadotropin-releasing hormone agonist plus estrogen-progestin or progestin “add-back” regimens for women with leiomyomata uteri. J Clin Endocrinol Metab 76:1439–1445 [DOI] [PubMed] [Google Scholar]
- Steinauer J, Pritts EA, Jackson R, Jacoby AF 2004 Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol 103:1331–1336 [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP 2001 Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell 8:269–280 [DOI] [PubMed] [Google Scholar]
- Ballare C, Vallejo G, Vicent GP, Saragueta P, Beato M 2006 Progesterone signaling in breast and endometrium. J Steroid Biochem Mol Biol 102:2–10 [DOI] [PubMed] [Google Scholar]
- Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier Joffe E, Schillaci R, Elizalde PV 2007 Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol 21:1335–1358 [DOI] [PubMed] [Google Scholar]
- Lengyel F, Vertes Z, Kovacs KA, Kornyei JL, Sumegi B, Vertes M 2007 Effect of estrogen and inhibition of phosphatidylinositol-3 kinase on Akt and FOXO1 in rat uterus. Steroids 72:422–428 [DOI] [PubMed] [Google Scholar]
- Vallejo G, Ballare C, Baranao JL, Beato M, Saragueta P 2005 Progestin activation of nongenomic pathways via cross talk of progesterone receptor with estrogen receptor β induces proliferation of endometrial stromal cells. Mol Endocrinol 19:3023–3037 [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME 1999 Cellular survival: a play in three Akts. Genes Dev 13:2905–2927 [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB 2005 Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4:988–1004 [DOI] [PubMed] [Google Scholar]
- Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H, Xue Q, Reierstad S, Innes J, Thung S, Kim JJ, Xu E, Bulun SE 2007 Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab 92:4459–4466 [DOI] [PubMed] [Google Scholar]
- Schroeder FC, Kau TR, Silver PA, Clardy J 2005 The psammaplysenes, specific inhibitors of FOXO1a nuclear export. J Nat Prod 68:574–576 [DOI] [PubMed] [Google Scholar]
- Jin X, Gossett DR, Wang S, Yang D, Cao Y, Chen J, Guo R, Reynolds RK, Lin J 2004 Inhibition of AKT survival pathway by a small molecule inhibitor in human endometrial cancer cells. Br J Cancer 91:1808–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tindall DJ 2006 FOXO factors: a matter of life and death. Future Oncol 2:83–89 [DOI] [PubMed] [Google Scholar]
- Kau TR, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, Clardy J, Sellers WR, Silver PA 2003 A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell 4:463–476 [DOI] [PubMed] [Google Scholar]
- Rein MS 2000 Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect 108(Suppl 5):791–793 [DOI] [PubMed] [Google Scholar]
- Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington MS, Erickson TE, Warner C, Keenan EJ, Clinton GM 1993 Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol 169:78–85 [DOI] [PubMed] [Google Scholar]
- Maruo T, Matsuo H, Samoto T, Shimomura Y, Kurachi O, Gao Z, Wang Y, Spitz IM, Johansson E 2000 Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids 65:585–592 [DOI] [PubMed] [Google Scholar]
- Wei JJ, Chiriboga L, Mittal K 2005 Expression profile of the tumorigenic factors associated with tumor size and sex steroid hormone status in uterine leiomyomata. Fertil Steril 84:474–484 [DOI] [PubMed] [Google Scholar]
- Chwalisz K, DeManno D, Garg R, Larsen L, Mattia-Goldberg C, Stickler T 2004 Therapeutic potential for the selective progesterone receptor modulator asoprisnil in the treatment of leiomyomata. Semin Reprod Med 22:113–119 [DOI] [PubMed] [Google Scholar]
- Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS 2006 Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol 108:1381–1387 [DOI] [PubMed] [Google Scholar]
- Rossi MJ, Chegini N, Masterson BJ 1992 Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology 130:1716–1727 [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Matsuo H, Samoto T, Maruo T 1998 Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab 83:2192–2198 [DOI] [PubMed] [Google Scholar]
- Ohara N, Morikawa A, Chen W, Wang J, DeManno DA, Chwalisz K, Maruo T 2007 Comparative effects of SPRM asoprisnil (J867) on proliferation, apoptosis, and the expression of growth factors in cultured uterine leiomyoma cells and normal myometrial cells. Reprod Sci 14:20–27 [DOI] [PubMed] [Google Scholar]
- Robey RB, Hay N 2006 Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25:4683–4696 [DOI] [PubMed] [Google Scholar]
- Kovacs KA, Lengyel F, Kornyei JL, Vertes Z, Szabo I, Sumegi B, Vertes M 2003 Differential expression of Akt/protein kinase B, Bcl-2 and Bax proteins in human leiomyoma and myometrium. J Steroid Biochem Mol Biol 87:233–240 [DOI] [PubMed] [Google Scholar]
- Walker CL, Hunter D, Everitt JI 2003 Uterine leiomyoma in the Eker rat: a unique model for important diseases of women. Genes Chromosomes Cancer 38:349–356 [DOI] [PubMed] [Google Scholar]
- Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ 2008 The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology 149:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades SN, Clardy J 2005 Total synthesis of psammaplysenes A and B, naturally occurring inhibitors of FOXO1a nuclear export. Org Lett 7:4091–4094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.