Abstract
Context: The typical polycystic ovary syndrome (PCOS) phenotype includes 17-hydroxyprogesterone (17OHP) hyperresponsiveness to GnRH agonist (GnRHag) testing. Functionally atypical PCOS lacks this feature.
Objective: The hypothesis was tested that the typical PCOS ovarian dysfunction results from intrinsically increased sensitivity to LH/human chorionic gonadotropin (hCG) due to a flaw in FSH action.
Participants/Design/Interventions/Main Outcome Measures: After phenotyping a cohort of 60 women, steroid and inhibin-B responses to gonadotropins were evaluated in representative typical (n = 7) and atypical (n = 5) PCOS and healthy controls (n = 8). Submaximal hCG testing before and after an FSH test dose was performed in random order before and after prolonged ovarian suppression by depot GnRHag.
Setting: The study was performed at a Clinical Research Center.
Results: Of our PCOS cohort, 68% were the typical type. Typical PCOS had 17OHP hyperresponsiveness and, unlike controls, significant androgen and estradiol responses to hCG. FSH increased inhibin-B and did not inhibit free testosterone or enhance estradiol responsiveness to hCG, all unlike controls. After ovarian suppression, 17OHP, androstenedione, and inhibin-B responsiveness to gonadotropin testing persisted. Atypical PCOS had significantly higher body mass index but lower ovarian volume and plasma free testosterone than typical PCOS. Steroid responses to hCG were insignificant and similar to controls. FSH suppressed free testosterone but stimulated inhibin-B. The estradiol level after combined hCG-FSH was subnormal. Free testosterone was less GnRHag suppressible than in typical PCOS.
Conclusions: Typical PCOS is characterized by intrinsic ovarian hypersensitivity to hCG to which excessive paracrine FSH signaling via inhibin-B may contribute. Atypical PCOS is due to a unique type of ovarian dysfunction that is relatively gonadotropin hyposensitive.
In contrast to the gonadotropin-hypersensitivity of typical PCOS, atypical PCOS is due to a unique type of ovarian dysfunction that is relatively gonadotropin-hyporesponsive.
Polycystic ovary syndrome (PCOS) is a syndrome of chronic hyperandrogenism and oligo-anovulation that may or may not be accompanied by a polycystic ovary (1,2). It is the most common endocrinopathy of reproductive-age women (3), and it has long-term health implications (4,5,6,7).
Most women who meet standard criteria for PCOS exhibit 17-hydroxyprogesterone (17OHP) hyperresponsiveness to LH, as elucidated by a GnRH agonist (GnRHag) or human chorionic gonadotropin (hCG) test (8,9). The precise mechanisms responsible for this steroidogenic response in such women with PCOS have not been established, although an intrinsic abnormality within the ovarian thecal cell has been favored by some (8,10). Furthermore, what distinguishes this functionally typical type of PCOS from the functionally atypical type of PCOS lacking this characteristic has not been addressed in prior studies.
We postulated that the ovarian dysfunction of typical PCOS is due to an intrinsic increase in sensitivity to LH resulting from a flaw in FSH paracrine action (Fig. 1) (8). If so, we expected that by characterizing a population of PCOS women with and without 17OHP hyperresponsiveness, we could identify clinical and/or biochemical features that would provide insights into the nature of this steroidogenic abnormality.
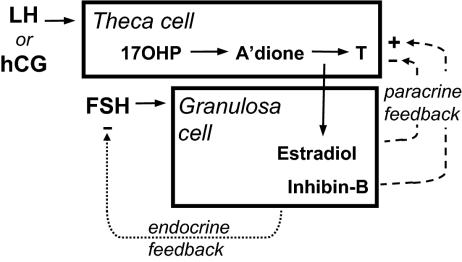
Figure 1.
Simplified version of the two-gonadotropin, two-cell model of the normal regulation of ovarian steroidogenesis during the early to midfollicular phase of the menstrual cycle. This diagram shows the major relationships evaluated in this study. LH (or the LH analog hCG) stimulates theca cells to form androgen. Androgens are used as substrates for estrogen formation by granulosa cells, a process requiring aromatase activity, which is normally up-regulated by FSH in accord with the state of maturation of granulosa cells. Gonadotrope secretion of FSH is more sensitive than LH to endocrine negative-feedback regulation by the granulosa cell products estradiol and inhibin (fine dotted line). FSH-dependent granulosa cell factors, including estradiol and inhibin-B, also act in an intraovarian paracrine manner to modulate the androgenic response to LH/hCG positively (+) or negatively (−) in accord with granulosa cell needs (heavy dotted lines). A’dione, Androstenedione; T, testosterone. Based on model of Ehrmann et al. (8).
Thus, we sought to characterize and compare these two functionally distinct types of PCOS. We first compared the baseline clinical and endocrine phenotype of cohorts of typical and atypical PCOS and healthy controls. We then tested representative subjects in each group for their sensitivity to hCG before and after FSH priming to determine the mechanism of the functional differences. Repeat gonadotropin sensitivity testing of these PCOS groups was performed after 3–4 months of gonadotropin suppression to determine whether ovarian dysfunction persisted in a crop of ovarian follicles that had developed in the absence of an abnormal hormonal milieu.
Subjects and Methods
Subjects
Thorough phenotyping was performed on the adult cohort of PCOS patients studied 2000–2007 in the University of Chicago Clinical Research Center who met National Institutes of Health criteria for the diagnosis of PCOS (1). They were oligo-amenorrheic as defined by fewer than eight menstrual periods in the previous 1 yr and hyperandrogenemic as defined by an elevated plasma free testosterone level more than 9 pg/ml; ovarian morphology was not a criterion for study. Hyperprolactinemia, congenital adrenal hyperplasia, Cushing’s syndrome, acromegaly, and thyroid dysfunction were excluded, as were unexpectedly preovulatory or luteal phase subjects. They were 18–38 yr of age; 51% were non-Hispanic Blacks, 47% were non-Hispanic whites, and 2% were non-Hispanic other. They were classified post hoc into a typical PCOS cohort (n = 30) or an atypical PCOS cohort (n = 14) based on whether or not their peak 17OHP response to the GnRHag test (see Phenotyping protocol) was greater than that of controls.
Phenotyping was similarly performed on a cohort of healthy adult volunteers who were contemporaneously recruited by advertisement after determining them to be eumenorrheic and to lack hirsutism or inflammatory acne. Controls were those with ultrasonographically normal ovaries (11) (n = 14). They were 19–40 yr of age; 57% were non-Hispanic whites, 36% were non-Hispanic Blacks, and 7% were Hispanic.
Self-selected representatives of these cohorts then underwent testing of ovarian sensitivity to gonadotropins to characterize further the mechanistic basis for their differences in ovarian function. Seven typical PCOS, five atypical PCOS, and eight control subjects volunteered to undergo the gonadotropin sensitivity protocol, commencing two or more months after being phenotyped.
Subjects were studied between January 2000 and November 2007. All studies were approved by the University of Chicago Institutional Review Board and were conducted in the University of Chicago General Clinical Research Center after obtaining informed consent.
Phenotyping protocol
All subjects underwent a previously described phenotyping protocol in which a GnRHag test (leuprolide acetate 10 μg/kg) was performed after short-term low-dose dexamethasone when they were amenorrheic for a minimum of 2 months or on d 4–10 of the menstrual cycle (12). This test followed sequential evaluation of baseline reproductive hormones, serum lipids, an oral glucose tolerance test, vaginal ultrasonography, and the response to low-dose ACTH (cosyntropin 1.0 μg/1.5 m2).
Gonadotropin sensitivity testing
Representatives from each cohort were again studied when amenorrheic or on d 3–10 of a cycle. Dexamethasone (0.25 mg/1.0 mg2) was administered at bedtime daily to attenuate coincidental adrenocortical secretion. An initial fasting “basal” post-dexamethasone level was obtained by pooling 0700 and 0800 h samples. Subjects then received either hCG alone (500 IU im) or recombinant FSH (300 IU sc), followed 24 h later by hCG; the order of the sequence (hCG vs. FSH-hCG) was randomized with a 1-month intervening washout period. Responses were evaluated by pooling 0700 and 0800 h samples 1 d after each gonadotropin injection. Pilot studies had indicated this hCG dose to yield approximately half-maximal 17OHP responses (13). This FSH dose was previously reported to approximately double levels of FSH (14) and to stimulate steroidogenic responses within 24 h in a woman with inactive FSH (15). It proved to increase FSH levels by 3.83 ± 0.79 U/liter, 24 h after injection across studies (P < 0.0001), with no significant differences among subsets.
In consenting PCOS subjects (five with typical, five with atypical PCOS), depot leuprolide acetate 7.5 mg im monthly was begun 3 months after their last hCG test, along with add-back transdermal estradiol 50 μg daily to prevent hot flashes and osteoporosis. One month after the third leuprolide depot injection, retesting of responses to hCG and/or FSH resumed 2 d after discontinuing estradiol replacement.
Assays
Plasma total testosterone and dehydroepiandrosterone (DHEA) sulfate (DHEAS) were measured by commercially available kits (Diagnostic Products Corp., Los Angeles, CA), as was estradiol (Pantex, Santa Monica, CA) (16,17). Free testosterone and SHBG binding capacity were calculated from a binding assay as previously reported (16,18). Cortisol was assayed by a Diagnostic Products kit until 2004 and thereafter by an automated immunometric method (Immulite 2000; Diagnostic Products); the data are expressed as Immulite equivalents according to the linear regression equation expressing the correlation of samples assayed by both methods. Steroid intermediates (17OHP, androstenedione, and DHEA) were measured by previously reported RIAs with sensitivities averaging 25 ng/dl and intraassay precision averaging 8% (17). All samples from an individual experiment for each individual were assayed in a single batch. LH and FSH were measured by immunochemiluminometric assay kits (Delphia, Wallach, Finland). Inhibin-B was assayed by a two-site sandwich immunoassay with antiinhibin β-B subunit and α-subunit antibodies (Diagnostic Systems Laboratories, Inc., Webster, TX) by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core with a sensitivity of 20 pg/ml. Plasma glucose was measured using a glucose analyzer (YSI Model 2300 STAT; Yellow Springs Instrument Inc., Yellow Springs, OH). Serum insulin was assayed by a double-antibody technique (19) until 2004 and thereafter by an automated immunometric method (Immulite 2000); the data are expressed as Immulite equivalents according to the linear regression equation expressing the correlation of samples assayed by both methods. Insulin resistance index was indexed by homeostatic model assessment (HOMA) from fasting glucose and insulin (20) and composite insulin sensitivity index (ISI) from glucose tolerance test data (21), excluding hemolyzed samples and data from one diabetic PCOS with fasting hyperglycemia. Serum lipids were measured by a homogenous enzymatic colorimetric assay (Roche, Indianapolis, IN).
Real-time intravaginal ultrasound imaging was performed using a General Electric Voluson 730 5-9 MHz transducer (General Electric Co., Fairfield, CT). A uniform report form was used for all studies. A PCO was defined by modified Rotterdam criteria (11,12).
Data analysis
Typical PCOS, atypical PCOS, and control data were compared with one-way ANOVA. Because free testosterone is a better index of testosterone production than total testosterone, the latter was a secondary outcome variable. Post hoc analyses were by Fisher’s protected least differences test; intragroup comparisons of responses to hCG and FSH were performed by paired Student’s t tests. Log transformations were performed as needed for normalization of data distribution. Results are reported as mean ± sem unless otherwise noted.
Results
Phenotypical characterization of typical and atypical PCOS cohorts
PCOS patients were classified as typical or atypical according to whether their peak 17OHP response to GnRHag was elevated compared with that of controls (Table 1). The peak 17OHP after GnRHag in controls was 77 ± 34, sd, ng/dl (observed range < 25–145 ng/dl). Of the PCOS cohort, 68% had peak responses above 145 ng/dl (154–1339) and were classified as typical PCOS, and the remaining 30% of the PCOS cohort were classified as atypical PCOS (peak 17OHP 31–125 ng/dl).
Table 1.
Phenotypes of typical PCOS, atypical PCOS, and control cohorts (mean ± sem)
| Typical PCOS (n = 30) | Atypical PCOS (n = 14) | Controls (n = 14) | |
|---|---|---|---|
| Age (yr) | 24.1 ± 1.0a | 25.3 ± 1.6 | 29.7 ± 1.7 |
| BMI (kg/m2) | 33.0 ± 1.6b | 44.3 ± 3.5a | 28.0 ± 2.3 |
| BP (mm Hg) | 116/66 ± 3/2 | 125/71 ± 4c /3 | 109/66 ± 4/2 |
| Ovarian volume (cc) | 19.9 ± 1.8a,b | 9.4 ± 1.0c | 6.7 ± 0.5 |
| Glucose 0 min (mg/d) | 95 ± 2.1 | 92 ± 2.1 | 90 ± 1.4 |
| Glucose 2-h GTT (mg/dl) | 122 ± 5.0 | 119 ± 4.0 | 116 ± 5.3 |
| Insulin 0 min (μU/ml) | 23.4 ± 4.2a | 22.6 ± 3.9a | 9.2 ± 1.7 |
| HOMA | 5.8 ± 1.1c | 5.2 ± 0.9c | 2.2 ± 0.5 |
| ISI composite | 3.8 ± 0.6c | 2.6 ± 0.5c | 5.1 ± 0.7 |
| HDL-cholesterol (mg/dl) | 50 ± 2.6 | 56 ± 8.9 | 63 ± 6.8 |
| Triglycerides (mg/dl) | 136 ± 37 | 90 ± 15 | 80 ± 6.6 |
| Testosterone (total) (ng/dl) | 73 ± 4.5a,b | 48 ± 3.3a | 29 ± 3.6 |
| Testosterone (free) (pg/ml) | 21 ± 2.0a | 16 ± 1.6a | 5.3 ± 0.5 |
| SHBG (nm) | 20.4 ± 2.2a,d | 11.2 ± 2.7a | 31.0 ± 2.8 |
| Androstenedione (ng/dl) | 211 ± 17a,b | 122 ± 12c | 89 ± 10 |
| 17OHP (ng/dl) | 73 ± 6.5a,b | 36 ± 4.5 | 39 ± 8.1 |
| DHEAS (μg/dl) | 137 ± 15 | 153 ± 24 | 89 ± 12 |
| GnRHag test | |||
| LH 0 h (U/liter) | 8.5 ± 0.9a,d | 5.6 ± 0.8 | 4.0 ± 0.4 |
| LH 0.5 h (U/liter) | 29 ± 3.5c | 20 ± 4.1 | 18 ± 2.8 |
| FSH 0 h (U/liter) | 4.9 ± 0.2 | 4.8 ± 0.3 | 5.8 ± 0.4 |
| FSH peak (U/liter) | 18.3 ± 1.4a | 18 ± 2.9a | 32 ± 2.9 |
| 17OHP peak (ng/dl) | 348 ± 42a,b | 84 ± 7.4 | 75 ± 8.7 |
| Estradiol 0 h (pg/ml) | 53 ± 2.9d | 44 ± 3.4 | 51 ± 6 |
| Estradiol peak (pg/ml) | 380 ± 20a,b | 171 ± 19 | 158 ± 17 |
| ACTH test peaks | |||
| 17OHP (ng/dl) | 126 ± 8.4b | 89 ± 8.7 | 109 ± 15 |
| DHEA (ng/dl) | 996 ± 68 | 1048 ± 192 | 697 ± 64 |
Multipliers for conversion to International System of Units: glucose 0.0556 (mm), cholesterol 0.0257 (mm), triglycerides 0.0113 (mm), testosterone 0.0347 (nm), androstenedione 0.0349 (nm), DHEAS 0.0271 (μm), estradiol 3.61 (pm), 17OHP 0.0303 (nm), and DHEA 0.0347 (nm). GTT, Glucose tolerance test; HDL, high-density lipoprotein.
P < 0.005 vs. controls.
P < 0.005 vs. atypical PCOS.
P < 0.05 vs. controls.
P < 0.05 vs. atypical PCOS.
The typical PCOS cohort differed from atypical PCOS in having a significantly lower body mass index (BMI) and in several other ways. 17OHP, testosterone, androstenedione, SHBG, and LH were significantly higher in typical PCOS, as was ovarian volume. Nevertheless, 36% (five of 14) of the atypical PCOS cohort met criteria for a polycystic ovary, 60% on the basis of enlargement alone. Typical PCOS also had higher estradiol (Table 1), androstenedione, and progesterone responses to GnRHag.
Both PCOS cohorts differed from controls in having: larger ovaries; higher androgens, insulin, and indexes of insulin resistance; lower SHBG; and lower FSH responsiveness to GnRHag. Although controls were slightly older, reanalysis after controlling for age did not alter the conclusions. Atypical PCOS also had a higher BMI and systolic blood pressure than controls.
Gonadotropin sensitivity testing
These PCOS study groups had similar characteristics to the others in the cohort they represented, except they were slightly older and substantially heavier, significantly so for the typical PCOS group (P < 0.05) (Table 2).
Table 2.
Phenotypical characteristics of test groups undergoing gonadotropin sensitivity testing (mean ± sem)
| Typical PCOS (n = 7) | Atypical PCOS (n = 5) | Controls (n = 8) | |
|---|---|---|---|
| Age (yr) | 28.5 ± 1.6 | 29.4 ± 1.1 | 30.8 ± 2.3 |
| BMI (kg/m2) | 39.8 ± 1.9a,b | 53.5 ± 3.0b | 25.9 ± 3.6 |
| Ovarian volume (cc) | 24 ± 4.5a,c | 7.9 ± 1.0 | 7.2 ± 0.5 |
| Glucose 0 min (mg/d) | 103 ± 2.9b | 96 ± 4.3 | 88 ± 2.1 |
| Insulin 0 min (μU/ml) | 32 ± 6.0b | 24 ± 8.7 | 7.5 ± 1.9 |
| HOMA | 8.3 ± 1.8b | 5.8 ± 2.2 | 1.8 ± 0.5 |
| ISI composite | 1.4 ± 0.3c | 1.4 ± 0.2c | 5.8 ± 1.2 |
| Testosterone (total) (ng/dl) | 65 ± 7.8b | 49 ± 4.7c | 28 ± 4.4 |
| Testosterone (free) (pg/ml) | 20 ± 4.2c | 17 ± 2.3c | 5.4 ± 0.9 |
| SHBG (nm) | 15.8 ± 3.8b | 9.8 ± 3.6b | 34.4 ± 3.7 |
| Androstenedione (ng/dl) | 149 ± 12c | 113 ± 26 | 89 ± 14 |
| 17OHP (ng/dl) | 61 ± 6.7a,c | 30 ± 8.6 | 42 ± 12 |
| DHEAS (μg/dl) | 109 ± 14 | 160 ± 45 | 106 ± 19 |
| GnRHag test | |||
| LH 0 hr (U/liter) | 5.3 ± 0.7 | 3.6 ± 0.4 | 4.0 ± 0.4 |
| LH 0.5 hr (U/liter) | 17 ± 3.7 | 14 ± 3.2 | 14 ± 3.3 |
| FSH 0 hr (U/liter) | 4.4 ± 0.3 | 4.8 ± 0.5 | 5.9 ± 0.6 |
| FSH peak (U/liter) | 18 ± 2.9c | 23 ± 8.1 | 29 ± 3.9 |
| 170OHP peak (ng/dl) | 395 ± 67b,d | 71 ± 12 | 69 ± 10 |
| Estradiol 0 hr (pg/ml) | 45 ± 5.0 | 43 ± 7.3 | 59 ± 6.9 |
| Estradiol peak (pg/ml) | 301 ± 28b,d | 108 ± 11 | 160 ± 25 |
| ACTH test peaks | |||
| 17OHP (ng/dl) | 128 ± 16 | 87 ± 11 | 108 ± 15 |
| DHEA (ng/dl) | 1015 ± 88 | 1495 ± 527 | 690 ± 104 |
P < 0.05 vs. atypical PCOS.
P < 0.005 vs. controls.
P < 0.05 vs. controls.
P < 0.005 vs. atypical PCOS.
Controls
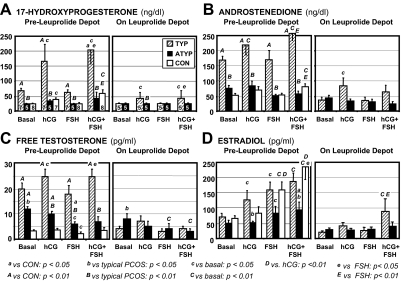
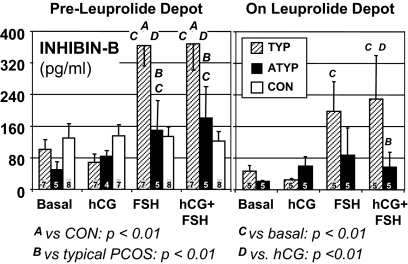
In normal volunteers, hCG stimulated 17OHP only slightly, but significantly (from 25.7 ± 0.8 to 41 ± 5.4 ng/dl), and did not cause a significant increase in androstenedione, free testosterone, or estradiol levels (Fig. 2). Although this dose of FSH stimulated estradiol, it decreased free testosterone (from its basal level of 3.4 ± 0.5 to 2.2 ± 0.5 pg/ml), whereas 17OHP and androstenedione remained unchanged. The hCG-FSH combination stimulated significant increases in testosterone precursors, restored free testosterone to its basal level, and stimulated estradiol significantly more than either hCG or FSH alone. The inhibin-B basal level was 129 ± 37 pg/ml and did not change significantly in response to any treatment (Fig. 3).
Figure 2.
Steroid responses to gonadotropin sensitivity tests. Steroidogenic responses (mean ± sem) to hCG, FSH, and hCG plus FSH before and after 3–4 months of leuprolide depot administration in typical (TYP) PCOS, atypical (ATYP) PCOS, and healthy volunteers (CON). The mean of basal samples obtained before administering hCG and before administering FSH is displayed. The number of subjects in each group before (n = 5–8) and after (n = 5) depot leuprolide treatment is indicated in the 17OHP panels.
Figure 3.
Inhibin-B responses to gonadotropin sensitivity testing. Subjects and study results are displayed as in Fig. 2.
Typical PCOS and comparison with controls
Basal free testosterone after dexamethasone was significantly higher in typical PCOS (20.4 ± 2.4 pg/ml) than controls (Fig. 2C), although adrenocortical function was similarly suppressed judging from DHEAS and cortisol levels (data not shown). In contrast to controls, hCG significantly stimulated both androstenedione and free testosterone increases in typical PCOS; the increases in 17OHP, androstenedione, and free testosterone were significantly greater than in controls (P < 0.05). hCG also caused an increase in estradiol that was significantly greater than that of controls (P < 0.05), to a level similar to that on FSH (Fig. 2). FSH did not exert its normal suppressive effect on free testosterone. Combined hCG/FSH did not lead to a significantly higher 17OHP or androgen levels than hCG alone, nor, in contrast to controls, did it lead to a significantly higher level of estradiol than FSH alone. FSH also stimulated a significant, 3.5-fold increase in inhibin-B (from 101 ± 24 to 365 ± 51 pg/ml; P < 0.01) (Fig. 3), unlike controls. SHBG binding was not changed by the treatments.
After prolonged leuprolide depot administration, basal LH decreased significantly to 0.25 ± 0.05 and FSH to 2.7 ± 0.6 U/liter in typical PCOS. Concomitantly all basal sex steroids decreased significantly (P < 0.05), e.g. free testosterone decreased by 19 ± 2 pg/ml to reach a normal pre-leuprolide basal level (3.7 ± 0.8 pg/ml); DHEAS (data not shown) also decreased slightly (from 96 ± 12 to 86 ± 12 μg/dl; P < 0.05) in the absence of a change in cortisol (1.4 ± 0.3 vs. 1.7 ± 0.9 μg/dl).
17OHP and androstenedione responses to hCG persisted, although lessened, after prolonged GnRHag suppression of endogenous gonadotropins (Fig. 2). Abnormal responsiveness of inhibin-B to FSH also persisted: the level increased 4-fold from a slightly lower basal level (not significant) to reach levels of 199 ± 70 and 230 ± 110 pg/ml on FSH and hCG-FSH, respectively (both P < 0.05), which were not significantly higher than pretreatment controls (Fig. 3). Notably, inhibin-B responded promptly to FSH alone, although estradiol did not.
Atypical PCOS and comparison with other groups
Basal free testosterone was significantly lower (11.5 ± 0.9 pg/ml) than in typical PCOS, whereas greater than in controls (Fig. 2C), in the presence of normal adrenocortical suppression by dexamethasone. 17OHP, androgens, and estradiol increases were not significant in response to hCG and were similar to those of controls. FSH alone significantly suppressed free testosterone (to 6.0 ± 1.1 pg/ml), as in controls. FSH tended to stimulate estradiol less than in typical PCOS and controls (P < 0.1). Notably, combining hCG with FSH administration did not elicit the significant further increase in estradiol found in controls, such that atypical PCOS achieved an estradiol level significantly less than that of either of the other groups. However, FSH consistently stimulated inhibin-B increases, unlike controls, but inhibin-B remained normal in most, differing from typical PCOS (Figs. 3 and 4).
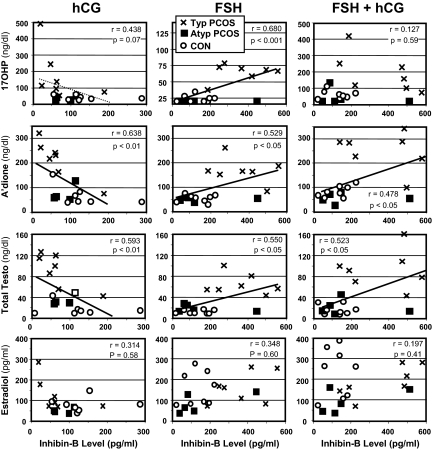
Figure 4.
Scatterplots showing changing relationships of inhibin-B to sex steroid levels in response to hCG, FSH, and their combination. Groups are designated as in Fig. 2. A’dione, Androstenedione; Testo, testosterone. Significant relationships are indicated by heavy linear regression lines; trend line is indicated by light dotted line. Note lower scales for inhibin on hCG and 17OHP on FSH. The correlations were virtually identical for total and free testosterone.
After prolonged leuprolide depot suppression, LH levels decreased significantly to levels similar to those in typical PCOS (0.5 ± 0.2 U/liter), but FSH was suppressed relatively more (decreasing to 1.4 ± 0.4 U/liter; P < 0.01). Basal plasma free testosterone decreased only slightly (to 8.1 ± 2.2 pg/ml, not significant) and, thus, remained higher than in typical PCOS (P < 0.05) and pre-leuprolide controls (P < 0.05) (Fig. 2C). All other steroids decreased significantly. FSH further suppressed free testosterone, both without and with concomitant hCG. The basal inhibin-B uniformly became virtually undetectable after gonadotropin suppression, and, with the exception of the subject with the highest pretreatment levels, was no longer responsive to FSH.
Relationship of inhibin-B and steroid responses to gonadotropins
Basal inhibin-B and androgen levels did not correlate significantly. However, in response to hCG, inhibin-B levels became inversely proportional to androgen levels (Fig. 4), although they were not significantly changed by hCG per se (Fig. 3). Conversely, in response to FSH, inhibin-B became positively correlated with these thecal steroid levels, especially with 17OHP (P < 0.001). When hCG stimulation followed FSH, the correlation between inhibin-B and 17OHP was negated.
Inhibin-B did not correlate with estradiol in response to hCG (Fig. 4), though estradiol correlated highly with androgen levels (P < 0.001 vs. 17OHP, P < 0.01 vs. androstenedione, P < 0.05 vs. testosterone). Inhibin-B also did not correlate significantly with estradiol or thecal steroid levels after FSH alone or with hCG.
Discussion
Our results support the hypothesis that the ovarian dysfunction of typical PCOS results from intrinsic thecal hypersensitivity to LH that is associated with inhibin-B hyperresponsiveness to FSH, suggesting a paracrine defect in FSH inhibition of theca cell function.
This study confirms the recently reported inhibitory effect of FSH on testosterone levels in normal volunteers (22). The responses to gonadotropins of normal women are consistent with expectations from a two-cell, two-gonadotropin model of ovarian secretion in which FSH exerts a paracrine effect to modulate LH/hCG stimulation of androgen secretion (Fig. 1) (8,23,24). Thus, normally, estradiol secretion is regulated by the combination of LH-dependent thecal provision of substrate (androgen) and FSH-regulated granulosa cell aromatase activity. In addition, FSH-dependent granulosa cell paracrine factors, both inhibitory and stimulatory, modulate LH-dependent androgen biosynthesis so as to coordinate it with granulosa cell needs for estrogen.
In typical PCOS, theca cell hypersensitivity to hCG/LH seems to result at least in part from inadequate FSH-dependent paracrine restraint of androgen production; thus, in response to hCG/LH, thecal androgenic substrate is allowed to increase sufficiently to drive inappropriate granulosa cell estradiol secretion, without the normal need for FSH up-regulation of aromatase. This dysregulation of estradiol secretion seems likely to contribute, via an endocrine negative-feedback effect, to the significant suppression of serum FSH levels in PCOS. We could not confirm the recently reported stimulatory effect of FSH on 17OHP and androstenedione in PCOS (22); this may be because of differences in study design and assay methodology.
The thecal hypersensitivity to hCG of typical PCOS was also manifest as a persistence of abnormal 17OHP and androstenedione responsiveness to submaximal hCG stimulation after ovarian suppression. Our study confirms and extends the findings of Gilling-Smith et al. (25) who obtained similar findings after testing the ovarian response to maximal hCG stimulation after short-term gonadotropin suppression by GnRHag treatment. We retested after a sufficiently long period of gonadotropin suppression to allow a new crop of follicles to mature (26) in the absence of excess LH and androgen (27). Our findings are compatible with the fundamental ovarian defect in typical PCOS being intrinsic to the ovary, as suggested by data demonstrating overexpression of most steroidogenic enzymes, particularly CYP17, in response to cAMP in passaged PCOS theca cells in culture (10,24).
In atypical PCOS, the absence of an elevated 17OHP response to a GnRHag test is but one feature of a distinctly different type of ovarian dysfunction. The phenotype of this atypical PCOS resembles that described by Pasquali et al. (28) in many respects: atypical PCOS had significantly lower baseline androgen levels, and tended to have lower 17OHP responses to ACTH, than typical PCOS. In addition, we found smaller ovaries, though significantly enlarged ones, in atypical PCOS. On the other hand, we reached some different conclusions than Pasquali et al. (28) about functionally atypical PCOS. For one, we found it to constitute a distinct minority (30%) of PCOS, rather than a small majority. The difference appears to be due to our defining 17OHP elevation in response to GnRHag on the basis of a considerably lower upper limit of normal than theirs because asymptomatic volunteers with a polycystic ovary were excluded from our control group since they are known to have an increased prevalence of ovarian dysfunction (12,29,30).
Our studies show that this lack of 17OHP hyperresponsiveness to GnRHag testing indicates a previously undescribed pathophysiological basis for PCOS. We demonstrate that although atypical PCOS has functional ovarian hyperandrogenism, as indicated by its increased plasma free testosterone level after adrenocortical suppression, the ovary is functioning in a partially gonadotropin-independent manner. Testosterone and its precursors were not significantly responsive to hCG, and plasma free testosterone was not completely normalized by long-term gonadotropin suppression. FSH decreased free testosterone normally, but it did not interact with hCG to stimulate estradiol to a normal extent, and it inappropriately stimulated inhibin-B, though to a lesser extent than it did in typical PCOS. Furthermore, whereas long-term suppression of gonadotropins lowered estradiol, it did not suppress plasma free testosterone to a normal extent.
Our cohort’s BMI was significantly above and ISIs were similar to those of typical PCOS, rather than being lower as reported by Pasquali et al. (28). Thus, our atypical group was as insulin resistant as typical PCOS, in which the compensatory hyperinsulinemia would be expected to synergize with LH to increase androgen production and sensitize responsiveness to hCG (8). On the other hand, their SHBG blood levels, which are partly determined by insulin, are lower in typical PCOS. Thus, we cannot exclude the possibility that insulin resistance or other obesity related factors (31) play a role in causing the relative gonadotropin-independent androgen production of atypical PCOS.
Inhibin-B was evaluated as a candidate mediator of FSH paracrine action. Inhibin-B is the product of the granulosa cells of small antral follicles (32), where it is normally regulated by FSH in a relatively sluggish negative feedback loop relationship (33). FSH exerts a paracrine effect on theca cells to stimulate androgen production at the level of CYP17 (34,35,36), and inhibin-B may mediate FSH’s permissive effect on the androgenic response to LH/hCG (15,37). Androgens in turn enhance FSH-stimulated granulosa cell inhibin production (38,39). This positive feedback loop appears to be counterbalanced by an inhibitory effect of hCG/LH (40).
The normal suppressive effect of FSH on testosterone levels indicates that the normal state of paracrine balance is one in which FSH-dependent inhibition of thecal androgen production predominates. FSH decreases free testosterone normally in atypical PCOS, in contrast to typical PCOS. The lack of free testosterone suppression by FSH in typical PCOS was associated with increased inhibin-B levels. The inhibin-B hyperresponsiveness to FSH in typical PCOS, in turn, seems to reflect their greater number of small antral follicles (32,41,42). On the other hand, atypical PCOS does not seem to generate sufficiently high inhibin-B levels to overcome the normal FSH-dependent inhibition of thecal androgen production.
Several granulosa cell factors are potential mediators of the normal paracrine inhibitory effect of FSH on thecal testosterone production (8,22,43,44,45). Estradiol seems unlikely to play this role because estradiol and androgenic responses to FSH did not correlate. Granulosa cell SHBG, particularly low in atypical PCOS, has emerged as a potential mediator of FSH action (46). Prime candidates are peptides of the TGF-β family, including activin, bone morphogenetic proteins, or TGF-β itself. All are inhibitory for cytochrome P450c17 expression. The activin to inhibin ratio has been relatively high in follicular fluid during midfollicular development (47). It is unknown which, if any, of these signaling systems undergo significant FSH-dependent intraovarian changes during human folliculogenesis.
The lack of a significant relationship between inhibin-B and estradiol responses to FSH is noteworthy. These finding are compatible with inhibin-B arising from small antral follicles at a stage before aromatase becomes highly inducible by FSH (48). Typical PCOS manifested a clear dissociation of inhibin-B responsiveness (significant) and estradiol responsiveness (insignificant) to acute FSH challenge after long-term suppression of gonadal function. The poor estradiol response appears to be normal and the inhibin-B response excessive for the induced hypogonadotropic state (49,50). These considerations suggest that in PCOS, an excessive number of small follicles is developing independently of an androgenic milieu, which itself can cause excessive follicular proliferation (51,52) and enhance inhibin-B production (38,39). Thus, these observations support the concept of an inherent defect in the regulation of early folliculogenesis in PCOS (42,53,54). The lower inhibin-B of atypical than typical PCOS may be due to lower follicle counts, consistent with their smaller baseline ovarian size. However, we cannot exclude the existence of a more fundamental defect in FSH signal transduction.
The main limitation of our study is the relatively small size of the groups that underwent gonadotropin sensitivity testing, which limits our statistical power. In addition, the subjects who chose to participate in this prolonged protocol were very obese, which may affect the generalizability of our results. Thus, our observations in atypical PCOS must be considered exploratory and require confirmation in larger studies.
In summary, typical PCOS subjects appear to have intrinsic theca cell hypersensitivity to LH/hCG stimulation that results in part from a deficit in a paracrine inhibitory effect of FSH that may in part involve inhibin-B signaling. Atypical PCOS is characterized by milder hyperandrogenemia and smaller ovaries, and their functional ovarian hyperandrogenism results from a different pathophysiological mechanism in which there is relative hyposensitivity to gonadotropin.
Acknowledgments
We thank Kristen Kasza for biostatistical analysis and Michael Snabes for assistance with setting up this protocol.
Footnotes
This research was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement [U54-041859] as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and RR-00055 and UL1RR024999 from the National Center For Research Resources.
Disclosure Summary: The authors have nothing to declare.
First Published Online February 24, 2009
Abbreviations: BMI, Body mass index; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; GnRHag, GnRH agonist; hCG, human chorionic gonadotropin; HOMA, homeostatic model assessment index of insulin resistance; 17OHP, 17-hydroxyprogesterone; ISI, insulin sensitivity index; PCOS, polycystic ovary syndrome.
References
- Zawadzki J, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens J, Haseltine F, Merriam G, eds. Polycystic ovary syndrome. Cambridge, MA: Blackwell Scientific Publications; 377–384 [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25 [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R 1998 Prevalence of the polycystic ovary syndrome in unselected Black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 83:3078–3082 [DOI] [PubMed] [Google Scholar]
- Dunaif A 1997 Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- Norman RJ, Dewailly D, Legro RS, Hickey TE 2007 Polycystic ovary syndrome. Lancet 370:685–697 [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ 2008 Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health-National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 93:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guzick DS 2008 Do cardiovascular risk factors in polycystic ovarian syndrome result in more cardiovascular events? J Clin Endocrinol Metab 93:1170–1171 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL 1995 Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 16:322–353 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Bellows AB, Gingrich MB, Hu Y, Evans WS, Marshall JC, Veldhuis JD 2004 Exaggerated 17-hydroxyprogesterone response to intravenous infusions of recombinant human LH in women with polycystic ovary syndrome. Am J Physiol Endocrinol Metab 286:E902–E908 [DOI] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss 3rd JF, McAllister JM 1999 Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 13:946–957 [DOI] [PubMed] [Google Scholar]
- Balen AH, Laven JS, Tan SL, Dewailly D 2003 Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 9:505–514 [DOI] [PubMed] [Google Scholar]
- Mortensen M, Ehrmann D, Littlejohn E, Rosenfield R 2009 Asymptomatic volunteers with a polycystic ovary are a functionally distinct but heterogeneous population. J Clin Endocrinol Metab 94:1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik DF, Barnes RB, Bieber E, Pickett K, Hirsch K, Wu E, Rosenfield RL hCG dose-response slopes indicate intrinsic abnormality in PCOS ovaries. Proc 83rd Annual Meeting of The Endocrine Society, Denver, CO, 2001 (Abstract OR19-15) [Google Scholar]
- Mannaerts B, Rombout F, Out H, Bennink H 1996 Clinical profiling of recombinant follicle stimulating hormone (rFSH; Puregon): relationship between serum FSH and efficacy. Hum Reprod 2:153–161 [DOI] [PubMed] [Google Scholar]
- Barnes RB, Namnoun A, Rosenfield RL, Layman LC 2000 Effect of follicle-stimulating hormone on ovarian androgen production in a woman with isolated follicle-stimulating hormone deficiency. N Engl J Med 343:1197–1198 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Perovic N, Ehrmann DA, Barnes RB 1996 Acute hormonal responses to the gonadotropin releasing hormone agonist leuprolide: dose-response studies and comparison to nafarelin—a clinical research center study. J Clin Endocrinol Metab 81:3408–3411 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Barnes RB, Ehrmann DA 1994 Studies of the nature of 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. J Clin Endocrinol Metab 79:1686–1692 [DOI] [PubMed] [Google Scholar]
- Moll Jr G, Rosenfield R 1979 Testosterone binding and free plasma androgen concentrations under physiological conditions: characterization by flow dialysis technique. J Clin Endocrinol Metab 49:730–736 [DOI] [PubMed] [Google Scholar]
- Morgan CR, Lazarow A 1963 Immunoassay of insulin: two antibody system. Plasma insulin levels of normal, subdiabetic, and diabetic rats. Diabetes 12:115–126 [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- Wachs DS, Coffler MS, Malcom PJ, Shimasaki S, Chang RJ 2008 Increased androgen response to follicle-stimulating hormone administration in women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:1827–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson GF, Magoffin DA, Dyer CA, Hofeditz C 1985 The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev 6:371–399 [DOI] [PubMed] [Google Scholar]
- Nelson VL, Qin Kn K, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss 3rd JF, McAllister JM 2001 The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab 86:5925–5933 [DOI] [PubMed] [Google Scholar]
- Gilling-Smith C, Story H, Rogers V, Franks S 1997 Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 47:93–99 [DOI] [PubMed] [Google Scholar]
- Gougeon A 1996 Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 17:121–155 [DOI] [PubMed] [Google Scholar]
- Goni M, Markussis V, Tolis G 1994 Efficacy of chronic therapy with the gonadotrophin releasing hormone agonist Decapeptyl in patients with polycystic ovary syndrome. Hum Reprod 9:1048–1052 [DOI] [PubMed] [Google Scholar]
- Pasquali R, Patton L, Pocognoli P, Cognigni GE, Gambineri A 2007 17-Hydroxyprogesterone responses to gonadotropin-releasing hormone disclose distinct phenotypes of functional ovarian hyperandrogenism and polycystic ovary syndrome. J Clin Endocrinol Metab 92:4208–4217 [DOI] [PubMed] [Google Scholar]
- Chang PL, Lindheim SR, Lowre C, Ferin M, Gonzalez F, Berglund L, Carmina E, Sauer MV, Lobo RA 2000 Normal ovulatory women with polycystic ovaries have hyperandrogenic pituitary-ovarian responses to gonadotropin-releasing hormone-agonist testing. J Clin Endocrinol Metab 85:995–1000 [DOI] [PubMed] [Google Scholar]
- Adams JM, Taylor AE, Crowley Jr WF, Hall JE 2004 Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab 89:4343–4350 [DOI] [PubMed] [Google Scholar]
- Barber TM, McCarthy MI, Wass JA, Franks S 2006 Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 65:137–145 [DOI] [PubMed] [Google Scholar]
- Welt CK, Taylor AE, Fox J, Messerlian GM, Adams JM, Schneyer AL 2005 Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab 90:5582–5587 [DOI] [PubMed] [Google Scholar]
- Welt CK, Smith ZA, Pauler DK, Hall JE 2001 Differential regulation of inhibin A and inhibin B by luteinizing hormone, follicle-stimulating hormone, and stage of follicle development. J Clin Endocrinol Metab 86:2531–2537 [DOI] [PubMed] [Google Scholar]
- Smyth C, Miro F, Whitelaw P, Howles C, Hillier S 1993 Ovarian thecal/interstitial androgen synthesis is enhanced by a follicle-stimulating hormone-stimulated paracrine mechanism. Endocrinology 133:1532–1538 [DOI] [PubMed] [Google Scholar]
- Nakhla A, Khan M, Romas N, Rosner W 1994 Estradiol causes the rapid accumulation of cAMP in human prostate. Proc Natl Acad Sci USA 91:5402–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawetawan C, Carr BR, McGee E, Bird IM, Hong TL, Rainey WE 1996 Inhibin and activin differentially regulate androgen production and 17 α-hydroxylase expression in human ovarian thecal-like tumor cells. J Endocrinol 148:213–221 [DOI] [PubMed] [Google Scholar]
- Barnes RB, Namnoum AB, Rosenfield RL, Layman LC 2002 The role of LH and FSH in ovarian androgen secretion and ovarian follicular development: clinical studies in a patient with isolated FSH deficiency and multicystic ovaries. Hum Reprod 17:88–91 [DOI] [PubMed] [Google Scholar]
- Henderson K, Franchimont P 1983 Inhibin production by bovine ovarian tissues in vitro and its regulation by androgens. J Reprod Fertil 67:291–298 [DOI] [PubMed] [Google Scholar]
- Hillier SG, Wickings EJ, Illingworth PI, Yong EL, Reichert Jr LE, Baird DT, McNeilly AS 1991 Control of inhibin production by human granulosa cells. Clin Endocrinol (Oxf) 35:71–78 [DOI] [PubMed] [Google Scholar]
- Welt CK, Taylor AE, Martin KA, Hall JE 2002 Serum inhibin B in polycystic ovary syndrome: regulation by insulin and luteinizing hormone. J Clin Endocrinol Metab 87:5559–5565 [DOI] [PubMed] [Google Scholar]
- Wachs DS, Coffler MS, Malcom PJ, Chang RJ 2006 Comparison of follicle-stimulating-hormone-stimulated dimeric inhibin and estradiol responses as indicators of granulosa cell function in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab 91:2920–2925 [DOI] [PubMed] [Google Scholar]
- Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D 2003 Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab 88:5957–5962 [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C 2006 TGF-β superfamily members and ovarian follicle development. Reproduction 132:191–206 [DOI] [PubMed] [Google Scholar]
- Lovell TM, Al-Musawi SL, Gladwell RT, Knight PG 2007 Gonadotrophins modulate hormone secretion and steady-state mRNA levels for activin receptors (type I, IIA, IIB) and inhibin co-receptor (βglycan) in granulosa and theca cells from chicken prehierarchical and preovulatory follicles. Reproduction 133:1159–1168 [DOI] [PubMed] [Google Scholar]
- Vanttinen T, Liu J, Hyden-Granskog C, Voutilainen R 2002 Biphasic regulation of activin A secretion by gonadotropins in cultured human ovarian granulosa-luteal cells leads to decreasing activin:inhibin ratios during continuing gonadotropin stimulation. J Endocrinol 172:557–563 [DOI] [PubMed] [Google Scholar]
- Forges T, Gerard A, Monnier-Barbarino P, Gerard H 2005 Immunolocalization of sex hormone-binding globulin (SHBG) in human ovarian follicles and corpus luteum. Histochem Cell Biol 124:285–290 [DOI] [PubMed] [Google Scholar]
- Schneyer AL, Fujiwara T, Fox J, Welt CK, Adams J, Messerlian GM, Taylor AE 2000 Dynamic changes in the intrafollicular inhibin/activin/follistatin axis during human follicular development: relationship to circulating hormone concentrations. J Clin Endocrinol Metab 85:3319–3330 [DOI] [PubMed] [Google Scholar]
- Jakimiuk AJ, Weitsman SR, Brzechffa PR, Magoffin DA 1998 Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol Hum Reprod 4:1–8 [DOI] [PubMed] [Google Scholar]
- Mannaerts B, Shoham Z, Schoot D, Bouchard P, Harlin J, Fauser B, Jacobs H, Rombout F, Coelingh Bennink H 1993 Single-dose pharmacokinetics and pharmacodynamics of recombinant human follicle-stimulating hormone (Org 32489*) in gonadotropin-deficient volunteers. Fertil Steril 59:108–114 [PubMed] [Google Scholar]
- Schoot DC, Harlin J, Shoham Z, Mannaerts BM, Lahlou N, Bouchard P, Bennink HJ, Fauser BC 1994 Recombinant human follicle-stimulating hormone and ovarian response in gonadotrophin-deficient women. Hum Reprod 9:1237–1242 [DOI] [PubMed] [Google Scholar]
- Hillier S, Tetsuka M 1997 Role of androgens in follicle maturation and atresia. Baillieres Clin Obstet Gynaecol 11:249–260 [DOI] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Bondy CA 1999 Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab 84:2951–2956 [DOI] [PubMed] [Google Scholar]
- Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, Themmen AP, Visser JA, Groome NP, Franks S 2005 Anti-müllerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab 90:5536–5543 [DOI] [PubMed] [Google Scholar]
- Stubbs SA, Stark J, Dilworth SM, Franks S, Hardy K 2007 Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab 92:4418–4426 [DOI] [PubMed] [Google Scholar]