Abstract
Context: The acute hypermetabolic response post-burn is associated with insulin resistance and hyperglycemia, significantly contributing to adverse outcome of these patients.
Objective: The aim of the study was to examine the persistence of abnormalities of various clinical parameters commonly utilized to assess the degree of insulin resistance in severely burned children for up to 3 yr after the burn injury.
Design, Setting and Patients: A total of 194 severely burned pediatric patients, admitted to our institute between 2002 and 2007, were enrolled in this prospective study and compared to a cohort of 95 nonburned, noninjured children.
Main Outcome Measures: Urinary cortisol, epinephrine, and norepinephrine, serum cytokines, and resting energy requirements were determined at admission and 1, 2, 6, 9, 12, 18, 24, and 36 months post-burn. A 75-g oral glucose tolerance test was performed at similar time points; serum glucose, insulin, and C-peptide were measured; and insulin sensitivity indices, such as ISI Matsuda, homeostasis model assessment, quantitative insulin sensitivity check index, and ISI Cederholm, were calculated. Statistical analysis was performed by ANOVA with Bonferroni correction with significance accepted at P < 0.05.
Results: Urinary cortisol and catecholamines, serum IL-7, IL-10, IL-12, macrophage inflammatory protein-1b, monocyte chemoattractant protein-1, and resting energy requirements were significantly increased for up to 36 months post-burn (P < 0.05). Glucose values were significantly augmented for 6 months post-burn (P < 0.05), associated with significant increases in serum C-peptide and insulin that remained significantly increased for 36 months compared to nonburned children (P < 0.05). Insulin sensitivity indices, ISI Matsuda, ISI quantitative insulin sensitivity check index, and homeostasis model assessment were abnormal throughout the whole study period, indicating peripheral and whole body insulin resistance. The insulinogenic index displayed physiological values, indicating normal pancreatic β-cell function.
Conclusions: A severe burn is associated with stress-induced insulin resistance that persists not only during the acute phase but also for up to 3 yr post-burn.
A burn injury is associated with stress-induced diabetes that persists not only for a short period of time but for up to 3 years post-burn.
Severe burns covering more than 40% of total body surface area (TBSA) are typically followed by a period of stress and hyperinflammation, characterized by marked and sustained increases in catecholamine, glucocorticoid, and cytokine secretion (1,2,3). Release of these mediators contributes significantly to the hypermetabolic response after injury, defined by increases in body temperature, oxygen consumption, and CO2 production and elevated basal energy requirements (4,5,6). Increased levels of epinephrine, norepinephrine, dopamine, and cortisol are further associated with enhanced hepatic gluconeogenesis and a reduced insulin-mediated glucose uptake into skeletal muscle and adipose tissue (7,8,9). These phenomena may lead to elevated blood glucose levels in association with normal or elevated serum insulin concentrations, a clinical state commonly defined as insulin resistance (10,11). Indeed, we recently showed in a large prospective clinical trial including 242 severely burned children that the hypermetabolic state during the acute phase post-burn strongly correlated with significantly elevated serum glucose levels and markedly increased serum insulin concentrations (12).
Advances in therapy strategies, based on better understanding of resuscitation, enhanced wound coverage, more appropriate infection control, improved treatment of inhalation injury, and better support of the hypermetabolic response to injury have significantly improved the clinical outcome of this unique patient population over the past few years (13). However, severe burns remain a devastating injury affecting nearly every organ system and leading to significant morbidity and mortality (4). One of the main contributors to adverse outcome of this patient population represents its profound metabolic changes associated with insulin resistance and hyperglycemia (4,14). Multiple studies have documented insulin resistance and its associated hyperglycemia after trauma, myocardial infarction, stroke, or surgery (14,15,16,17,18). Hyperglycemia is of serious clinical concern because it has been frequently linked to impaired wound healing (19), increased skin graft loss (20), increased muscle protein catabolism (21), increased incidence of infections (22,23), and mortality (14,22,23,24,25,26). Thus, recent studies have focused on elucidating potential treatment options to overcome insulin resistance-induced hyperglycemia in the acute period after surgery or medical illness (17,27). However, it is currently unknown how long these conditions persist beyond the acute phase after injury.
Recent studies found that metabolic alterations in response to burn injury may last for more than 12 months after the initial event (1,2,28). Thus, we decided to conduct a prospective study in a large cohort of pediatric patients to examine extent and persistence of abnormalities of various clinical parameters to define the hypermetabolic response and the degree of insulin resistance in a large pediatric population post-burn for up to 3 yr.
Patients and Methods
Patients
A total of 194 severely burned pediatric patients, admitted to the Shriners Hospitals for Children (Galveston, TX) between 2002 and 2007, were enrolled in this prospective study. Patients were included if they were 0 to 18 yr of age, admitted to our institute within 7 d post-burn injury, and had burns covering more than 40% of their TBSA. Permission for conducting the study was obtained from the Institutional Review Board of the University of Texas Medical Branch, Galveston, Texas.
Admission data
On admission, the extent and degree of burn was assessed and recorded on a standard Lund and Browder chart by the attending burns surgeon present. Information also recorded at the time of admission included burn-related (date and mechanism) as well as demographic data (age and gender). All patients were treated in our pediatric burns intensive care unit according to standardized protocols.
Patients were resuscitated if needed according to the Galveston formula with 5000 cc/m2 TBSA burned + 2000 cc/m2 TBSA lactated Ringer’s solution given in increments over the first 24 h. Within 24 h of admission, all patients underwent total burn wound excision, the wounds were covered with available autograft skin, and any remaining open areas were covered with homograft. After the first operative procedure, it took 5–10 d until the donor site was healed and patients were taken back to the operation theater. This procedure was repeated until all open wound areas were covered with autologous skin.
All patients underwent the same nutritional treatment according to a standardized protocol. The intake is calculated as 1500 kcal/m2 body surface + 1500 kcal/m2 area burned, or we assessed the need by measuring the resting energy expenditure (REE) and multiplying by 1.4, with weekly adjustments as previously published (1,2,29).
Urinary catecholamine and cortisol measurements
Twenty-four-hour urine collections were taken regularly throughout acute hospital stay and during admissions for reconstructive operations and rehabilitation services. These samples were collected and chilled by the bedside before transport to our clinical lab for processing using HPLC techniques. Briefly, specimens were acidified to pH 2.0 with hydrochloric acid and 1 ml of acidified urine and were extracted on an HLB Oasis cartridge previously conditioned with 1 ml of methanol and 1 ml of distilled water. The cartridge was washed with a solution of 25% methanol in water (pH 10.9) and eluted with 100% methanol (pH 10.9 with ammonium hydroxide). The eluent was evaporated under a gentle stream of air to dryness and reconstituted with 50% methanol in HPLC grade water (pH 2.9) and submitted to HPLC analysis. HPLC analysis was performed using a symmetry shield 3.5 μm, 4.6 × 150 mm column (Waters Corporation, Milford, MA), with a mobile phase consisting of 30–35% methanol in water with 0.1% trifluoroacetic acid, with UV detection at 245 nm. Urinary catecholamines were analyzed using HPLC techniques. Extraction of the catecholamines from acidified urine samples was performed using a Bio-Rad kit (Bio-Rad, Hercules, CA), according to the manufacturer’s instructions.
Serum cytokine and hormone measurements
Blood was collected from the burn patients at the time of admission, preoperatively, and 5 d postoperatively, as well as during subsequent stays for surgical and rehabilitation services for serum cytokine and hormone analysis for up to 36 months post-burn. Blood was drawn in a serum-separator collection tube and centrifuged for 10 min at 1320 rpm; the serum was removed and stored at −70 C until assayed.
Serum hormones were determined using HPLC and ELISA techniques. The Bio-Plex Human Cytokine 17-Plex panel was used with the Bio-Plex Suspension Array System (Bio-Rad) to profile expression of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL- 12p70, IL-13, IL-17, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interferon-γ, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1b (MIP-1b), and TNF. The assay was performed according to the manufacturer’s instructions.
Indirect calorimetry
As part of our routine clinical practice, all patients underwent REE measurements within 1 wk after hospital admission, at 2 to 4 wk after hospital admission, at discharge, and during admissions for reconstructive operations for up to 18 months post-burn. REE was measured using a Sensor-Medics Vmax 29 metabolic cart (Sensor-Medics, Yorba Linda, CA) as previously published (2). REE was calculated from the oxygen consumption and carbon dioxide production by equations described before (2). For statistical comparison, measured energy expenditure was expressed as the percentage of the basal metabolic rate predicted compared with predicted norms based upon the Harris-Benedict equation and to body mass index (BMI) (2).
Growth measurements
Heights and weights were measured at admission and during subsequent stays for reconstructive purposes and were plotted on standard growth charts (30) to obtain the individual height and weight percentiles for age and gender. Data are presented as percentage change from individual height and weight percentiles at admission. Percentages of the population plotted within each percentile ranking were then calculated.
Glucose, insulin, C-peptide, and glycosylated hemoglobin (HbA1c) measurements before and during oral glucose tolerance testing (OGTT)
Each patient had OGTT performed during both the acute hospital admission and subsequent stays for reconstructive purposes. Studies were carried out after an overnight fast before scar revision surgery. This approach enabled us to use peripheral iv catheters placed for medical treatment. Standard procedure consisted of a baseline blood draw for the measurement of glucose, C-peptide, and insulin levels (fasting values), followed by the glucose load (adjusted for weight by the formula: weight × 1.75 × 2.96 ml of Glucola (up to 300 ml, one full bottle) and subsequent measurements of serum glucose and insulin at 30, 60, 90, and 120 min after the glucose load.
Serum glucose concentrations were quantified using a hexokinase assay on a Dimension Instrument (Dade Behring/Siemens Healthcare Diagnostics, Rockville, MD). Serum insulin and C-peptide concentrations were determined by common ELISA techniques (Diagnostic Systems Laboratories/Beckmann-Coulter, Webster, TX).
HbA1c levels were measured using the A1cNow*InView test (Metrika, Sunnyvale, CA).
Area under the curve (AUC) for glucose and insulin
Total glucose and insulin secretion was assessed from the area under the 120 min curve of glucose (AUCglucose) and insulin (AUCinsulin) concentration using the trapezoid rule.
Insulin sensitivity scores
Four indices for the assessment of insulin resistance were calculated for the above-mentioned time periods using glucose and insulin values during OGTT: 1) the homeostasis model assessment of insulin resistance index (HOMA-IR) [fasting glucose (mmol/liter) × fasting insulin (mU/liter)/22.5, according to Matthews et al. (31)]; 2) the quantitative insulin sensitivity check index (QUICKI) [1/log fasting insulin (μU/ml) + log fasting glucose (mg/dl), according to Uwaifo et al. (32)]; 3) the Matsuda insulin sensitivity index (ISI) [10,000/√G0 × I0 × Gmean × Imean, where G and I represent the plasma glucose (mg/dl) and insulin (mU/liter) concentrations, respectively, expressing fasting (0) and mean OGTT concentrations, as described by Matsuda and DeFronzo (33)]; and 4) the insulinogenic index (IGI) [δ insulin (0–30 min) (mU/liter)/δ glucose (0–30 min) (mg/dl), according to Yeckel et al. (34)].
Time points
Results obtained during the 3-yr period were divided into eight different time phases: <1, 1 to 2, 2 to 6, 6 to 9, 9 to 12, 12 to 18, 18 to 24, and 24 to 36 months post-burn. Data presented include 42 to 194 different measurements at each time point. If any patient had more than one measurement performed during each time period, results were averaged to give a single mean result for each patient at each time period. For subsequent calculations and additional information, burn patients and controls were divided into three age groups, 0–9.9, 10–14.9, and 15–18 yr of age.
Ninety-five nonburned children, admitted to the Yale University School of Medicine (New Haven, Connecticut) and the University of Texas Medical Branch (Galveston, TX) that had at least one OGTT performed or required blood and/or 24-h urine collections, were used as normal cohort. All these nonburned patients had an OGTT performed because they were obese, had a suspicion of gestational diabetes, or had a family history of diabetes.
Statistical analysis
ANOVA with post hoc Bonferroni correction, paired and unpaired Student’s t test, and χ2 analysis were used where appropriate. Data are expressed as means ± sd or sem, where appropriate. Significance was accepted at P < 0.05.
Results
Patient demographics
Characteristics of burn patients and controls at the time of acute hospitalization are depicted in Table 1. Patients were on average 9 yr of age and suffered from severe burn injury of 58% TBSA burn and a third-degree burn of 46% TBSA. Average length of intensive care unit stay was 33 d. There were considerably more male patients than female patients in the burn group. Burned patients were significantly younger and had a significantly lower BMI compared with nonburned children (Table 1).
Table 1.
Patient demographics
| Patients | Nonburned controls | |
|---|---|---|
| n | 194 | 95 |
| Age (yr) | 8.8 ± 5.3a | 13.2 ± 3.6 |
| Gender (female/male) (%) | 26/74a | 68/32 |
| TBSA (%) | 57.9 ± 14.7 | N/A |
| TBSA 3rd-degree (%) | 45.5 ± 23.1 | N/A |
| LOS (d) | 32.9 ± 23.1 | N/A |
| Type of burn (electrical/flame/electrical-flame/scald) (n) | 13/151/3/27 | N/A |
| Mortality (%) | 0 | N/A |
| Inhalation injury (Y/N) (%) | 13/87 | N/A |
| BMI (kg/m2) | 18.1 ± 3.9a | 24.3 ± 5.6 |
Data are presented as means ± sd or percentage. LOS, Length of stay; N/A, not available.
Statistically significant difference between patients vs. nonburned controls, P < 0.05.
Urinary catecholamine and cortisol measurements
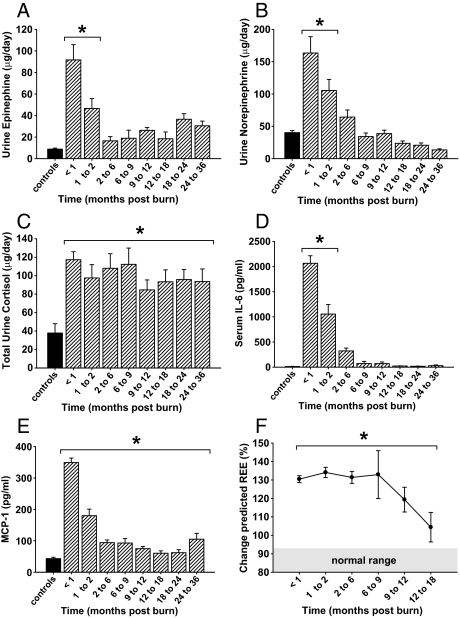
Norepinephrine and epinephrine increased by 4- and 10-fold (P < 0.05), respectively, upon burn trauma before decreasing to normal levels at 2 to 6 months after injury (Fig. 1, A and B). Total urine cortisol levels increased initially to 139 ± 11 μg/24 h and then gradually decreased for the remainder of the study, although they remained significantly elevated compared with normal values (Fig. 1C).
Figure 1.
Severe burn injury leads to significant alterations of the metabolic response. A and B, Urinary epinephrine and norepinephrine increase initially after burn injury and then decrease to normal levels at around 2 months after injury. C, Twenty-four-hour total urine cortisol levels increase upon burn injury and remain significantly elevated for up to 36 months. D and E, Proinflammatory cytokines are significantly elevated for up to 36 months in response to burn injury. Histograms depict serum concentrations of IL-6 or MCP-1 at steady-state levels. F, REE % predicted increases upon burn injury and decreased over time but remains significantly elevated up to 18 months after injury. Normal range depicted at base of graph (shaded area). Bars represent means; error bars correspond to sem. Asterisks denote statistical difference between burned children vs. nonburned children, P < 0.05.
Serum cytokines
Serum levels of IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, TNF, MIP-1b, and MCP-1 were significantly increased for up to 6 months post-burn compared with values of nonburned children. Although levels of IL-5, IL-6, IL-8, and TNF decreased to normal values at approximately 9 months after injury, the serum levels of IL-7, IL-10, IL-12, MIP-1b, and MCP-1 remained significantly elevated for up to 36 months post-burn when compared with values of controls (Fig. 1, D and E). Levels of IL-2, IL-4, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and interferon-γ did not differ from values of nonburned children (data not shown).
Indirect calorimetry
Predicted REE increased significantly post-burn, gradually decreased over time, but remained significantly elevated for 18 months after burn injury, indicating marked hypermetabolism (Fig. 1F).
Hormones
Serum levels of both IGF-I and IGF binding protein-3 (IGFBP-3) decreased significantly immediately post-burn and remained diminished for 2 months post-burn before increasing to normal levels at 9 months after injury (Table 2). Human GH (hGH) decreased by 2-fold in response to burn injury and remained significantly decreased for 36 months after injury (Table 2). We did not find any statistically significant differences for IGF-I, IGFBP-3, and hGH between the three age groups characterized above (data not shown).
Table 2.
Hormones
| Nonburn controls | <1 month | 1 to 2 months | 2 to 6 months | 6 to 9 months | 9 to 12 months | 12 to 18 months | 18 to 24 months | 24 to 36 months | |
|---|---|---|---|---|---|---|---|---|---|
| IGF-I (ng/ml) | 183.09 ± 178.22 | 72.01 ± 60.51a | 124.97 ± 126.23a | 234.57 ± 262.94 | 224.37 ± 181.35 | 248.45 ± 192.71 | 218.35 ± 161.83 | 197.48 ± 146.82 | 229.27 ± 160.84 |
| IGFBP-3 (ng/ml) | 3788.04 ± 1391.14 | 1752.32 ± 978.80a | 2289.49 ± 1503.46a | 3845.65 ± 2314.34 | 3953.09 ± 2186.86 | 4023.22 ± 1947.12 | 4114.04 ± 1965.65 | 3695.35 ± 1835.72 | 3861.62 ± 2081.45 |
| hGH (ng/ml) | 3.92 ± 5.23 | 1.74 ± 1.10a | 1.48 ± 1.65a | 1.27 ± 1.52a | 1.15 ± 1.65a | 0.94 ± 1.41a | 1.18 ± 1.49a | 1.07 ± 1.58a | 0.86 ± 1.50a |
Data are presented as means ± sd.
Statistically significant difference between patients vs. nonburned controls, P < 0.05.
Heights and weights
On admission, the patient population fell essentially within a normal distribution pattern for both height and weight (Table 3). Thirty-six percent of the patient population fell below the 50th percentile (the mean) for height at admission, whereas the percentage of burned children that fell below the 50th percentile for height was significantly greater for up to 2 yr post-burn (Table 3), indicating a profound growth delay in this patient population. Forty-two percent of the patients included into this study were below the mean for weight at admission, whereas the percentage of burned children that fell below the 50th percentile for weight was significantly greater for up to 3 yr post-burn (Table 3).
Table 3.
Heights and weights
| Admit | <1 month | 1 to 2 months | 2 to 6 months | 6 to 9 months | 9 to 12 months | 12 to 18 months | 18 to 24 months | 24 to 36 months | |
|---|---|---|---|---|---|---|---|---|---|
| % Change in heights from admit | −27 | −32 | −41 | −23 | −28 | 40 | −48 | −42 | |
| Below mean height (%) | 36 | 60a | 69a | 78a | 61 | 63a | 70a | 81a | 76 |
| % Change in weights from admit | −49 | −47 | −34 | −19 | −24 | −21 | −20 | −27 | |
| Below mean weight (%) | 42 | 80a | 75a | 69a | 51 | 53a | 56 | 50a | 58a |
Data are presented as means ± sd.
Statistically significant difference between patients vs. nonburned controls, P < 0.05.
Fasting serum glucose, insulin, C-peptide, and HbA1c measurements
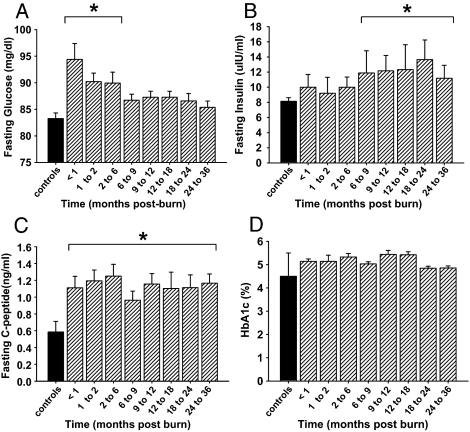
Fasting serum glucose increased significantly post-burn and decreased to normal levels at 6 months after injury (Fig. 2A). Fasting insulin levels displayed a lag phase after burn injury before gradually increasing to significant levels of 12 ± 3 μIU/ml at 6 to 9 months post-burn and remained significantly elevated for 36 months after the burn injury (Fig. 2B). Fasting C-peptide levels increased upon burn injury and remained significantly elevated throughout the time period studied compared with values of nonburned children (Fig. 2C). HbA1c levels of patients included in this study did not differ from values of nonburned children (Fig. 2D).
Figure 2.
Burn trauma leads to hyperglycemia and elevated fasting serum insulin concentrations, indicating insulin resistance. Histograms depict fasting serum concentrations of glucose (A), insulin (B), and C-peptide (C). D, HbA1c in burned pediatric patients is not statistically significant from nonburned children. Bars represent means; error bars correspond to sem. Asterisks denote statistical difference between burned children vs. nonburned children, P < 0.05.
OGTT
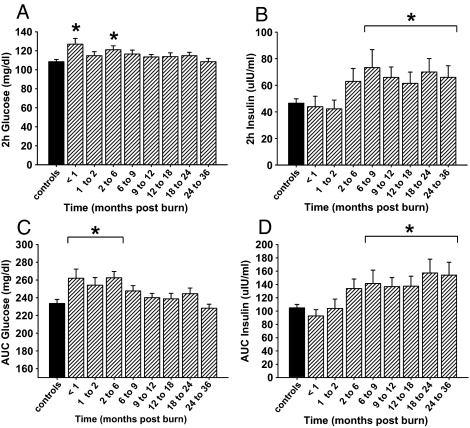
Two-hour glucose levels initially increased to a significant level of 127 ± 6 mg/dl upon burn injury and remained significantly elevated for 9 months post-burn, with a dip at 2 months after injury (Fig. 3A). Calculations of the area under the 120-minute curve of glucose showed a similar pattern by increasing upon burn injury and displaying significant elevated values for 6 months post-burn (Fig. 3C).
Figure 3.
OGTT reveals impaired insulin sensitivity for up to 36 months after burn injury. Histograms depict 2-h serum concentrations of glucose (A) and insulin (B), as well as the AUC for glucose (C) and insulin (D) upon glucose challenge during OGTT. Bars represent means; error bars correspond to sem. Asterisks denote statistical difference between burned children vs. nonburned children, P < 0.05.
Both 2-h insulin levels and calculations of the area under the 120-minute curve of insulin during OGTT displayed a lag phase after burn injury before gradually increasing to significant levels of 73 ± 3 and 141 ± 20, respectively, at 6 to 9 months post-burn and remained significantly increased during the remainder of the study (Fig. 3, B and D). No significances were detected for 2-h glucose and insulin levels between the three age groups characterized above (data not shown).
Insulin sensitivity scores
Insulin sensitivity indices, such as HOMA-IR and QUICKI, were significantly elevated throughout the entire length of the study, indicating that insulin resistance persists for up to 36 months after injury (Table 4). ISI Matsuda values significantly increased upon burn injury and remained significantly elevated for 36 months post-burn compared with values of nonburned children (Table 4). Calculations for the IGI revealed normal values during the time period studied (Table 4).
Table 4.
Insulin sensitivity scores
| Non-burn controls | <1 month | 1 to 2 months | 2 to 6 months | 6 to 9 months | 9 to 12 months | 12 to 18 months | 18 to 24 months | 24 to 36 months | |
|---|---|---|---|---|---|---|---|---|---|
| ISI Matsuda | 4.44 ± 1.56 | 7.12 ± 5.20a | 6.90 ± 4.66a | 6.76 ± 5.69a | 7.66 ± 8.00a | 10.55 ± 12.46a | 7.82 ± 8.27a | 5.55 ± 3.53a | 5.88 ± 4.17a |
| HOMA-IR | 0.44 ± 0.17 | 1.18 ± 1.78a | 3.47 ± 11.52a | 5.65 ± 15.98a | 5.49 ± 14.18a | 7.10 ± 16.44a | 3.07 ± 10.96a | 2.70 ± 10.73 | 2.18 ± 8.68 |
| QUICKI | 0.34 ± 0.02 | 0.37 ± 0.08a | 0.40 ± 0.18a | 0.43 ± 0.24a | 0.43 ± 0.23a | 0.46 ± 0.25a | 0.40 ± 0.17a | 0.38 ± 0.16a | 0.38 ± 0.14a |
| IGI | 1.61 ± 2.07 | 0.97 ± 1.03 | 0.82 ± 1.87 | 1.45 ± 1.30 | 1.34 ± 2.55 | 1.37 ± 1.15 | 2.00 ± 3.73 | 2.08 ± 2.00 | 2.43 ± 2.44 |
Data are presented as means ± sd.
Statistically significant difference between patients vs. nonburned controls, P < 0.05.
Discussion
Severe burn injury leads to significant metabolic alterations, characterized by a hyperdynamic circulatory response associated with increased body temperature, glycolysis, proteolysis, lipolysis, and futile substrate cycling (1,35,36). These responses are present in all trauma, surgical, or critically ill patients, but the severity, length, and magnitude is unique for burn patients (4). Several studies have indicated that these metabolic phenomena occur in a timely manner post-burn (37); however, current understanding has been that these metabolic alterations resolve soon after complete wound closure. Now, recent studies found that the hypermetabolic response to burn injury may last for more than 12 months after the initial event (1,2,28,38) and may lead to persistent hyperglycemia and insulin resistance, significantly contributing to the incidence of morbidity and mortality in this patient population (4,10,11,14).
Several methods are available to evaluate insulin sensitivity in humans. Although hyperinsulinemic-euglycemic clamp studies are considered to be the “gold standard” for quantifying peripheral and hepatic insulin sensitivity (39), clamp studies are, due to their risks, costs, and invasiveness, frequently avoided in a pediatric population. The OGTT is the most commonly used method to evaluate whole body glucose tolerance in vivo and has been shown to correlate well with results from the hyperinsulinemic-euglycemic clamp (33), and it was thus chosen for our study.
Significantly, we found in this study that burned children displayed markedly elevated levels of fasting glucose for 6 months post-burn. However, HbA1c values of our patients were within the normal range at admission and throughout the study, suggesting that these patients did not suffer from persistently elevated blood glucose levels before or during the burn injury. Fasting insulin levels displayed a lag phase before increasing to significant levels at 6 to 9 months, potentially contributing to this state of hyperglycemia post-burn. Fasting C-peptide serum levels, however, increased immediately upon burn injury, indicating enhanced proinsulin secretion in response to elevated glucose levels. The IGI, a commonly used index for β-cell function, also revealed normal values for severely burned children throughout the time period studied, indicating normal pancreatic β-cell function (34). Increased levels of secreted proinsulin in combination with lacking elevations of serum insulin levels upon burn injury may suggest enhanced insulin breakdown during the acute hypermetabolic response to burn (40). Interestingly, although glucose levels returned to normal values subsequently, the levels of insulin and C-peptide remained significantly elevated throughout the entire length of the study. Total glucose and insulin secretion upon glucose challenge, assessed from the AUC during OGTT as well as the 2-h values for serum glucose and serum insulin, showed similar patterns of their secretion profile during the fasted state. ISI Matsuda, recently found to be a good predictor for the development of diabetes (41), displayed pathological values for 3 yr post-burn when compared with nonburned children. Values obtained from HOMA and QUICKI indicated peripheral insulin resistance throughout the whole time period studied (33).
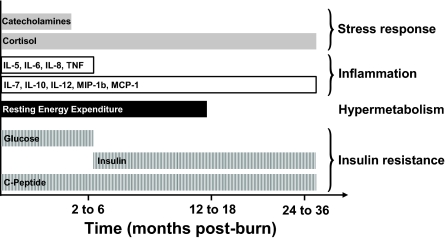
The exact cause of post-burn insulin resistance is still inadequately understood, and the biochemical pathways are poorly defined. Marked increases in endogenous stress hormones and inflammatory mediators have been causally associated with injury-induced insulin resistance (28,42,43,44,45,46). To provide glucose, a major fuel source to vital organs, release of these stress mediators opposes the anabolic actions of insulin (47). By enhancing adipose tissue lipolysis (48) and skeletal muscle proteolysis (49), they increase gluconeogenic substrates, including glycerol, alanine, and lactate, thus contributing to augmented hepatic glucose production in burned patients (7,9,50). Catecholamine-mediated enhancement of hepatic glycogenolysis, as well as direct sympathetic stimulation of glycogen breakdown, may further aggravate the hyperglycemia in response to stress (7). Catecholamines have also been shown to impair glucose disposal via alterations of the insulin signaling pathway and glucose transporter-4 translocation in muscle tissue, resulting in peripheral insulin resistance (9,51). Cree et al. (52) showed an impaired activation of insulin receptor substrate-1 at its tyrosine binding site and an inhibition of AKT in muscle biopsies of children at 7 d post-burn. Catecholamines may also stimulate p38 MAPK, causing the release of inflammatory cytokines, such as TNF (53). Inflammatory cytokines such as TNF, IL-6, and MCP-1 have been shown to activate nuclear inhibitor of nuclear factor-κB-kinase-β (53), which in turn may lead to inhibition of insulin action through modification of signaling properties of insulin receptor substrates, contributing to liver and skeletal muscle insulin resistance (54,55,56). However, in this study, exact causative association and timely correlation between increases of the respective mediators and their effects on glucose metabolism remains challenging. Increases of urinary catecholamine levels and serum levels of IL-5, IL-6, IL-8, and TNF were most elevated within the first 2 months post-burn and thus correlated closely with the observed initially elevated glucose values. Levels of urinary cortisol and serum levels of IL-7, IL-10, IL-12, MIP-1b, and MCP-1, however, remained significantly elevated for 36 months post-burn and may thus considerably contribute to persistently increased insulin and C-peptide levels (Fig. 4).
Figure 4.
The metabolic response to burn injury over time. Bars depict significantly elevated values for inflammatory serum cytokines, urinary catecholamines and cortisol, REE, as well as serum glucose, insulin and C-peptide levels during the acute hospital stay and for 36 months after the burn injury when compared with nonburned children.
Elevated levels of these cytokines and urinary cortisol were accompanied by significant increases in REE for 18 months, indicating a sustained hypermetabolic response and decreases of endogenous anabolic hormones, including IGF-I, IGFBP-3, and GH. Aside from the suggested mechanisms underlying impaired insulin sensitivity post-burn, the decreases in muscle mass, during both the acute and convalescent phases after injury, may significantly contribute to this persistent insulin resistance because skeletal muscle has been shown to be responsible for 70–80% of whole body insulin-stimulated glucose uptake (57). In contrast to starvation, in which lipolysis and ketosis provide energy and protect muscle reserves, burn injury considerably reduces the ability of the body to use fat as an energy source. Skeletal muscle is thus the major source of fuel in the burned patient, which leads to marked wasting of lean body mass within days after injury (4,58) as shown in our burned patients. The correlation between hyperglycemia and muscle protein catabolism has been supported by the study of Flakoll et al. (59) in which an isotopic tracer of leucine was used to index whole-body protein flux in normal volunteers. The group showed a significant increase in proteolysis rates occurring without any alteration in either leucine oxidation or nonoxidative disposal (an estimate of protein synthesis), suggesting a hyperglycemia-induced increase in protein breakdown. Flakoll et al. (59) further demonstrated that elevations of plasma glucose levels resulted in a marked stimulation of whole body proteolysis during hyperinsulinemia. Impaired efficacy of insulin as a muscle protein anabolic agent post-burn, may in turn contribute to this persistent protein catabolism, subsequently leading to the weight loss and growth delay observed in our pediatric patient population for up to 3 yr after thermal injury.
Burn patients included in this study were significantly younger than children in the control group; however, it is unlikely that this age difference is clinically or biologically important because fasting glucose and insulin levels in our normal cohort fall within the reference range reported for children of this age (60,61). Indeed, we stratified our burn and control patients by age and did not find any statistically significant differences regarding GH serum levels, glucose and insulin values during OGTT, as well as the calculated insulin sensitivity scores between the three age groups characterized above.
Because substantially more male patients were included in the present study, we stratified the parameters measured by gender and did not find any statistical significance between the two gender groups. Burned children also displayed a significantly lower BMI compared with our nonburned controls. A current study in 946 patients, however, just demonstrated that increasing BMI was significantly associated with insulin resistance (62).
Conclusion
Burn injury encompassing more than 40% of the TBSA leads to a period of increased hypermetabolism, catabolism, and marked inflammation accompanied by alterations in insulin sensitivity that persist for up to 3 yr after the initial burn injury. Although glucose values of our study patients returned to levels of nonburned children 6 months after injury, these children displayed characteristics of impaired insulin sensitivity for up to 3 yr post-burn. We therefore suggest that burned children should be carefully monitored for the development of diabetes mellitus for a prolonged time after the recovery from injury because medical intervention may be warranted. Given that metabolic alterations and persistent protein catabolism may result in growth delay and may be associated with post-burn insulin resistance, thus significantly contributing to adverse outcome of this patient population, therapeutic intervention to reverse these metabolic alterations post-burn may significantly improve the clinical outcome of this unique patient population.
Acknowledgments
The authors thank Eileen Figueroa and Steve Schuenke for their help in the preparation of this manuscript.
Footnotes
This work was supported by grants from Shriners Hospitals for Children (8660, 8760, 9145 and 8640), the National Institutes of Health (R01 GM56687, T32 GM008256, and P50 GM60338), the National Institute on Disability and Rehabilitation Research (H133A020102), and the American Surgical Association Foundation.
Trial Registration: clinicaltrials.gov Identifier: NCT00673309.
Disclosure Summary: G.G.G., D.N.H., G.A.K., W.J.M., and M.G.J. have no disclosures to declare.
First Published Online February 24, 2009
Abbreviations: AUC, Area under the curve; BMI, body mass index; HbA1c, hemoglobin A1c; hGH, human GH; HOMA-IR, homeostasis model assessment of insulin resistance index; IGFBP-3, IGF binding protein-3; IGI, insulinogenic index; ISI, insulin sensitivity index; MCP-1, monocyte chemoattractant protein-1; MIP-1b, macrophage inflammatory protein-1b; OGTT, oral glucose tolerance test; QUICKI, quantitative insulin sensitivity check index; REE, resting energy expenditure; TBSA, total body surface area.
References
- Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN 2000 Persistence of muscle catabolism after severe burn. Surgery 128:312–319 [DOI] [PubMed] [Google Scholar]
- Mlcak RP, Jeschke MG, Barrow RE, Herndon DN 2006 The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg 244:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, Chinkes DL, Mlcak RP, Herndon DN 2006 Body composition changes with time in pediatric burn patients. J Trauma 60:968–971 [DOI] [PubMed] [Google Scholar]
- Herndon DN, Tompkins RG 2004 Support of the metabolic response to burn injury. Lancet 363:1895–1902 [DOI] [PubMed] [Google Scholar]
- Wilmore DW, Long JM, Mason Jr AD, Skreen RW, Pruitt Jr BA 1974 Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg 180:653–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore DW, Aulick LH 1978 Metabolic changes in burned patients. Surg Clin North Am 58:1173–1187 [DOI] [PubMed] [Google Scholar]
- Robinson LE, van Soeren MH 2004 Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues 15:45–62 [DOI] [PubMed] [Google Scholar]
- Jahoor F, Herndon DN, Wolfe RR 1986 Role of insulin and glucagon in the response of glucose and alanine kinetics in burn-injured patients. J Clin Invest 78:807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart MM, Parbhoo SK 2006 Hyperglycemia in the critically ill patient. AACN Clin Issues 17:50–55 [DOI] [PubMed] [Google Scholar]
- Xin-Long C, Zhao-Fan X, Dao-Feng B, Jian-Guang T, Duo W 2007 Insulin resistance following thermal injury: an animal study. Burns 33:480–483 [DOI] [PubMed] [Google Scholar]
- Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Schiefermeier M, Ratheiser K, Schneeweiss B, Zauner C 2007 Severity of insulin resistance in critically ill medical patients. Metabolism 56:1–5 [DOI] [PubMed] [Google Scholar]
- Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN 2008 Pathophysiologic response to severe burn injury. Ann Surg 248:387–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon DN 2007 Total burn care. 3rd ed. Philadelphia: Saunders Elsevier [Google Scholar]
- McCowen KC, Malhotra A, Bistrian BR 2001 Stress-induced hyperglycemia. Crit Care Clin 17:107–124 [DOI] [PubMed] [Google Scholar]
- Kagansky N, Levy S, Knobler H 2001 The role of hyperglycemia in acute stroke. Arch Neurol 58:1209–1212 [DOI] [PubMed] [Google Scholar]
- Mizock BA 2001 Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab 15:533–551 [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R 2001 Intensive insulin therapy in the critically ill patients. N Engl J Med 345:1359–1367 [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Gerstein HC 2000 Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 355:773–778 [DOI] [PubMed] [Google Scholar]
- McMurry Jr JF 1984 Wound healing with diabetes mellitus. Better glucose control for better wound healing in diabetes. Surg Clin North Am 64:769–778 [DOI] [PubMed] [Google Scholar]
- Mowlavi A, Andrews K, Milner S, Herndon DN, Heggers JP 2000 The effects of hyperglycemia on skin graft survival in the burn patient. Ann Plast Surg 45:629–632 [DOI] [PubMed] [Google Scholar]
- Gore DC, Chinkes DL, Hart DW, Wolf SE, Herndon DN, Sanford AP 2002 Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med 30:2438–2442 [DOI] [PubMed] [Google Scholar]
- Guvener M, Pasaoglu I, Demircin M, Oc M 2002 Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J 49:531–537 [DOI] [PubMed] [Google Scholar]
- Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M 2001 Association of hyperglycemia with increased mortality after severe burn injury. J Trauma 51:540–544 [DOI] [PubMed] [Google Scholar]
- Thorell A, Efendic S, Gutniak M, Haggmark T, Ljungqvist O 1993 Development of postoperative insulin resistance is associated with the magnitude of operation. Eur J Surg 159:593–599 [PubMed] [Google Scholar]
- Garcia-Avello A, Lorente JA, Cesar-Perez J, Garcia-Frade LJ, Alvarado R, Arevalo JM, Navarro JL, Esteban A 1998 Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis after burn trauma. Thromb Res 89:59–64 [DOI] [PubMed] [Google Scholar]
- Christiansen C, Toft P, Jorgensen HS, Andersen SK, Tonnesen E 2004 Hyperglycaemia and mortality in critically ill patients. A prospective study. Intensive Care Med 30:1685–1688 [DOI] [PubMed] [Google Scholar]
- Ingels C, Debaveye Y, Milants I, Buelens E, Peeraer A, Devriendt Y, Vanhoutte T, Van Damme A, Schetz M, Wouters PJ, Van den Berghe G 2006 Strict blood glucose control with insulin during intensive care after cardiac surgery: impact on 4-years survival, dependency on medical care, and quality-of-life. Eur Heart J 27:2716–2724 [DOI] [PubMed] [Google Scholar]
- Norbury WB, Herndon DN 2007 Modulation of the hypermetabolic response after burn injury. In: Herndon DN, ed. Total burn care. 3rd ed. New York: Saunders Elsevier; 420–433 [Google Scholar]
- Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, Obeng MK, Lal S, Gold WF, Wolfe RR, Herndon DN 2000 Determinants of skeletal muscle catabolism after severe burn. Ann Surg 232:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM 1979 Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr 32:607–629 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA 2002 Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care 25:2081–2087 [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, Tamborlane WV, Caprio S 2004 Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab 89:1096–1101 [DOI] [PubMed] [Google Scholar]
- Reiss E, Pearson E, Artz CP 1956 The metabolic response to burns. J Clin Invest 35:62–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YM, Tompkins RG, Ryan CM, Young VR 1999 The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr 23:160–168 [DOI] [PubMed] [Google Scholar]
- Wolfe RR 1981 Review: acute versus chronic response to burn injury. Circ Shock 8:105–115 [PubMed] [Google Scholar]
- Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, Herndon DN 2007 Burn size determines the inflammatory and hypermetabolic response. Crit Care 11:R90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Cree MG, Wolfe RR 2008 Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab 294:E1–E9 [DOI] [PubMed] [Google Scholar]
- Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, Fagot-Campagna A, Pettitt DJ, Bennett PH, Knowler WC 2000 Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 151:190–198 [DOI] [PubMed] [Google Scholar]
- Dolecek R 1989 Endocrine changes after burn trauma–a review. Keio J Med 38:262–276 [DOI] [PubMed] [Google Scholar]
- Jeffries MK, Vance ML 1992 Growth hormone and cortisol secretion in patients with burn injury. J Burn Care Rehabil 13:391–395 [DOI] [PubMed] [Google Scholar]
- Klein GL, Bi LX, Sherrard DJ, Beavan SR, Ireland D, Compston JE, Williams WG, Herndon DN 2004 Evidence supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int 15:468–474 [DOI] [PubMed] [Google Scholar]
- Goodall M, Stone C, Haynes Jr BW 1957 Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg 145:479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes EJ, Batstone GF 1982 Urine cortisol levels after burn injury. Burns Incl Therm Inj 8:333–337 [DOI] [PubMed] [Google Scholar]
- Khani S, Tayek JA 2001 Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci (Lond) 101:739–747 [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M 1987 Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med 317:403–408 [DOI] [PubMed] [Google Scholar]
- Gore DC, Jahoor F, Wolfe RR, Herndon DN 1993 Acute response of human muscle protein to catabolic hormones. Ann Surg 218:679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GL 2001 Insulin resistance and glucose-induced thermogenesis in critical illness. Proc Nutr Soc 60:381–388 [DOI] [PubMed] [Google Scholar]
- Hunt DG, Ivy JL 2002 Epinephrine inhibits insulin-stimulated muscle glucose transport. J Appl Physiol 93:1638–1643 [DOI] [PubMed] [Google Scholar]
- Cree MG, Zwetsloot JJ, Herndon DN, Qian T, Morio B, Fram R, Sanford AP, Aarsland A, Wolfe RR 2007 Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg 245:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Xia ZF, Yu YX, Wei D, Wang CR, Ben DF 2005 p38 Mitogen-activated protein kinase inhibition attenuates burn-induced liver injury in rats. Burns 31:320–330 [DOI] [PubMed] [Google Scholar]
- Fan J, Li YH, Wojnar MM, Lang CH 1996 Endotoxin-induced alterations in insulin-stimulated phosphorylation of insulin receptor, IRS-1, and MAP kinase in skeletal muscle. Shock 6:164–170 [PubMed] [Google Scholar]
- del Aguila LF, Claffey KP, Kirwan JP 1999 TNF-α impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol 276:E849–E855 [DOI] [PubMed] [Google Scholar]
- Sell H, Dietze-Schroeder D, Kaiser U, Eckel J 2006 Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology 147:2458–2467 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP 1981 The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007 [DOI] [PubMed] [Google Scholar]
- Saffle JR, Graves C 2007 Nutritional support of the burned patient. In: Herndon DN, ed. Total burn care. 3rd ed. London: Saunders Elsevier; 398–419 [Google Scholar]
- Flakoll PJ, Hill JO, Abumrad NN 1993 Acute hyperglycemia enhances proteolysis in normal man. Am J Physiol 265:E715–E721 [DOI] [PubMed] [Google Scholar]
- Feigerlova E, Pruhova S, Dittertova L, Lebl J, Pinterova D, Kolostova K, Cerna M, Pedersen O, Hansen T 2006 Aetiological heterogeneity of asymptomatic hyperglycaemia in children and adolescents. Eur J Pediatr 165:446–452 [DOI] [PubMed] [Google Scholar]
- Baldelli R, Bellone S, Castellino N, Petri A, Rapa A, Vivenza D, Bellone J, Broglio F, Ghigo E, Bona G 2006 Oral glucose load inhibits circulating ghrelin levels to the same extent in normal and obese children. Clin Endocrinol (Oxf) 64:255–259 [DOI] [PubMed] [Google Scholar]
- Pieracci F, Hydo L, Eachempati S, Pomp A, Shou J, Barie PS 2008 Higher body mass index predicts need for insulin but not hyperglycemia, nosocomial infection, or death in critically ill surgical patients. Surg Infect (Larchmt) 9:121–130 [DOI] [PubMed] [Google Scholar]