Abstract
Asthma is an inflammatory disorder caused by airway exposures to allergens and chemical irritants. Studies focusing on immune, smooth muscle, and airway epithelial function revealed many aspects of the disease mechanism of asthma. However, the limited efficacies of immune-directed therapies suggest the involvement of additional mechanisms in asthmatic airway inflammation. TRPA1 is an irritant-sensing ion channel expressed in airway chemosensory nerves. TRPA1-activating stimuli such as cigarette smoke, chlorine, aldehydes, and scents are among the most prevalent triggers of asthma. Endogenous TRPA1 agonists, including reactive oxygen species and lipid peroxidation products, are potent drivers of allergen-induced airway inflammation in asthma. Here, we examined the role of TRPA1 in allergic asthma in the murine ovalbumin model. Strikingly, genetic ablation of TRPA1 inhibited allergen-induced leukocyte infiltration in the airways, reduced cytokine and mucus production, and almost completely abolished airway hyperreactivity to contractile stimuli. This phenotype is recapitulated by treatment of wild-type mice with HC-030031, a TRPA1 antagonist. HC-030031, when administered during airway allergen challenge, inhibited eosinophil infiltration and prevented the development of airway hyperreactivity. Trpa1−/− mice displayed deficiencies in chemically and allergen-induced neuropeptide release in the airways, providing a potential explanation for the impaired inflammatory response. Our data suggest that TRPA1 is a key integrator of interactions between the immune and nervous systems in the airways, driving asthmatic airway inflammation following inhaled allergen challenge. TRPA1 may represent a promising pharmacological target for the treatment of asthma and other allergic inflammatory conditions.
Keywords: airway hyperreactivity, TRP channel, TRPA1
The dramatic increase in the number of asthma cases over the last decades is of great concern for public health in the United States and world-wide (1, 2). The inflammatory response in asthma is orchestrated by CD4 Th2 cells inducing eosinophil infiltration and mast cell activation, followed by tissue remodeling, mucus hypersecretion, and airway hyperresponsiveness (3). While it is clear that immune mechanisms play a significant role in the development and maintenance of asthma, the limited efficacy of immune therapies suggests the involvement of additional mechanisms and physiological systems in the disease process (4).
The airways are densely innervated by peripheral sensory neurons expressing specific receptors activated by noxious chemicals contained in the inhaled air (5). Over the last decades, evidence has mounted for bi-directional feedback between immunogenic and neurogenic mechanisms in airway inflammation (6, 7). Neuronal activation causes pain and irritation, neurogenic inflammation, mucus secretion, and reflex responses, such as cough, sneezing, and bronchoconstriction (8, 9). Members of the transient receptor potential (TRP) superfamily of ion channels play a key role in the response of sensory neurons to inflammatory mediators (10–12). The 2 major pro-inflammatory TRP ion channels in sensory neurons are TRPV1, the capsaicin receptor, and TRPA1, activated by mustard oil (13–16).
Agonists of TRPV1 and TRPA1, such as capsaicin, acrolein, or chlorine, are potent tussive agents and have been associated with allergic and occupational asthma and reactive airway dysfunction syndrome (RADS) (12, 17–23). Potential endogenous TRPA1 agonists include reactive oxygen species, hypochlorite, and lipid peroxidation products (18, 24–26). Similar to TRPV1, TRPA1 is activated or sensitized downstream of inflammatory PLC-coupled receptor pathways and mediates inflammatory pain sensitization (12–14, 27). In animal models, TRPA1 antagonists block chemically induced inflammatory thermal and mechanical hyperalgesia, neuropathic pain, and diminish acute airway responses to chemical exposures (17, 19, 28).
The roles of TRPV1 and TRPA1 in asthmatic airway inflammation remain unknown. Using a murine model of acute asthma, we identify a critical role of TRPA1 in this disease. We show that genetic deletion of TRPA1 or pharmacological channel inhibition diminishes allergen-induced inflammatory leukocyte infiltration, mucus production, cytokine and chemokine levels, and airway hyperreactivity. Trpa1−/− mice also show impaired acute and inflammatory neuropeptide release in the airways. In contrast, all aspects of asthmatic airway inflammation were normal in Trpv1−/− mice. These results suggest that TRPA1 is a major neuronal mediator of allergic airway inflammation and may represent a promising target for suppression of inflammation and airway hyperreactivity in asthma.
Results
Diminished Leukocyte Airway Infiltration and Airway Hyperreactivity in OVA-Challenged Trpa1−/− Mice.
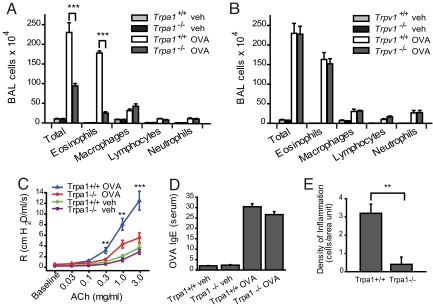
We used the ovalbumin (OVA) mouse model of asthma to induce a Th2-directed allergic response, comparing leukocyte levels in the bronchoalveolar lavage fluid (BALF) of OVA-challenged wild-type, Trpa1−/−, and Trpv1−/− mice (Fig. 1 A and B). Leukocyte numbers were greatly elevated in BALF of OVA-challenged wild-type C57BL/6 mice (Fig. 1A). Eosinophils represented the majority of leukocytes (Fig. 1A). In OVA-challenged TRPA1-deficient mice, we observed a remarkable reduction in BALF leukocyte numbers (>50%), with eosinophilia reduced by >80% (Fig. 1A). In contrast, OVA-challenged Trpv1−/− mice showed robust leukocyte infiltration, with BALF cell counts indistinguishable from those of wild-type mice (Fig. 1B).
Fig. 1.
Decreased inflammatory response to inhaled OVA in TRPA1-deficient mice. (A) Reduced leukocyte infiltration in airways of OVA-challenged Trpa1−/− mice. Cell differentials are shown for total cells, eosinophils, macrophages, lymphocytes, and neutrophils in BALF collected from vehicle (veh, PBS)- and OVA-challenged Trpa1+/+ and Trpa1−/− mice. Animal groups: Trpa1+/+ OVA: n = 8, Trpa1−/− OVA: n = 8, Trpa1+/+ veh: n = 7, Trpa1−/− veh: n = 6. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. (B) Normal inflammatory leukocyte infiltration in OVA-challenged Trpv1−/− mice. BALF leukocyte cell differentials are shown for vehicle (veh, PBS)- and OVA-challenged Trpv1+/+ and Trpv1−/− mice. Animal groups: Trpv1+/+ OVA: n = 6, Trpv1−/− OVA: n = 4, Trpv1+/+ veh: n = 6, Trpv1−/− veh: n = 4. (C) Comparison of airway resistance (R) in OVA-challenged Trpa1+/+ (blue) and Trpa1−/− mice (red), as well as vehicle (veh)-treated Trpa1+/+ (green) and Trpa1−/− (purple) mice, measured by forced oscillation in response to increasing dosages of acetylcholine. Animal groups: Trpa1+/+ OVA: n = 4, Trpa1−/− OVA: n = 4, Trpa1+/+ veh: n = 6, Trpa1−/− veh: n = 6. (∗, α = 0.05; ∗∗, α = 0.01; ∗∗∗, α = 0.001). (D) Induction of OVA-reactive Ig E in OVA-challenged wild-type and TRPA1-deficient mice, as determined by ELISA. Animal groups as in Fig. 1A. (E) Density of inflammation in H&E-stained airway sections from OVA-challenged Trpa1+/+ and Trpa1−/− mice, scored by counting of inflammatory cells near bronchial bundles (n = 4 mice per group).
Airway hyperreactivity (AHR) is another important hallmark of asthma. Airway resistance was measured by forced oscillation in response to i.v. administration of increasing concentrations of acetylcholine (Fig. 1C). OVA-challenged wild-type C57BL/6 mice developed robust AHR (Fig. 1C). In OVA-challenged Trpa1−/− mice, AHR was very mild, only differing from control animals at the highest doses of acetylcholine. Basal reactivity of vehicle-treated wild-type and Trpa1−/− mice was identical. We conclude that TRPA1 plays an essential role in asthma-related airway hyperreactivity.
Airway eosinophilia and hyperreactivity are consequences of an allergen-induced Th2-lymphocyte response leading to the production of allergen-specific IgE antibodies. We measured OVA-reactive IgE in serum by EIA to verify whether Trpa1−/− mice produce a normal Th2-response following immunization and airway challenge with OVA (Fig. 1D). OVA-reactive IgE serum levels in Trpa1−/− mice were indistinguishable from those in wild-type C57BL/6 mice, suggesting a normal Th2-dependent systemic immune response to OVA (Fig. 1D). These data indicate that TRPA1 has a crucial role in later events leading to airway inflammation following allergen challenge.
Quantitative comparison of inflammatory cell densities near airways in lung sections of OVA-challenged mice confirmed diminished eosinophilia in Trpa1−/− mice and reduced hyperplasia of mucus-producing goblet cells (Fig. 1E and supporting information (SI) Fig. S1A).
Reduced Mucus Production and Th2 Cytokine Levels in Airways of OVA-Challenged Trpa1−/− Mice.
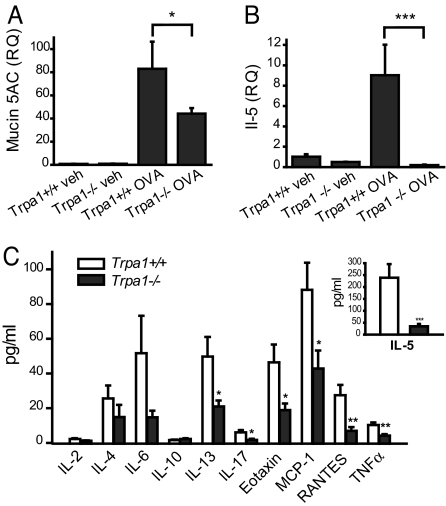
Using quantitative Taqman PCR, we compared the transcriptional levels of the muc5ac mucin genes in whole lung cDNA (Fig. 2A). Mucins are mucus proteins highly expressed in asthmatic airways. OVA-challenged wild-type mice displayed robust induction of muc5ac transcription (Fig. 2A). In contrast, muc5ac levels were reduced by 50% in lungs of OVA-challenged Trpa1−/− mice (Fig. 2A). Mucin5ac induction was normal in OVA-challenged Trpv1−/− mice (Fig. S1B).
Fig. 2.
Impaired induction of mucin, cytokines, and chemokines in OVA-challenged airways of Trpa1-deficient mice. (A) Relative quantities (RQ) of mucin5ac gene transcript, determined by Taqman qPCR of whole mouse lung cDNA. Mucin5ac induction is diminished in Trpa1−/− OVA mice. GAPDH transcript levels were used for normalization as endogenous control. Animal groups: Trpa1+/+ veh: n = 4, Trpa1−/− veh: n = 4, Trpa1+/+ OVA: n = 6, Trpa1−/− OVA: n = 7. ∗, P < 0.05. (B) Relative quantities (RQ) of interleukin 5 (IL-5) gene transcript, as determined by Taqman real-time quantitative PCR of whole mouse lung cDNA. OVA-challenged Trpa1−/− mice show no significant changes in IL-5 transcription compared with vehicle-treated mice. GAPDH transcript levels were used for normalization as endogenous control. Animal groups: Trpa1+/+ veh: n = 4, Trpa1−/− veh: n = 4, Trpa1+/+ OVA: n = 6, Trpa1−/− OVA: n = 6. ∗∗∗, P < 0.001. (C) Comparison of cytokine and chemokine levels in BALF of OVA-challenged Trpa1+/+ (white) and Trpa1−/− (black) mice, as measured by Luminex peptide analysis. Groups: Trpa1+/+ n = 8–10, Trpa1−/− n = 8–10 for each analyte. ∗∗, P < 0.01; ∗∗∗, P < 0.001
Th2 leukocytes orchestrate the allergic inflammatory response in the airways through the release of cytokines, such as interleukin 5 (IL-5). We examined transcriptional activity of the IL-5 gene by Taqman PCR of whole lung cDNA from wild-type, Trpa1−/−, and Trpv1−/− mice as a measure for Th2 leukocyte infiltration and activity (Fig. 2B). Strikingly, while OVA-challenged wild-type mice showed a robust increase in IL-5 transcriptional activity, IL-5 levels in OVA-challenged Trpa1−/− mice were indistinguishable from those in vehicle-treated mice (Fig. 2B). Trpv1−/− mice showed normal induction of IL-5 expression (Fig. S1C).
A systematic comparison of peptide concentrations of cytokines and chemokines was performed using Luminex multiplex protein analysis of BAL fluid (Fig. 2C). As predicted from our qPCR analysis, IL-5 protein levels in BALF of OVA-challenged Trpa1−/− mice were much lower (<20%) than in wild-type mice (Fig. 2C, Inset). Trpa1−/− mice also showed significantly diminished levels of IL-13, IL-17, eotaxin, MCP-1, RANTES, and TNFα, suggesting a profound defect in the Th2-directed local inflammatory response in the airways (Fig. 2C). Levels of IFN γ, an indicator for a Th1 leukocyte activity, were below the detection limit in all mouse groups, showing that the observed reduction in airway eosinophilia was not due to a shift toward a Th1-directed immune response in Trpa1−/− mice. Cytokine levels were normally elevated in OVA-challenged Trpv1−/− mice (Fig. S1D).
A TRPA1 Antagonist Reduces Airway Inflammation and Hyperreactivity when Administered During OVA Airway Challenge.
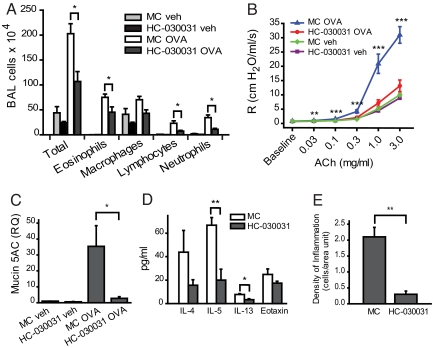
TRPA1-antagonists showed efficacy in animal models of acute and inflammatory pain and diminished the noxious effects of TRPA1 agonists known to cause asthma-related conditions (17, 19, 20, 28, 29). We asked whether a TRPA1 antagonist would prevent or diminish airway inflammation when administered to OVA-sensitized Balb/C mice during the airway challenge phase of the OVA protocol. HC-030031, the most thoroughly studied TRPA1 antagonist, was injected i.p. into OVA-sensitized animals on the day before (200 mg/kg) and twice daily (100 mg/kg) during the 4 days of OVA airway challenge. While vehicle (methyl cellulose, MC)-treated mice showed robust OVA-induced increases in BALF leukocyte numbers, cell numbers were diminished in OVA-challenged HC-030031-treated mice (Fig. 3A). Moreover, treatment with HC-030031 led to a nearly complete suppression of airway hyperreactivity in OVA-challenged Balb/C mice (Fig. 3B). Mucin5ac transcription was strongly suppressed by HC-030031 treatment, indicating diminished production of airway mucus (Fig. 3C). Antagonist-injected mice also showed diminished levels of Th2 cytokines IL-5 and IL-13 in BALF (Fig. 3D). Histological sections of airways from antagonist-treated mice showed much lower densities of inflammatory cells (Figs. 3E and S1E). Similar to Trpa1−/− mice, treatment with HC-030031 did not affect serum levels of OVA-specific IgE in OVA-challenged wild-type BALB/C mice, indicating a normal Th2-directed systemic inflammatory response (Fig. S1F). These data suggest that TRPA1 plays a crucial role in the development of asthma during airway allergen challenge, enabling inflammatory leukocyte infiltration, airway hyperreactivity, and mucus production.
Fig. 3.
Decreased inflammatory response in Balb/C mice treated with the TRPA1 antagonist HC-030031 during the OVA airway challenge phase. (A) Cell differentials for total leukocytes, eosinophils, macrophages, lymphocytes, and neutrophils in BALF collected from vehicle (veh, PBS)- or OVA-challenged mice injected i.p. with HC-030031 or with methyl cellulose (MC) during the airway challenge phase. Animal groups: MC veh: n = 8, HC-030031 veh: n = 9, MC OVA, n = 10, HC-030031 OVA: n = 8. ∗, P < 0.05. (B) Comparison of airway hyperresponsiveness to i.v. acetylcholine in vehicle (veh, PBS) - or OVA-challenged mice injected i.p. with HC-030031 or with just methyl cellulose (MC) during OVA airway challenge. Animal groups: MC veh: n = 7, HC-030031 veh: n = 7, MC OVA, n = 7, HC-030031 OVA: n = 6 (∗, α = 0.05; ∗∗, α = 0.01; ∗∗∗, α = 0.001). (C) Decreased lung mucin5ac transcription in OVA-challenged mice treated with TRPA1 antagonist HC-030031, determined by Taqman q PCR. RQ of mucin5ac transcript are shown for vehicle (veh.) or OVA-challenged mice injected i.p. with HC-030031 or with just methyl cellulose (MC) during OVA airway challenge. GAPDH transcript levels were used for normalization as endogenous control. Animal groups: MC veh: n = 4, HC-030031 veh: n = 4, MC OVA, n = 8, HC-030031 OVA: n = 8. ∗, P < 0.05. (D) Cytokine and eotaxin levels in bronchoalveolar lavage fluid (BALF) of OVA-challenged Balb/C mice treated with TRPA1 antagonist HC-030031 (black) or carrier methyl cellulose (white). (n = 4 mice/group) ∗, P < 0.05; ∗∗, P < 0.01. (E) Density of inflammation in H&E-stained airway sections from OVA-challenged HC-030031-treated and −untreated (MC) Balb/C mice, scored by counting of inflammatory cells near bronchial bundles (n = 4 mice per group).
Trpa1 is Essential for Chemically Induced and Inflammatory Neuropeptide Release in the Airways.
It is unclear whether the pro-inflammatory action of TRPA1 in asthma can be explained through purely neurogenic effects. TRPA1 may play an as yet undetected role in cells of the immune system or in airway tissue. To assess this possibility, we used Taqman quantitative PCR to compare TRPA1 transcript levels in cDNA derived from spleen harboring a large variety of leukocyte precursors, Th2 lymphocytes, whole mouse lung and BALF leukocytes of OVA-challenged mice, and DRG. Relative transcript quantities in spleen, Th2 cells, whole lung, and leukocytes were minimal, with DRG expression several 100-fold higher (Fig. S1G). Additional qPCR experiments using cDNA prepared from primary leukocytes and leukocyte cell lines failed to detect the presence of TRPA1 cDNA. These results point to a key role for sensory neuronal TRPA1 channels in allergic airway inflammation.
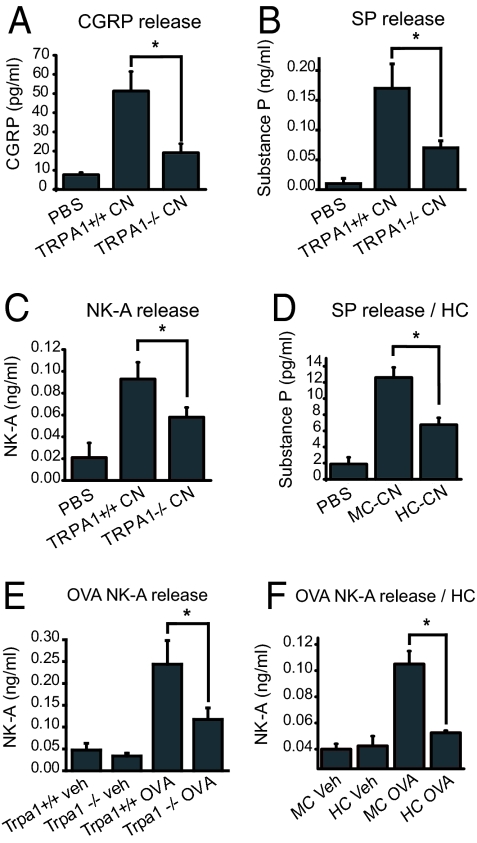
TRPA1 may be a critical trigger for neuropeptide release crucial for leukocyte infiltration and inflammatory progression in asthmatic airways. To investigate this possibility, we compared neuropeptide release in airways of wild-type and and Trpa1 −/− mice in response to 2-chloroacetophenone (CN), a potent inflammatory TRPA1 agonist (20). We performed a 30-s BAL in mice with CN (4 mM) contained in the BAL buffer (PBS) and measured the resultant release of CGRP, substance P (SP) and neurokinin A (NKA) using EIA (Fig. 4 A–C). CN induced strong increases in the levels of all 3 neuropeptides in BALF of wild-type C57BL/6 mice (Fig. 4 A–C). In Trpa1−/− mice CN-induced peptide release was clearly diminished (<50% of wild-type levels), supporting a specific and essential role for TRPA1 in chemically stimulated neurogenic peptide release in the airways (Fig. 4 A–C). Acute CN-induced neuropeptide release was suppressed by prior treatment with HC-030031 in Balb/C mice (Fig. 4D).
Fig. 4.
Role of TRPA1 in chemically induced and inflammatory neuropeptide release in the airways, measured by EIA. (A) Diminished chemically induced release of CGRP in Trpa1−/− mice following lung exposure to CN (2-chloroacetophenone) during BAL. Averaged CGRP levels in BAL fluid of TRPA1+/+ mice, treated with PBS (n = 4) or 4 mM CN (n = 6), and TRPA1−/− mice treated with 4 mM CN (n = 5) are shown. ∗, P < 0.05. (B) Diminished chemically induced release of Substance P (SP) in Trpa1−/− mice following lung exposure to CN (2-chloroacetophenone) during BAL. Treatments and mouse groups as in Fig. 4A. (C) Diminished chemically induced release of neurokinin A (NK-A) in Trpa1−/− mice following lung exposure to CN (2-chloroacetophenone) during BAL. Treatments and mouse groups as in Fig. 4A. (D) Diminished CN-induced release of SP in mice injected i.p. with TRPA1 antagonist HC-030031. Averaged SP concentrations are shown in BAL fluid of Balb/C mice treated with PBS (n = 4), 200 μM CN and methylcellulose vehicle (MC CN, n = 4), or with 200 μM CN and HC-030031 (HC CN, n = 4) are shown. ∗, P < 0.05. (E) Reduced level of neurokinin A in BAL fluid of OVA-challenged Trpa1−/− mice. NK-A levels were compared by EIA in BAL fluid of vehicle-treated wild-type mice (Trpa1+/+ veh, n = 11), vehicle-treated Trpa1−/− mice (n = 7), OVA-challenged wild-type mice (Trpa1+/+ OVA, n = 12), and OVA-challenged Trpa1−/− mice (n = 12). ∗, α<0.05. (F) Reduction of NK-A in BAL fluid of OVA-challenged Balb/C mice due to injection of TRPA1-antagonist HC-030031. NK-A levels were compared by EIA in BAL fluid of methyl cellulose and vehicle-treated mice (MC veh, n = 4), HC-030031- and vehicle-treated (HC veh, n = 4), methyl cellulose treated and OVA-challenged (MC OVA, n = 7), and HC-030031-treated and OVA-challenged mice (HC OVA, n = 8). ∗, α<0.05
Exogenous TRPA1 agonists such as CN are likely to mimic the actions of endogenous reactive products and inflammatory signaling pathways activating TRPA1 (16). Since Trpa1−/− mice showed clear deficiencies in acute neurogenic peptide release in the airways, we asked whether neuropeptide levels would also be reduced during airway allergen challenge. We compared neuropeptide levels in BALF of OVA-challenged wild-type and Trpa1−/−-deficient C57/BL6 mice, and in antagonist treated Balb/C mice, focusing on NK-A, the most abundant neuropeptide in airway lining fluid (30) (Fig. 4 E and F). Indeed, we observed that the OVA-induced increase in BALF NK-A levels was clearly diminished in Trpa1−/− mice and in antagonist-treated Balb/C mice (Fig. 4 E and F).
Discussion
Our results reveal a crucial role for the sensory neuronal ion channel TRPA1 in experimental asthma. TRPA1-deficient mice showed profound deficits in airway infiltration by inflammatory leukocytes in the OVA model of allergic airway inflammation, accompanied by a reduction in inflammatory Th2 cytokines, such as IL-5 and IL-13, and pro-inflammatory chemokines, such as TNFα and the eosinophil attractant, eotaxin. As a consequence, OVA-induced mucus production is impaired in Trpa1−/− mice. Airway hyperreactivity, another important hallmark of asthma, was strongly reduced. Pharmacological inhibition of TRPA1 during the airway challenge phase of the OVA protocol confirmed the essential function of this receptor, blocking leukocyte infiltration, cytokine and neuropeptide release, mucus production, and abolishing airway hyperreactivity.
Trpa1−/− mice are deficient in the neuronal detection of multiple pro-inflammatory exogenous and endogenous agents. These include asthma-inducing agents such as chlorine, unsaturated aldehydes in smoke and smog, chloramines, tear gas agents, and industrial isocyanates, as well as endogenous reactive oxidative species and lipid mediators (12, 17–20, 24, 25). Some of these endogenous mediators are produced by infiltrating leukocytes or inflamed airway tissue and can reach concentrations high enough to chronically activate TRPA1 in airway nerve endings (31). Chemosensory deficiency in Trpa1−/− mice may cause a lack of neuronal excitation and Ca2+ influx activated by these reactive compounds during inflammatory progression in asthma, resulting in reduced reflex hyperreactivity and neuropeptide release. Indeed, we find that sensory neuropeptide release, a prerequisite for inflammatory leukocyte infiltration in mice, is impaired in the airways of Trpa1−/− mice. This defect applies to both acute neuropeptide release, induced by a TRPA1 agonist, and inflammatory peptide release following OVA challenge in the airways.
The ability of a TRPA1 antagonist to recapitulate the knock-out phenotype suggests that TRPA1 fulfills an acute role in promoting local inflammation in the airways, rather than causing a developmental defect in immune system function. Administration of HC-030031 during the OVA airway challenge phase was sufficient to potently suppress airway leukocyte infiltration, mucus production and hyperreactivity. The role of TRPA1 in local inflammatory responses to allergen challenge is also supported by the observation that genetic deletion of TRPA1, or treatment with the TRPA1 antagonist HC-030031, did not seem to affect the systemic Th2-mediated response to allergen immunization, as evidenced by normal serum levels of OVA-reactive IgE. Future experiments are needed to address the detailed mechanistic role of TRPA1 in neurogenic responses affecting Th2 lymphocyte migration into the airways, cytokine production, leukocyte recruitment, as well as in airway hyperreactivity.
Our experiments clearly show that TRPV1, the capsaicin receptor, is not required for allergic airway inflammation in the OVA mouse model of asthma. A TRPA1-specific stimulus, possibly a reactive TRPA1-specific mediator, appears to be required to drive the pro-inflammatory activity of airway C-fibers in asthma. Nevertheless, TRPV1 is clearly involved in the symptomatic consequences of airway inflammation, as evidenced by a recent report showing anti-tussive activity of a TRPV1 antagonist in a model of chronic cough (32).
The data in our present study support the idea that TRPA1 may function as an integrator of chemical and immunological stimuli modulating inflammation in the airways. This integrative activity can explain the pro-inflammatory effects of chemical exposures in asthma patients (16). By activating TRPA1, chemical irritants may trigger the release of neuropeptides and chemokines in the airways, thereby exacerbating the cellular and tissue inflammatory response observed in our present study. Our study opens an avenue for asthma pharmacology, revealing TRPA1 as a potential target for anti-asthmatic drugs. Future studies will address the action of TRPA1 antagonists in additional animal models of asthma and in other allergic inflammatory conditions.
Materials and Methods
Animals.
Experimental procedures were approved by the Institutional Animal Care and Use Committees of Yale University, the University of California, San Francisco, and Hydra Biosciences. Mice were housed at facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care in standard environmental conditions (12-h light-dark cycle and 23 °C). Food and water were provided ad libitum. Trpa1−/− mice were a gift from David Julius (University of California, San Francisco). The Trpa1-knockout allele was backcrossed into the C57BL/6 background (>99.5%) by marker assisted accelerated backcrossing (Charles River Laboratories). Trpv1−/− mice were purchased from Jackson Laboratories and C57/Bl6 and BALB/c mice from Charles River Laboratories. For experiments on C57BL/6, Trpv1−/−, and Trpa1−/− mice, animals were matched for age (12–22 weeks) and gender. Six- to 8-week-old BALB/c mice were used for OVA sensitization and antagonist studies.
Sensitization and Airway Challenge Procedure.
Mice were sensitized on days 0, 7, and 14 by i.p. injection of 50 μg ovalbumin (OVA) (Sigma-Aldrich) adsorbed in 2 mg alum gel (Sigma-Aldrich) in a total volume of 200 μL PBS. Control animals received alum only. Subsequently, lightly anesthetized mice (isoflurane) were intranasally challenged with OVA [100 μg in 40 μL PBS (PBS)] or with PBS alone on days 21, 22, and 23. For therapeutic intervention with TRP channel antagonist HC-030031, mice were given the compound (200 mg/kg mouse body weight) i.p. once on day 20 and 100 mg/kg twice a day on days 21, 22, and 23. On the day of lung mechanics measurement, all mice received 100 mg/kg compound once in the morning and subject to the end point of the experiment. All measurements and sample collection were performed 24 h after the final intranasal challenge. Following lung mechanics measurements plasma levels of HC-030031 along with samples of dose solutions were analyzed by LC/MS/MS using a standard protein precipitation extraction with the addition of an internal standard.
Measurement of Airway Reactivity.
Twenty-four hours following the last OVA challenge, mice were anesthetized with pentobarbital (60 mg/kg of body weight) and urethane (1 g/kg). A tracheostomy was performed, and the trachea was cannulated. Mice were attached to a Flexivent pulmonary mechanics analyzer (SCIREQ) and ventilated at a tidal volume of 9 mL/kg, at a frequency of 150 bpm. Positive end-expiratory pressure was set at 2 cm H2O. Mice were paralyzed with pancuronium (0.1 mg/kg i.p.). A 27-gauge needle was used to administer acetylcholine (0.03, 0.1, 0.3, 1.0, and 3.0 mg/mL) through the subclavial/tail vein to generate a concentration-response curve. Measurements of airway mechanics were made continuously applying the single-compartment model.
Quantitative Analysis of Cytokines, Chemokines, and Neuropetides in BAL Fluid.
Cytokines and chemokines in BAL fluid (50 μL) were measured using a Milliplex MAP Mouse Cytokine/Chemokine Kit (Millipore) on Luminex 200 analyzer (Luminex), following the manufacturer's recommendations. Neuropeptide levels in BAL were measured by EIA (CGRP: Cayman Chemical; Substance P: Phoenix Pharmaceuticals; Neurokinin A: Bachem). To measure acute peptide release, 4 mM CN (2-chloroacetophenone) was added to the BAL buffer. Lungs were inflated with CN-BAL buffer for 30 sec. HC-030031 was injected i.p. in Balb/C mice at 200 mg/kg 24 h, 100 mg/kg 6 h, and 100 mg/kg 30 min before CN challenge (200 μM).
Supplementary Material
Acknowledgments.
This work was supported by grants from the American Asthma Foundation (Grant 07–0212 to S.J.), the National Institute of Environmental Health Sciences (ES015056 and ES017218 to S.J.), a Spanish Ministry of Science and Innovation (MICINN)/Fulbright fellowship to A.I.C. (2007–0480), a fellowship from the German Research Foundation to M. B. (BR 3773/1–1.) and National Institutes of Health (NIH) training grant 5T32GM07205 to M. D. E. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or federal government. S. J. serves on the Scientific Advisory Board of Hydra Biosciences. All members of Hydra Biosciences receive options. We are grateful to Aiwei Sui and Lesley Devine for technical support.
Footnotes
Conflict of interest statement: S.-E.J. is serving on the scientific advisory board of Hydra Biosciences, Cambridge, MA. Hydra Biosciences developed the TRPA1-antagonist, HC-030031, used in the present study. D.d.C., M.D., J.S.W., C.M.F., J.A.C., N.J.H., and M.M.M. are employees of Hydra Biosciences, and receive options.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900591106/DCSupplemental.
References
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–498. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- 3.Cohn L, Elias JA, Chupp GL. Asthma: Mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 4.Flood-Page P, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 5.Undem BJ, Carr MJ. The role of nerves in asthma. Curr Allergy Asthma Rep. 2002;2:159–165. doi: 10.1007/s11882-002-0011-4. [DOI] [PubMed] [Google Scholar]
- 6.Dakhama A, et al. Regulation of airway hyperresponsiveness by calcitonin gene-related peptide in allergen sensitized and challenged mice. Am J Respir Crit Care Med. 2002;165:1137–1144. doi: 10.1164/ajrccm.165.8.2109058. [DOI] [PubMed] [Google Scholar]
- 7.White FA, Wilson NM. Chemokines as pain mediators and modulators. Curr Opin Anaesthesiol. 2008;21:580–585. doi: 10.1097/ACO.0b013e32830eb69d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis B, Roberts AM, Coleridge HM, Coleridge JC. Reflex tracheal gland secretion evoked by stimulation of bronchial C-fibers in dogs. J Appl Physiol. 1982;53:985–991. doi: 10.1152/jappl.1982.53.4.985. [DOI] [PubMed] [Google Scholar]
- 9.Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy. 2004;59):1139–1152. doi: 10.1111/j.1398-9995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 11.Basbaum AI, Julius D. Toward better pain control. Sci Am. 2006;294:60–67. doi: 10.1038/scientificamerican0606-60. [DOI] [PubMed] [Google Scholar]
- 12.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 14.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 15.Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta. 2007;1772:915–927. doi: 10.1016/j.bbadis.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Bessac BF, Jordt SE. Breathtaking TRP Channels: TRPA1 and TRPV1 in Airway Chemosensation and Reflex Control. Physiology (Bethesda) 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNamara CR, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessac BF, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andre E, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bessac BF, et al. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 2009;23:1102–1114. doi: 10.1096/fj.08-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2008–0292OC. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shakeri MS, Dick FD, Ayres JG. Which agents cause reactive airways dysfunction syndrome (RADS)? A systematic review. Occup Med (Lond) 2008;58:205–211. doi: 10.1093/occmed/kqn013. [DOI] [PubMed] [Google Scholar]
- 23.Redlich CA, Karol MH. Diisocyanate asthma: Clinical aspects and immunopathogenesis. Int Immunopharmacol. 2002;2:213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- 24.Trevisani M, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz-Orengo L, et al. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain. 2008;4:30. doi: 10.1186/1744-8069-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Y, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eid SR, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrus M, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heaney LG, et al. Neurokinin A is the predominant tachykinin in human bronchoalveolar lavage fluid in normal and asthmatic subjects. Thorax. 1998;53:357–362. doi: 10.1136/thx.53.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 32.McLeod RL, et al. TRPV1 antagonists attenuate antigen-provoked cough in ovalbumin sensitized guinea pigs. Cough. 2006;2:10. doi: 10.1186/1745-9974-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.