Abstract
Context: Inadequate dietary protein intake has been implicated in sarcopenia.
Objective and Design: The objectives of this study were to determine whether: 1) chronic essential amino acid (EAA) supplementation improves postabsorptive muscle protein fractional synthesis rate (FSR), lean body mass (LBM), and one-repetition maximum muscle strength, and androgen receptor and IGF-I muscle protein expression; and 2) the acute anabolic response to EAA ingestion is preserved after a 3-month supplementation period. Using a randomized, double-blinded, placebo-controlled design, older women (68 ± 2 yr) were assigned to receive either placebo (n = 7), or 15 g EAA/d [supplemented treatment group (SUP)] (n = 7) for 3 months. Metabolic outcomes were assessed in association with stable isotope studies conducted at 0 and 3 months.
Setting: The study was performed at The University of Texas Medical Branch General Clinical Research Center.
Results: Ingestion of 7.5 g EAA acutely stimulated FSR in both groups at 0 months (P < 0.05). Basal FSR at 3 months was increased in SUP only. The magnitude of the acute response to EAA was unaltered after 3 months in SUP. LBM increased in SUP only (P < 0.05). One-repetition maximum strength remained unchanged in both groups. Basal IGF-I protein expression increased in SUP after 3 months (P = 0.05), with no changes in androgen receptor or total and phosphorylated Akt, mammalian target of rapamycin, S6 kinase, and 4E-binding protein.
Conclusions: EAA improved LBM and basal muscle protein synthesis in older individuals. The acute anabolic response to EAA supplementation is maintained over time and can improve LBM, possibly offsetting the debilitating effects of sarcopenia.
Twice daily between-meal ingestion of essential amino acid for 3 months increased lean body mass and the basal synthetic rate of muscle protein in healthy older women.
Older age is often associated with a series of physical, hormonal, and nutritional alterations that facilitate the loss of contractile protein, lean body mass (LBM), and the ability to perform the activities of daily living. Although properly balanced nutrition is paramount in providing adequate dietary protein for the maintenance of muscle mass and strength during healthy aging, such requirements are often not met. Early indications of the need for additional protein intake among elderly came after a study showing that homebound elderly consume well below (<0.7 g mixed protein · kg−1 · d−1) the recommended daily intake of protein (0.8 g · kg−1 · d−1) (1), an amount also considered low based on more recent evidence (2,3). Ideally, such discrepancies in dietary protein can be corrected with modest changes in dietary habits or perhaps increases in total dietary intake. However, although some nutritional supplement regimens result in increased protein and energy intake (4) and are well tolerated by assisted-living elders (5), subsequent voluntary food consumption is often negatively affected (6).
Age-related changes in muscle metabolism are evident even in healthy individuals. Thus, the rationale for undertaking an outcome study such as this in healthy older individuals is based on considerable data showing the anabolic benefit of acute ingestion of essential amino acids (EAAs) (7,8,9,10,11). We recently demonstrated that acute ingestion of 15 g EAA increased arterial amino acid concentrations by 300%, and resulted in a 60% increase in the fractional synthetic rate (FSR) of mixed muscle protein in younger and older individuals (8). More recently, we showed that an acute ingestion of EAAs (i.e. 6.7 g) resulted in a diminished accretion of muscle protein in older compared with younger individuals, despite similar increases in blood EAA concentrations (12). Viewed together with the evidence from Bohe et al. (13) showing that the muscle protein synthetic response is a saturable process, it seems apparent that there is an optimal dose of EAA (e.g. 6.7–15 g) needed to maximally stimulate muscle protein synthesis in elderly, with too few EAAs potentially dampening the synthetic process, and too many EAAs saturating the synthetic system. However, the critical question remains whether chronic EAA administration will significantly impact LBM and function. Nutritional therapies intended to slow down the process of age-related sarcopenia will only be effective if the documented short-term effects persist over a longer-term administration.
The purposes of this study were to determine whether: 1) chronic EAA supplementation improves postabsorptive muscle protein fractional synthetic rate (FSR), LBM, and one-repetition maximum (1RM) strength, and androgen receptor (AR) and IGF-I muscle protein expression; and 2) the acute anabolic response to EAA is preserved after 3 months supplementation. We hypothesized that repeated exposure to EAAs given in a twice-daily between-meal fashion (7.5 g twice a day for a total of 15 g/d) for 3 months would enhance the FSR of mixed muscle protein and promote net muscle protein accretion in older individuals, without diminishing the acute anabolic effects of EAAs. Confirming this hypothesis is crucial for future nutritional supplementation paradigms to treat sarcopenia.
Subjects and Methods
Subjects
Healthy older women (Table 1) were randomized to receive placebo, or 15 g EAA [supplemented treatment group (SUP)] administered in soft capsules for 3 months in a between-meal fashion (1000 and 1400 h). Placebo and EAA capsules were prepared by a compounding pharmacy. Subjects took 20 capsules in the morning and 20 in the evening for a total of 40 daily. Subjects and researchers were blinded to the intervention.
Table 1.
Subject characteristics
| Placebo (n = 7) | SUP (n = 7) | |||
|---|---|---|---|---|
| Age (yr) | 69 ± 3 | 67 ± 1 | ||
| Height (cm) | 163 ± 2 | 165 ± 3 | ||
| Month 0 | Month 3 | Month 0 | Month 3 | |
|---|---|---|---|---|
| Weight (kg) | 71 ± 4 | 72 ± 5 | 73 ± 6 | 74 ± 6 |
| LBM (kg) | 40.7 ± 2.4 | 41.0 ± 2.8 | 43.5 ± 2.8 | 45.2 ± 3.0a |
| Fat (%) | 40 ± 1 | 40 ± 1 | 38 ± 2 | 38 ± 2 |
| Serum creatinine (mg/dl) | 0.81 ± 0.06 | 0.83 ± 0.04 | 0.76 ± 0.03 | 0.74 ± 0.04 |
Significantly different from 0 months (P < 0.05).
Volunteers were recruited through The University of Texas Medical Branch (UTMB) Sealy Center on Aging Volunteer Registry. All subjects gave informed, written consent according to guidelines established by the institutional review board at UTMB, and the study was conducted according to Declaration of Helsinki principles. Subject eligibility was assessed by a battery of medical screening tests, including a history and physical examination, an electrocardiogram, blood count, plasma electrolytes, and liver and renal function tests. Exclusion criteria included the presence of a metabolically unstable medical condition, vascular disease, hypertension, cardiac abnormality, estrogen supplementation within the last 3 months, or currently aerobically or resistance exercise trained. All subjects were living independently. Older men were not studied to avoid any potential interaction of testosterone with the nutritional supplementation.
Experimental protocol
Before beginning the 3-month supplementation period, all subjects underwent a 1RM strength assessment, dual energy x-ray absorptiometry for LBM, and 7-h stable isotope infusion study. 1RM tests of bicep curl, triceps extension, leg extension, and leg curl were performed on traditional Cybex weight machines (Cybex, Medway, MA) in UTMB Alumni Field House 1 wk before the stable isotope study, as previously described (14). Subjects were familiarized on the machines after screening and selection.
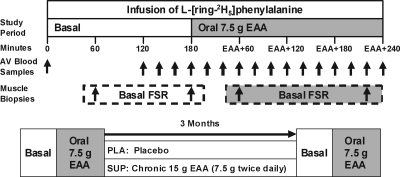
Stable isotope infusion studies were performed in UTMB General Clinical Research Center (GCRC). The isotope infusion protocol is depicted in Fig. 1. Volunteers were instructed to maintain their usual diet during the weeks preceding each metabolic study. Subjects were admitted to the GCRC at noon the day before each study, and fed a standardized lunch and dinner prepared by the GCRC Metabolic Kitchen. After a 2200-h snack, subjects were fasted overnight for approximately 10 h.
Figure 1.
Baseline and 3-month infusion protocol. Arterial and venous (AV) blood samples were obtained at 15-min intervals during an infusion of l-[ring 2H5]phenylalanine. Basal muscle biopsies from the vastus lateralis were obtained at 60 and 180 min. Post-EAA muscle biopsies were obtained at 60 and 240 min after a drink containing 7.5 g EAA.
At 0700 h, an 18-guage polyethylene catheter (Insyte-W; Becton Dickinson, Sandy, UT) was inserted into an antecubital vein. Blood samples were drawn for the analysis of background amino acid enrichment and again every 15 min throughout the 180-min basal period. A second 18-guage polyethylene catheter was placed in the contralateral wrist for a 7-h primed-(2 μmol/kg−1) continuous infusion (0.05 μmol/kg−1 · min−1) of [l-ring-2H5]phenylalanine.
Two muscle biopsies were taken in the basal state using local anesthesia (1% lidocaine) over the lateral portion of the vastus lateralis approximately 10–15 cm above the knee using a 5-mm Bergstrom biopsy needle at 60 and 180 min. Samples (∼100 mg) were immediately rinsed, blotted, and quick frozen in liquid nitrogen. After the second biopsy, all subjects consumed a drink containing 7.5 g EAA to assess the acute response to EAA. Two additional biopsies were taken at 60 and 240 min after EAA ingestion, respectively. After the fourth biopsy, all catheters were removed, and subjects were fed. Before discharge, subjects were dispensed 1 month worth of EAA or placebo capsules by an independent third party and instructed to bring their empty capsule container back each month for assessment of compliance. Subjects were asked to maintain their usual dietary habits and daily activities during the 3-month intervention period. Subjects were instructed to take the capsules at 1000 h and then again at 1400 h every day for 3 months. EAA supplement composition (Table 2) was the same as used in our comparison study of acute amino acid supplementation in the young and elderly (8). Placebo capsules were filled with lactose, and were equal to the EAA supplement in weight and energy content. Each month, subjects returned to the GCRC to collect their next month’s capsules. At month 3, subjects returned to the GCRC to undergo their second and final stable isotope infusion study, dual energy x-ray absorptiometry, and 1RM assessment. The final dose of the supplement was ingested at 1400 h the day before the final isotope study.
Table 2.
Composition of EAA supplements
| Amino acid | 7.5 g EAA | Total (%) |
|---|---|---|
| Histidine | 0.82 | 10.9 |
| Isoleucine | 0.78 | 10.4 |
| Leucine | 1.39 | 18.6 |
| Lysine | 1.17 | 15.5 |
| Methionine | 0.23 | 3.1 |
| Phenylalanine | 1.17 | 15.5 |
| Threonine | 1.10 | 14.7 |
| Valine | 0.86 | 11.5 |
| Total | 7.52 | 100 |
The composition of the EAA mixture was identical to that used by Paddon-Jones et al. (8). The 7.5-g EAA supplement was used as the acute bolus during the kinetic studies at 0 and 3 months for both groups. The SUP group received 7.5 g EAA twice a day to total 15 g EAA/d.
Analytical methods
Blood and muscle samples were precipitated in sulfosalicylic acid and internal standard, and analyzed as previously described (8). Amino acids were extracted by cation exchange chromatography (Dowex AG 50W-8X, 100–200 mesh H+ form; Bio-Rad Laboratories, Inc., Richmond, CA) and dried under vacuum (Savant Instruments, Farmingdale, NY). Blood and skeletal muscle intracellular phenylalanine enrichment and concentrations were determined using the tert-butyldimethylsilyl derivative using GCMS (Hewlett-Packard Model 5989; Hewlett-Packard Co., Palo Alto, CA) with electron impact ionization as previously described (15,16,17,18). Skeletal muscle protein bound l-[ring-2H5]phenylalanine enrichment was determined using the standard curve approach using GCMS (19).
Basal plasma amino acids at months 0 and 3 were measured by the Biomolecular Resource Facility and UTMB Protein Chemistry Lab using ion exchange HPLC (Hitachi L8800 Amino Acid Analyzer; Hitachi, Inc., Pleasanton, CA).
Western blot analyses
Blots (80 μg protein) were incubated overnight in the presence of rabbit primary antibodies to AR (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or IGF-I (Santa Cruz Biotechnology). Total and phosphorylated Akt (Ser473), mammalian target of rapamycin (mTOR) (Ser2448), p70S6K (Thr389), and 4E-binding protein (4E-BP1) (Thr37/46) (Cell Signaling Technology, Inc., Danvers, MA) were determined as a secondary outcome using unused aliquots of muscle tissue. Antibodies for actin and glyceraldehyde-3-phosphate dehydrogenase were used to verify for consistency in protein loading.
Calculations
A stable isotope of phenylalanine was selected because it is neither produced nor metabolized in skeletal muscle. Indices of protein synthesis (Rd) and protein breakdown (Ra) were calculated as follows:
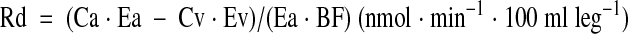 |
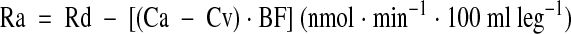 |
where Ca and Cv represent the phenylalanine concentrations in the femoral artery and vein, respectively (20). BF represents leg blood flow, as determined by the indocyanine green dye dilution method (21). Leg volume was determined anthropometrically (22).
The FSR of mixed muscle protein was calculated by measuring the direct incorporation of l-[ring-2H5]phenylalanine into protein, using the precursor-product model:
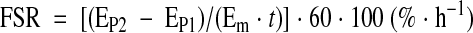 |
where EP1 and EP2 are the enrichments of bound l-[ring-2H5] phenylalanine in sequential muscle biopsies, t is the time interval between biopsies, and Em is the mean l-[ring-2H5]phenylalanine enrichment in the muscle intracellular pool (23).
Statistical analysis
Data are presented as means ± se. Effects of supplementation on LBM, basal FSR, and other outcome parameters were assessed by examining the interaction effect of the factors time and treatment using repeated measures ANOVA, followed by the F test to determine whether interaction was significantly different than zero. Whether or not EAA stimulated FSR before and/or after treatment was assessed by looking at the main effects and interaction effects of time × treatment × EAA dose using repeated measures ANOVA followed by the F test to determine whether interactions and/or main effects were significantly different than zero. Differences were considered significant at P ≤ 0.05.
Results
Physical characteristics
There were no differences in age, height, weight, or LBM between the groups (Table 1). Renal function was not affected by chronic EAA supplementation, as evidenced by unaltered serum creatinine concentrations. Our primary outcome measure, LBM, was significantly increased in SUP at month 3 (P < 0.05). Upper and lower body 1RM strength remained unchanged in both groups (data not shown).
Plasma amino acids
Basal plasma essential and non-EAAs were similar in placebo and SUP at months 0 and 3.
Phenylalanine enrichment and concentration
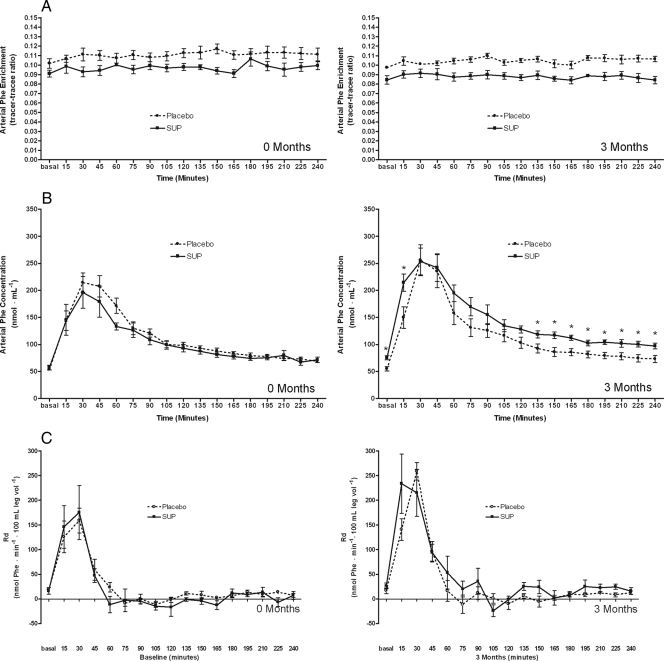
Arterial phenylalanine enrichment remained stable across basal and postprandial periods in both groups at months 0 and 3 (Fig. 2A). Basal arterial phenylalanine concentrations after 3 months were higher in SUP than placebo but increased to similar levels after EAA ingestion in both groups (Fig. 2B).
Figure 2.
Phenylalanine (Phe) enrichment (A), concentration (B), and Rd (C) throughout the infusion studies at months 0 and 3.
Rd and Ra
Basal phenylalanine Rd was similar between groups and increased immediately after acute EAA ingestion in both groups at 0 and 3 months (Fig. 2C). Phenylalanine Ra did not change after acute EAA ingestion at 0 or 3 months and was similar between groups (data not shown).
Mixed muscle fractional synthesis rate (FSR)
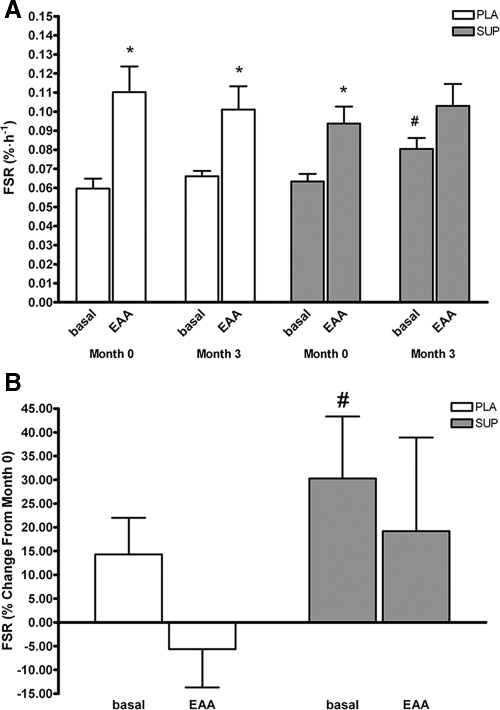
Both groups showed similar increases in FSR in response to acute 7.5 g EAA at month 0 (P < 0.05) (Fig. 3). Basal mixed muscle FSR was increased in the SUP group after month 3 (P < 0.05). The magnitude of the increase in FSR in response to acute EAA ingestion at month 3 was similar between placebo and SUP (P = 0.13).
Figure 3.
A, Basal-mixed muscle FSR at months 0 and 3. Dotted lines are representative of pooled averages from both groups at month 0. B, Percent change in FSR from months 0–3. #, Significant increase in FSR from baseline to 3 months (P < 0.05). *, Significant increase in FSR after ingestion of an EAA bolus (P < 0.05). PLA, Placebo.
Plasma insulin
Both groups elicited similar acute insulin responses after EAA ingestion at month 0 (from 6.1 ± 1.6 to 15.0 ± 4.4, and from 5.5 ± 2.0 to 16.4 ± 5.6 μIU/ml, placebo and SUP, respectively; P < 0.05), which remained unaltered after 3 months. Insulin returned to basal concentrations within 60 min in both groups. Net insulin release (calculated from area under the curve over the entire 240 min periods) at months 0 and 3 was 6.8 ± 1.2 vs. 6.7 ± 1.4 and 7.6 ± 2.3 vs. 7.1 ± 2.6 μIU/ml−1 · min−1 for placebo and SUP, respectively.
Western immunoblots
Basal skeletal muscle IGF-I protein expression was significantly increased after 3 months in the SUP group only (Fig. 4; P = 0.05). There were no changes in expression of AR or in total protein, phosphorylated protein, or the ratio of phosphorylated to total protein for Akt, mTOR, p70 S6 kinase (S6K1), or 4E-BP1 in either group at any time (data not shown).
Figure 4.
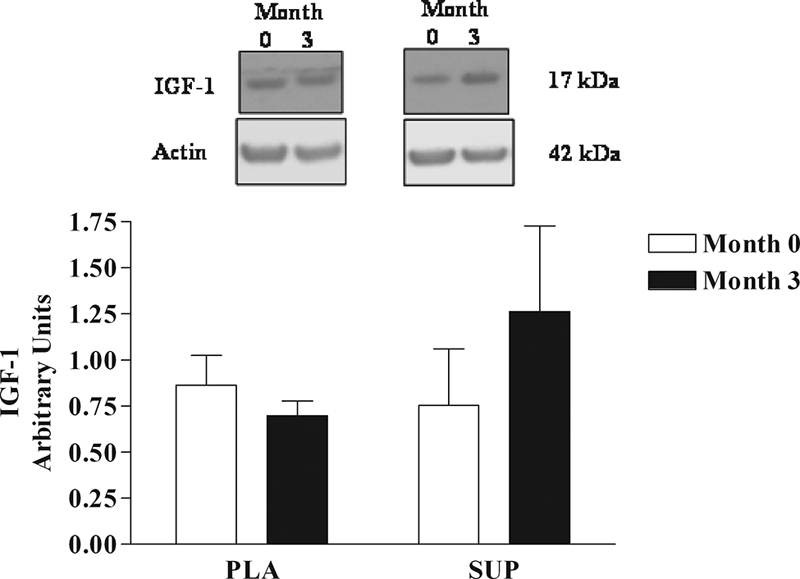
IGF-I protein expression in skeletal muscle at months 0 and 3 of EAA supplementation. *, Significant increase in IGF-I expression in the SUP group (P = 0.05). Top, Representative Western blot from a placebo (PLA) and SUP subject. Bottom, Mean densitometry data from the seven subjects receiving 3 months placebo and seven subjects receiving 3 months 15 g EAA (SUP). Months 0 (white bars) and 3 (black bars). The arbitrary units result from the IGF-I densitometric data expressed as a ratio with the densitometric data for the actin control.
Discussion
Our positive findings are essential for future nutritional recommendations and supplementation paradigms to prevent sarcopenia. The persistence of EAA stimulation on FSR is reassuring for longer-term studies, and suggestive that nutritional interventions could significantly impact the morbidity and mortality associated with sarcopenia without affecting kidney function. Moreover, the significance of our results should stimulate the food science industry to develop more practical methods of delivering dietary or supplemental EAA than the multiple capsules used in our study.
Specifically, this study demonstrates that chronic EAA supplementation enhances LBM and the basal rate of muscle protein synthesis in healthy older women. Furthermore, we demonstrate that the acute anabolic effects of EAA ingestion do not diminish over time. These results indicate that chronic EAA supplementation has the potential to retard or reverse the muscle loss associated with aging, perhaps in part, by stimulating muscle protein synthesis in the period between meals when amino acid concentrations are normally on the decline. Mechanistically, increased expression of IGF-I in basal muscle samples of the amino acid group suggests a strong role for the IGF-I/phosphatidylinositol 3-kinase (PI3K)/Akt pathway in amino acid-induced anabolism and hypertrophy signaling in skeletal muscle. Our LBM data suggest that chronic EAA supplementation conferred a cumulative anabolic effect on muscle mass during the 3-month period. This finding is supported by our data from a recent bed rest study that showed that the acute stimulatory effect of EAA supplementation was preserved throughout the 28 d bed rest, and resulted in the maintenance of LBM and partial preservation of muscle strength (8).
In the present study, EAA supplementation alone was not sufficient to enhance muscle strength. The lack of increase in muscle strength is not surprising considering that these subjects were habitually sedentary and were asked to refrain from making any drastic changes to their normal physical habits throughout the 3 months. In addition, 1RM assessment may not be sufficiently sensitive to detect subtle changes in muscle strength. It is likely that positive strength changes would occur in a more integrated healthy lifestyle involving both regular exercise and adequate daily EAA ingestion, and may take longer than 3 months to confer strength gains. Future studies conducted over longer periods of time and perhaps including scripted exercise programs are warranted and may be more appropriate to demonstrate functional improvements.
Acute ingestion of amino acids primarily acts to stimulate muscle protein synthesis in healthy populations, without altering protein breakdown (24,25). Such acute studies are necessary because they provide direct evidence of the magnitude and duration of the anabolic potential of a particular nutritional supplement, however, they do not provide insight into the potential interactive or confounding effect of these supplements on prior or subsequent meal ingestion. The basis for this research design comes from our recent data in healthy young showing that ingestion of a supplement containing 15 g EAA and 30 g carbohydrate provided a strong anabolic stimulus to skeletal muscle, without interfering with the anabolic response to a mixed nutrient meal provided 3 h later (26). These data provided the necessary rationale and optimism for the current study’s examination of the prolonged benefits of twice-daily between-meal EAA supplementation in older individuals. Our results provide novel data that chronic nutritional EAA therapy for the treatment of sarcopenia holds promise if it can be delivered in a palatable and practical formulation.
The primary goal of amino acid supplementation is to facilitate an increase or maintenance in muscle mass and strength. To be an effective supplement in aging or clinical populations, the selected dose of EAA must first be acutely anabolic to skeletal muscle (8). Our rationale for using a 7.5-g EAA supplement was based on our previous acute findings that 15 g whey protein (containing 6.5 g EAA) is strongly anabolic to skeletal muscle in healthy older individuals (7) but less effective than an isocaloric supplement containing only EAA. Next, the volume and energy content of the supplement should not be excessively large that it slows gastric emptying, increases satiety, and impacts subsequent ad libitum food intake (6,27). Our solution to this important issue was to encapsulate the amino acids rather than mixing them with a sugar-free soda such as done in our acute studies, thus preventing the issue of gastric distension. This also made them palatable and enabled capsule counting to monitor compliance. Whether supplement compositions that include both EAA and nonessential amino acids can yield similar results is a topic outside the scope of this study.
Among EAAs, the anabolic potential of leucine has received the greatest attention (28,29). Retrospectively, the leucine content in this study was relatively low (1.39 g, Table 2) by comparison to amounts used in recently published studies (1.7 g) (12). Indeed, Katsanos et al. (30) showed that 7 g EAA was anabolic to muscle protein synthesis in older individuals only after increasing the leucine content from 1.7–2.8 g. Similarly, Dardevet et al. (31) recently demonstrated in vitro that the response in S6K activity to low concentrations of leucine is diminished in muscle of old rats when compared with younger adult rats. However, this age difference disappeared when concentrations of leucine were increased. Nevertheless, the daily amount of EAA that our SUP group received (2 × 7.5 g = 15 g EAA) was sufficient to increase basal levels of muscle protein synthesis over a 3-month period. Furthermore, the absence of statistical significance in the increase in post-EAA FSR in the SUP group at month 3 was due to the increased basal FSR levels in these subjects and not due to a diminished acute anabolic response because there were no differences in post-EAA FSR between the placebo and SUP groups. In addition, this increase in basal FSR was not due to an increase in amino acid availability in the SUP group because basal amino acid concentrations were not affected by chronic amino acid supplementation.
Measurements of signaling molecules were performed as a secondary outcome on unused muscle tissue after sample collection had taken place. The major pathways through which EAAs are known to induce protein synthesis involve activation of mTOR and its downstream effectors: eukaryotic initiation factor, 4E-BP1, and the ribosomal protein S6K1. This pathway is downstream and sensitive to Akt signaling, and insulin is believed to play at least a permissive role in the activation of the mTOR pathway (32). Despite acute increases in insulin concentrations in responses to oral EAA, Akt phosphorylation in skeletal muscle was unchanged, suggestive of impaired insulin sensitivity in skeletal muscle. In addition, phosphorylation of mTOR, S6K1, and 4E-BP1 did not increase significantly in either group after acute or 3 months twice-daily EAA ingestion. Although reports in the literature vary (33,34,35,36), there is an increasing body of evidence suggesting that EAA stimulation of the mTOR pathway is blunted in older compared with younger individuals.
Although our results are consistent with a blunted signaling response in skeletal muscle of older individuals, it is also possible that we missed the signaling event that may have occurred between 30 and 60 min after EAA ingestion. However, the primary factor dictating the timing of the muscle biopsies in our study was for the assessment of skeletal muscle protein kinetics. Second, we purposefully studied an otherwise healthy free-living older population using a minimalist nutritional approach by enhancing their normal dietary intake. Although inclusion of a controlled exercise component could have further benefitted muscle mass and strength, our positive results show that a chronic stand-alone nutritional intervention to a largely sedentary older population can contribute to improvements in the protein synthetic response. Finally, we intentionally omitted a strict dietary regimen as part of our protocol to avoid forced changes in dietary habits, thereby influencing muscle protein anabolism and muscle strength. We acknowledge that changes in basal FSR could have been attributed to EAA deficiencies in the unsupplemented diet. However, we measured no between-group differences in basal plasma amino acid concentrations in either group before or after 3 months daily EAA. Regardless, such findings would have further highlighted the need for nutritional support in older, free-dwelling, populations.
An enhanced basal rate of muscle protein synthesis in our EAA treated group was consistent with the increase in LBM and increased protein expression of skeletal muscle IGF-I. It is well known that the major signal that regulates postnatal growth of skeletal muscle is the IGF-I/PI3K/Akt pathway (37). This pathway promotes protein synthesis and the resulting hypertrophy by activating translation and retarding proteolysis and expression of various atrogenes (38,39). In the current study, the combination of elevated FSR with increased IGF-I protein expression found in the chronic EAA treated group demonstrates that the IGF-I/PI3K/Akt pathway was activated by chronic EAA exposure. Further confirmation comes from a recent study examining the effects of 1 wk of dietary carbohydrate restriction and increased protein intake on muscle protein metabolism in young (40). In that study, 1 wk of increased protein intake elevated approximately 2-fold the basal FSR of muscle protein and skeletal muscle IGF-I mRNA expression, respectively. Thus, elevation of basal muscle protein synthesis can be accomplished with both shorter (i.e. 1 wk) and longer-term (i.e. 3 months) exposure to amino acids, and may be mediated via the IGF/PI3K/Akt pathway.
In conclusion, twice-daily between-meal ingestion of EAA increased LBM and the basal synthetic rate of muscle protein in healthy older women. These data provide the rationale for embarking on targeted nutritional interventions in aging and other chronic disease populations undergoing progressive or acute losses in skeletal muscle mass.
Acknowledgments
We thank the volunteers that participated in this project, and Aaron Matlock and Jennifer Jones Amerine for their invaluable assistance with data collection and sample processing. We also thank Darren W. Lackan, M.D., for his assistance with clinical oversight, and The University of Texas Medical Branch General Clinical Research Center nursing and dietary staff for assisting with the conduct of these studies.
Footnotes
This work was supported by National Institutes of Health (NIH)/National Institute on Aging Claude D. Pepper Older Americans Independence Center Grant P30 AG024832 (to R.J.U.), NIH/National Institute on Aging R01 AG21539 (to M.S.-M.), NIH/National Center for Research Resources, United States Public Health Service, and General Clinical Research Center Grant M01 RR00073.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 10, 2009
For editorial see page 1524
Abbreviations: AR, Androgen receptor; 4E-BP1, 4E-binding protein; EAA, essential amino acid; FSR, fractional synthesis rate; GCRC, General Clinical Research Center; LBM, lean body mass; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; Ra, model derived protein breakdown; Rd, model derived protein synthesis; 1RM, one repetition maximum; S6K1, S6 kinase; SUP, supplemented treatment group; UTMB, The University of Texas Medical Branch.
References
- Bunker VW, Lawson MS, Stansfield MF, Clayton BE 1987 Nitrogen balance studies in apparently healthy elderly people and those who are housebound. Br J Nutr 57:211–221 [DOI] [PubMed] [Google Scholar]
- Campbell WW, Evans WJ 1996 Protein requirements of elderly people. Eur J Clin Nutr 50(Suppl 1):S180–S183 [PubMed] [Google Scholar]
- Campbell WW, Trappe TA, Wolfe RR, Evans WJ 2001 The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci 56:M373–M380 [DOI] [PubMed] [Google Scholar]
- Arnaud-Battandier F, Malvy D, Jeandel C, Schmitt C, Aussage P, Beaufrere B, Cynober L 2004 Use of oral supplements in malnourished elderly patients living in the community: a pharmaco-economic study. Clin Nutr 23:1096–1103 [DOI] [PubMed] [Google Scholar]
- Lauque S, Arnaud-Battandier F, Mansourian R, Guigoz Y, Paintin M, Nourhashemi F, Vellas B 2000 Protein-energy oral supplementation in malnourished nursing-home residents. A controlled trial. Age Ageing 29:51–56 [DOI] [PubMed] [Google Scholar]
- Fiatarone Singh MA, Bernstein MA, Ryan AD, O'Neill EF, Clements KM, Evans WJ 2000 The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J Nutr Health Aging 4:5–12 [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR 2006 Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol 41:215–219 [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR 2004 Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286:E321–E328 [DOI] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR 1998 Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101:2000–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR 2003 Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolf SE, Wolfe RR 1999 Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol 277(3 Pt 1):E513–E520 [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR 2005 Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82:1065–1073 [DOI] [PubMed] [Google Scholar]
- Bohe J, Low A, Wolfe RR, Rennie MJ 2003 Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 552(Pt 1):315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ 2002 Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:E601–E607 [DOI] [PubMed] [Google Scholar]
- Zhang X, Chinkes DL, Sakurai Y, Wolfe RR 1996 An isotopic method for measurement of muscle protein fractional breakdown rate in vivo. Am J Physiol 270(5 Pt 1):E759–E767 [DOI] [PubMed] [Google Scholar]
- Patterson BW 1997 Use of stable isotopically labeled tracers for studies of metabolic kinetics: an overview. Metabolism 46:322–329 [DOI] [PubMed] [Google Scholar]
- Wolfe RR 1992 Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley-Liss [Google Scholar]
- Bergstrom J, Furst P, Noree LO, Vinnars E 1974 Intracellular free amino acid concentration in human muscle tissue. J App Physiol 36:693–697 [DOI] [PubMed] [Google Scholar]
- Calder AG, Anderson SE, Grant I, Menurlan MA, Garlick PJ 1992 The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 6:421–424 [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Chinkes DL 2005 Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. Hoboken, NJ: Wiley-Liss [Google Scholar]
- Jorfeldt L, Wahren J 1971 Leg blood flow during exercise in man. Clin Sci 41:459–473 [DOI] [PubMed] [Google Scholar]
- Katch V, Weltman A 1975 Predictability of body segment volumes in living subjects. Hum Biol 47:203–218 [PubMed] [Google Scholar]
- Baumann PQ, Stirewalt WS, O'Rourke BD, Howard D, Nair KS 1994 Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol 267(2 Pt 1):E203–E209 [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR 1997 An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 273(1 Pt 1):E122–E129 [DOI] [PubMed] [Google Scholar]
- Wolfe RR 2002 Regulation of muscle protein by amino acids. J Nutr 132:3219S–3224S [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA 2005 Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am J Physiol Endocrinol Metab 288:E761–E767 [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ 1994 Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330:1769–1775 [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Rasmussen BB 2008 Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care 11:222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS 2006 Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 136(Suppl):227S–231S [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR 2006 A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291:E381–E387 [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M, Grizard J 2000 Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr 130:2630–2635 [DOI] [PubMed] [Google Scholar]
- Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS 2002 Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab 282:E1092–E1101 [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ 2005 Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19:422–424 [DOI] [PubMed] [Google Scholar]
- Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y 2004 Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 18:1586–1587 [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E 2006 Insulin resistance of muscle protein metabolism in aging. FASEB J 20:768–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR 2000 The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 85:4481–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD 2001 Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019 [DOI] [PubMed] [Google Scholar]
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ 2005 Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280:2737–2744 [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ 2004 The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403 [DOI] [PubMed] [Google Scholar]
- Harber MP, Schenk S, Barkan AL, Horowitz JF 2005 Effects of dietary carbohydrate restriction with high protein intake on protein metabolism and the somatotropic axis. J Clin Endocrinol Metab 90:5175–5181 [DOI] [PubMed] [Google Scholar]