Abstract
Context: Compared with glucose-sweetened beverages, consumption of fructose-sweetened beverages with meals elevates postprandial plasma triglycerides and lowers 24-h insulin and leptin profiles in normal-weight women. The effects of fructose, compared with glucose, ingestion on metabolic profiles in obese subjects has not been studied.
Objective: The objective of the study was to compare the effects of fructose- and glucose-sweetened beverages consumed with meals on hormones and metabolic substrates in obese subjects.
Design and Setting: The study had a within-subject design conducted in the clinical and translational research center.
Participants: Participants included 17 obese men (n = 9) and women (n = 8), with a body mass index greater than 30 kg/m2.
Interventions: Subjects were studied under two conditions involving ingestion of mixed nutrient meals with either glucose-sweetened beverages or fructose-sweetened beverages. The beverages provided 30% of total kilocalories. Blood samples were collected over 24 h.
Main Outcome Measures: Area under the curve (24 h AUC) for glucose, lactate, insulin, leptin, ghrelin, uric acid, triglycerides (TGs), and free fatty acids was measured.
Results: Compared with glucose-sweetened beverages, fructose consumption was associated with lower AUCs for insulin (1052.6 ± 135.1 vs. 549.2 ± 79.7 μU/ml per 23 h, P < 0.001) and leptin (151.9 ± 22.7 vs. 107.0 ± 15.0 ng/ml per 24 h, P < 0.03) and increased AUC for TG (242.3 ± 96.8 vs. 704.3 ± 124.4 mg/dl per 24 h, P < 0.0001). Insulin-resistant subjects exhibited larger 24-h TG profiles (P < 0.03).
Conclusions: In obese subjects, consumption of fructose-sweetened beverages with meals was associated with less insulin secretion, blunted diurnal leptin profiles, and increased postprandial TG concentrations compared with glucose consumption. Increases of TGs were augmented in obese subjects with insulin resistance, suggesting that fructose consumption may exacerbate an already adverse metabolic profile present in many obese subjects.
Compared to glucose-sweetened beverages, consumption of fructose-sweetened beverages increases postprandial triglycerides in obese subjects, potentially exacerbating the known adverse metabolic profile associated with obesity.
Consumption of fructose has increased dramatically over the past 4 decades due to its widespread use in many foods and beverages sweetened with sucrose (50% fructose) or high fructose corn syrup (42–55% fructose) (1). The increase of fructose consumption parallels the increased incidence of obesity in the U.S. population leading some investigators to suggest that dietary fructose contributes to increased body weight (2,3). Fructose does in fact have specific metabolic characteristics that could potentially contribute to a metabolic profile associated with increased body adiposity and the insulin resistance (metabolic) syndrome. For example, fructose does not stimulate insulin secretion (4) and plasma glucose and insulin responses are blunted when fructose compared with glucose-sweetened beverages are consumed with meals (5). As we previously demonstrated, these reductions in meal-induced insulin secretion result in attenuated diurnal circulating leptin profiles (6) due to the dependence of leptin production on insulin-mediated glucose metabolism (7). Reduced postprandial insulin levels also may contribute to blunted postprandial suppression of the putative orexigenic hormone ghrelin after ingestion of fructose-containing beverages compared with glucose-containing beverages with meals (5). Thus, fructose ingestion elicits an endocrine profile that could potentially favor increased energy intake and weight gain by attenuating levels of insulin and leptin, two hormones that inhibit food intake and contribute to the long-term regulation of energy balance and body adiposity through actions in the central nervous system (8). Furthermore, the fructose-induced impairment in postprandial suppression of ghrelin, a hormone that increases hunger and food intake and has been proposed to be involved in meal initiation in humans may also contribute to increased food intake (9,10).
The second characteristic of fructose that has important metabolic implications is the site and mechanism of fructose metabolism. Fructose metabolism occurs primarily in the liver in which it is phosphorylated to fructose-1-phosphate and metabolized to produce the triose phosphates, glyceraldehyde, and dihydroxyacetone and via conversion of pyruvate to acetyl-CoA by the mitochondria, which exports citrate to the cytosol for fatty acid synthesis (11). In contrast to glucose metabolism, which is controlled by the rate-limiting enzyme phosphofructokinase and subject to negative feedback control by elevated cytosolic levels of ATP and citrate, fructose metabolism is relatively unregulated. Thus, after ingestion of large amounts of fructose, increases of hepatic acetyl-CoA lead to increased production of very low-density lipoprotein and triglycerides (TGs) (12). We previously reported that circulating TG concentrations were substantially elevated over a 24-h period in normal-weight, healthy women after consumption of fructose-sweetened beverages compared with isocaloric glucose-sweetened beverages, ingested with mixed nutrient meals (5). Furthermore, other studies demonstrated that fructose promotes lipogenesis (12,13). A direct relationship between fructose ingestion and elevated postprandial triglyceride levels has important clinical implications because elevated postprandial TG concentrations are known predictors of cardiovascular disease (CVD) (14). If obese individuals already at risk for CVD, exhibit a similarly increased postprandial TG response to fructose consumption as in normal weight subjects, then overconsumption of dietary fructose could exacerbate their predisposition to development of metabolic syndrome and CVD. In the present study, using a within-subject crossover design, we compared the endocrine and metabolic consequences of consuming fructose-sweetened beverages to glucose-sweetened beverages consumed with mixed-nutrient meals on circulating concentrations of glucose, lactate, insulin, leptin, ghrelin, uric acid, free fatty acids, and TGs over a 24-h period in obese men and women. We hypothesized that compared with glucose, ingestion of fructose would elicit greater increases of postprandial TG concentrations and that this effect would be exacerbated in insulin-resistant subjects.
Subjects and Methods
Subjects
A total of 17 obese subjects participated in the study. This included eight obese women with a mean age of 27 ± 2 yr (range 18–36) and mean body mass index of 34.7 ± 1.0 kg/m2 (range 31–38) and nine obese men with a mean age of 39 ± 3 yr (range 25–49) and body mass index of 34.5 ± 1.0 kg/m2 (range 30–38). Subjects were recruited through advertisements in newspapers and on the Internet. Prospective subjects were screened during an initial telephone interview and then scheduled for an appointment at the Monell Chemical Senses Center, at which they gave informed consent to participate in the study and were weighed and percent body fat determined using bioelectrical impedance analysis (RJL Systems, Mt. Clemens, MI). After the initial interview and consenting, subjects were given a physical examination at the Clinical and Translational Research Center of the University of Pennsylvania (CTRC), including an electrocardiogram and a medical history to ensure the absence of chronic illnesses. The women were given a pregnancy test and excluded from the study if they were pregnant. During the physical examination, a blood sample was drawn and a standardized oral glucose tolerance test was administered. Only subjects with fasting triglyceride concentrations less than 200 mg/dl, fasting glucose less than 90 mg/dl, hemoglobin 12 or greater, and blood pressure less than 150/99 mm Hg and with no history of chronic illness including diabetes were enrolled in the study. The only medication permitted for participation in the study was hormonal birth control. The study was approved by the Committees on Studies Involving Human Subjects at the University of Pennsylvania (Philadelphia, PA) and the Institutional Review Board at the University of California, Davis (Davis, CA). One male subject dropped out of the study without completing both trials and his data were not included in the results.
Experimental protocol
Each subject was studied under two separate experimental conditions, administered in a random order. Each experimental condition involved a 2-night stay at the CTRC and was spaced 1 month apart. During each inpatient stay, blood was sampled over a 24-h period during which the diet was controlled. The experimental conditions were identical except for the sweetener used in the beverages. Subjects consumed identical mixed-nutrient meals (described below) and either a glucose-sweetened beverage (HGl) or a fructose-sweetened beverage (HFr). Female subjects were studied during the first 7 d of their menstrual cycle.
The participants were instructed to maintain their usual dietary and exercise habits during the month between the study sessions. During each experimental session, subjects arrived at the CTRC of the Hospital of the University of Pennsylvania at 1700 h, during which time another pregnancy test was administered to all of the female subjects. After a standardized dinner at 1800 h, the subjects remained fasted until the following morning. At 0730 h the next morning, an iv catheter was inserted in the antecubital vein and kept patent with a slow saline infusion. Blood sampling then commenced at 0800 h and continued for 24 h, until the following day (d2) at 0800 h. Blood samples for hormone and metabolic substrate measurements were collected before and while the subjects consumed three standardized meals, each accompanied by either the HGl or HFr beverages.
Diets
Each subject ingested three meals per day. The energy content of the meals was based on an estimate of that individual’s daily energy requirements estimated by the Mifflin equation with an activity factor of 1.2. Caloric distribution over the day was 25% of kilocalories for breakfast, 35% for lunch and 40% for dinner. The meals provided 30% energy from fat, 15% from protein, and 55% from carbohydrate. Of the 55% of total energy intake provided by carbohydrate, 25% was derived from complex carbohydrate and 30% for either glucose or fructose in the form of the beverages. Beverages were prepared as 15% solutions and flavored with a popular unsweetened drink mix. As an example, for an individual requiring 1800 kcal/d, the volume of each beverage at each meal would be 300 ml. Diets and beverages were identical with those used in our previous study (5).
Blood sampling
Blood sampling began at 0800 h of d 1 of the study and continued for 24 h until 0800 h of d 2 of the study. Three baseline samples, collected at 0800, 0830, and 0900 h, were drawn, and the remaining samples were taken every hour, except after meals and during the anticipated nocturnal peak of circulating leptin concentrations (which typically occurs 5–9 h after the evening meal). For the 2 h after each meal (0900–1100, 1300–1500, and 1800–2000 h) and the 4 h around the nocturnal leptin peak (2300–0300 h), blood samples were collected every half-hour, for a total of 36 blood samples collected over the 24-h period. Each sample consisted of the withdrawal of 1 ml of blood to clear the catheter tubing, and 5 ml of blood collected into Vacutainer tubes (Becton, Dickinson and Co., Franklin Lakes, NJ) containing EDTA. To inhibit proteolysis, each blood sample was then mixed with an inhibitor of dipeptidyl peptidase IV (Linco Research, Inc., St. Charles, MO), and protease inhibitor cocktails containing Trasylol and leupeptin (10 μl/ml of whole blood of the protease inhibitor cocktails and dipeptidyl peptidase IV inhibitor) were added as recommended by the distributor. The samples were kept on ice for no more than 1 h and were then centrifuged, aliquoted, and stored at −70 C until assayed.
Assays and data analysis
Plasma glucose and lactate were measured in duplicate using a YSI 2300 StatPlus glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured in duplicate by double antibody RIA with human specific antibodies (Linco Research). The insulin assays were performed by the Diabetes Research Center of the University of Pennsylvania. Plasma leptin was measured with a RIA for human leptin with reagents (Linco Research). Plasma TG was measured in triplicate with reagents from Sigma (St. Louis, MO), and free fatty acid was measured with reagents from Wako (Richmond, VA). Total plasma ghrelin was measured without extraction with an RIA (Phoenix Pharmaceuticals, Inc., Burlingame, CA). Uric acid was measured with reagents from Thermo Scientific (Pittsburgh, PA).
A plasma fructose assay was developed to measure plasma fructose concentrations through a modification of a commercial glucose/fructose analysis kit (Megazyme, catalog no. K-FRUGL; Xygen Diagnostics Inc., Burgessville, Ontario, Canada). Details of the methodology are reported in the study by Adams et al. (15).
The area under the curve (AUC) was calculated for all variables using the Origin Software (version 7; Northhampton, MA). For glucose, lactate, leptin, TGs, and uric acid, the mean of the three baseline values was determined, and net AUC was calculated by subtracting the areas below baseline from AUC values above baseline. AUCs for glucose, lactate, leptin, TGs, and uric acid are expressed as units per 23 h above each subject’s fasting baseline levels because the first hour of sampling determined the baseline levels. For free fatty acids and ghrelin, because these two variables drop below baseline, the AUC below baseline is included to avoid negative numbers. The nadirs and peaks for plasma leptin were determined as the mean of two lowest consecutive morning concentrations before 1200 h and the mean of the two highest consecutive nighttime concentrations after 2000 h, respectively. The AUCs for leptin are therefore expressed as units above each subject’s nadir over 24 h. Differences between AUCs for the two diets were determined with paired t tests.
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from the means of the glucose and insulin concentrations in three baseline samples using the original model (16) [fasting plasma insulin (microunits per milliliter) × fasting plasma glucose (millimoles/22.5)].
To determine the effects of insulin resistance and interactions between insulin resistance and the experimental conditions, treatment × time × insulin resistance interactions were evaluated using repeated-measures ANOVA. Post hoc analysis was conducted using Tukey’s test. Sex effects were also evaluated using sex as a factor in place of insulin resistance. Statistical significance was considered at P < 0.05.
Results
Plasma glucose, fructose, lactate, and free fatty acids
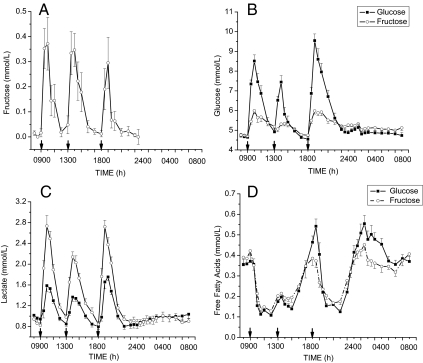
Fructose was undetectable in plasma samples collected on the day of HGl consumption. However, on the day that HFr beverages were consumed, plasma fructose concentration increased by approximately 0.5 mm after consumption of the fructose beverages with meals (Fig. 1A). Baseline fasting glucose concentrations were not significantly different between the HGl and HFr days averaging (4.6 ± 0.1, 4.7 ± 0.1 mm). After ingestion of fructose beverages with the meals, plasma glucose concentrations increased significantly by approximately 1 mm but were significantly lower when compared with after glucose-sweetened beverages were consumed (Fig. 1B). The AUC for glucose was reduced by approximately 50% on the HFr compared with the HGl day (Table 1). Plasma lactate concentrations increased much more after ingestion of the HFr beverages (Fig. 1C) compared with the HGl beverages, with the lactate AUC being more than 5 times higher than when glucose-sweetened beverage were consumed (Table 1). No significant differences were observed between plasma free fatty acid responses (Fig. 1D), although a trend toward a lower AUC for free fatty acids was observed after Hfr (Table 2), as we have previously reported in normal-weight women (5).
Figure 1.
Effect of glucose-sweetened beverages (solid lines) and fructose-sweetened beverages (dashed lines) on plasma concentrations of fructose (A), glucose (B), lactate (C), and free fatty acid levels (D) in obese men and women consuming beverages with meals (mean ± sem, n = 17). Fructose was undetectable on the day glucose-sweetened beverages were consumed. Plasma glucose levels were significantly lower (F = 9.0, P < 0.00001, time × treatment), and plasma lactate levels were elevated (F = 13.7 P < 0.00001, time × treatment) after fructose compared with glucose consumption. No significant differences were observed in plasma free fatty acid concentrations between treatments.
Table 1.
AUC calculated above baseline (0800–0900 h) for the 23 h after the three morning baseline samples (glucose, lactate, insulin, TG, uric acid) or including baseline (free fatty acids and ghrelin)
| High glucose | High fructose | P | |
|---|---|---|---|
| Glucose (mg/dl per 23 h) | 430.6 ± 32.0 | 221.4 ± 25.3 | 0.0001a |
| Lactate (mmol/liter per 23 h) | 112.3 ± 64.2 | 609.0 ± 67.1 | 0.0001a |
| Insulin (uU/ml per 23 h) | 1052.6 ± 135.1 | 549.2 ± 79.7 | 0.0001a |
| Leptin (ng/ml per 24 h) | 151.9 ± 22.7 | 107.0 ± 15.0 | 0.03a |
| TG (mg/dl per 23 h) | 242.2 ± 96.8 | 704.3 ± 124.4 | 0.0001a |
| Free fatty acids (mmol/liter per 23 h) | 411.3 ± 73.1 | 383.5 ± 64.9 | 0.21 |
| Ghrelin (pg/ml per 23 h) | 10532.3 ± 663.1 | 10405.4 ± 597.2 | 0.53 |
| Uric acid (mg/dl per 23 h) | −0.10 ± 1.9 | 1.1 ± 1.3 | 0.55 |
Leptin was calculated as the 24-h AUC above morning trough concentrations. Mean ± sem (n = 17), paired t test.
P values are statistically significant.
Table 2.
Effect of sex on metabolic responses to glucose- and fructose-sweetened beverages consumed with a meal
| Male
|
Female
|
PS | PT×S | |||
|---|---|---|---|---|---|---|
| Glucose | Fructose | Glucose | Fructose | |||
| Glucose (mg/dl per 23 h) | 429.1 ± 45.7 | 228.4 ± 35.9 | 432.1 ± 46.5 | 213.5 ± 38.1 | 0.90 | 0.80 |
| Lactate (mmol/liter per 23 h) | 165.8 ± 88.9 | 542.9 ± 96.4 | 52.0 ± 94.3 | 579.4 ± 102.3 | 0.60 | 0.35 |
| Insulin (μU/ml per 23 h) | 1156.9 ± 187.6 | 731.6 ± 94.8 | 935.3 ± 199.1 | 343.8 ± 100.6 | 0.15 | 0.39 |
| Leptin (ng/ml per 24 h) | 109.1 ± 27.9 | 80.0 ± 19.2 | 200.0 ± 29.6 | 139.0 ± 20.4 | 0.02a | 0.43 |
| TG (mg/d per 23 h) | 408.5 ± 122.3 | 774.9 ± 145.0 | 55.0 ± 129.7 | 473.2 ± 153.8 | 0.09 | 0.74 |
| Free fatty acids (mmol/liter per 23 h) | 403.1 ± 24.9 | 425.2 ± 15.0 | 420.6 ± 26.5 | 336.6 ± 16.1 | 0.17 | 0.08 |
| Ghrelin (pg/ml per 23 h) | 9735.6 ± 892.1 | 9594.5 ± 790.8 | 11428.5 ± 946.3 | 11317.6 ± 838.8 | 0.18 | 0.94 |
| Uric acid (mg/dl per 23 h) | −4.8 ± 2.1 | 0.65 ± 1.9 | 5.1 ± 2.2 | 1.6 ± 1.9 | 0.04a | 0.02a |
AUC calculated above baseline (0800–0900 h) for the 23 h after the three morning baseline samples (glucose, lactate, insulin, TG, uric acid) or including baseline (free fatty acids and ghrelin). Leptin AUC was calculated as the 24-h AUC above morning trough concentrations. Mean se (male, n = 9; female, n = 8). PS, P value for effect of sex; PT×S, P value for treatment × sex interaction, statistical significance if P < 0.05.
P values are statistically significant.
Plasma insulin, leptin, and ghrelin
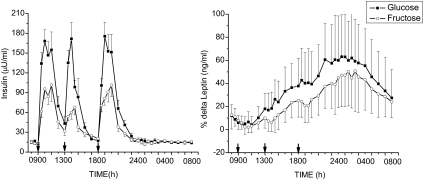
Baseline fasting insulin levels were not significantly different on the HFr and HGl days averaging 14.8 ± 7.9 and 13.9 ± 8.6 μU/ml, respectively. Peak plasma insulin concentrations were significantly reduced after consumption of the HFr beverages with meals compared with levels after ingestion of the HGl beverages (Fig. 2A). The AUC for insulin was reduced by approximately 50%, reflecting a similar reduction as observed for glucose concentrations (Table 1). Fructose ingestion also reduced the diurnal circulating leptin profile compared with glucose (Fig. 2B). The AUC for leptin was reduced by approximately 30% on the day when HFr beverages compared with HGl beverages were consumed (Table 1), consistent with what we have previously reported in normal-weight women (5). Whereas leptin levels were significantly higher in women, no sex-by-treatment interaction was observed (Table 2). In contrast with what we have reported in normal-weight subjects (5), 24-h ghrelin AUCs were not different on the HGl and HFr days, averaging 10532.3 ± 663.1 and 10405.4 ± 597.2 pg/ml, respectively.
Figure 2.
Effect of glucose-sweetened beverages (solid lines) andfructose-sweetened beverages (dashed lines) on plasma concentrations of insulin (left panel) and the percent change of plasma leptin from baseline (right panel) in obese human subjects (mean ± sem, n = 17). Plasma insulin (F = 2.15, P < 0.003, time × treatment) and leptin levels (F = 2.55, P < 0.00001, time × treatment) were significantly lower after fructose compared with glucose consumption. Baseline leptin values were 20.7 ± 10.69 (HGl) and 20.3 ± 11.0 (HFr).
Plasma uric acid and TG
Baseline fasting uric acid concentrations were 4.9 ± 0.2 mg/dl in women and were slightly higher in men averaging 5.5 ± 0.2 mg/dl. Although there was a tendency for higher postmeal peaks of uric acid of approximately 0.5 mg/dl after HFr compared with HGl, there was no significant difference in the AUC for uric acid concentrations between the HGl and HFr days (Table 1). Men and women exhibited differential responses to the dietary treatments because AUC for uric acid was higher after fructose ingestion in men but lower in women compared with after glucose ingestion (Table 2).
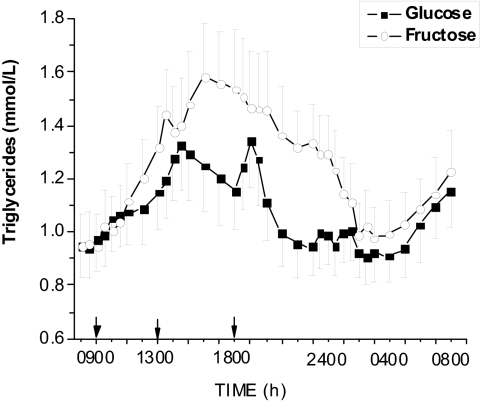
Baseline fasting TG concentrations were not different on the HGl (84.5 ± 10.8 mg/dl) and HFr study days (84.0 ± 10.5 mg/dl). Plasma TG concentrations were significantly elevated after ingestion of fructose-sweetened beverages with meals (Fig. 3). The AUC for TGs was almost 200% higher on the HFr compared with the HGl days in men and women together (Table 1). Fasting TG concentrations were higher in men (94 ± 15 mg/dl) compared with women (69 ± 10) as were the postprandial AUCs for the 24 h after fructose ingestion (Table 2), but the differences did not reach statistical significance (P < 0.09).
Figure 3.
Effect of glucose-sweetened beverages (solid lines) and fructose-sweetened beverages (dashed lines) on plasma TG concentrations in obese subjects (mean ± sem, n = 17). Plasma TG levels were significantly elevated after fructose compared with glucose consumption. (F = 4.99 P < 0.0001, treatment × time interaction).
Effect of insulin sensitivity/resistance on metabolic and endocrine responses
To determine whether the endocrine metabolic responses to glucose and fructose ingestion might differ as a function of insulin resistance, subjects were classified as insulin sensitive if HOMA-IR was less than 2.5 and insulin resistant if HOMA-IR was greater than 2.5. Using these criteria, 10 subjects were categorized as insulin sensitive (four men, six women) with mean HOMA-IR of 1.93 ± 0.29 on the HGl day and 2.05 ± 0.47 on the HFr day. Seven subjects were classified as insulin resistant (five men, two women) with a mean HOMA-IR of 4.97 ± 0.38 on the HGl day and 4.49 ± 0.61 on the HFr day (P < 0.02 vs. insulin sensitive subjects). Baseline fasting TGs tended to be higher in the insulin-resistant compared with the insulin-sensitive subjects (107.0 ± 48.7 vs. 68.8 ± 37.5 mg/dl insulin resistant vs. insulin sensitive, HGl day, P < 0.09). Table 3 presents the AUCs for the measured parameters in subjects classified as insulin sensitive vs. insulin resistant. As would be predicted, a significant effect of insulin resistance on insulin AUCs was observed. There were significant interactions between the effects of treatment and insulin resistance for both insulin and leptin (Table 3). In both cases, the effect of fructose to reduce insulin and leptin profiles relative to glucose was greater in the presence of insulin resistance.
Table 3.
Effect of insulin resistance on metabolic responses to glucose- and fructose-sweetened beverages consumed with a meal
| Insulin sensitive
|
Insulin resistant
|
PR | PT×R | |||
|---|---|---|---|---|---|---|
| Glucose | Fructose | Glucose | Fructose | |||
| Glucose (mg/dl per 23 h) | 425.2 ± 43.3 | 225.6 ± 34.1 | 438.1 ± 51.7 | 215.3 ± 40.7 | 0.97 | 0.75 |
| Lactate (mmol/liter per 23 h) | 124.1 ± 86.4 | 635.4 ± 90.4 | 95.4 ± 103.3 | 573.4 ± 108.0 | 0.69 | 0.83 |
| Insulin (μU/ml per 23 h) | 706.9 ± 117.2 | 412.9 ± 90.6 | 1546.4 ± 140.0 | 760.4 ± 108.3 | 0.01a | 0.01a |
| Leptin (ng/ml per 24 h) | 131.8 ± 29.5 | 119.3 ± 20.2 | 180.6 ± 35.3 | 91.2 ± 24.1 | 0.77 | 0.03a |
| TG (mg/dl per 23 h) | 73.1 ± 111.2 | 460.2 ± 129.1 | 483.6 ± 132.9 | 879.7 ± 154.3 | 0.03a | 0.95 |
| Free fatty acids (mmol/liter per 23 h) | 415 ± 23.8 | 369.5 ± 20.4 | 405.6 ± 28.7 | 403.4 ± 24.4 | 0.65 | 0.33 |
| Ghrelin (pg/ml per 23 h) | 10,322.2 ± 888.8 | 10,366.9 ± 865.3 | 10,832.4 ± 1062.4 | 10,460.2 ± 960.9 | 0.81 | 0.51 |
| Uric acid (mg/dl per 23 h) | −0.19 ± 2.6 | 0.71 ± 1.7 | 0.02 ± 3.1 | 1.6 ± 2.1 | 0.84 | 0.86 |
AUC calculated above baseline (0800–0900 h) for the 23 h after the three morning baseline samples (glucose, lactate, insulin, TG, uric acid) or including baseline (free fatty acids and ghrelin). Leptin was calculated as the 24-h AUC above morning trough concentrations. Mean ± sem (insulin sensitive: HOMA < 2.5, n = 10; insulin resistant: HOMA > 2.5, n = 7). PR, P value for effect of resistance; PT×R, P value for treatment × insulin resistance interaction.
P values are statistically significant.
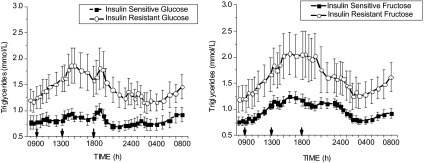
Subjects with insulin resistance had significantly increased TG responses after consumption of both glucose- and fructose-sweetened beverages (Fig. 4). No significant interaction between insulin resistance and treatment was observed, suggesting that the effect of fructose compared with glucose to increase postprandial triglyceride profiles is consistent across insulin-sensitive and insulin-resistant individuals (Table 3).
Figure 4.
TG responses to consumption of glucose-sweetened (left panel) and fructose-sweetened beverages (right panel) with meals in insulin-sensitive (n = 10, dashed line, round symbol) and insulin-resistant obese subjects (n = 7, solid line, square symbol). Plasma TG concentrations were significantly elevated after fructose consumption in both insulin-sensitive and insulin-resistant subjects (F = 5.1, P < 0.0001, treatment × time).
Discussion
Glucose, insulin, and leptin profiles
The overall objective of this study was to compare 24-h endocrine and metabolic profiles after the consumption of either fructose-sweetened or glucose-sweetened beverages in conjunction with isocaloric meals in obese individuals. Consumption of fructose-sweetened beverages with meals results in smaller postmeal increases of plasma glucose and reduced meal-induced insulin responses. This decrease of insulin secretion has further metabolic consequences because insulin-mediated glucose metabolism leading to specificity protein-1-mediated transcription in adipocytes is an important mechanism in the regulation of leptin production (17). After fructose ingestion, the lower glucose and insulin excursion result in decreased leptin production and a reduction in the diurnal circulating leptin profile compared with consumption of the same amount of glucose. The decreased insulin responses also underlie the dramatic increases of circulating lactate. Reduced insulin action leads to less pyruvate entering the mitochondria for oxidation and a corresponding increase of anaerobic metabolism to lactate, independent of increasing leptin production (18). Increases in lactate are typically associated with increased lipogenesis (19,20), which has been demonstrated after fructose administration in humans.
The amplitude of the diurnal leptin pattern has been implicated in the effects of substituting dietary carbohydrate for fat to induce weight loss under ad libitum conditions (21). In the present study, we found that the reduction of the diurnal leptin profile after fructose ingestion was of comparable magnitude with that reported in our previous studies conducted in normal-weight subjects comparing the effects of high fructose and glucose diets (5) as well as high-carbohydrate and high-fat diets (22). Taken together, these studies indicate that 24-h circulating leptin concentrations can be reduced by approximately 30–40% through dietary manipulations. Whereas the nadir of leptin remains similar and leptin still peaks during the late evening hours, the rate of increase and the amplitude of the peak are blunted after consumption of the meal high in fructose. Both high-fat and high-fructose diets reduce insulin secretion and leptin production as well as increase triglyceride levels relative to glucose-containing carbohydrate. Because insulin and leptin are two critical endocrine signals involved in the medium to long-term regulation of body adiposity, it has been hypothesized that diets high in fat and/or fructose may lead to increased energy intake and weight gain due to reductions of insulin secretion and leptin production (23).
Ghrelin
In support of the hypothesis that high-fructose diets elicit a metabolic/endocrine profile that would promote increased food intake, in our previous study, we reported an attenuated postprandial suppression of the orexigenic hormone ghrelin after high-fructose meals compared with high-glucose meals in normal-weight women (5). The decreased suppression of ghrelin after fructose consumption was most likely also a consequence of the smaller glucose and insulin excursions because insulin-mediated glucose metabolism has been implicated in the postprandial suppression of ghrelin and the differential effects of dietary macronutrients on this suppression (24,25). In the present study conducted in obese subjects, there were no significant differences in 24-h circulating ghrelin concentrations when comparing fructose- with glucose-sweetened beverage consumption. The effects of meal ingestion to suppress plasma ghrelin levels has been reported to be blunted in obese subjects (26,27), and it is possible that this is why differences between the effects of glucose and fructose were not observed (28,29) as occurs in normal-weight subjects. In normal-weight subjects, plasma ghrelin was reduced by approximately 35% after each high-glucose meal, but in the current study, the postmeal suppression of ghrelin averaged only around 15% in obese subjects. Although some investigators have reported blunted ghrelin levels in obese insulin-resistant subjects but not obese insulin sensitive subjects (30), 24-h ghrelin profiles did not differ between insulin-sensitive and -resistant subjects in the current study.
TGs
Circulating TG concentrations were significantly increased after fructose compared with glucose consumption. Increased circulating TGs after ingestion of diets high in fructose has been reported previously in animal studies as well as normal-weight humans (23) and reflects the absence of regulatory controls involved in the uptake and metabolism of fructose by the liver. The rapid clearance and metabolism of fructose to lactate as demonstrated by relatively low levels of circulating fructose and marked increases of lactate after fructose iv administration has also been recently reported in rhesus monkeys (15). The hepatic metabolism of fructose results in increased availability of triose phosphate for glycerol and acetyl Co-A of precursors for fatty acid and TG production and export to the circulation (23). Increases in postprandial fructose may also result from increased reesterification of TGs derived from the diet (31).
Postprandial TGs were increased by fructose compared with glucose in both insulin-sensitive and insulin-resistant subjects. Subjects with insulin resistance had significantly increased TG responses, consistent with the larger postprandial TG responses reported in insulin-resistant subjects and those with elevated fasting TGs. HOMA-IR is derived from fasting levels of glucose and insulin concentrations, and elevated HOMA-IR values are considered to reflect hepatic insulin resistance. Thus, subjects with hepatic insulin resistance, and associated increases in basal lipogenesis, have exacerbated hypertriglyceridemic responses to acute fructose and glucose ingestion. Because elevated postprandial TGs are associated with increased CVD (14,32), these results suggest that obese individuals, particularly those with insulin resistance or elevated TG levels, should avoid overconsumption of foods and beverages containing fructose.
Dietary fructose has also been shown to increase circulating concentrations of uric acid, a purine metabolite independently associated with the metabolic syndrome (33,34,35). The hypothesized mechanism mediating the increase in uric acid after fructose ingestion is thought to be due to an increase of intracellular ADP levels resulting from the phosphorylation of fructose-1-phosphate by fructokinase. In the present study, we did not find significant differences in the 24 h uric acid profiles (AUCs) between the days when fructose and glucose-sweetened beverages were consumed (Table 1), although there was a trend for small postmeal increases of around 0.5 mg/dl with fructose but not glucose. However, there were statistically significant sex differences and sex-by-treatment interactions (P < 0.01). The AUC for uric acid in men after glucose was −4.8 ± 2.1 mg/dl (23 h) compared with 0.65 ± 1.9 mg/dl (23 h) after fructose. In women, AUC after glucose was 5.1 ± 2.2 mg/dl (23 h in women, P < 0.04) and 1.56 ± 1.9 (after the HFr condition). Sex differences in the effects of added sugars or sugar-sweetened beverages on uric acid concentrations have been reported with significant effects observed in men but not women (36). Other studies have also reported relationships between uric acid and TG levels. For example, inhibiting increases in uric acid by administration of allopurinol also inhibits the increase in TG levels in rats on a high-fructose diet; however, this effect has not been observed in some human studies (37,38). Whereas the mechanism of the relationship among fructose, TGs, and uric acid has not been established, some investigators have hypothesized that uric acid elevates TGs by inhibiting TG clearance. In the present study, uric acid and TG levels were not correlated during either the high-glucose or high-fructose study days. It is possible that elevations in uric acid levels require prolonged and sustained increases in plasma fructose, which did not occur with this study design.
Very few studies have reported the plasma fructose concentrations after ingestion of dietary fructose, and measurements of fructose can facilitate understanding of the metabolic fate of fructose in the postprandial state. In this study, increases of plasma fructose when a large fructose sweetened beverage was consumed in the context of a mixed meal averaged 0.15–0.3 mm and were only approximately 5% of the increase of circulating glucose levels after ingestion of the same amount of glucose. Within 3 h after consumption of the high-fructose beverages, fructose concentrations were below the detection limit of the assay. As expected, fructose was undetectable in the circulation on the day that glucose-sweetened beverages were consumed. We recently reported low levels of fructose during in a study comparing the effects of iv fructose and glucose infusions in rhesus monkeys (15), again reflecting the rapid metabolism of fructose by the liver, even when fructose is administered systemically rather than entering via the portal circulation.
It has been argued that the results from studies examining the effect of fructose alone may not be applicable to the effects of fructose in a normal diet, which typically contains fructose in the form of sucrose, a disaccharide composed of 50% glucose and 50% fructose and high-fructose corn syrup composed of 55% glucose and 45% fructose. However, recently published data from our laboratory suggest that even when the amount of fructose is diluted by the presence of glucose such as occurs in the form of sucrose or high-fructose corn syrup, 24-h TG profiles are elevated to the same extent as with fructose alone (39). Thus, fructose, in the presence of glucose, appears to increase hepatic TG production and postprandial TG concentrations. Additional short-term and long-term dose-response studies in both metabolically normal and at-risk subjects will be required to determine the amounts of dietary fructose that have adverse effects on lipid metabolism in different populations.
In summary, we compared the effect of the two monosaccharides, glucose and fructose, consumed in the form of sweetened beverages with isocaloric mixed nutrient meals on circulating hormones and lipids in obese men and women. In comparison with glucose, consumption of fructose-sweetened beverages results in decreased insulin secretion, a reduced diurnal leptin profile, and increased postprandial TG concentrations in obese individuals, independent of insulin sensitivity. In addition, the effect of fructose to increase TGs was augmented in obese subjects with insulin resistance. Epidemiological studies support an association between fructose intake and increased risk of type 2 diabetes mellitus (40). Together with the present results, these data suggest that overconsumption of dietary fructose may exacerbate the adverse metabolic profiles in obese individuals, particularly those with existing insulin resistance and may therefore increase the risks for developing diabetes and CVD.
Footnotes
This work was supported by Grant DK-58003 (to K.L.T.), Grants DK-19525 and M01-RR00042 from the University of Pennsylvania; the National Institutes of Health Grants DK-58108, AT-002599, AT-003645, HL-075675, and HL-091333; and the American Diabetes Association (to P.J.H.); and intramural U.S. Department of Agriculture- Agricultural Research Service Current Research Information System Grant 5306-51530-016-00D (to S.H.A.).
First Published Online February 10, 2009
Abbreviations: AUC, Area under the curve; CTRC, Clinical and Translational Research Center of the University of Pennsylvania; CVD, cardiovascular disease; HFr, fructose-sweetened beverage; HGl, glucose-sweetened beverage; HOMA-IR, homeostasis model assessment of insulin resistance; TG, triglyceride.
References
- Putnam JJ, Allshouse JE 1999 Food consumption, prices and expenditures, 1970–1997. Economic Research Service. Washington, DC: U.S. Department of Agriculture [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM 2004 Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543 [DOI] [PubMed] [Google Scholar]
- Basciano H, Federico L, Adeli K 2005 Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D 1989 Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas 4:2–9 [DOI] [PubMed] [Google Scholar]
- Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D'Alessio D, Havel PJ 2004 Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 89:2963–2972 [DOI] [PubMed] [Google Scholar]
- Anderson G, Teff K, Young S 1987 Serotonin in cisternal cerebrospinal fluid of the rat: measurement and use as an index of functionally active serotonin. Life Sci 40:2253–2260 [DOI] [PubMed] [Google Scholar]
- Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Stern JS, Havel PJ 1998 Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology 139:551–558 [DOI] [PubMed] [Google Scholar]
- Porte DJ, Baskin D, Schwartz MW 2002 Leptin and insulin action in the central nervous system. Nutr Rev 60:S20–S29 [DOI] [PubMed] [Google Scholar]
- Williams DL, Cummings DE 2005 Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr 135:1320–1325 [DOI] [PubMed] [Google Scholar]
- Cummings D, Purnell J, Frayo R, Schmidova K, Wisse B, Weigle D 2001 A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- Mayes PA 1993 Intermediary metabolism of fructose. Am J Clin Nutr 58:754S–765S [DOI] [PubMed] [Google Scholar]
- Park OJ, Cesar D, Faix D, Wu K, Shackleton CH, Hellerstein MK 1992 Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem J 282(Pt 3):753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks EJ, Skokan LE, Timlin MT, Dingfelder CS 2008 Dietary sugars stimulate fatty acid synthesis in adults. J Nutr 138:1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A 2007 Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298:299–308 [DOI] [PubMed] [Google Scholar]
- Adams SH, Stanhope KL, Grant RW, Cummings BP, Havel PJ 2008 Metabolic and endocrine profiles in response to systemic infusion of fructose and glucose in rhesus macaques. Endocrinology 149:3002–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ 2008 Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294:E15–E26 [DOI] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Swarbrick MM, Lorente-Cebrian S, Stanhope KL, Havel PJ, Martinez JA 2007 Sp1-mediated transcription is involved in the induction of leptin by insulin-stimulated glucose metabolism. J Mol Endocrinol 38:537–546 [DOI] [PubMed] [Google Scholar]
- Mueller WM, Stanhope KL, Gregoire F, Evans JL, Havel PJ 2000 Effects of metformin and vanadium on leptin secretion from cultured rat adipocytes. Obes Res 8:530–539 [DOI] [PubMed] [Google Scholar]
- Carmona A, Freedland RA 1989 Comparison among the lipogenic potential of various substrates in rat hepatocytes: the differential effects of fructose-containing diets on hepatic lipogenesis. J Nutr 119:1304–1310 [DOI] [PubMed] [Google Scholar]
- Walli RA 1978 Interrelation of aerobic glycolysis and lipogenesis in isolated perfused liver of well-fed rats. Biochim Biophys Acta 539:62–80 [DOI] [PubMed] [Google Scholar]
- Weigle DS, Cummings DE, Newby PD, Breen PAFRS, Matthys CC, Callahan HS, Purnell JQ 2003 Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab 88:1577–1586 [DOI] [PubMed] [Google Scholar]
- Havel PJ, Townsend RCL, Teff K 1999 High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes 48:334–341 [DOI] [PubMed] [Google Scholar]
- Havel PJ 2005 Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 63:133–157 [DOI] [PubMed] [Google Scholar]
- Griffen SC, Oostema K, Stanhope KL, Graham J, Styne DM, Glaser N, Cummings DE, Connors MH, Havel PJ 2006 Administration of Lispro insulin with meals improves glycemic control, increases circulating leptin, and suppresses ghrelin, compared with regular/NPH insulin in female patients with type 1 diabetes. J Clin Endocrinol Metab 91:485–491 [DOI] [PubMed] [Google Scholar]
- Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE 2008 Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab 93:1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML 2001 Circulating ghrelin levels are decreased in human obesity. Diabetes 50:707–709 [DOI] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S 2002 Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 87:240–244 [DOI] [PubMed] [Google Scholar]
- Abraha A, Humphreys SM, Clark ML, Matthews DR, Frayn KN 1998 Acute effect of fructose on postprandial lipaemia in diabetic and non-diabetic subjects. Br J Nutr 80:169–175 [PubMed] [Google Scholar]
- Jeppesen J, Chen YI, Zhou MY, Schaaf P, Coulston A, Reaven GM 1995 Postprandial triglyceride and retinyl ester responses to oral fat: effects of fructose. Am J Clin Nutr 61:787–791 [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE 2004 Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab 89:1630–1635 [DOI] [PubMed] [Google Scholar]
- Chong MF, Fielding BA, Frayn KN 2007 Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 85:1511–1520 [DOI] [PubMed] [Google Scholar]
- Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM 2007 Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298:309–316 [DOI] [PubMed] [Google Scholar]
- Miller A, Adeli K 2008 Dietary fructose and the metabolic syndrome. Curr Opin Gastroenterol 24:204–209 [DOI] [PubMed] [Google Scholar]
- Heinig M, Johnson RJ 2006 Role of uric acid in hypertension, renal disease, and metabolic syndrome. Cleve Clin J Med 73:1059–1064 [DOI] [PubMed] [Google Scholar]
- Hallfrisch J, Ellwood K, Michaelis OE, Reiser S, Prather ES 1986 Plasma fructose, uric acid, and inorganic phosphorus responses of hyperinsulinemic men fed fructose. J Am Coll Nutr 5:61–68 [DOI] [PubMed] [Google Scholar]
- Gao X, Qi L, Qiao N, Choi HK, Curhan G, Tucker KL, Ascherio A 2007 Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in U.S. men and women. Hypertension 50:306–312 [DOI] [PubMed] [Google Scholar]
- Jitapunkul S, Chalaprawat M, Bunnag S, Bhuvapanich S, Kangkaya V, Pasatrat S, Vajanamarhutue C 1991 The relationship between glucose and uric acid metabolism: influence of short term allopurinol on glucose metabolism. J Med Assoc Thai 74:80–86 [PubMed] [Google Scholar]
- Gibson T, Kilbourn K, Horner I, Simmonds HA 1979 Mechanism and treatment of hypertriglyceridaemia in gout. Ann Rheum Dis 38:31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ 2008 Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr 87:1194–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A 2007 Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr 137:1447–1454 [DOI] [PubMed] [Google Scholar]