Abstract
Approximately 50% of hospitalized elders have cognitive impairment (CI) that increases their vulnerability to hospital-acquired complications. Matching geriatric evaluation and recommendations to the true pace of hospital care may improve the care of elders in general, in particular those with CI. Integrating information technology into geriatric services (gero-informatics) might allow reduction of the time to implementation of geriatric recommendations and prevent the initiation of potentially harmful medications and procedures during the critical first 48 hours of hospitalization. This paper reviews our local gero-informatics early experience of developing a computerized decision support system (CDSS) to enhance hospital care for elders with CI by reducing inappropriate use of anticholinergic medications, urinary catheters, and physical restraints.
Keywords: gero-informatics, cognitive impairment, hospital, decision support
Overview
In 2001, approximately 12.6 million individuals aged 65 years and older were discharged from US hospitals, following an average length of stay of 5.8 days (Graves and Gillum 1997). It is estimated that up to 56% of these hospitalized elders had cognitive impairment (CI) during their hospital stay (Harwood et al 1997). Hospitalized elders with CI are more prone to falls, injuries, pressure ulcers, restraints, and delirium (Bynum et al 2004). These complications contribute to mortality, decreased functional status, limited rehabilitation, prolonged length of stay, increased institutionalization, and higher health care costs (Lyketsos et al 2000; Marcantonio et al 2000; McCusker et al 2003; Bynum et al 2004).
Although there is ample room for quality improvement, evidence suggests that interdisciplinary inpatient geriatrics services may improve care for hospitalized elders without CI. However, their effectiveness among elders with CI is less clear (Inouye et al 1999; Marcantonio et al 2001; Baldwin et al 2004; Mador et al 2004). One cause of limited effectiveness of inpatient geriatrics consultation may be the ever-quickening pace of care in the hospital setting. This rapid pace limits the window of opportunity for input from the geriatrics team and for communication and timely implementation of geriatrics recommendations. More importantly, recommendations may come “after the fact”; suggestions to avoid potentially inappropriate care often come after that care has been initiated (eg, bladder catheterization, anticholinergic, or other psychoactive medications). Thus, matching geriatrics evaluation and recommendations to the true pace of hospital care may be one mechanism to improve the care of elders with CI. Increasing the pace of geriatrics care may provide improved capacity to provide patient-specific warnings to avoid potentially inappropriate care at the time of medical decision-making. A recent report from the Institute of Medicine (2001) suggested that integrating medical informatics into healthcare of elders (“gero-informatics”) is the best route to improving the overall safety and quality of the healthcare system. This paper provides a real world practical example of using informational technology (or specifically, Gero-informatics) as a tool to improve the care of hospitalized elders with CI.
Vulnerability of hospitalized elders with CI
CI in hospitalized older adults includes a variety of disorders, such as mild cognitive deficits, delirium, and advanced dementia. Delirium and dementia are the underlying causes of CI among most hospitalized older adults (Harwood et al 1997). They occur together in approximately 20% to 60% of this population (Fick et al 2002). It is estimated that the prevalence of CI (induced by dementia, delirium, or other disorders) in hospitalized older adults ranges from 14% to 66%, depending on the method used to measure cognition, the definition of CI, and the type of hospital unit (eg, surgical, medical, geriatrics) (Harwood et al 1997; Inouye et al 1999; Lyketsos et al 2000; Maracantonio et al 2000, 2001; Fick et al 2002; McCusker et al 2003; Baldwin et al 2004; Bynum et al 2004; Mador et al 2004). Hospitalized older adults with CI are vulnerable to physical or chemical restraints and hospital-acquired complications, such as urinary incontinence, urinary catheters, falls, injuries, pressure ulcers, and delirium (Lyketsos et al 2000; Marcantonio et al 2000; McCusker et al 2003; Bynum et al 2004). In addition, the management of medical or surgical illnesses of hospitalized older adults with CI requires avoiding certain medications with central nervous system properties that might worsen cognition. Such medications include barbiturates, anticholinergic drugs, antispasmodics, and skeletal muscle relaxants. Furthermore, CI may delay diagnostic and therapeutic procedures, impede informed consent, and result in difficulty in adherence to medical care. The special needs and vulnerability of hospitalized elders with CI lead to more demands on nursing staff, prolonged length of stay, increased risk of post-discharge institutionalization, and higher health care costs (Lyketsos et al 2000; Marcantonio et al 2000; McCusker et al 2003; Bynum et al 2004) (see Table 1).
Table 1.
Impact of cognitive impairment on hospitalized elders
|
Making the case for the local hospital leadership
In order to justify the local resource reallocation to our proposed program (see next sections) and get the buy-in from the local hospital leadership, our local research team conducted a secondary data analysis of all Medicare enrollees who resided in Central Indiana counties (including the local hospital) and were hospitalized between January 1995 and December 1999. The main reason for this secondary data analysis was to demonstrate the impact of elders with CI on the hospital performance regarding safety, length of stay, and cost. We used ICD-9 codes to identify hospitalized Medicare beneficiaries with CI, including dementia-related codes, delirium-related codes, and any codes that indicated the presence of cognitive deficit. Among the 105,361 patients hospitalized in any of the Central Indiana counties during the study period, 18% had evidence of CI (dementia, delirium, or mild CI), 12.5% had dementia, and 10.3% had delirium with or without dementia. In comparison to patients with no evidence of CI and after adjusting for the patients’ age, gender, race, and comorbidity, hospitalized elders with CI stayed in the hospital for one additional day (mean length of stay 7.7 days vs. 6.7 days), cost Medicare over the study period US$6,648 more, and had two times the odds of dying during the study period (odds ratio 2.073; 95% confidence interval 2.000–2.149).
The current national state of hospital-based geriatric services
Hospital-based geriatric services include two types of care delivery models. One model targets older surgical or medical patients who meet frailty criteria and provides care on a specialized unit. The other model provides a geriatric consultation. Both models of care are usually delivered via interaction between the primary care provider and the interdisciplinary geriatrics team. Published controlled clinical trials that evaluated the effectiveness of these models of care report conflicting results (Stuck et al 1993; Landefeld et al 1995; Slaets et al 1997; Huusko et al 1999; Asplund et al 2000; Counsell et al 2000; Cohen et al 2002; Cole et al 2002). Caring for hospitalized elders in a specialized geriatric unit may lead to improvement in patients’ physical function and decrease length of stay without affecting overall mortality (Inouye et al 1999; Counsell et al 2000; Cohen et al 2002). Such positive results indicate that changing the system of care may improve outcomes. Unfortunately, inpatient geriatrics units will reach only a small number of the large and growing segment of older hospitalized patients. In contrast to the positive effect of inpatient geriatrics units, studies of geriatrics consultation have generally failed to demonstrate efficacy across a range of health outcomes (Stuck et al 1993; Landefeld et al 1995; Slaets et al 1997; Cole et al 2002). One exception is a study of patients undergoing surgical repair of hip fracture. In that study geriatrics consultation decreased the incidence of delirium without affecting other outcomes, such as length of stay, mortality, or functional status (Marcantonio et al 2001). Nevertheless, both models of inpatient geriatrics care improve prescribing for hospitalized elders, such as decreasing polypharmacy and the use of potentially inappropriate medications.
Despite the high prevalence of hospitalized elders with CI and the numerous studies indicating that they are considered a vulnerable population with special needs, few studies have attempted to develop models of care to accommodate their specific needs. Recently, a randomized controlled trial targeting the management of behavioral problems among older medically ill patients with CI compared traditional geriatric consultation delivered via a geriatrician with a geriatrics consultation enhanced with specialized nursing interventions. Mador and colleagues (2004) found that the nursing-based intervention had no effect on agitation, sleep, restraint and psychotropic drug use, length of stay, falls, discharge plans, nursing satisfaction, or next-of-kin satisfaction. However, there was a trend toward more appropriate use of medications. In another recent randomized controlled trial of medically ill hospitalized older adults with CI and in comparison to usual care, a nurse-led mental health liaison service did not improve length of stay, mortality, re-hospitalization, cognition, or psychotropic use. This service included an assessment component, recommendations for management of mental illnesses, and educational supports for nursing staff (Baldwin et al 2004).
There are four potential explanations for the modest impact of inpatient geriatrics services among elders in general and the lack of efficacy among those with CI in particular. First, currently available management strategies may simply be ineffective. Second, limited efficacy may be explained by the low and incomplete adherence to the recommendations of the geriatrics team. Third, the short and delayed exposure to geriatrics recommendations, even when the primary team accepts such recommendations, may limit their potential impact. Fourth, implementation after an adverse event or exposure may have less impact than an intervention that might have prevented the event. Given these findings from the literature, using new, systems-based, gero-informatics interventions to improve the safety and care of hospitalized elders with CI could reach older adults earlier in the hospital course, be available 24 hours per day, and better integrate inpatient with outpatient services. Computerized decision support systems integrated with available expertise of geriatrics consultants offer an innovative solution to accommodate the pace and complexity of care needed by patients with CI.
Gero-informatics and computerized decision support systems (CDSS)
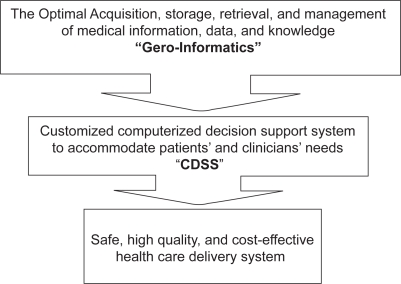
Gero-informatics is the study and application of medical informatics in caring for older adults. Medical informatics refers to the acquisition, storage, retrieval, management, and optimal use of medical information, data, and knowledge (Weiner et al 2003). We can use gero-informatics to customize CDSS through tools to accommodate patients’ and clinicians’ needs. A CDSS can retrieve relevant, individualized, and updated information from a health system’s data repository across various settings and then feed these data directly to clinicians at the time of decision making. In many environments, this requires the presence of both electronic medical records and electronic physician order entry. Physicians have a low adherence rate to guidelines, and passive educational initiatives are often ineffective in changing physicians’ behaviors (Weiner et al 2003). Even when physicians accept a given general guideline, they may not recognize that a particular patient is eligible for the actions indicated by that guideline. Numerous personal and systems factors might explain these findings in a hospital setting where, following admission, physicians spend on average 3.5 minutes per day interacting with a patient and 2.5 minutes with the patient’s caregiver (Weiner et al 2003). Equipped with clinical data such as cognitive status, evidence-based guidelines, and the ability of merging patient-specific data with the most relevant guideline, the CDSS is considered a valuable tool to support physicians’ medical decisions (Kawamoto and Lobach 2003; Kaushal et al 2003; Handler et al 2004).
Over the last three decades, numerous clinical trials (Kawamoto and Lobach 2003; Kaushal et al 2003; Handler et al 2004) have demonstrated that CDSS can improve processes of care, lead to better clinical outcomes, reduce medical errors, and decrease health care expenditures (Kawamoto and Lobach 2003; Kaushal et al 2003; Weiner et al 2003; Handler et al 2004). The first of these studies was published in 1976 and showed that CDSS could improve physicians’ adherence to ideal practice standards (Weiner et al 2003). Since that early trial, evidence has continued to accumulate supporting the effectiveness of CDSS. CDSS improves physicians’ performance in ordering mammography and fecal occult blood testing, managing diabetes mellitus, monitoring use of warfarin and digoxin, preventing narcotic-induced constipation, discussing and completing advance directives, prophylactic inpatient prescription of heparin and aspirin, and administering inpatient pneumonia and influenza vaccinations among older adults (Kawamoto and Lobach 2003; Kaushal et al 2003; Weiner et al 2003; Handler et al 2004). CDSS has reduced inpatient and out-patient charges and length of stay and can restore time for clinical care (Kawamoto and Lobach 2003; Kaushal et al 2003; Weiner et al 2003; Handler et al 2004).
Rationale for the use of gero-informatics and CDSS to enhance the care of hospitalized elders with CI
A growing body of evidence demonstrates that older patients with CI who are hospitalized for the management of their severe illnesses are especially vulnerable to adverse events. Even our ability to detect CI among hospitalized patients is quite limited. Because detection of CI is low, and CI has been linked to adverse outcomes for older hospitalized patients, screening for CI is considered an inpatient quality indicator. However, few studies have developed and evaluated steps that might follow the results of screening for CI in hospitalized elders, and the results of these studies have been unimpressive (Cole et al 2002; Baldwin et al 2004; Mador et al 2004). Inpatient geriatrics models of care have not been effective among those with CI (Inouye et al 1999; Marcantonio et al 2001; Cole et al 2002; Fick et al 2002; Baldwin et al 2004; Mador et al 2004). Missed, delayed, post-hoc, and incomplete implementation of the recommendations are significant factors explaining the poor outcomes among hospitalized elders with CI, even in the setting of prior clinical trials. Leape outlined four mechanisms for redesigning health care systems to reduce complications and improve safety: reduce reliance on memory, improve access to information, standardize, and train (Weiner et al 2003). The CDSS provides access to patient-specific guidelines at the point of care, offers standardization through suggested therapeutic and diagnostic recommendations, and presents a valuable matrix for training. At Wishard Memorial Hospital and the Regenstrief Institute, we are using gero-informatics and an inpatient geriatrics unit with a consultative service (Acute Care for Elders [ACE]), to test the impact of CDSS in accommodating the special needs of hospitalized elders with CI. We believe that this application of the CDSS signals a new type of geriatrics service that is continuously available during the hospital stays of older adults, “The virtual ACE/ACE version II”.
Acute care for the elderly service at Wishard Memorial Hospital (ACE version I)
The ACE service was first implemented at Wishard Memorial Hospital in 1998 with the primary goal of this service of combating functional decline that may occur as a result of an acute illness and hospitalization. The geriatric team includes a geriatrician, gerontological clinical nurse specialist, social worker, pharmacist, physical therapist, occupational therapist, and administrator assistant. The service receives, on average, 15 new referrals from both medical and surgical wards every week. The team discusses and develops care plans for each referral and provides recommendations for the primary care team that facilitate the management of various geriatric syndromes, including discharge planning. The effectiveness of this ACE version I model was evaluated in a randomized controlled trial conducted in a community hospital (Counsell et al 2000).
Reviewing the medical chart of 194 patients referred to ACE version I service over a three-month period in 2004 revealed that the average patient age was 75.9 years and the average hospital stay was 5.2 days. Using mini mental status examination, functional status assessment, and interview with caregiver and staff, the team identified the presence of CI due to dementia, delirium, or other disorders in 52% of the referred patients. On average, it took two days from the admission for the primary care team to enter an order requesting the geriatric service. Following this order, it took an average of 12 hours for the geriatric team to evaluate the referred patient. Approximately 47% of patients referred to the geriatric consultation service returned home, 11% were discharged to a long term care facility, and 42% were discharged into a short-term rehabilitation facility. These real world data suggest that more than half of ACE version I patients suffered from CI; that there is a more than 48-hour delay in implementing geriatric services for this vulnerable group, and that there is significant room for improving the pace and penetration of geriatric consultations and recommendations.
Enhancing care for hospitalized elders with CI (ACE version II)
In order to upgrade the current ACE service at our local hospital to accommodate the special needs of hospitalized elders with CI, we are conducting a randomized controlled clinical trial that is evaluating the efficacy of a screening program combined with a CDSS in 1. reducing the use of potentially inappropriate medications and procedures (urine catheters and physical restraints) and 2. decreasing the time to geriatric consultation. In addition, the trial is testing the feasibility of using such a system to decrease hospital acquired complications among this vulnerable population. This trial will set the stage for the long-term vision of developing an enhanced geriatric hospital system that will detect hospitalized older adults with CI, decrease hospital acquired complications including delirium, facilitate transition back to the community, rehabilitation, or long-term care settings, and integrate care for these patients across the continuum of care including formal follow-up evaluation of the patient’s CI in the outpatient setting.
The entire necessary information technology infrastructure for applying the CDSS to CI is currently in place at Wishard Memorial Hospital (Weiner et al 2003). The CDSS in the current trial is focusing on informing the physicians first of the probable presence of CI; second of the need to consider geriatric services for further evaluation and treatment of patient’s CI; and third of specific targeted recommendations. The specific recommendations of the CDSS were developed using national guidelines (Fick et al 2001) as the basic reference, then transferring such guidelines into a locally accepted recommendations using a reflective adaptive process method of developing consensus among a team of local users and experts (Stroebel et al 2005).
The local users and experts included medical informaticians, hospitalists, general internists, pharmacists, and geriatricians. This team selected the list of prohibited anticholinergic medications with their alternatives and the process of eliminating physical restraints (see Tables 2 and 3). In brief, the following steps outline the process of interaction between the physician and the CDSS:
each time a patient aged 65 or older is admitted to a medicine service, they are screened for CI and delirium by the study staff;
those patients screening positive for CI and/or delirium are randomized to the intervention or control arms by means of the computerized order entry system;
each time a physician enters an order for a patient randomized to the intervention arm, the CDSS will notify the physician of the presence of CI;
the system will recommend referral to geriatric service in reference to the CI;
if the physician orders a urinary catheter and/or at least once each day while the catheter order is active, the CDSS will suggest avoiding use of the catheter or discontinuing the catheter as early as possible;
if the physician orders physical restraints, the CDSS will recommend avoiding the use of restraints or substituting physical restraints with the use of a professional sitter; and
the CDSS will offer specific recommendations regarding appropriate substitutes for any of the inappropriate medications. The CDSS might recommend stopping the drug, suggest an alternative, or recommend dose modification. The physician could accept, reject, or modify these orders with just a few keystrokes.
Table 2.
The list of anticholinergic medications to avoid in hospitalized elders with cognitive impairment
| Drug | Alternatives |
|---|---|
| Meperidine | Morphine sulfate, oxycodone (or hydrocodone) with Acetaminophen |
| Promethazine | Dolasetron, metoclopramide |
| Diphenhydramine | Loratadine for allergic reactions, itching, or urticaria
Trazodone for assistance with sleeping Acetaminophen, low dose diphenhydramine, or hydrocortisone for blood transfusion |
| Hydroxyzine | Loratadine for allergic reactions, itching, or urticaria Trazodone for assistance with sleeping |
| Chlorpheniramine | Loratadine for allergic reactions, itching, or urticaria Trazodone for assistance with sleeping |
| Meclizine | Hold while patient in the hospital |
| Cyclobenzaprine | Acetaminophen or oxycodone with acetaminophen |
| Methocarbamol | Acetaminophen or oxycodone with acetaminophen |
| Hyoscyamine | Morphine sulfate for painful cramps Proton pump inhibitor for reflux disorders |
| Oxybutynin | Hold while patient in the hospital |
| Tolterodine | Hold while patient in the hospital |
| Paroxetine | Sertraline |
| Amitriptyline | Trazodone for assistance with sleeping
Referral to ACE for depression Gabapentin (or Duloxetine?) for neuropathic pain |
| Amoxapine | Referral to ACE for depression |
| Doxepin | Trazodone for assistance with sleeping Referral to ACE for depression Loratadine for itching |
| Imipramine | Trazodone for assistance with sleeping Referral to ACE for depression |
| Nortriptyline | Trazodone for assistance with sleeping
Referral to ACE for depression Gabapentin (or duloxetine?) for neuropathic pain |
| Benztropine | Hold while in the hospital and observe for extrapyramidal sign |
Abbreviations: ACE, acute care for elders.
Table 3.
Alternatives for the use of physical restraint and Foley catheterization among patients with cognitive impairment
| Physical restraints |
|
| Foley catheterization | Reconsider the need for Foley catheter in the presence of cognitive impairment. |
Although the efficacy and the effectiveness of this new ACE version II are currently being evaluated, the process of integrating IT within the local hospital care is complicated and requires a direct involvement of the program developers with both the future users of the program and the hospital leadership. Such a process demands conducting a local need assessment, reviewing the national solutions to accommodate the local needs, converting such a solution into a locally acceptable and sensitive program, and finally assessing the impact of such a program.
In conclusion, the current study is a first step in using gero-informatics to build an enhanced system to improve care and safety of hospitalized elders with CI by integrating active CI screening and CDSS with the existing geriatric service. This new ACE version II could be the answer to accommodate the need of hospitalized elders and set the stage for We hope that this new ACE version II will.
Figure 1.
The impact of gero-informatics on healthcare.
Acknowledgments
Dr. Boustani was supported by the Paul A. Beeson Career Development Award in Aging (K23 AG 26770–01).
References
- Asplund K, Gustafson Y, Jacobsson C, et al. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. J Am Geriatr Soc. 2000;48:1381–8. doi: 10.1111/j.1532-5415.2000.tb02626.x. [DOI] [PubMed] [Google Scholar]
- Baldwin R, Pratt H, Goring H, et al. Does a nurse-led mental health liaison service for older people reduce psychiatric morbidity in acute general medical wards? A randomised controlled trial. Age Ageing. 2004;33:472–8. doi: 10.1093/ageing/afh154. [DOI] [PubMed] [Google Scholar]
- Bynum JP, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–94. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Feussner JR, Weinberger M, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346:905–12. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- Cole MG, McCusker J, Bellavance F, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167:753–9. [PMC free article] [PubMed] [Google Scholar]
- Counsell SR, Holder CM, Liebenauer LL, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48:1572–81. doi: 10.1111/j.1532-5415.2000.tb03866.x. [DOI] [PubMed] [Google Scholar]
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50:1723–32. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- Fick DM, Waller JL, Maclean JR, et al. Potentially inappropriate medication use in a Medicare managed care population: association with higher costs and utilization. J Managed Care Pharm. 2001;7:407–13. [Google Scholar]
- Graves EJ, Gillum BS. National hospital discharge survey: annual summary, 1994. Vital Health Stat. 1997;13:1–50. [PubMed] [Google Scholar]
- Handler JA, Feied CF, Coonan K, et al. Computerized physician order entry and online decision support. Acad Emerg Med. 2004;11:1135–41. doi: 10.1197/j.aem.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Harwood DM, Hope T, Jacoby R. Cognitive impairment in medical inpatients. I: Screening for dementia--is history better than mental state? Age Ageing. 1997;26:31–5. doi: 10.1093/ageing/26.1.31. [DOI] [PubMed] [Google Scholar]
- Huusko T, Karppi P, Avikainen V, et al. Significant changes in the surgical methods and length of hospital stay of hip fracture patients occurring over 10 years in Central Finland. Ann Chir Gynaecol. 1999;88:55–60. [PubMed] [Google Scholar]
- Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–16. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Lobach DF. Clinical decision support provided within physician order entry systems: a systematic review of features effective for changing clinician behavior. AMIA Annu Symp Proc. 2003:361–5. [PMC free article] [PubMed] [Google Scholar]
- Landefeld CS, Palmer RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–44. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Sheppard JM, Rabins PV. Dementia in elderly persons in a general hospital. Am J Psychiatry. 2000;157:704–7. doi: 10.1176/appi.ajp.157.5.704. [DOI] [PubMed] [Google Scholar]
- Mador JE, Giles L, Whitehead C, et al. A randomized controlled trial of a behavior advisory service for hospitalized older patients with confusion. Int J Geriatr Psychiatry. 2004;19:858–63. doi: 10.1002/gps.1165. [DOI] [PubMed] [Google Scholar]
- Marcantonio ER, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–24. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–22. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- McCusker J, Cole MG, Dendukuri N, et al. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51:1539–46. doi: 10.1046/j.1532-5415.2003.51509.x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Crossing the quality chasm. Washington, DC: Institute of Medicine Pr; 2001. [Google Scholar]
- Slaets JP, Kauffmann RH, Duivenvoorden HJ, et al. A randomized trial of geriatric liaison intervention in elderly medical inpatients. Psychosom Med. 1997;59:585–91. doi: 10.1097/00006842-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Stroebel CK, McDaniel RR, Jr, Crabtree BF, et al. How complexity science can inform a reflective process for improvement in primary care practices. Jt Comm J Qual Patient Saf. 2005;31:438–46. doi: 10.1016/s1553-7250(05)31057-9. [DOI] [PubMed] [Google Scholar]
- Stuck AE, Siu AL, Wieland GD, et al. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342:1032–6. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- Weiner M, Callahan CM, Tierney WM, et al. Using information technology to improve the health care of older adults. Ann Intern Med. 2003;139:430–6. doi: 10.7326/0003-4819-139-5_part_2-200309021-00010. [DOI] [PubMed] [Google Scholar]