Abstract
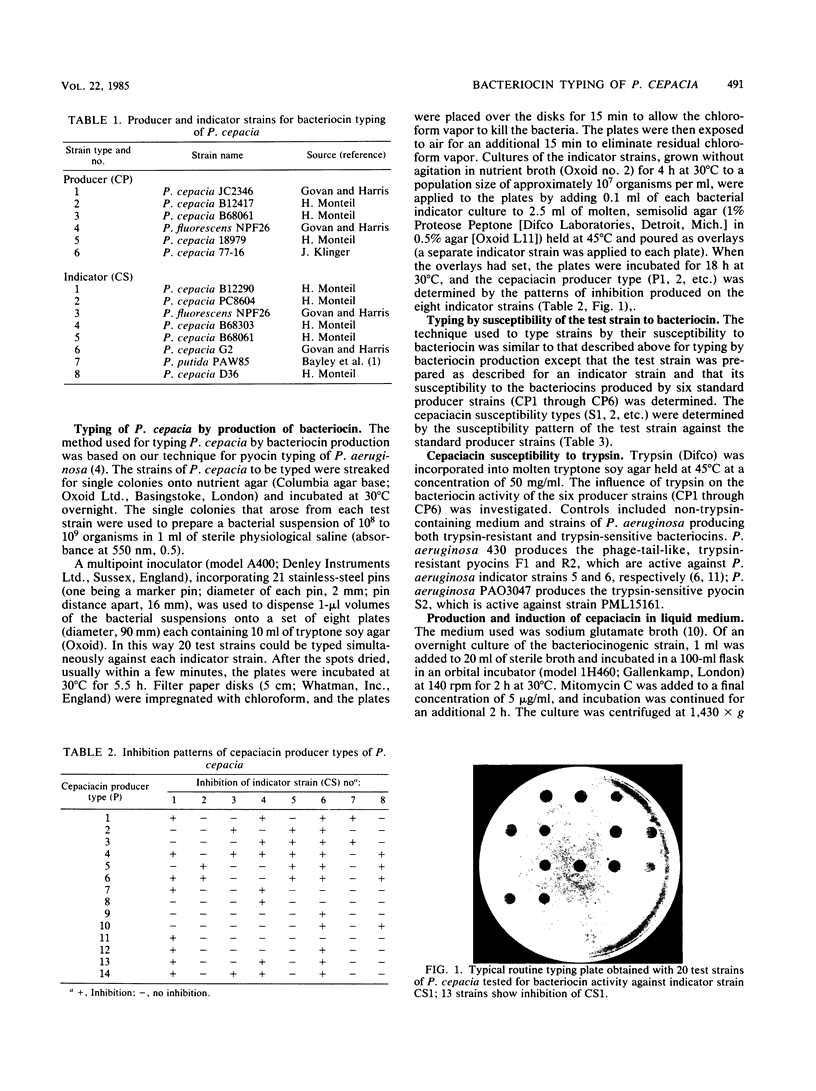
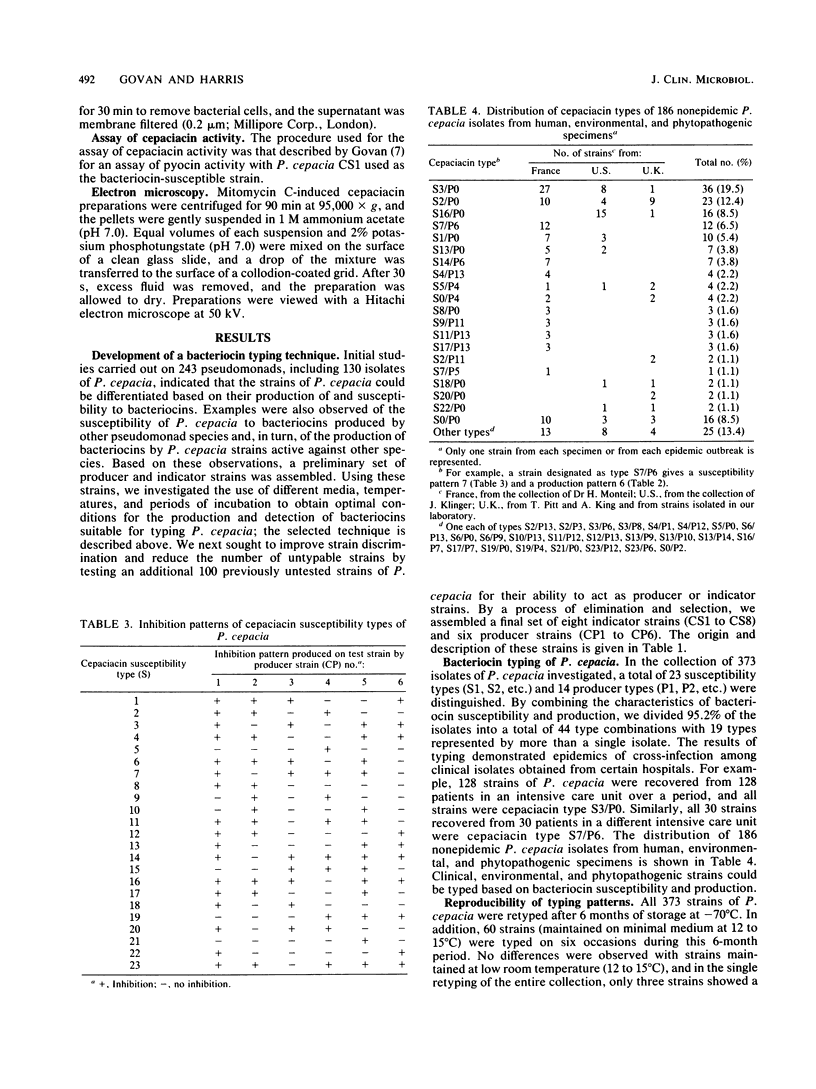
The significance of Pseudomonas cepacia as an opportunistic pathogen in immunocompromised patients has become increasingly recognized. Particularly disturbing is its increased incidence, reported by several North American centers, in respiratory tract cultures from patients with cystic fibrosis. Epidemiological studies of P. cepacia have been hampered by a lack of typing methods. In this paper we report the development of a typing scheme based on bacteriocin production and susceptibility. For bacteriocin production, test isolates of P. cepacia were rapidly applied to the surfaces of agar plates with a multiple inoculator. After incubation of these test isolates for 5.5 h and their exposure to chloroform, indicator strains were applied in agar overlays without prior removal of the test strain growth. After 18 h of incubation, inhibition zones caused by bacteriocin activity were recognized. A similar procedure was used to examine the bacteriocin susceptibility of the test strain. The bacteriocin type of the test strain was defined based on its bacteriocin production as judged by zones of inhibition against a set of eight indicator strains and by susceptibility or resistance of the test strain to bacteriocin produced by six producer strains. Of 373 strains of P. cepacia, 95.2% were typed into a total of 44 type combinations. Bacteriocin typing provided a suitable procedure for epidemiological studies of colonization or infection by P. cepacia. The technique described in this paper was simple to perform, gave a result within 24 h, provided good strain discrimination, and was suitable for clinical, environmental, and phytopathogenic strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley S. A., Duggleby C. J., Worsey M. J., Williams P. A., Hardy K. G., Broda P. Two modes of loss of the Tol function from Pseudomonas putida mt-2. Mol Gen Genet. 1977 Jul 20;154(2):203–204. doi: 10.1007/BF00330838. [DOI] [PubMed] [Google Scholar]
- Beckman W., Lessie T. G. Response of Pseudomonas cepacia to beta-Lactam antibiotics: utilization of penicillin G as the carbon source. J Bacteriol. 1979 Dec;140(3):1126–1128. doi: 10.1128/jb.140.3.1126-1128.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. E., Kaslow R. A., Mackel D. C., Fulkerson C. C., Mallison G. F. Aqueous quaternary ammonium antiseptics and disinfectants. Use and misuse. JAMA. 1976 Nov 22;236(21):2415–2417. [PubMed] [Google Scholar]
- Gonzalez C. F., Vidaver A. K. Bacteriocin, plasmid and pectolytic diversity in Pseudomonas cepacia of clinical and plant origin. J Gen Microbiol. 1979 Jan;110(1):161–170. doi: 10.1099/00221287-110-1-161. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: morphology and mode of action of contractile pyocins. J Gen Microbiol. 1974 Jan;80(1):1–15. doi: 10.1099/00221287-80-1-1. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: production of contractile and flexuous pyocins in Pseudomonas aeruginosa. J Gen Microbiol. 1974 Jan;80(1):17–30. doi: 10.1099/00221287-80-1-17. [DOI] [PubMed] [Google Scholar]
- Heidt A., Monteil H., Richard C. O and H serotyping of Pseudomonas cepacia. J Clin Microbiol. 1983 Sep;18(3):738–740. doi: 10.1128/jcm.18.3.738-740.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984 Feb;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- Moody M. R., Young V. M., Kenton D. M. In vitro antibiotic susceptibility of pseudomonads other than Pseudomonas aeruginosa recovered from cancer patients. Antimicrob Agents Chemother. 1972 Nov;2(5):344–349. doi: 10.1128/aac.2.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallent L. J., Hugo W. B., Grant D. J., Davies A. Pseudomonas cepacia as contaminant and infective agent. J Hosp Infect. 1983 Mar;4(1):9–13. doi: 10.1016/0195-6701(83)90059-2. [DOI] [PubMed] [Google Scholar]
- Randall C. The problem of Pseudomonas cepacia in a hospital. Can J Public Health. 1980 Mar-Apr;71(2):119–123. [PubMed] [Google Scholar]
- Sobel J. D., Hashman N., Reinherz G., Merzbach D. Nosocomial Pseudomonas cepacia infection associated with chlorhexidine contamination. Am J Med. 1982 Aug;73(2):183–186. doi: 10.1016/0002-9343(82)90176-0. [DOI] [PubMed] [Google Scholar]
- Stewart D. J. A composite arginine glucose medium for the characterization of Pseudomonas aeruginosa and other gram negative bacilli. J Appl Bacteriol. 1971 Dec;34(4):779–786. doi: 10.1111/j.1365-2672.1971.tb01015.x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]




