Abstract
Context: Germline mutation in the MEN1 gene is the usual cause of multiple endocrine neoplasia type 1 (MEN1). However, the prevalence of identifiable germline MEN1 mutations in familial MEN1 cases is only 70%. Some cases may have a germline mutation in another gene such as the p27 cyclin-dependent kinase inhibitor (CDKI).
Objective: The aim of the study was to investigate cases of MEN1 or related states for germline mutations in all CDKI genes.
Methods: A total of 196 consecutive index cases were selected with clear or suspected MEN1 and no identifiable germline MEN1 mutation. Every case was analyzed for germline mutation in each of the seven CDKI genes.
Results: We identified benign polymorphisms of the CDKI genes and also 15 other initially unclassified sequence variants. After detailed gene/protein analysis, seven of these 15 variants were classified as probably pathological mutations. Three of these seven were probable mutations of p27. The remaining four were probable pathological mutations in three of the other CDKI genes, thereby implicating these three genes in the germline of human tumors. The identification rates for probably pathological mutations among the 196 index cases were similarly low for each of four CDKI genes: p15 (1%), p18 (0.5%), p21 (0.5%), and p27 (1.5%). No characteristic clinical subtype related to MEN1 was identified among the seven index cases and their families.
Conclusion: Rare germline mutation in any among four (p15, p18, p21, and p27) of the seven CDKIs is a probable cause of MEN1 or of some related states.
Some patients who have MEN1 or related states and who test negative for germline mutations in the MEN1 gene are now shown to have germline mutations in one of several cyclin-dependent kinase inhibitor genes.
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant syndrome with tumors of many characteristic endocrine and nonendocrine tissues. The standard clinical criteria for a case of MEN1 are tumors in two of the three main associated endocrine tissues (parathyroid, duodenopancreas, and anterior pituitary) (1). By extension, familial MEN1 is defined clinically as an index case with MEN1 and at least one first-degree relative with tumor in one or more of these three tissues. The prevalence of identifiable heterozygous germline mutations in the MEN1 gene is only 70% in index cases with familial MEN1 and considerably lower in sporadic MEN1 (2). Similarly, some other related states have only rare germline MEN1 mutations. For instance, MEN1 mutations have been identified in only 10% of index cases with familial primary hyperparathyroidism (1°HPT) and in less than 1% of index cases with familial pituitary tumor (3,4,5). Some MEN1 and MEN1-like patients without an identifiable germline MEN1 mutation may have an MEN1 mutation that is undetectable by the methods used. However, among the possible mechanisms for nondetection, promoter mutations of the MEN1 gene have not been reported, and large deletions of MEN1 seem rare (6,7). An important possibility is germline mutation of a different gene.
In mouse models, interactions of some cell cycle regulators can cause endocrine tumors. In particular, double knockouts combining germline losses of p27 or p21 with loss of p18 resulted in endocrine tumors characteristic of both MEN1 and MEN2 (8) (Fig. 1). Despite their central roles in the cell cycle and in tumors of genetically engineered mice, most of these CDKIs have rarely been shown to cause tumors by germline mutation in man (see Discussion) [Online Mendelian Inheritance in Man (OMIM) numbers 116899, 600778, 600856, 600431, 603369, and 600927).
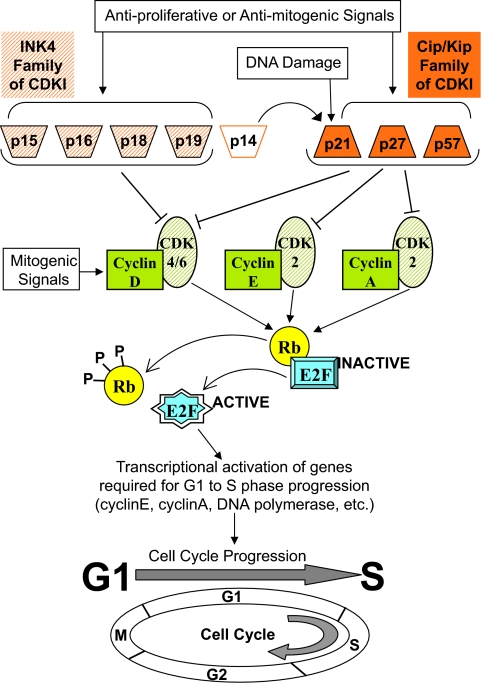
Figure 1.
Molecular pathways distal to CDKIs. Mitogenic signals promote entry into the G1 phase through assembly and activation of cyclinD-CDK4 or cyclinD-CDK6 complexes. Subsequent progression through G1/S transition and S phase is dependent on active cyclinE-CDK2 complexes and active cyclinA-CDK2 complexes. The complexes of cyclin (green solid) and CDK (green striped) phosphorylate members (only one shown) of the Rb (the retinoblastoma gene product) family. This releases from them the E2F family of transcription factors (only one factor shown). The E2F factors in turn transactivate genes for G1 to S phase progression. Some other substrates (data not shown) of active cyclin-CDK complexes during G1 and S phase are: for cyclinE-CDK2, NPAT (nuclear protein mapped to ATM locus), p27, and histone-H1; for cyclinA-CDK2, DNA polymerase α primase. Members of two families of CDKIs, INK4 family (orange striped) and Cip/Kip family (orange solid), negatively regulate the cell cycle. p14 is from an alternative reading frame of the p16 gene (see Supplemental Fig. 4); it does not bind to any cyclin-CDK complex, and it is not a CDKI. p14 inhibits the cell cycle through activating the inhibitory functions of p21 (9). Arrows, Activation; ⊣, inhibition.
Recently, a syndrome that combined MEN1 and MEN2 (named MENX) was reported in a strain of rat (10); a germline homozygous frameshift mutation in p27 caused this phenotype (11). A heterozygous germline nonsense mutation in p27 was in parallel identified as the cause of MEN1 in one human index case and in two of his other affected family members (11). A heterozygous germline frameshift mutation in p27 was later reported in another MEN1 case (12). Among these four affected human cases, two had parathyroid tumor and three had pituitary tumor, secreting GH or ACTH. p27 germline mutation was not found in three subsequent studies analyzing 34, 69, and 21 MEN1 or MEN1-like index cases without identified MEN1 mutations, emphasizing the rarity of p27 mutation in MEN1 (2,13,14). MEN1-like state caused by p27 mutation in man was termed MEN4 (OMIM no. 610755). We have now tested for germline mutation in all of the CDKI genesa, and we have examined these in a larger series of index cases with MEN1 and related states.
Patients and Methods
Patients
The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each patient gave written informed consent.
DNA from each case had been referred for MEN1 sequencing. Each of the 196 index cases [including the 34 cases analyzed previously for p27 mutation (2)] had been tested through the National Institutes of Health (NIH) between 1997 and 2007, and each had no identified germline MEN1 mutation. The criteria for MEN1 testing were broad. In general, a sporadic case or a familial index case was tested if there was likely multigland parathyroid hyperfunction or parathyroid hyperfunction plus another likely MEN1-related tumor. Each case was triaged by one of the same six senior staff members. Most cases were characterized from available clinical information. The amount of additional testing among cases depended partly on whether they were ever evaluated as in-patients or out-patients at NIH or elsewhere. No more than one member of a family was accepted as an index case. Extra effort was made in cases and relatives of cases with probable mutation of a CDKI gene. During this time, a total of 347 index cases suspected of MEN1 had sequencing of MEN1 plus or minus other genes. CASR or HRPT2 tests were used selectively. The prevalences of identified mutations were: MEN1, 128 of 347 (37%), CASR, 13 of 347 (4%), and HRPT2, 10 of 347 (3%). Index cases with identified mutation of MEN1, CASR, or HRPT2 were excluded from the analysis of the CDKI genes; analyses of CDKI germline mutation in this separate group will be of interest in the future but seem unlikely to uncover pathological CDKI mutations.
The distribution of nonoverlapping pretest diagnoses among the 196 index cases was: MEN1, 35%; familial MEN1, 7%; 1°HPT, 11%; multigland 1°HPT, 8%; familial 1°HPT, 17%; HPT-jaw tumor syndrome or parathyroid cancer, 7%; pituitary tumor, 1%; familial pituitary tumor, 4%; and other, 10%.
Isolation of genomic DNA and total RNA
Genomic DNA was isolated from peripheral blood, using the blood and cell culture genomic DNA maxi kit (QIAGEN, Valencia, CA). Total RNA from blood was isolated using the PaxGene blood RNA isolation kit (QIAGEN).
Parathyroid tumors (n = 5) were slowly frozen, stored under liquid nitrogen, and sectioned (8–10 μm thickness) (15). Portions were scraped from slides for DNA and RNA isolation using the Picopure system for DNA or RNA (Arcturus, MountainView, CA).
DNA sequence analysis
Primers were designed to amplify and sequence 16 coding exons, including the exon-intron boundaries, of CDKIs p15, p16/p14, p18, p19, p21, p27, and p57 (Supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). The 3′ part of the first coding exon of p57 was not analyzed due to its highly variable PAPA (proline and alanine) repeats. PCR products were amplified using standard conditions with AmpliTaq Gold (ABI, Foster City, CA), purified, and sequenced (MWG Biotech, High Point, NC). Sequences were analyzed for variants by comparison with sequences obtained from normal control individuals (and with CDKI sequences deposited in GenBank), using MacVector (Oxford Molecular, Ltd., Cambridge, UK). Each sequence trace was visually analyzed.
Each DNA sample with a sequence variant was reamplified and resequenced. Sequence variants were also confirmed by independent PCR amplification using restriction enzyme digest (Supplemental Table 2).
Evaluation of CDKI sequence variants in controls
Two human random control DNA panels (HRC1 and HRC2), each consisting of genomic DNA from 96 healthy UK Caucasian blood donors were used (ECACC/Sigma-Aldrich, St. Louis, MO). These 192 control DNA samples were analyzed by PCR and sequencing for each of the 15 uncategorized sequence variants identified in the CDKI genes (Polymorphic DNA Technologies, Inc., Alameda, CA). For each of the 15 sequence variants, the same single nucleotide polymorphism was also searched in the HapMap database (hapmap.org), in the JSNPs database (snp.ims.u- tokyo.ac.jp), and in the GeneSNPs database (www.genome.utah.edu/genesnps).
Function analysis of CDKI variants
Quantitative analysis of protein expression (Western blot) and glutathione S-transferase (GST) pull-down assay was carried out using Alpha Innotech quantitation software. Further analyses of CDKI variants were with other methods (see Supplemental Methods, included in supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
We only scored a mutation as probably pathological if at least one relevant test showed a substantial defect in a function of the encoded CDKI protein. The criterion for such an effect to be judged probably pathological was a decrease to less than 11% (median = 0.1%) of control in a relevant bioassay and similar results at least three separate times.
Results
CDKI sequence variants and control sequences
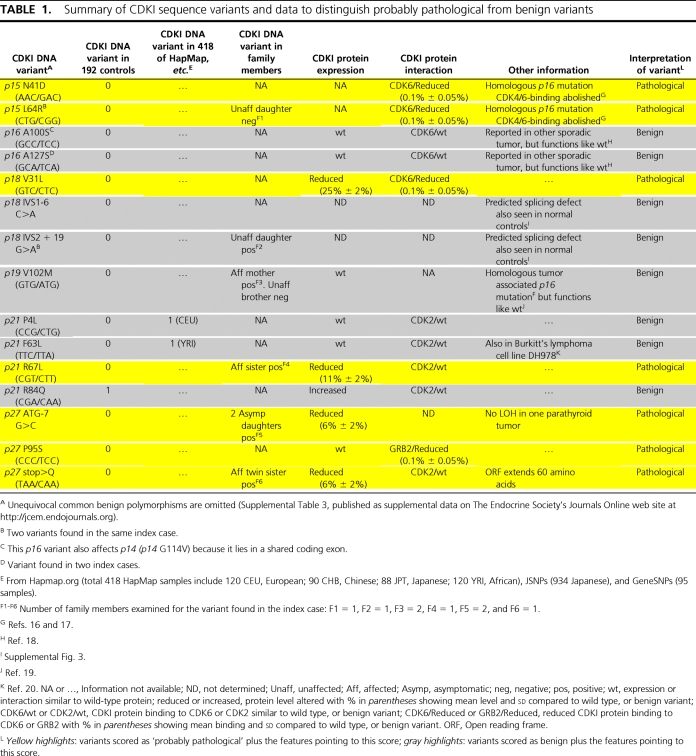
Among the 196 index cases tested for the seven CDKI genes, we identified 15 germline heterozygous sequence variants (initially and temporarily scored as unclassified) (Table 1) and 15 common benign polymorphisms (10 previously known) (Supplemental Table 3).
Table 1.
Summary of CDKI sequence variants and data to distinguish probably pathological from benign variants
Twelve of the 15 unclassified variants were not found in any among 192 control DNA samples, or in the HapMap database of 418 DNA samples, or in the JSNPs database of 934 DNA samples, or in the GeneSNPs database of 95 DNA samples.
However, the p21 missense variant R84Q was seen in one of 192 controls, and two of the p21 missense variants P4L and F63L were each found once in the HapMap database. Each of the three CDKI variants found in one among many apparently normal persons (an in-house control or a case in the HapMap database) was thus scored as a rare benign polymorphism.
Sequence homologies among CDKI variants
The sequence variants in the INK4 CDKI subfamily were compared with known germline or somatic disease-causing mutations of p16 (Table 1, and Supplemental Table 4) (18). Also, see supplemental Results (supplemental Table 5). No comparable analysis was possible in the Cip/Kip subfamily because analogous mutations were not reported previously for these. Both of the p15 variants (n = 2) and the p19 variant (n = 1) corresponded to previously identified and probably pathological somatic mutations in homologous amino acids of p16 (18). Similar to our data about the two p15 variants, the p16 variants had also been shown previously to impair the binding of p16 to CDK4/CDK6 (18). This abnormality was confirmed by us for both p15 variants in the GST pull-down assays here, thereby extending evidence (see CKDI binding by CDKI variants) that both p15 variants were probably pathological. The p16 mutation that was similar to the p19 variant has not been shown to affect p16 activity (19). Therefore, this p19 variant was of uncertain significance and was classified conservatively as benign.
Defects in functions of p27 due to sequence variants
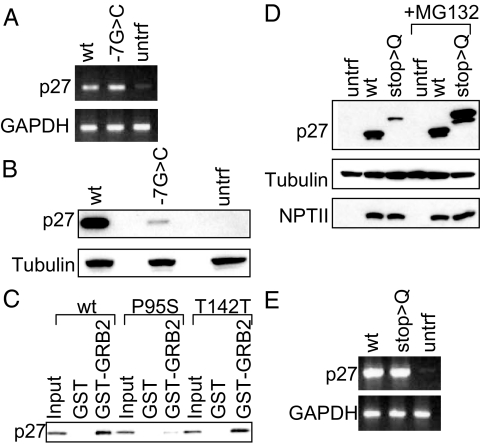
The nucleotide at the −7 position in the Kozak sequence affected by one p27 DNA variant (ATG-7G>C) is conserved among vertebrate species (Supplemental Fig. 1). RT-PCR revealed similar expression of p27 RNA from cells transfected with wild-type or variant-containing plasmid (Fig. 2A). However, the concentration of p27 protein was reduced (6 ± 2%) in lysates of cells transfected with the variant-containing plasmid, suggesting deficient translation of p27 protein (Fig. 2B). Thus this variant was scored as a probably pathological mutation.
Figure 2.
Function assays of p27 variants among index cases. A, mRNA. RT-PCR of HEK293 cells untransfected or transfected with wild-type or p27 variant (ATG-7G>C) genomic constructs. Top panel shows RT-PCR products (at 30 cycles) with p27 primers, and lower panel shows products with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers. B, Protein. Western blot of whole cell lysates from HEK293 cells untransfected or transfected with wild-type or p27 variant (ATG-7G>C) genomic constructs. Top panel was probed with anti-p27 antibody and lower panel with antitubulin antibody. C, Binding to GRB2. GST pull-down assay for binding with GST-GRB2 of 35S labeled in vitro translated (IVT) wild-type p27 or missense variant P95S or benign polymorphism T142T. The bound IVT protein was detected by SDS-PAGE and autoradiography. Input lane contained 10% of the IVT product incubated with GST or GST-GRB2. D, Protein. Western blot of whole cell lysates from HEK293 cells transfected with wild-type or p27 (stop>Q) expression constructs, untreated or treated with 20 μm MG132 (proteasome inhibitor). Top panel was probed with anti-p27 antibody, middle panel with antitubulin antibody, and lower panel with anti-NPTII antibody (index of transfection efficiency). E, mRNA. RT-PCR of HEK293 cells from panel D. Top panel shows RT-PCR products (at 25 cycles) with p27 primers, and lower panel shows RT-PCR products with GAPDH primers. untrf, Untransfected; wt, wild-type.
p27 inhibits Ras activation by p27 binding to growth factor receptor-bound protein 2 (GRB2), an adaptor protein involved in activation of Ras (21). p27 contains a proline-rich region at amino acids 91 to 95 similar to that used by son of sevenless to bind the SH3 domain of GRB2. p27 competes with SOS for binding to GRB2 at this region. In GST pull-down assays, the p27 missense variant P95S had reduced (0.1 ± 0.05%) binding to GRB2 (Fig. 2C). Thus this variant was scored as a probably pathological mutation.
Expression analysis of the p27 stop>Q variant protein showed a higher molecular weight than wild-type p27, as predicted from the extension of the open reading frame by 60 amino acids to the next in-frame stop codon; however, the protein was expressed at a lower level (6 ± 2%) (Fig. 2D). RT-PCR revealed similar expression of p27 RNA from plasmids expressing wild-type or stop>Q variant (Fig. 2E). MG132, which blocks proteasomal degradation, brought expression of stop>Q protein to wild-type levels (Fig. 2D). Therefore, the p27 stop>Q variation results in an unstable protein in vitro, prone to degradation by the proteasomal pathway. Thus, it was scored as a probably pathological mutation.
Protein expression from 11 other CDKI variants
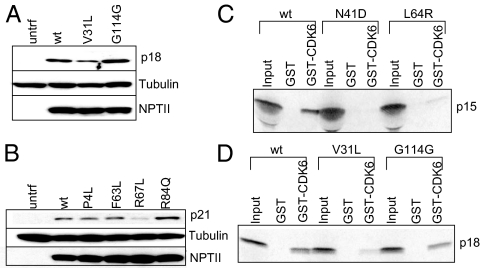
Protein expression of missense variants of other CDKIs was analyzed as for two variants of p27 (ATG-7G>C and stop>Q) in transiently transfected HEK293 cells. No abnormality in protein expression was detected for missense variants of p16 (and its alternative reading frame p14), p19, two for p21, and one for p27 (Fig. 3B, and Supplemental Fig. 2, A–D). The protein expression of p18 V31L and p21 R67L was decreased (25 ± 2% and 11 ± 2%) (Fig. 3, A and B) indicating likely underexpression or instability. Thus, protein expression analysis for two among these 11 missense variants contributed to their scores as probably pathological mutations.
Figure 3.
Expression and interaction assays of several CDKI missense variants. A and B, Protein. Western blot of whole cell lysates from HEK293 cells transfected with wild-type or mutagenized cDNA expression constructs. Top panels were probed with the indicated specific anti-CDKI antibody, middle panels with antitubulin antibody, and lower panels with anti-NPTII antibody. C and D, Protein. Binding of 35S-labeled IVT wild-type or variants of p15 or p18 to CDK6 in GST pull-down assay. The bound IVT protein was detected by SDS-PAGE and autoradiography. Input lane contained 10% of the IVT product incubated with GST or GST-CDK6.
The expression of p21 R84Q was increased (Fig. 3B), indicating that the variant was more stable or was produced more efficiently than the wild-type protein. More importantly, this was not scored as a probably pathological mutation because this sequence change was also detected in one of 192 in-house control samples (Table 1).
Cyclin-dependent kinase (CDK) binding by CDKI variants
To examine the possible consequence of CDKI sequence change on binding to CDKs, GST pull-down assays were performed. The variants of p21 and p27 were analyzed for binding to GST-CDK2, and the variants of p15, p16, p18, and p19 were analyzed for binding to GST-CDK6. No abnormality in binding was observed for the missense variants of p16, p21, or p27 (Supplemental Fig. 2, E–I). However, missense variants of p15 (N41D and L64R) and p18 (V31L) were deficient in binding to GST-CDK6 (0.1 ± 0.05%) (Fig. 3, C and D). Thus these three were scored as probably pathological mutations. This confirmed and strengthened the protein expression data of p18 V31L.
CDKI variants in family members
Germline DNA from five first-degree relatives of four index cases with probably pathological CDKI variants was sequenced. Two affected relatives aged 47 and 48 had the appropriate variant. No affected relative tested negative for the appropriate variant. Two unaffected relatives tested positive for a variant, and one tested negative for a variant. Small size of each family precluded testing for genetic linkage to any of the probably pathological CDKI variants (Fig. 4). Thus family patterns were not used as a main criterion for assigning probably pathological roles of the CDKI variants.
Figure 4.
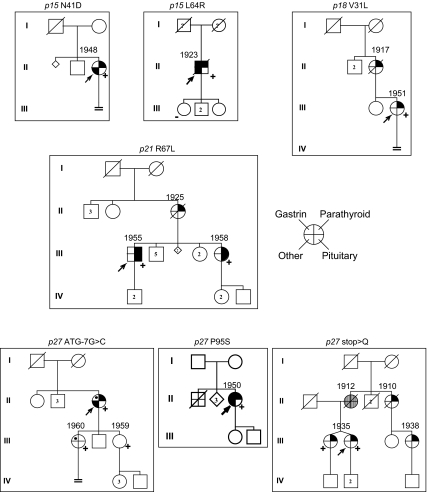
Pedigrees and tumors of index cases with probably pathological CDKI mutations, their affected relatives, and all of their other first-degree relatives (see Table 2 for details). Germline heterozygous CDKI mutation is shown above each pedigree. Roman numerals indicate generation number. Arrows indicate index case. Circle, Female; square, male; diamond, sex not known; small diamond, miscarriage of unknown gender; double line below descent line, no offspring; number in symbol, number of unaffected offspring of that gender; empty symbol, unaffected family member; slashed symbol, deceased; stippled circle, obligate carrier by family position; question mark in symbol, offspring is adopted; question mark near symbol, offspring information not known; filled quadrant in symbol, tumor; dot in quadrant, likely tumor; upper right quadrant, parathyroid tumor; upper left quadrant, gastrinoma; lower right quadrant, pituitary tumor; lower left quadrant, other MEN1-associated tumor [e.g. adrenal cortex (n = 2), meningioma (n = 1), uterine fibroids (n = 1)] (see also Table 2); (+), CDKI mutation positive; (−), CDKI mutation negative; numbers on top of symbols, year of birth for all carriers. First-degree relatives without CDKI mutation information were shown as affected syndrome carrier if they expressed at least one of the three main tumors of MEN1.
Clinical characteristics
Based on biochemical analyses of CDKI variants, seven index cases and their families had probably pathological mutations that supported the reported tumorigenic implication of p27, or that newly implicated three other CDKI genes in endocrine tumors (Table 1). The seven index cases and five of their relatives (two affected, one unaffected, and two asymptomatic carriers) were tabulated separately by CDKI gene and were also examined clinically as one large group. We did not tabulate data about skin tumors of MEN1 because these data were too incomplete. Cases were classified as four index cases with sporadic or familial MEN1, two index cases with familial isolated 1°HPT, and one index case with the MEN1-associated combination of sporadic multigland 1°HPT and meningioma (22) (Fig. 4 and Table 2). Endocrine tumor onset ages seemed similar to those for MEN1. We did not encounter pituitary tumor from p27 germline mutation although it had been reported in three of four affected cases with proven or likely p27 mutation previously (11,12). There were two prolactinomas in one family (with p21 R67L). Two cases (p15 L64R and p27 P95S) had Zollinger-Ellison syndrome that had not been previously reported with p27 mutation. Tumors of uncertain importance in affected cases or in their possibly affected first-degree relatives included esophageal carcinoid, prostate cancer, and cutaneous schwannoma.
Table 2.
Clinical characteristics with probably pathological CDKI mutations in index cases and families, including two published
| Gene | Mutation | Syndrome summary | Hormone excess, and other known or suspected tumorsa of index case | Others affected in family | Additional possible tumor | |
|---|---|---|---|---|---|---|
| 1 | p15 | N41D | 1°HPT | 1°HPT (3 parathyroid tumors)B1, skin schwannoma, meningioma, liver hemangioma | 0 | |
| 2 | p15 | L64R | MEN1 | 1°HPT (3 parathyroid tumors)C1, ZESC2, adrenal massC3, prostate cancer | 0 | Son (age 56) has prostate cancer (not tested for p15 variant). Unaffected daughter (age 52) is negative for the same p15 variant. |
| 3 | p18 | V31L | 1°HPT (familial) | 1°HPT (2 parathyroid tumors)D1, breast cancer | 1 | Mother had 1°HPT (1 parathyroid tumor)D2 (not tested for p18 variant). |
| 4 | p21 | R67L | MEN1 (familial) | 1°HPTE1, macroprolactinomaE2 | 2 | Mother had 1°HPTE3 (not tested for p21 variant). Sister has 1°HPTE4, macroprolactinomaE5, and is positive for same p21 variant. |
| 5 | p27 | ATG-7G>C | MEN1 | 1°HPT (1 parathyroid tumor)F1, stomach problems, bilateral adrenal mass nonfunctioningF2, uterine fibroids | 0 | Two asymptomatic daughters (ages 47 and 48); one has stomach problems, and both are positive for same p27 variant. |
| 6 | p27 | P95S | MEN1 | 1°HPT (2 parathyroid tumors)G1, ZESG2, masses in duodenum and tail of pancreasG3 | 0 | Family was outside United States and not reachable. |
| 7 | p27 | Stop>Q | 1°HPT (familial) | 1°HPT (3 parathyroid tumors)H1 | 3 | Identical twin sister 1°HPT (1 parathyroid tumor)H2, and is positive for same p27 variant. AuntH3 and cousinH4 have 1°HPT (not tested for p27 variant). |
| 8 | p27 | W76X | MEN1 (familial) | 1°HPT, GH-pituitary tumor | 2 | 3 family members tested positive for same p27 variant (phenotype of two undetermined). Father had acromegaly (not tested for the p27 variant). Sister had renal angiomyolipoma (positive for same p27 variant); her son has testicular cancer. Published (Ref. 11). |
| 9 | p27 | K25fs | MEN1 | 1°HPT, ACTH-pituitary tumor, carcinoid tumor of uterine cervix | 0 | Published (Ref. 12). |
Number of parathyroid tumors was estimated for cases with one to three parathyroidectomies. ZES, Zollinger-Ellison syndrome, including high basal acid output, and high gastrin (more than 3-fold over upper limit of normal gastrin).
We did not tabulate data about skin tumors of MEN1 because these data were too incomplete.
B–H Onset age of endocrine tumor (age in years in parentheses): B1(49), C1(59), C2(55), C3(55), D1(44), D2(69), E1(44), E2(43), E3(?), E4(?), E5(?), F1(61), F2(63), G1(50), G2(50), G3(50), H1(50), H2(66), H3(52), H4(?). Question mark indicates that the age information is not available. Onset age for an endocrine tumor was the age of first symptom or first sign.
Discussion
CDKI mutations
Among 196 index cases of MEN1 or related states, without identifiable germline MEN1 mutations, seven showed probably pathological germline mutations in four CDKI genes (Supplemental Fig. 1); identification ratesb of probably pathological mutation among these 196 index cases were similarly low for each of these four CDKIs: p15 (1%), p18 (0.5%), p21 (0.5%), and p27 (1.5%). Eight CDKI variants of the initially unclassified 15 were scored here as benign variants (Table 1); it remains possible that one or more of these eight might eventually prove to be a pathological mutation.
No mutation herein of a CDKI gene predicted truncation of a CDKI protein, so possible pathology from mutation needed careful scrutiny. Easton et al. (23) analyzed 1433 such BRCA1 and BRCA2 variants of unknown clinical significance. Typically, the DNA variations judged to be pathological predicted defective splicing, were located in highly conserved positions, were located in likely functional domains, segregated with disease in pedigrees, or affected the protein in assays of function. If the assay of function is relevant and the measured impairment is significant, then the defect caused by the missense mutation is probably important and pathological. Our expression studies were done in a heterologous nonendocrine cell line (HEK293 cells); human islet or parathyroid cell lines could be even more informative, but such lines are not available.
Prior reports of the effects of CDKI mutation offered aspects for comparisons to our study. In vitro CDK binding studies using amino acid substitutions in CDKIs have identified amino acids critical for optimal CDKI-CDK binding and for kinase inhibitor activity (24,25). Furthermore, several missense variants of p16 associated with a tumor phenotype have been shown to impair its CDK binding activity or to affect its expression/stability in vitro (16,17,18). Results from similar assays helped us to identify several variants of homologous CDKIs (p15 and p18) as probably pathological. We also analyzed variants by sequence homology to p16 mutations. The many sequence homologies in the INK4 family and the extended data on mutations of p16 combined to help categorize two variants (p15 N41D and p15 L64R) as probably pathological and to categorize another (p19 V102M) as probably benign.
Our analyses of CDKI function cannot be exhaustive because some aspects of CDKI function are unknown. Furthermore, some known aspects were not studied, e.g. interaction with CDK-cyclin complex or effect on CDK activity (26,27).
The seven probably pathological CDKI gene mutations predict functional impairment by combinations among protein expression, protein interaction, and absence of mutation in controls. This adds three new CDKI genes as a likely cause in MEN1 and related states. Six of the seven CDKI genes are now implicated by germline mutation in one tumor syndrome (see Clinical features of tumors associated with CDKI mutation). With likely pathological CDKI gene mutation identified in only 4% of our index cases, other unidentified genes/proteins in the cell cycle pathway may also cause a similar syndrome among some of the remaining cases. Another interpretation of the 4% rate of total identified mutations is that additional mutations of these CDKI genes were not identified by our methods. In particular, large deletions are undetected by sequencing, and this is a major mechanism for mutation of p16 (28).
The implication of germline mutations in four CDKIs in MEN1 and related syndromes (see Clinical features of tumors associated with CDKI mutation) raises the possibility that one or more CDKI could contribute by mutation or inactivation mechanisms in common sporadic endocrine tumors. It is thus relevant that some CDKIs (p16, p18, p21, and p27) have previously been analyzed for mutation and expression in sporadic endocrine tumors (Refs. 29,30,31 and references therein). Expression changes of uncertain importance were identified, but mutations were not found.
Loss of wild-type CDKI allele in tumors
Although tumorigenesis herein seems likely to be caused by the loss of CDKI gene function (32), three of four CDKIs affected by probably pathological mutations could not be tested for the loss of the wild-type allele due to the unavailability of tumors. Unfortunately, tumor was available from only one case with a probably pathological germline variant p27 (ATG-7G>C); there was no loss of the wild-type DNA copy in that tumor. For this variant, there was a reduction in p27 protein amount (analyzed by transient transfection assay in HEK293 cells; Fig. 2B) that might initiate tumors by a haploinsufficiency expression (33).
Prior mutation analyses of p27 in human and rodent tumors, in particular those from germline heterozygous knockout mice, have usually not shown biallelic p27 inactivation in the tumor (33,34). This is similar to much data on p27 somatic mutations in several nonendocrine human tumors (35).
Inactivation of a single allele of p18, p21, or p27 has been shown to be sufficient to sensitize mice to radiation-induced or carcinogen-induced tumors (33,36,37). Among the CDKIs, only p16 was found previously to have consistent biallelic inactivation in a variety of human cancers (32,38). Among other CDKIs, mutation with a parallel loss of heterozygosity (LOH) has seldom been seen in human tumors (28,38). Considering prior reports, CDKIs may sometimes function as tumor suppressor genes via haploinsufficiency or via silencing of the wild-type allele without LOH, e.g. by promoter methylation.
Clinical features of tumors associated with CDKI mutation
The clinical phenotype of previously reported human tumors from proven or likely germline p27 mutation has so far been based on only four affected cases (and two silent carriers). We have now extended this via six more cases (four affected and two asymptomatic carriers) with probable p27 mutation. We also identified five cases affected with probable mutation in any one of three other CDKI genes (p15, p18, and p21). Because no specific phenotype for mutation of any one CDKI gene was evident, these were all pooled with p27 for preliminary examinations of phenotype in the CDKI gene family. Further studies will be required to determine age-related penetrance of tumors for each CDKI gene. In particular, penetrance could be determined in the future in part from larger kindreds than we have encountered. The overall features here and in two previous reports with germline p27 mutation (11,12) were those of MEN1, including that of the closely related familial or multigland 1°HPT. Among the pretest diagnoses for index cases, probable CDKI mutations were most prevalent (10%; P < 0.05; χ2) in familial 1°HPT. Such features may typify the full tumor spectrum of the mutant CDKIs; however, they might also reflect our own referral biases toward MEN1 or 1°HPT.
The current phenotype (MEN1 or related states) from mutation in one of four CDKI genes contrasts with two other phenotypes from other CDKI genes (Supplemental Fig. 4). First, familial melanomas with pancreatic ductal adenocarcinomas can arise from inactivating mutation of p16. Secondly, tumors (Wilms’ tumor, adrenocortical carcinoma, and rhabdomyosarcoma) in the Beckwith-Wiedemann syndrome arise from inactivation by mutation or other changes of p57 (39,40). Tumor specificity in the latter two phenotypes is robust from the p16 or p57 gene, respectively, although with unknown details of the relationship to that gene. It may explain why a mutation in the p16 or the p57 CDKI gene was not found in any of our index cases that had been selected for a different or MEN1-related tumor spectrum. The predominance of MEN1-associated tumors in our series may also explain why CDKI mutations (except p16) were not previously found in many common nonendocrine tumors (32,38). It is notable that we did not find C cell or chromaffin tumors of MEN2 in any family with probable CDKI mutation, although tumors of MEN2 and MEN1 were seen in both rat and mouse models of CDKI mutation (8,11).
In conclusion, we confirm and extend reports that germline p27 mutations account for a small but important fraction of cases with MEN1 and related states. Germline mutations in three of six other CDKI genes (p15, p18, and p21) are also shown for the first time as a probable cause of MEN1 or related states. The rarity of p15, p18, and p21 mutations in these cases and the lack of unequivocal pathological features of the identified mutations indicate that further research will be needed before applying these findings to the clinic. The involvement of three previously unimplicated CDKIs may indicate a broad relevance of this gene family to endocrine tumors.
Supplementary Material
Acknowledgments
The authors thank the patients, referring physicians, and staff of the National Institutes of Health Inter-Institute Endocrine Training Program. We thank Dr. H. C. Shen and Dr. S. K. Libutti for providing parathyroid tumor DNA and RNA, and Dr. A. B. Hickman, Dr. A. Horvath, and Dr. C. A. Stratakis for advice. We also express our gratitude to Dr. A. M. Spiegel and Dr. F. S. Collins for guidance and suggestions.
Footnotes
This work was supported by the intramural program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 13, 2009
For editorial see page 1518
Abbreviations: CDK, Cyclin-dependent kinase; CDKI, CDK inhibitor; GRB2, growth factor receptor-bound protein 2; GST, glutathione S-transferase; 1°HPT, primary hyperparathyroidism; LOH, loss of heterozygosity; MEN1, multiple endocrine neoplasia type 1.
The INK4 CDKI family members specifically bind and inhibit cyclinD-CDK4 and cyclinD-CDK6; the genes in this family include CDKN2A, CDKN2B, CDKN2C, and CDKN2D encoding p16Ink4a, p15Ink4b, p18Ink4c, and p19Ink4d, respectively. The INK4 members are referred to here as p16, p15, p18, and p19. An alternate reading frame (ARF) of CDKN2A encodes p14ARF (p19ARF in mouse); this is not a CDKI and is referred to here as p14.
The Cip/Kip family members of CDKIs bind and inhibit a broad spectrum of cyclins-CDKs (cyclinD-CDK4, cyclinD-CDK6, cyclinE-CDK2, and cyclinA-CDK2); the genes in this family include CDKN1A, CDKN1B, and CDKN1C encoding p21Cip1, p27Kip1, and p57Kip2, respectively. The Cip/Kip members are referred to here as p21, p27, and p57.
The overall identification rates of probably pathological mutations in the CDKI genes among all of our 347 MEN1 or MEN1-like index cases with or without identified MEN1 mutation were the following: p15 (0.58%), p18 (0.29%), p21 (0.29%), and p27 (0.86%).
References
- Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells Jr SA, Marx SJ 2001 Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86:5658–5671 [DOI] [PubMed] [Google Scholar]
- Ozawa A, Agarwal SK, Mateo CM, Burns AL, Rice TS, Kennedy PA, Quigley CM, Simonds WF, Weinstein LS, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ 2007 The parathyroid/pituitary variant of multiple endocrine neoplasia type 1 usually has causes other than p27Kip1 mutations. J Clin Endocrinol Metab 92:1948–1951 [DOI] [PubMed] [Google Scholar]
- Marx SJ 2002 Multiple endocrine neoplasia type 1. In: Vogelstein B, Kinzler KW, eds. The genetic basis of human cancer. 2nd ed. New York: McGraw Hill; 450–475 [Google Scholar]
- Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA 2006 Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 312:1228–1230 [DOI] [PubMed] [Google Scholar]
- Simonds WF, James-Newton LA, Agarwal SK, Yang B, Skarulis MC, Hendy GN, Marx SJ 2002 Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 81:1–26 [DOI] [PubMed] [Google Scholar]
- Lemos MC, Thakker RV 2008 Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat 29:22–32 [DOI] [PubMed] [Google Scholar]
- Owens M, Ellard S, Vaidya B 2008 Analysis of gross deletions in the MEN1 gene in patients with multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 68:350–354 [DOI] [PubMed] [Google Scholar]
- Franklin DS, Godfrey VL, O'Brien DA, Deng C, Xiong Y 2000 Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol 20:6147–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Sherr CJ 2003 Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev 13:77–83 [DOI] [PubMed] [Google Scholar]
- Fritz A, Walch A, Piotrowska K, Rosemann M, Schäffer E, Weber K, Timper A, Wildner G, Graw J, Höfler H, Atkinson MJ 2002 Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Res 62:3048–3051 [PubMed] [Google Scholar]
- Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Graw J, Atkinson MJ 2006 Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA 103:15558–15563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, Vierimaa O, Mäkinen MJ, Tuppurainen K, Paschke R, Gimm O, Koch CA, Gündogdu S, Lucassen A, Tischkowitz M, Izatt L, Aylwin S, Bano G, Hodgson S, De Menis E, Launonen V, Vahteristo P, Aaltonen LA 2007 Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab 92:3321–3325 [DOI] [PubMed] [Google Scholar]
- Igreja S, Chahal HS, Akker SA, Gueorguiev M, Popovic V, Damjanovic S, Wass JA, Quinton R, Grossman AB, Korbonits M 15 August 2008 Assessment of p27 (cyclin-dependent kinase inhibitor 1B) and AIP (aryl hydrocarbon receptor-interacting protein) genes in MEN1 syndrome patients without any detectable MEN1 gene mutations. Clin Endocrinol (Oxf) 70:259–264 [DOI] [PubMed] [Google Scholar]
- Owens M, Stals K, Ellard S, Vaidya B 31 July 2008 Germline mutations in the CDKN1B gene encoding p27(Kip1) are a rare cause of multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 10.1111/j.1365-2265.2008.03363.x [DOI] [PubMed] [Google Scholar]
- Rosen JE, Costouros NG, Lorang D, Burns AL, Alexander HR, Skarulis MC, Cochran C, Pingpank JF, Marx SJ, Spiegel AM, Libutti SK 2005 Gland size is associated with changes in gene expression profiles in sporadic parathyroid adenomas. Ann Surg Oncol 12:412–416 [DOI] [PubMed] [Google Scholar]
- Greenblatt MS, Beaudet JG, Gump JR, Godin KS, Trombley L, Koh J, Bond JP 2003 Detailed computational study of p53 and p16: using evolutionary sequence analysis and disease-associated mutations to predict the functional consequences of allelic variants. Oncogene 22:1150–1163 [DOI] [PubMed] [Google Scholar]
- Ruas M, Brookes S, McDonald NQ, Peters G 1999 Functional evaluation of tumour-specific variants of p16INK4a/CDKN2A: correlation with protein structure information. Oncogene 18:5423–5434 [DOI] [PubMed] [Google Scholar]
- Baumgartner R, Fernandez-Catalan C, Winoto A, Huber R, Engh RA, Holak TA 1998 Structure of human cyclin-dependent kinase inhibitor p19INK4d: comparison to known ankyrin-repeat-containing structures and implications for the dysfunction of tumor suppressor p16INK4a. Structure 6:1279–1290 [DOI] [PubMed] [Google Scholar]
- Arap W, Knudsen ES, Wang JY, Cavenee WK, Huang HJ 1997 Point mutations can inactivate in vitro and in vivo activities of p16(INK4a)/CDKN2A in human glioma. Oncogene 14:603–609 [DOI] [PubMed] [Google Scholar]
- Bhatia K, Fan S, Spangler G, Weintraub M, O'Connor PM, Judde JG, Magrath I 1995 A mutant p21 cyclin-dependent kinase inhibitor isolated from a Burkitt’s lymphoma. Cancer Res 55:1431–1435 [PubMed] [Google Scholar]
- Moeller SJ, Head ED, Sheaff RJ 2003 p27Kip1 inhibition of GRB2-SOS formation can regulate Ras activation. Mol Cell Biol 23:3735–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgharian B, Chen YJ, Patronas NJ, Peghini PL, Reynolds JC, Vortmeyer A, Zhuang Z, Venzon DJ, Gibril F, Jensen RT 2004 Meningiomas may be a component tumor of multiple endocrine neoplasia type 1. Clin Cancer Res 10:869–880 [DOI] [PubMed] [Google Scholar]
- Easton DF, Deffenbaugh AM, Pruss D, Frye C, Wenstrup RJ, Allen-Brady K, Tavtigian SV, Monteiro AN, Iversen ES, Couch FJ, Goldgar DE 2007 A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet 81:873–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon TK, Nordin AA 1998 Identification of cdk2 binding sites on the p27Kip1 cyclin-dependent kinase inhibitor. Oncogene 16:755–762 [DOI] [PubMed] [Google Scholar]
- Noh SJ, Li Y, Xiong Y, Guan KL 1999 Identification of functional elements of p18INK4C essential for binding and inhibition of cyclin-dependent kinase (CDK) 4 and CDK6. Cancer Res 59:558–564 [PubMed] [Google Scholar]
- Russo AA, Jeffrey PD, Patten AK, Massagué J, Pavletich NP 1996 Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382:325–331 [DOI] [PubMed] [Google Scholar]
- Jeffrey PD, Tong L, Pavletich NP 2000 Structural basis of inhibition of CDK-cyclin complexes by INK4 inhibitors. Genes Dev 14:3115–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Ortega S, Barbacid M 2000 Genetic analysis of mammalian cyclin-dependent kinases and their inhibitors. Biol Chem 381:827–838 [DOI] [PubMed] [Google Scholar]
- Buchwald PC, Akerström G, Westin G 2004 Reduced p18INK4c, p21CIP1/WAF1 and p27KIP1 mRNA levels in tumours of primary and secondary hyperparathyroidism. Clin Endocrinol (Oxf) 60:389–393 [DOI] [PubMed] [Google Scholar]
- Duerr EM, Chung DC 2007 Molecular genetics of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab 21:1–14 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Quereda V 2008 Cell cycle control of pituitary development and disease. J Mol Endocrinol 10.1677/JME-08-0146 [DOI] [PubMed] [Google Scholar]
- Santamaria D, Ortega S 2006 Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci 11:1164–1188 [DOI] [PubMed] [Google Scholar]
- Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ 1998 The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396:177–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Valentine MB, Rubnitz JE, Saito M, Raimondi SC, Carroll AJ, Yi T, Sherr CJ, Look AT 1999 p27KIP1 deletions in childhood acute lymphoblastic leukemia. Neoplasia 1:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp-Staheli J, Payne SR, Kemp CJ 2001 p27(Kip1): regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp Cell Res 264:148–168 [DOI] [PubMed] [Google Scholar]
- Bai F, Pei XH, Godfrey VL, Xiong Y 2003 Haploinsufficiency of p18(INK4c) sensitizes mice to carcinogen-induced tumorigenesis. Mol Cell Biol 23:1269–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Engelman RW, Coppola D, Cantor AB, Wharton W, Pledger WJ 2003 p21Cip1 nullizygosity increases tumor metastasis in irradiated mice. Cancer Res 63:3021–3025 [PubMed] [Google Scholar]
- Ruas M, Peters G 1998 The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta 1378:F115–77 [DOI] [PubMed] [Google Scholar]
- Goldstein AM 2004 Familial melanoma, pancreatic cancer and germline CDKN2A mutations. Hum Mutat 23:630 [DOI] [PubMed] [Google Scholar]
- Lam WW, Hatada I, Ohishi S, Mukai T, Joyce JA, Cole TR, Donnai D, Reik W, Schofield PN, Maher ER 1999 Analysis of germline CDKN1C (p57KIP2) mutations in familial and sporadic Beckwith-Wiedemann syndrome (BWS) provides a novel genotype-phenotype correlation. J Med Genet 36:518–523 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.