Abstract
Context: Symptomatic uterine leiomyoma is associated with irregular uterine bleeding, anemia, and recurrent pregnancy loss. African-American women develop uterine leiomyomas at an earlier age and with higher frequency compared with Caucasian-American women or other races; however, the underlying mechanism for this discrepancy is unknown.
Objective: Our objective was to determine whether gene targets of emerging leiomyoma therapeutics such as aromatase inhibitors and antiprogestins, which reduce tumor size and symptoms, are differentially expressed in tissues of African-American (n = 31), Caucasian-American (n = 34), and Japanese women (n = 36).
Results: We found strikingly higher aromatase mRNA levels in leiomyoma compared with adjacent myometrium in African-American (83 fold), Caucasian-American (38 fold), and Japanese women (33 fold). Among the four major promoters that regulate aromatase expression in leiomyoma, the proximal promoter II accounted for higher aromatase mRNA levels in tissues from African-American women. Estrogen receptor subtype α mRNA levels were significantly, and 1.8- to 2.6-fold, higher in leiomyoma compared with adjacent myometrium in all groups, whereas leiomyoma estrogen receptor subtype β mRNA levels were significantly elevated only in Japanese women. Leiomyoma progesterone receptor mRNA levels were significantly higher in Japanese women compared with African-American or Caucasian-American women.
Conclusions: Leiomyoma tissues from African-American women contained the highest level of aromatase expression, which may result in elevated tissue concentrations of estrogen, and account for the higher prevalence and earlier incidence. Analysis of leiomyoma tissue for biomarkers may predict the response to hormonal treatments such as aromatase inhibitors.
Aromatase in uterine leiomyoma tissues from African-American women is expressed at significantly higher levels compared with those from Japanese women.
Uterine leiomyomas (fibroids) are benign smooth muscle tumors originating from the uterus, and affect up to 77% of all reproductive-age women in the United States. Uterine leiomyoma is a major cause of morbidity, which results in direct costs of approximately $2 billion to our health care system (1,2). No effective treatments other than myomectomy or hysterectomy exist, and approximately 200,000 hysterectomies are performed for leiomyoma annually in the United States (3). The prevalence of uterine leiomyoma is much higher in African-American women compared with Caucasian-American women or other races (1,4). Compared with Caucasian-American women, African-American women develop leiomyomas at an earlier age, and have more numerous and symptomatic tumors (1). Earlier menarche and higher body mass index (BMI) in African-American women have been reported as possible risk factors for the higher incidence of uterine leiomyoma. Moreover, polymorphisms in genes involved in estrogen synthesis and/or metabolism may be related to a higher incidence of leiomyoma in African-American women (5); however, the underlying molecular mechanisms accounting for this racial discrepancy are not fully understood.
Recently, aromatase inhibitors were reported to reduce the uterine leiomyoma size, underscoring the biological role of aromatase in this disease (6,7). Aromatase, the key enzyme for estrogen production, is encoded by the CYP19A1 gene and expressed in strikingly higher levels in uterine leiomyoma compared with adjacent myometrium (8,9). Estrogen locally produced via aromatase activity in leiomyoma contributed to tumor growth (10). Aromatase gene expression is regulated by the activation of a number of promoters via alternative splicing (11). We previously demonstrated that aromatase expression in vivo in leiomyoma tissue is primarily regulated by the promoter I.3/II region rather than I.4 in African-American and Caucasian-American women (8). On the other hand, promoter I.4 may play a more prominent role for aromatase expression in leiomyoma tissue of Japanese women (12).
Circulating estrogen and progesterone secreted from the ovary are also believed to play key roles in the pathophysiology of uterine leiomyoma (13). Estrogen or progesterone action is primarily mediated by these specific nuclear receptors: estrogen receptor subtypes α (ERα) and β (ERβ) and progesterone receptor (PR). ERα and/or ERβ may mediate estrogen-dependent growth of leiomyomas, and PR may mediate the effects of progesterone and antiprogestins in leiomyomas. In fact, the antiprogestin mifepristone (RU486) is clinically useful for reducing the size of leiomyoma and improving associated symptoms (14).
Here, we compared the mRNA levels of aromatase, ERα, ERβ, and the estrogen responsive gene, PR, in leiomyomas of women with different racial/ethnic backgrounds. This represents the molecular-based evidence for a race-specific biological difference in uterine leiomyomas. We suggest that this type of analysis provides critical translational evidence and opens an avenue for identifying subsets of patients who are more likely to respond to hormonal treatments such as aromatase inhibitors or antiprogestins.
Subjects and Methods
Tissue acquisition and patient background
Human uterine leiomyoma and adjacent normal appearing-matched myometrial tissues were collected from women undergoing hysterectomy. Specimens from African-American (n = 31) and Caucasian-American women (n = 34) were obtained at the hospitals of Northwestern University (Chicago, IL). Specimens of Japanese women (n = 36) were obtained at the hospitals of Kanazawa University (Kanazawa, Japan) and Chiba University (Chiba, Japan). All specimens were collected after obtaining informed consent from subjects following protocols approved by the Institutional Review Board for Human Research of the corresponding university. Subjects using GnRH analog, oral contraceptive, or progestin up to 3 months before surgery were excluded. Cycle phase was estimated by the last menstrual period. In the case of multiple leiomyomas, we sampled the largest tumor. Leiomyomas were sampled consistently at 1 cm from the outer capsule. The adjacent myometrial tissue was sampled at a 2-cm distance from a leiomyoma.
RNA extraction and quantitative real-time RT-PCR
Total RNA from tissue was extracted using the QIA shredder followed by the RNeasy mini kit (QIAGEN, Inc., Valencia, CA), and subsequent quantitative real-time RT-PCR was performed as described previously (15). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts were quantified as an internal control. The following primer pairs were used for PCR: GAPDH, forward 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse 5′-GAAGATGGTGATGGGATTTC-3′; aromatase, forward 5′-CACATCCTCAATACCAGGTCC-3′ and reverse 5′-CAGAGATCCAGACTCGCATG-3′; ERα, forward 5′-AAGAGCTGCCAGGCCTGCC-3′ and reverse 5′-TTGGCAGCTCTCATGTCTCC-3′; ERβ, forward 5′-CCATGATCCTGCTCAATTCC-3′ and reverse 5′-CTCTTGGCAATCACCCAAAC-3′; and PR, forward 5′-TCAGTGGGCAGATGCTGTATTT-3′ and reverse 5′-GCCACATGGTAAGGCATAATGA-3′.
For the detection of aromatase mRNA levels, we used a double dose of cDNA as a template for PCR compared with other genes. All mRNA levels were normalized to GAPDH mRNA levels, and levels in leiomyoma mRNA were shown as a fold difference of matched myometrium. Alternatively, we used multiplex RT-PCR using exon-specific primers to quantify promoter-specific mRNA species of aromatase, as described previously (15,16). Primers used for multiplex PCR are shown in supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org.
Statistical analysis
Statistical analysis for comparison of gene expression in leiomyoma compared with adjacent myometrium was performed by the Wilcoxon signed-rank test. Comparison between each racial group was performed by ANOVA followed by pairwise t tests. All values were given as the mean, with bars indicating sem, and a P value less than 0.05 was considered significant.
Results
High aromatase gene expression and multiple promoter usage in uterine leiomyoma
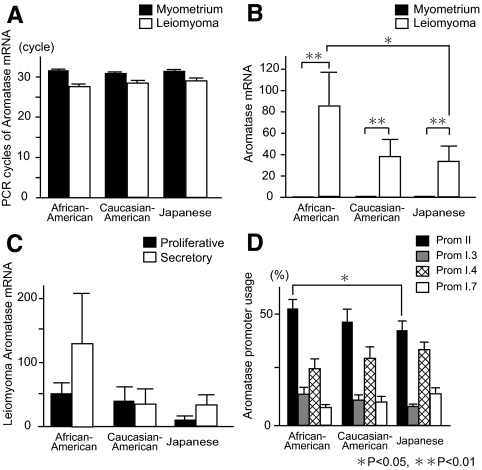
Clinical characteristics of each group are summarized in Table 1. There was no significant difference regarding age or cycle phase at surgery among groups. BMI was significantly higher in African-American subjects compared with Japanese subjects. The number of PCR cycles for detectable aromatase mRNA (not adjusted by GAPDH mRNA) in myometrial tissues was over 30 in all groups (Fig. 1A). In contrast, levels of leiomyoma aromatase mRNA (normalized to GAPDH mRNA) were strikingly higher compared with adjacent myometrium: 83-fold in African-American, 38-fold in Caucasian-American, and 33-fold in Japanese subjects. Moreover, leiomyoma aromatase mRNA levels in African-American subjects were significantly higher compared with Japanese subjects (Fig. 1B). Leiomyoma aromatase mRNA levels in samples obtained during the secretory phase appeared to be higher than those obtained during the proliferative phase in African-American and Japanese subjects, although these trends were not significant (Fig. 1C).
Table 1.
Clinical features and racial distribution of women with leiomyoma
| Characteristic | African-American (n = 31) | Caucasian (n = 34) | Japanese (n = 36) | P value |
|---|---|---|---|---|
| Age (range) | 43.3 ± 2.92 yr (39–48) | 44.3 ± 3.26 yr (38–49) | 41.5 ± 6.96 yr (27–52) | 0.08 |
| BMI (range)a | 31.0 ± 6.7 kg/m2 (19.5−46.8) | 27.9 ± 7.5 kg/m2 (19.3–50.1) | 21.4 ± 3.3 kg/m2 (17.9–30.9) | <0.0001 |
| Menstrual phase at surgery: proliferative/secretory/unknownb | 16/10/5 | 17/8/9 | 11/18/7 | 0.16 |
Data are shown as mean ± sd. P values are provided by one-way ANOVA or Fisher’s exact test.
BMI was calculated as weight (kg) divided by height squared (m2).
Cycle phases were determined by the last menstrual period in subjects with regular cycles. Subjects with unknown cycle phases include those who had irregular cycles or an unknown last menstrual period.
Figure 1.
Expression of aromatase mRNA and promoter (Prom) usage in uterine leiomyomas of African-American, Caucasian-American, and Japanese women. A, Cycle threshold (Ct) values for aromatase mRNA are shown. Aromatase mRNA levels (not adjusted to GAPDH mRNA) in leiomyoma and adjacent-matched myometrial tissues were measured by quantitative real-time RT-PCR, and the required PCR cycles (Ct values) for aromatase mRNA are shown. B, Aromatase mRNA levels in leiomyoma, which were normalized to GAPDH mRNA levels, were described as fold differences compared with matched myometrium. C, Leiomyoma aromatase mRNA levels assorted with menstrual cycle are shown. They were subdivided as proliferative phase and secretory phase. D, Exon-specific multiplex PCR, as described in Subjects and Methods, was used to quantify aromatase mRNA species specific for each of the promoters II, I.3, I.4, or I.7. Each promoter-specific aromatase mRNA level was represented as a percentage of the sum of all four promoter-specific mRNA levels. *, P < 0.05 and **, P < 0.01 by the Wilcoxon signed-rank test for leiomyoma vs. myometrium, and by ANOVA with pairwise t tests for African-American subjects vs. Japanese subjects, Caucasian-American subjects vs. Japanese subjects, or African-American subjects vs. Caucasian-American subjects.
With respect to the promoter usage, we found alternatively used promoters II, I.3, I.4, and I.7 that regulated aromatase expression in leiomyoma, and a significantly higher use of promoter II in African-American subjects compared with Japanese subjects (Fig. 1D). Because obesity is considered a possible risk factor for leiomyomas, we investigated but did not find a significant correlation between BMI and leiomyoma tissue aromatase mRNA levels. These results suggest that the strikingly high expression of aromatase mRNA levels in leiomyoma may affect leiomyoma growth, and the higher leiomyoma aromatase mRNA levels in African-American women may be in part due to the significantly higher use of promoter II.
Race-related expression of ERα, ERβ, and PR in uterine leiomyoma
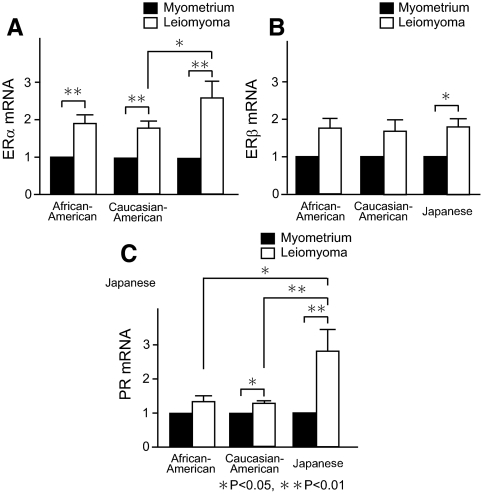
In contrast to strikingly high aromatase expression, ERα, ERβ, and PR mRNA levels in leiomyoma tissue were less than 3-fold higher compared with matched myometrium in all groups. Levels of ERα mRNA in leiomyoma were significantly higher compared with matched myometrium without any racial differences; leiomyoma ERα mRNA levels in Japanese subjects were significantly higher compared with those in Caucasian-American subjects (Fig. 2A). In contrast, leiomyoma ERβ mRNA levels were significantly higher only in Japanese subjects (Fig. 2B). Leiomyoma PR mRNA levels were significantly higher compared with matched myometrium in Caucasian-American and Japanese subjects; leiomyoma PR mRNA levels in Japanese subjects were significantly higher compared with those in African-American or Caucasian-American subjects (Fig. 2C). Thus, leiomyoma tissue may be more responsive to the sex-steroid hormones compared with myometrium.
Figure 2.
Expression of estrogen receptor and PR mRNA in uterine leiomyomas of African-American, Caucasian-American, and Japanese women. ERα (A), ERβ (B), or PR (C) mRNA levels in uterine leiomyoma and matched myometrium were measured by quantitative real-time RT-PCR and were normalized to GAPDH mRNA levels. All values in leiomyoma were described as fold differences compared with those measured in matched myometrium. *, P < 0.05 and **, P < 0.01 by Wilcoxon signed-rank test for leiomyoma vs. myometrium, and by ANOVA with pairwise t tests for African-American subjects vs. Japanese subjects, Caucasian-American subjects vs. Japanese subjects, or African-American subjects vs. Caucasian-American subjects.
Discussion
African-American women have a higher incidence, earlier growth, and larger volume of uterine leiomyomas compared with Caucasian-American women or other racial origins in the United States (1). However, the underlying mechanisms for this discrepancy remain unknown. Clinical trials suggested roles for aromatase inhibitors and antiprogestins in the treatment of uterine leiomyoma. Thus, we focused on the targets of these emerging therapeutics, namely aromatase, ERs and PR. We found that aromatase mRNA levels in leiomyoma tissues were strikingly higher compared with adjacent myometrium in African-American, Caucasian-American, and Japanese women. Intriguingly, the highest levels of aromatase mRNA were found in leiomyoma tissues from African-American women compared with Japanese women with statistical significance. Although aromatase mRNA levels in the adjacent myometrial tissues are low, it is likely that once tumorigenesis is initiated, higher aromatase expression in leiomyoma cells of African-American women may account for a higher incidence of clinically detectable tumors in this group.
The importance of aromatase for the leiomyoma growth and pathophysiology has been recently recognized both in vivo and in vitro. Clinically, aromatase inhibitors reduce leiomyoma volume and improve associated symptoms in premenopausal women (6,7). We recently reported that the cAMP-responsive proximal promoter I.3/II region was the primary regulator of aromatase expression in leiomyoma tissue in vivo (8), and cAMP-induced binding of the transcription factor CCAAT/enhancer binding protein-β to multiple motifs in the promoter I.3/II region is a critical mechanism regulating aromatase expression in leiomyoma smooth muscle cells (15).
Aromatase is a unique enzyme encoded by a unique gene, which contributes to sufficient biological activity at very low levels of expression (11). Useful antibodies are not available commercially to detect low levels of aromatase in human tissue samples. On the other hand, we have previously shown that aromatase mRNA levels are closely related with aromatase enzyme activity in primary leiomyoma smooth muscle cells (15). Together, aromatase mRNA levels closely reflect aromatase enzyme activity in leiomyoma tissue. In the current study, we found that the proximal promoter II was primarily used in vivo, regardless of racial backgrounds. Moreover, a significantly higher use of promoter II in African-American women compared with Japanese women suggested that this promoter may in part account for the strikingly higher leiomyoma aromatase expression observed in African-American women. Because the analysis of data for the use of four separate promoters in each sample requires stringent statistical methods, larger sample sizes are required to detect possible additional differences.
Although levels of ERα, ERβ, and PR mRNA in leiomyoma compared with adjacent myometrium were also higher, the fold differences were much smaller than those for aromatase mRNA. Leiomyoma ERα mRNA levels were significantly higher in all groups with no racial differences, whereas leiomyoma PR mRNA levels were significantly higher compared with matched myometrium only in Caucasian-American and Japanese women. These findings are interesting because antiprogestins or selective PR modulators significantly reduce leiomyoma size and associated uterine bleeding (17,18). Our results provide support for a selective effect of antiprogestins on uterine leiomyoma tissue compared with myometrium.
In conclusion, our most striking finding is the presence of the highest aromatase expression in leiomyoma tissue from African-American women. This finding provides plausible molecular evidence as to the higher incidence of uterine leiomyoma that becomes symptomatic at an earlier age in African-American women. It is tempting to investigate whether the response of leiomyomas to treatment with an aromatase inhibitor is different in African-American women compared with Caucasian-American or Japanese women. Identification of clinically useful biomarkers may permit us predict the response to hormonal treatment with higher accuracy.
Supplementary Material
Footnotes
This work was supported by grants from the National Institutes of Health (HD46260) and Friends of Prentice (to S.E.B.), and the International Training Program of Japan Society for the Promotion of Science (to H.I.).
Disclosure Summary: H.I., S.R., M.D., A.W.R., T.K., M.I., H.U., and M.S. have nothing to declare. S.E.B. serves as a consultant for Meditrina Phamaceuticals, Inc., GlaxoSmithKline, and Novartis.
First Published Online February 24, 2009
Abbreviations: BMI, Body mass index; ERα, estrogen receptor subtype α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PR, progesterone receptor.
References
- Parker WH 2007 Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 87:725–736 [DOI] [PubMed] [Google Scholar]
- Flynn M, Jamison M, Datta S, Myers E 2006 Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol 195:955–964 [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Steiner CA 2002 Hysterectomy rates in the United States 1990–1997. Obstet Gynecol 99:229–234 [DOI] [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ 1997 Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 90:967–973 [DOI] [PubMed] [Google Scholar]
- Othman EE, Al-Hendy A 2008 Molecular genetics and racial disparities of uterine leiomyomas. Best Pract Res Clin Obstet Gynaecol 22:589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario SG, Bozzini N, Borsari R, Baracat EC 2009 Action of aromatase inhibitor for treatment of uterine leiomyoma in perimenopausal patients. Fertil Steril 91:240–243 [DOI] [PubMed] [Google Scholar]
- Varelas FK, Papanicolaou AN, Vavatsi-Christaki N, Makedos GA, Vlassis GD 2007 The effect of anastrazole on symptomatic uterine leiomyomata. Obstet Gynecol 110:643–649 [DOI] [PubMed] [Google Scholar]
- Imir AG, Lin Z, Yin P, Deb S, Yilmaz B, Cetin M, Cetin A, Bulun SE 2007 Aromatase expression in uterine leiomyomata is regulated primarily by proximal promoters I.3/II. J Clin Endocrinol Metab 92:1979–1982 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Simpson ER, Word RA 1994 Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. J Clin Endocrinol Metab 78:736–743 [DOI] [PubMed] [Google Scholar]
- Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M 2000 In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 141:3852–3861 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S 2005 Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev 57:359–383 [DOI] [PubMed] [Google Scholar]
- Shozu M, Sumitani H, Segawa T, Yang HJ, Murakami K, Kasai T, Inoue M 2002 Overexpression of aromatase P450 in leiomyoma tissue is driven primarily through promoter I.4 of the aromatase P450 gene (CYP19). J Clin Endocrinol Metab 87:2540–2548 [DOI] [PubMed] [Google Scholar]
- Stewart EA 2001 Uterine fibroids. Lancet 357:293–298 [DOI] [PubMed] [Google Scholar]
- Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS 2006 Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol 108:1381–1387 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Fencki V, Marsh EE, Yin P, Chen D, Cheng YH, Reisterd S, Lin Z, Bulun SE 2008 CCAAT/enhancer binding protein β regulates aromatase expression via multiple and novel cis-regulatory sequences in uterine leiomyoma. J Clin Endocrinol Metab 93:981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura M, Reierstad S, Innes JE, Bulun SE 2008 Novel promoter I. 8 and promoter usage in the CYP19 (aromatase) gene. Reprod Sci 15:1044–1053 [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA 2007 A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril 87:1399–1412 [DOI] [PubMed] [Google Scholar]
- Jirecek S, Lee A, Pavo I, Crans G, Eppel W, Wenzl R 2004 Raloxifene prevents the growth of uterine leiomyomas in premenopausal women. Fertil Steril 81:132–136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.