Abstract
Overproduction of reactive oxygen species in aging tissues has been implicated in the pathogenesis of aging-associated cardiovascular dysfunction. Oxidant-induced DNA-damage activates the poly(ADP-ribose) polymerase (PARP) pathway, leading to tissue injury. In this study we investigated the acute effects of the PARP inhibitor INO-1001 on aging-associated cardiac and endothelial dysfunction. Using a pressure-volume conductance catheter, left ventricular pressure-volume analysis of young and aging rats was performed before and after a single injection of INO-1001. Endothelium-dependent and -independent vasorelaxation of isolated aortic rings were investigated by using acethylcholine and sodium nitroprusside. Aging animals showed a marked reduction of myocardial contractility and endothelium-dependent relaxant responsiveness of aortic rings. Single dose INO-1001-treatment resulted in acute improvement in their cardiac and endothelial function. Immunohistochemistry for nitrotyrosine and poly(ADP-ribose) confirmed enhanced nitro-oxidative stress and PARP-activation in aging animals. Acute treatment with INO-1001 decreased PARP-activation, but did not affect nitrotyrosine-immunoreactivity. Our results demonstrate that the aging-associated chronic cardiovascular dysfunction can be improved, at least, short term, by a single treatment course with a PARP-inhibitor, supporting the role of the nitro-oxidative stress–PARP–pathway in the age-related functional decline of the cardiovascular system. Pharmacological inhibition of PARP may represent a novel therapeutic utility to improve aging-associated cardiovascular dysfunction.
Keywords: aging, nitro-oxidative stress, poly(ADP-ribose) polymerase, cardiac function, endothelial function, immunohistochemistry
Introduction
There is emerging evidence that aging is an important risk factor in the development of ischemic heart disease. This may be due to an age-associated increase in coronary vascular resistance leading to reduced myocardial blood supply and flow reserve (Hachamovitch et al., 1989). Numerous studies suggest that aging is associated with impaired function of endothelium in laboratory animals (Tschudi et al., 1996) and humans (Egashira et al., 1993) and this endothelial dysfunction predisposes the aging population to cardiovascular complications and micro-thrombus formation. Recent studies demonstrate that the cardiovascular dysfunction associated with advanced aging is related to the local formation of reactive oxygen and nitrogen species in the myocardium and coronary vasculature (Bejma et al., 2000; van der Loo et al., 2000; Csiszar et al., 2002).
Aging organisms are exposed to continuous oxidative injury, due to the higher rate of superoxide and other free radical production from the mitochondrial electron-transport chain (Sohal & Sohal, 1991). Increases in reactive oxidant species (ROS) and other oxidants at old age can elicit oxidative modifications of various cell components, such as lipid, protein and particularly DNA (de la Asuncion et al., 1996).
Various oxygen and nitrogen species (peroxynitrite, hydrogen peroxide, hydroxyl radical and nitroxyl anion) have been established as pathophysiological relevant endogenous triggers of DNA single-strand breakage and activation of the poly(ADP-ribose) polymerase (PARP) enzyme (Virag & Szabo, 2002). When activated by DNA single strand breaks, PARP initiates an energy-consuming metabolic cycle by transferring ADP-ribose units from NAD+ to nuclear proteins. This process results in rapid depletion of intracellular ATP-pools and impaired mitochondrial respiration, eventually leading to cellular energetic crisis, dysfunction and death via the necrotic route (Virag & Szabo, 2002).
Pharmacological attempts against nitro-oxidative stress using classic antioxidants, such as vitamin E (which works by scavenging toxic oxidation products), ascorbate or glutathione (which react with peroxynitrite, albeit at a relatively slow rate) resulted in conflicting results in experimental models of disease (Ceriello, 2003). Based on recent studies, pharmacological inhibition of PARP (Szabo C et al., 2004b; Soriano et al., 2001a; Szabo et al., 2003; Beller et al., 2006) or decomposition of peroxynitrite (Szabo et al., 2002; Pacher et al., 2003; Radovits et al., 2006), which block the peroxynitrite - DNA injury - poly(ADP-ribose) polymerase pathway emerge as potent novel antioxidant therapeutic possibilities in multiple pathophysiological conditions.
Chronic treatment with PARP-inhibitors PJ34 and INO-1001 for 2 months in a rodent model has been demonstrated to improve endothelial and cardiac dysfunction associated with aging (Pacher et al., 2002e, Pacher et al., 2004b) showing the involvement of the nitro-oxidative stress - PARP - pathway in the pathophysiology of cardiac and vascular aging.
Considering the theoretical possibility of acutely interrupting this pathway by pharmacological inhibition of the PARP enzyme, thereby quickly restoring the ATP-pools and the normal energy supply of the cells, we investigated in this study whether cardiac and vascular dysfunction at old age may be beneficially affected even by a single treatment course with a potent pharmacological inhibitor of PARP.
Materials and Methods
Animals, treatment protocols
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). All procedures and handling of animals during the investigations were reviewed and approved by the Ethical Committee of the Land Baden-Württemberg for Animal Experimentation.
Young adult (3 month old, 200–250g) and aging (20 month old, 450–600g) male Lewis rats (Charles River, Sulzfeld, Germany) were housed in a room at a constant temperature of 22 ± 2 °C with 12 hours light/dark cycles and fed a standard laboratory rat diet and water ad libitum.
Hemodynamic measurements
Young (n=7) and aging (n=7) rats were anesthetized with thiopentone sodium (60 mg kg−1 i.p.), tracheotomized, intubated and artificially ventilated. Animals were placed on controlled heating pads and core temperature measured via a rectal probe was maintained at 37 °C. The thoracic cavity was opened to permit access to the apex of the heart. All incisions were kept to a minimum to avoid major blood loss. The left ventricle was punctured by a 20 G plastic cannula, through which a 2F microtip pressure-volume catheter (SPR-838, Millar Instruments, Houston, TX, USA) was inserted into the left ventricular cavity. Mean arterial pressure was measured via the right femoral artery. After stabilization for 5 minutes, the signals were continuously recorded using a pressure-volume conductance system (Leycom, Zoetermeer, The Netherlands) coupled to an A/D converter (EMKA Technologies, Paris, France) at a sampling rate of 1000 s−1, stored and displayed on a computer by the IOX Software System (EMKA Technologies, Paris, France). With the help of a special pressure-volume analysis program (EMKA Technologies, Paris, France) mean arterial pressure (MAP), maximal left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and developed pressure (DP) were computed and calculated. Left ventricular pressure-volume relations were measured by transiently compressing the inferior vena cava. The slope (Ees) of the left ventricular end-systolic pressure-volume relationships (ESPVR), preload recruitable stroke work (PRSW) and maximal slope of systolic pressure increment – end-diastolic volume relation (+dP/dt-EDV) were calculated as load-independent indexes of left ventricular contractility. The above detailed left ventricular pressure-volume analysis was performed before and 60 minutes after a single dose iv. injection of the potent PARP-inhibitor INO-1001 (5mg kg−1). Myocardial INO-1001 concentrations as high as 100–400 nM can be achieved by dosing of the compound in doses similar to this one in rodents. These tissue concentrations are sufficient to provide a substantial inhibition of the cellular activity of PARP (Xiao et al. 2005). Myocardial sections of the rats used for the hemodynamic measurements were removed for immunohistochemical processing immediately after completing the left ventricular pressure-volume analysis.
In vitro organ bath experiments
In additional experiments, young and aging rats received a single intraperitoneal injection of vehicle (control groups) or the PARP-inhibitor INO-1001 (5 mg kg−1) (treatment groups) intraperitoneally. 2 hours after the injection, animals were anesthetized with thiopentone sodium (60 mg kg−1 i.p.), the descending thoracic aorta was carefully removed and placed in cold (+4 °C), oxigenized Krebs-Henseleit solution (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.77 mM CaCl2, 25 mM NaHCO3, 11.4 mM glucose; pH=7.4). The aortae were prepared and cleaned from periadventitial fat and surrounding connective tissue and cut transversely into 4-mm width rings using an operation microscope. An additional thoracic aortic segment from each animal was prepared and separated for immunohistochemical processing.
Isolated aortic rings were mounted on stainless steel hooks in individual organ baths (Radnoti Glass Technology, Monrovia, CA, USA), containing 25 ml of Krebs-Henseleit solution at 37 °C and aerated with 95% O2 and 5% CO2. Special attention was paid during the preparation to avoid damaging the endothelium.
Isometric contractions were recorded using isometric force transducers (Radnoti Glass Technology, Monrovia, CA, USA), digitized, stored and displayed with the IOX Software System (EMKA Technologies, Paris, France).
The aortic rings were placed under a resting tension of 2 g and equilibrated for 60 minutes. During this period, tension was periodically adjusted to the desired level and the Krebs-Henseleit solution was changed every 30 minutes. Maximal contraction forces to potassium chloride (KCl, 100mM) were determined and aortic rings were washed until resting tension was again obtained. Phenylephrine (10−6 M) was used to precontract the rings until a stable plateau was reached, and relaxation responses were examined by adding cumulative concentrations of endothelium-dependent dilator acethylcholine (ACh, 10−9–10−4 M) and endothelium-independent dilator sodium nitroprusside (SNP, 10−10–10−5 M). Contractile responses are expressed as grams of tension, relaxation is expressed as percent of contraction induced by phenylephrine (10−6 M).
Immunohistochemical analysis
Myocardial sections and aortic segments separated for immunohistochemical processing were fixed immediately after excision in buffered paraformaldehyde solution (4%) for 1 day. Three adjacent sections were processed for both of the following types of immunohistochemical labelling. According to the methods previously described (Liaudet et al., 2000), we performed immunohistochemical staining for nitrotyrosine (NT, a marker of nitrosative stress in general (Halliwell, 1997)), and for poly(ADP-ribose) (PAR, the enzymatic product of PARP). Primary antibodies used for the stainings were polyclonal sheep anti-nitrotyrosine antibody (Upstate, Chicago, IL, USA) and mouse monoclonal anti-poly(ADP-ribose) antibody (Calbiochem, San Diego, CA, USA).
Statistical analysis
All data are expressed as means ± s.e.mean. Intergroup comparisons were performed by using one-way analysis of variance followed by Student’s unpaired t-test with Bonferroni’s correction for multiple comparisons. Differences were considered significant when p<0.05.
Drugs
Phenylephrine, acethylcholine and sodium nitroprusside (Sigma-Aldrich, Germany) were dissolved in normal saline, INO-1001, the potent indeno-isoquinolinone-based poly(ADP)-ribose polymerase inhibitor (Inotek Pharmaceuticals Corporation, Beverly, MA, USA; Jagtap et al., 2005) was dissolved in 5% glucose solution.
Results
Immunohistochemical analysis
Immunohistochemical staining showed increased immunoreactivity for nitrotyrosine and poly(ADP-ribose) - indicative of nitrosative stress and enhanced activation of PARP - in the left ventricular myocardium and in the aortic wall (mainly in the endothelium) of aging rats. (Fig. 1–2.)
Figure 1. Photomicrographs of nitrotyrosine immunohistochemistry.
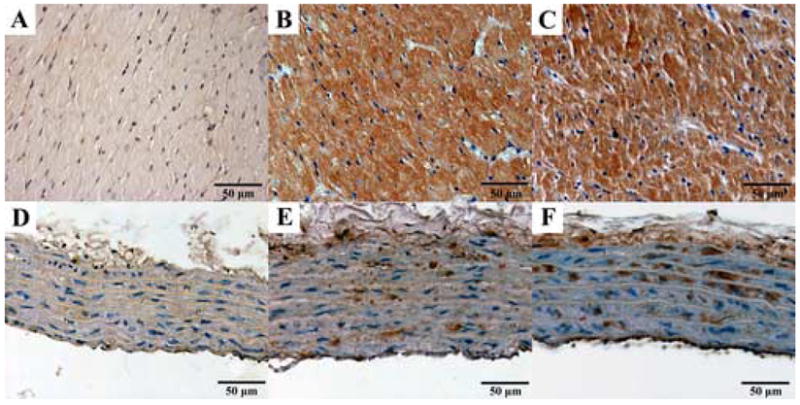
Representative immunohistochemical stainings for nitrotyrosine (NT, brown staining) in the myocardium (A–C) and aortic wall (D–F). Young control group: A, D; aging control group: B, E; and aging INO-1001 treatment groups: C, F (magnification: 400X, scale bar: 50μm).
Figure 2. Photomicrographs of poly(ADP-ribose) immunohistochemistry.
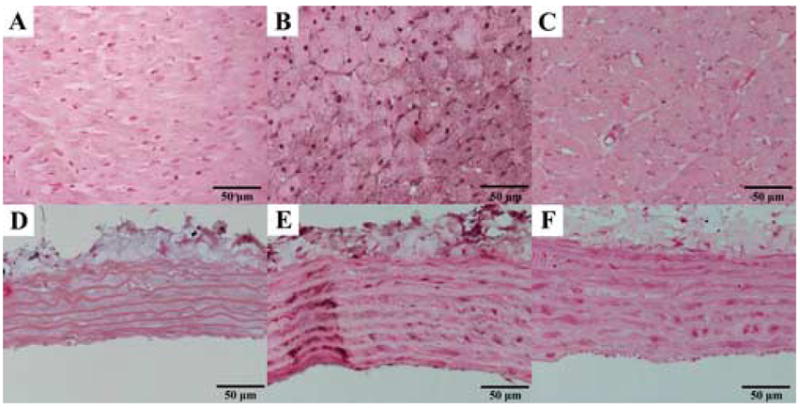
Representative immunohistochemical stainings for poly(ADP-ribose) (PAR, dark brown/black staining mainly in cell nuclei) in the myocardium (A–C) and aortic wall (D–F). Young control group: A, D; aging control group: B, E; and aging INO-1001 treatment groups: C, F (magnification: 400X, scale bar: 50μm).
Single dose treatment with the potent PARP-inhibitor INO-1001 notably decreased PAR formation both in the myocardium and the aortic wall. Immunoreactivity for nitrotyrosine was not affected by acute PARP-inhibition. Figure 1. and 2. show representative stainings for NT and PAR in the young control, aging control and INO-1001 treatment groups.
Vascular function
Similar to previous studies, the impairment of endothelial function in aging rats was demonstrated in our in vitro organ bath experiments. The aging-associated endothelial dysfunction was indicated by the reduced maximal relaxation of isolated aortic rings to ACh (61.2 ± 2.19% aging control vs. 80.8 ± 1.99% young control, p<0.05), and the rightward shift of the dose-response curve as compared with the young control group. (Fig. 3. A). Single dose treatment with PARP-inhibitor INO-1001 significantly improved the ACh-induced, endothelium-dependent, nitric oxide mediated vasorelaxation in aging animals (maximal relaxation: 69.1 ± 2.25% aging treatment group vs. 61.2 ± 2.19% aging control, p<0.05). The same treatment had no effect in young rats. (Fig. 3. A)
Figure 3. Rapid reversal of aging-associated vascular dysfunction by treatment with INO-1001 in rat aortic rings.
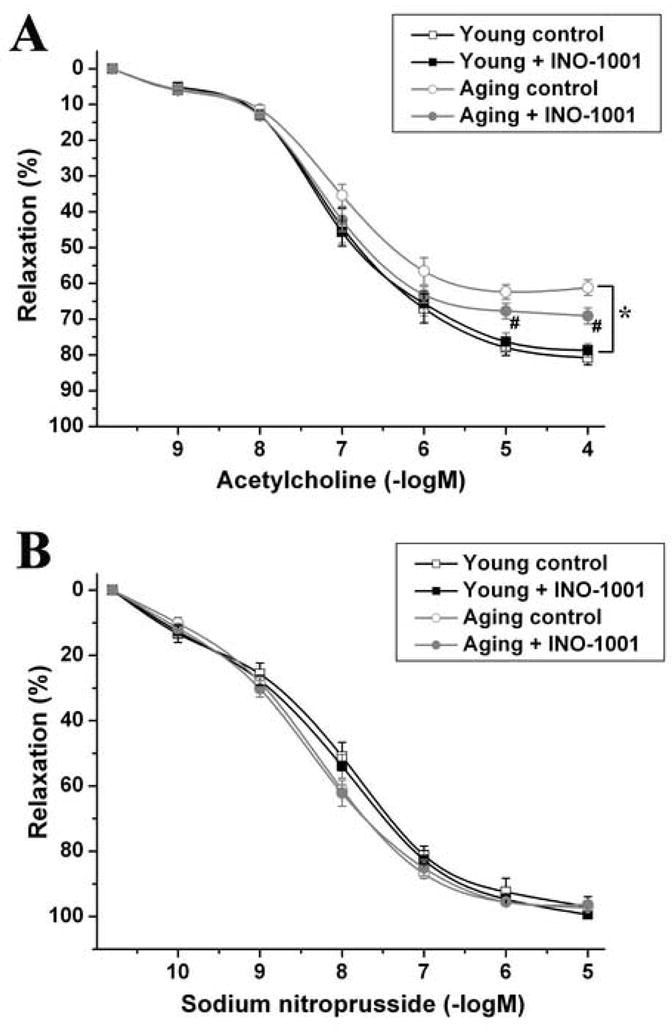
ACh-induced endothelium-dependent relaxation (A), and SNP-induced endothelium-independent relaxation (B). Each point of the curve represents mean ± s.e.mean of 12 experiments with thoracic aortic rings in all groups. *, P<0.05 versus young control; #, P<0.05 versus aging control.
The endothelium-independent vascular smooth muscle function indicated by the vasorelaxation of aortic rings to SNP was not impaired in aging rats and was also unaffected by acute INO-1001 treatment. (Fig. 3. B)
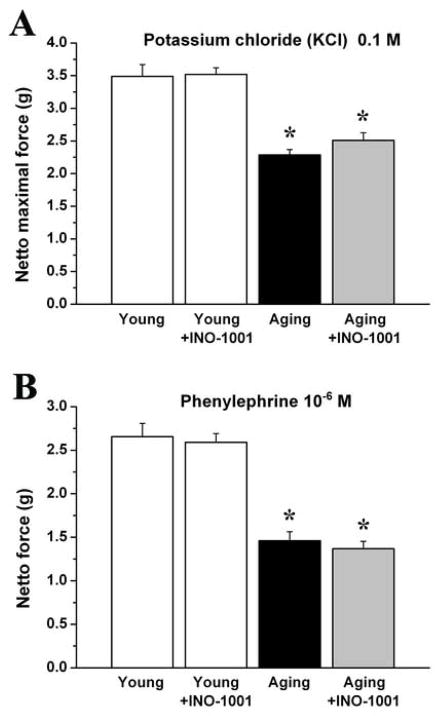
Maximal isometric forces produced by the isolated aortic rings precontracted by potassium chloride (100mM) and phenylephrine (10−6 M) were significantly lower in the aging control group as compared with young animals, which was not influenced by acute PARP-inhibition. (Fig. 4.)
Figure 4. The effect of aging and acute PARP-inhibition on contraction of rat aortic rings.
Contraction forces induced by potassium chloride (KCl; 0.1M) (A) and phenylephrine (PE; 10−6 M) (B). Each column represents mean ± s.e.mean of 12 experiments with thoracic aortic rings in all groups. *, P<0.05 versus young control.
Cardiac function
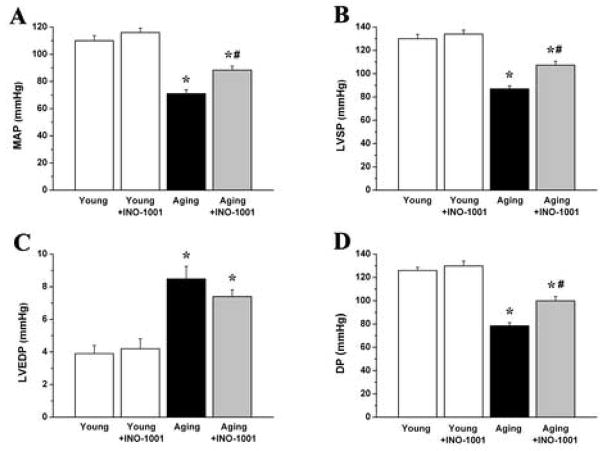
In the aging control group we found significantly decreased mean arterial pressure (MAP), maximal left ventricular systolic pressure (LVSP), developed pressure (DP) and increased left ventricular end-diastolic pressure (LVEDP). Single dose treatment with INO-1001 in aging rats significantly improved the hemodynamic parameters MAP, LVSP and DP (Fig. 5.).
Figure 5. The effect of aging and acute PARP-inhibition on arterial and left ventricular blood pressure.
Mean arterial pressure (MAP), maximal left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and developed pressure (DP) are shown in young adult, young treated with INO-1001, aging and aging treated with INO-1001 male rats. Values are mean ± s.e.mean of 7 experiments in each group. *, P<0.05 versus young control; #, P<0.05 versus aging control.
When compared to the young group, aging in rats was associated with significantly decreased left ventricular contractility. The load independent, PV-loop derived contractility indexes (Emax, PRSW, +dP/dt-EDV) showed a marked reduction in aging animals. After acute PARP-inhibition, we observed significantly increased Emax and PRSW, indicating the rapid improvement in left ventricular contractility (Fig. 6.). Treatment with INO-1001 in young rats had no effect on any of the hemodynamic parameters studied (Fig. 5–6.).
Figure 6. The effect of aging and acute PARP-inhibition on cardiac contractility.
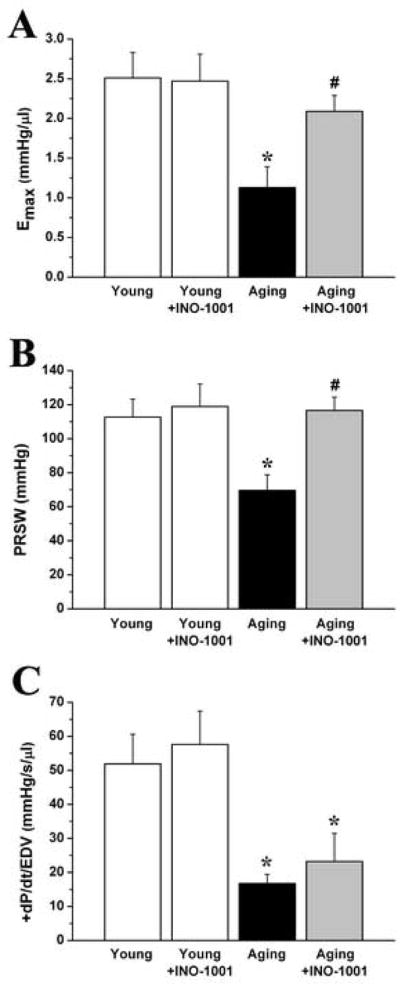
The slope (Ees) of the left ventricular end- systolic pressure-volume relationships (ESPVR), preload recruitable stroke work (PRSW) and maximal slope of systolic pressure increment – end diastolic volume relation (+dP/dt-EDV) are shown in young adult, young treated with INO-1001, aging and aging treated with INO-1001 male rats. Values are mean ± s.e.mean of 7 experiments in each group. *, P<0.05 versus young control; #, P<0.05 versus aging control.
Discussion
In the current study we demonstrate that a single injection of PARP-inhibitor INO-1001 effectively decreases the age-related myocardial and vascular PARP-activation, resulting in acute improvement of left ventricular contractility and enhanced endothelium-dependent vasorelaxation in a rat model of aging-associated cardiovascular dysfunction.
Recent studies elucidated numerous cellular and molecular mechanisms responsible for the functional decline of the cardiovascular system at old age (Csiszar et al., 2005). The oxidative stress hypothesis (or free radical theory, as it was originally proposed) (Harman, 1956) is currently one of the most favored explanations for how aging leads to progressive cellular damage at the biochemical level. According to this theory, age-related loss of physiological function and aging is caused by the deleterious effects of progressive and irreversible accumulation of oxidative damage. Several previous studies have demonstrated that aging organisms have a higher rate of free radical production (O2−. and H2O2) due to the incomplete terminal oxidation in the mitochondria at older age (Sohal & Sohal, 1991; Nohl et al., 1978; Kim et al., 1996). Superoxide anions interact with the physiological mediator nitric oxide, forming the potent oxidant peroxynitrite. Hydroxyl radicals are formed mainly from hydrogen-peroxide in the Haber-Weiss reaction catalysed by iron. Nitro-oxidative stress accompanied by increased formation of hydrogen peroxide, hydroxyl radical, and peroxynitrite are endogenous inducers of DNA single strand breakage, that is the obligatory trigger of activation of the nuclear enzyme poly(ADP-ribose) polymerase (PARP), which mediates the cellular response to DNA injury (Virag & Szabo, 2002).
Depending on the severity of DNA damage, different cellular pathways can be triggered. In the case of mild DNA damage, PARP facilitates DNA repair and thus cell survival. Severe DNA injury causes excessive PARP activation that initiates an energy-consuming futile repair cycle by transferring ADP-ribose units from NAD+ to nuclear proteins. The excessive nuclear poly(ADP-ribose) formation results in rapid depletion of intracellular NAD+ and ATP-pools, slowing the rate of glycolysis and mitochondrial respiration, eventually leading first to cellular energetic crisis and dysfunction, then to cell necrosis (Soriano et al., 2001b). By this route, PARP activation in cardiomyocytes and endothelial cells causes functional impairment of contractile function at the cellular level and reduced ability of endothelial cells to produce nitric oxide when stimulated by an endothelium-dependent relaxant agonist, such as acetylcholine (Soriano et al., 2001b; Szabo et al., 1997; Pacher et al., 2002a; Pacher et al., 2002b; Szabo et al., 2004a; Pacher et al., 2002d; Pacher et al., 2002e; Szabo & Bahrle, 2005). Impairment of endothelial function in the coronary arteries may lead to regional or global myocardial ischaemia, which secondarily impairs cardiac performance. The increased ROS-formation and PARP activation together with the above discussed downstream molecular and intracellular mechanisms are considered to play an important role in the pathogenesis of various forms of chronic heart failure (Pacher et al., 2002d; Pacher et al., 2006).
Similar to other studies (Radovits et al., 2006; van der Loo et al., 2000; Csiszar et al., 2002; Csiszar et al., 2005), we demonstrated increased immunoreactivity for nitrotyrosine and activation of PARP in the left ventricular myocardium and aortic wall of aging rats (Fig. 1–2.), which confirms the nitro-oxidative stress and the activation of the peroxynitrite-poly(ADP-ribose) polymerase pathway, and are consistent with the above discussed “free radical theory” of aging (Pacher et al., 2005; Ungvari et al., 2005).
INO-1001 effectively decreased PAR-formation in various models of disease, but there are conflicting data about the effect of PARP-inhibitors on tyrosine-nitration (Beller et al., 2006; Farivar et al., 2005; Pacher et al., 2002c). After single dose treatment with INO-1001 in aging rats we found decreased PAR staining (reflecting decreased PARP activity) both in the myocardium and the aortic intima, however, the immunoreactivity for protein-nitrotyrosine was unaffected. These findings are consistent with the hypothesis that short-term PARP inhibition is sufficient to affect the imbalance between the rapid and reversible polymerization and degradation of ADP-ribose units (by poly(ADP-ribose) polymerase (PARP) and poly(ADP-ribose) glycohydrolase (PARG); Davidovic et al., 2001), but it does not affect upstream processes such as peroxynitrite generation and action (a marker of which is nitrotyrosine), and/or it might exert feedback effects on the generation of oxidants, but this is not reflected in the staining for nitrotyrosine, as this is a rather stable product.
In aging vessels, the nitro-oxidative damage of the vascular smooth muscle layers were found to be less pronounced, when compared to the endothelium. (Fig. 1.) These immunohistochemical findings are in line with recent reports demonstrating that the vascular superoxide-overproduction at old age occurs mainly in the endothelial cells (van der Loo et al., 2000), which are in addition more vulnerable to oxidative injury.
Endothelial dysfunction associated with advanced aging is a well-known phenomenon and can be explained by the reduced nitric oxide (NO) production of endothelial cells (Soriano et al., 2001b); or by the increased NO-inactivation by superoxide anions (peroxynitrite formation) resulting in altered NO bioavailibility (van der Loo et al., 2000). The underlying intracellular pathways and molecular mechanisms have been subject of intensive investigations recent years (van der Loo et al., 2000; Pacher et al., 2004b; Pacher et al., 2002e; Pacher et al., 2002f). In accordance with these studies we report here impaired endothelium-dependent acetylcholine-induced relaxation of isolated aortic rings of aging rats. The endothelium-independent relaxation induced by the exogenously administered NO-donor SNP was unaffected by aging, indicating the normal dilative capacity of the vascular smooth muscle. These functional data are consistent with our immunohistochemical findings showing signs of severe nitro-oxidative damage mainly in the endothelium of aging vessels. In contrast with a previous work using epinephrine for precontraction (Pacher et al., 2002e), we found a significant decrease in contraction forces induced by phenylephrine and potassium chloride in aging animals which was in line with the results of another study investigating vascular function of diabetic rats (Soriano et al., 2001a) and may be due to alterations in receptor density and/or receptor/effector coupling.
Previous studies report acute amelioration of endothelial dysfunction in chronic diseases and pathophysiological conditions: single doses of antioxidants (such as vitamin C), tetrahydrobiopterin and L-arginine were shown to improve endothelial dysfunction associated with hypertension, diabetes, chronic smoking or atherosclerosis (Pieper, 1997; Heitzer et al., 2000; Taddei et al., 1998; Quyyumi, 1998). All of these therapeutical attempts focuses on the restoration of nitric oxide bioavailibility either by directly supplying the endothelial nitric oxide synthase with substrate/co-factor (and reversing eNOS-uncoupling) or by decreasing NO-removal by reducing nitro-oxidative stress. As previously reported, in vitro incubation of blood vessels with PARP-inhibitors resulted in rapid reversal of endothelial dysfunction in aortae from diabetic mice (Soriano et al., 2001a) and also in the early stage of atherosclerosis (Benko et al., 2004). Similarly to the results of these studies we report here significantly enhanced endothelium-dependent vasorelaxation (improved endothelial function) in aortic rings of rats with advanced aging after a single dose injection of PARP-inhibitor INO-1001.
We propose that the aging-related overproduction of ROS represents a continuous trigger of DNA single strand breakage that, in turn keeps PARP in an activated state, which continuously depletes the ATP-pools of endothelial cells, thereby impairing the endothelium-dependent relaxant responsiveness. Under this continuing nitro-oxidative stress the endothelium is likely to exist in a state of chronic energy starvation (metabolic suppression) associated with dysfunction. Due to the reversibility of the rapid polymerization of poly(ADP-ribose) units, pharmacological interruption of this metabolic cycle by acute inhibition of PARP can lead to normalization of the energy balance of endothelial cells by rapid restoration of endothelial ATP-levels to normal levels (Szabo G et al. 2002), and according to some reports even to higher levels (Csordas et al. 2006). Considering the fact that - similarly to former studies - our results on young control rats show no direct vasodilatory effects of INO-1001, the found acute effect of PARP-inhibition on aging-associated endothelial dysfunction can be explained by this intracellular mechanism. However, most of the studies on ATP-levels supporting our hypothesis were conducted on cultured endothelial cells, obvious evidence for the proposed mechanism could be provided by directly measuring in vivo/ex vivo tissue ATP-levels, but this was not conducted in the present study, and therefore alternative explanations for the current findings are also theoretically possible.
Recent studies performing invasive hemodynamic measurements in aging rats report decreased cardiac performance and development of progressive heart failure after the age of 20 months (Radovits et al., 2006; Pacher et al., 2004a; Pacher et al., 2004b; Anversa et al., 1989). A recent study provided detailed echocardiographic evidence of a progressive decrement in multiple aspects of systolic and diastolic LV function in aging rats (Boluyt et al., 2004). Consistent with these results, we demonstrated that advanced aging in rats is associated with impaired myocardial contractility, as reflected by the PV-loop-derived load-independent contractility-indexes Emax, PRSW or +dP/dt-EDV. These indexes are widely used as sensitive cardiac contractile parameters, because they are independent from changes in loading conditions and therefore especially informative in assessing cardiac contractility in models, where preload and afterload are altered.
The possible molecular mechanism responsible for the rapid improvement of cardiac dysfunction are supposed to be the energy restoration of myocardial cells, similarly to that discussed detailed above. In other words, similar to the situation in blood vessels, it is possible (although remains to be directly confirmed) that PARP inhibition acutely affects cardiac contractility via improvement of cardiac high energy phosphate status. An acute improvement in the myocardial blood supply (due to improved endothelial function in the coronary microvasculature) may also be feasible.
In accordance with our previous work with other PARP-inhibitors (Szabo G et al., 2002) we found that INO-1001 did not affect cardiac function of young control rats. Thus the improved cardiac function seen in the aging treatment group is a specific phenomenon, reflecting a reversal of the aging-associated suppressed myocardial performance, rather than the consequence of some nonspecific direct cardiac effects of INO-1001.
This is the first study reporting rapid improvement of the chronic cardiac and vascular dysfunction associated with advanced aging by acute inhibition of the PARP enzyme. The current findings indicate the importance of the nitro-oxidative stress - PARP - pathway, especially its quickly reversible energy depleting aspects in the pathogenesis of myocardial and endothelial dysfunction at old age. The current work further supports the concept that PARP-inhibition may represent a potential therapy approach to improve cardiovascular dysfunction associated with aging. There are many studies and reviews about the clinical treatment perspectives of PARP-inhibitors (Jagtap and Szabo, 2005; Graziani and Szabo, 2005). We probably already know enough about the safety of PARP inhibitors to consider them suitable for short-term treatment of acute situations where the inhibition of PARP may provide considerable benefit, e.g. in myocardial reperfusion injury. However, chronic use of PARP inhibitors may be more challenging because of the unknown potential long-term side effects. Whether or not chronic PARP inhibition is safe, there is no clear answer, as chronic safety studies have not yet been conducted with any of the orally bioavailable PARP inhibitors. Depending on the outcome of such studies, chronic PARP inhibition may or may not turn out to be clinically sustainable. It also remains to be tested as to how long is the period for which a single dose of the PARP inhibitor provides cardiovascular benefit. In chronic heart failure models there is evidence that the beneficial effect of PARP inhibition is sustained after discontinuation of the treatment (Pacher et al., 2002c), therefore the duration of the beneficial effect seen in the current study needs to be delineated.
Acknowledgments
This work was supported by a Grant from the German Research Foundation (SFB 414) to G.S. and by the Hungarian Research Fund (OTKA AT049488) and the National Institutes of Health (R01 GM060915) to C.S. The expert technical assistance of Anne Schuppe and Heike Ziebart are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ANVERSA P, PUNTILLO E, NIKITIN P, OLIVETTI G, CAPASSO JM, SONNENBLICK EH. Effects of age on mechanical and structural properties of myocardium of Fischer 344 rats. Am J Physiol. 1989;256:H1440–9. doi: 10.1152/ajpheart.1989.256.5.H1440. [DOI] [PubMed] [Google Scholar]
- DE LA ASUNCION JG, MILLAN A, PLA R, BRUSEGHINI L, ESTERAS A, PALLARDO FV, SASTRE J, VINA J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–8. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- BEJMA J, RAMIRES P, JI LL. Free radical generation and oxidative stress with aging and exercise: differential effects in the myocardium and liver. Acta Physiol Scand. 2000;169:343–51. doi: 10.1046/j.1365-201x.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- BELLER CJ, RADOVITS T, KOSSE J, GERÖ D, SZABO C, SZABO G. Activation of the peroxynitrite-poly(adenosine diphosphate-ribose) polymerase pathway during neointima proliferation: a new target to prevent restenosis after endarterectomy. J Vasc Surg. 2006;43:824–30. doi: 10.1016/j.jvs.2005.11.021. [DOI] [PubMed] [Google Scholar]
- BENKO R, PACHER P, VASLIN A, KOLLAI M, SZABO C. Restoration of the endothelial function in the aortic rings of apolipoprotein E deficient mice by pharmacological inhibition of the nuclear enzyme poly(ADP-ribose) polymerase. Life Sci. 2004;75:1255–61. doi: 10.1016/j.lfs.2004.04.007. [DOI] [PubMed] [Google Scholar]
- BOLUYT MO, CONVERSO K, HWANG HS, MIKKOR A, RUSSELL MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96:822–8. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- CERIELLO A. New insights on oxidative stress and diabetic complications may lead to a „causal” antioxidant therapy. Diabetes Care. 2003;26:1589–96. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- CSISZAR A, PACHER P, KALEY G, UNGVARI Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol. 2005;3:285–91. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSISZAR A, UNGVARI Z, EDWARDS JG, KAMINSKI P, WOLIN MS, KOLLER A, KALEY G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–66. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- CSORDAS A, WICK G, BERNHARD D. Hydrogen peroxide-mediated necrosis induction in HUVECs is associated with an atypical pattern of caspase-3 cleavage. Exp Cell Res. 2006;312:1753–64. doi: 10.1016/j.yexcr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- DAVIDOVIC L, VODENICHAROV M, AFFAR EB, POIRIER GG. Importance of poly(ADP-ribose) glycohydrolase in the control of poly(ADP-ribose) metabolism. Exp Cell Res. 2001;268:7–13. doi: 10.1006/excr.2001.5263. [DOI] [PubMed] [Google Scholar]
- EGASHIRA K, INOU T, HIROOKA Y, KAI H, SUGIMACHI M, SUZUKI S, KUGA T, URABE Y, TAKESHITA A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- FARIVAR AS, MCCOURTIE AS, MACKINNON-PATTERSON BC, WOOLLEY SM, BARNES AD, CHEN M, JAGTAP P, SZABO C, SALERNO CT, MULLIGAN MS. Poly (ADP) ribose polymerase inhibition improves rat cardiac allograft survival. Ann Thorac Surg. 2005;80:950–6. doi: 10.1016/j.athoracsur.2005.02.035. [DOI] [PubMed] [Google Scholar]
- GRAZIANI G, SZABO C. Clinical perspectives of PARP inhibitors. Pharmacol Res. 2005;52:109–18. doi: 10.1016/j.phrs.2005.02.013. [DOI] [PubMed] [Google Scholar]
- HACHAMOVITCH R, WICKER P, CAPASSO JM, ANVERSA P. Alterations of coronary blood flow and reserve with aging in Fischer 344 rats. Am J Physiol. 1989;256:H66–73. doi: 10.1152/ajpheart.1989.256.1.H66. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–60. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- HEITZER T, BROCKHOFF C, MAYER B, WARNHOLTZ A, MOLLNAU H, HENNE S, MEINERZT T, MUNZEL T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers : evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- JAGTAP PG, BALOGLU E, SOUTHAN GJ, MABLEY JG, LI H, ZHOU J, VAN DUZER J, SALZMAN AL, SZABO C. Discovery of potent poly(ADP-ribose) polymerase-1 inhibitors from the modification of indeno[1,2-c]isoquinolinone. J Med Chem. 2005;48:5100–3. doi: 10.1021/jm0502891. [DOI] [PubMed] [Google Scholar]
- JAGTAP P, SZABO C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- KIM JD, MCCARTER RJ, YU BP. Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging (Milano) 1996;8:123–9. doi: 10.1007/BF03339566. [DOI] [PubMed] [Google Scholar]
- LIAUDET L, SORIANO FG, SZABO E, VIRAG L, MABLEY JG, SALZMAN AL, SZABO C. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc Natl Acad Sci U S A. 2000;97:10203–8. doi: 10.1073/pnas.170226797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER LOO B, LABUGGER R, SKEPPER JN, BACHSCHMID M, KILO J, POWELL JM, PALACIOS-CALLENDER M, ERUSALIMSKY JD, QUASCHNING T, MALINSKI T, GYGI D, ULLRICH V, LUSCHER TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–44. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOHL H, BREUNINGER V, HEGNER D. Influence of mitochondrial radical formation on energy-linked respiration. Eur J Biochem. 1978;90:385–90. doi: 10.1111/j.1432-1033.1978.tb12615.x. [DOI] [PubMed] [Google Scholar]
- PACHER P, CZIRAKI A, MABLEY JG, LIAUDET L, PAPP L, SZABO C. Role of poly(ADP-ribose) polymerase activation in endotoxin-induced cardiac collapse in rodents. Biochem Pharmacol. 2002a;64:1785–91. doi: 10.1016/s0006-2952(02)01421-1. [DOI] [PubMed] [Google Scholar]
- PACHER P, LIAUDET L, BAI P, VIRAG L, MABLEY JG, HASKO G, SZABO C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002b;300:862–7. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- PACHER P, LIAUDET L, MABLEY J, KOMJATI K, SZABO C. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J Am Coll Cardiol. 2002c;40:1006–16. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- PACHER P, LIAUDET L, SORIANO FG, MABLEY JG, SZABO E, SZABO C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002d;51:514–21. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- PACHER P, MABLEY JG, SORIANO FG, LIAUDET L, KOMJATI K, SZABO C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002e;135:1347–50. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACHER P, MABLEY JG, SORIANO FG, LIAUDET L, SZABO C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med. 2002f;9:659–64. [PubMed] [Google Scholar]
- PACHER P, LIAUDET L, BAI P, MABLEY JG, KAMINSKI PM, VIRAG L, DEB A, SZABO E, UNGVARI Z, WOLIN MS, GROVES JT, SZABO C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- PACHER P, MABLEY JG, LIAUDET L, EVGENOV OV, MARTON A, HASKO G, KOLLAI M, SZABO C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004a;287:H2132–7. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACHER P, VASLIN A, BENKO R, MABLEY JG, LIAUDET L, HASKO G, MARTON A, BATKAI S, KOLLAI M, SZABO C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004b;311:485–91. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACHER P, SCHULZ R, LIAUDET L, SZABO C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–10. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACHER P, LIAUDET L, MABLEY JG, CZIRAKI A, HASKO G, SZABO C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med. 2006;17:369–75. [PMC free article] [PubMed] [Google Scholar]
- PIEPER GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- QUYYUMI AA. Does acute improvement of endothelial dysfunction in coronary artery disease improve myocardial ischemia? A double-blind comparison of parenteral D- and L-arginine. J Am Coll Cardiol. 1998;32:904–11. doi: 10.1016/s0735-1097(98)00323-4. [DOI] [PubMed] [Google Scholar]
- RADOVITS T, SERES L, GERÖ D, LIN LL, BELLER CJ, CHEN SH, ZOTKINA J, BERGER I, GROVES JT, SZABO C, SZABO G. The peroxynitrite decomposition catalyst FP15 improves aging-associated cardiac and vascular dysfunction. Mech Aging Dev. 2006 doi: 10.1016/j.mad.2006.09.005. In Press. [DOI] [PubMed] [Google Scholar]
- SOHAL RS, SOHAL BH. Hydrogen peroxide release by mitochondria increases during aging. Mech Aging Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- SORIANO FG, PACHER P, MABLEY J, LIAUDET L, SZABO C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001a;89:684–91. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- SORIANO FG, VIRAG L, SZABO C. Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly(ADP-ribose) polymerase activation. J Mol Med. 2001b;79:437–48. doi: 10.1007/s001090100236. [DOI] [PubMed] [Google Scholar]
- SZABO C, CUZZOCREA S, ZINGARELLI B, O’CONNOR M, SALZMAN AL. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Invest. 1997;100:723–35. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO C, MABLEY JG, MOELLER SM, SHIMANOVICH R, PACHER P, VIRAG L, SORIANO FG, VAN DUZER JH, WILLIAMS W, SALZMAN AL, GROVES JT. FP 15, a novel potent peroxynitrite decomposition catalyst: in vitro cytoprotective actions and protection against diabetes mellitus and diabetic cardiovascular complications. Mol Med. 2002;8:571–80. [PMC free article] [PubMed] [Google Scholar]
- SZABO G, BAHRLE S. Role of nitrosative stress and poly(ADP-ribose) polymerase activation in myocardial reperfusion injury. Curr Vasc Pharmacol. 2005;3:215–20. doi: 10.2174/1570161054368599. [DOI] [PubMed] [Google Scholar]
- SZABO G, BAHRLE S, STUMPF N, SONNENBERG K, SZABO EE, PACHER P, CSONT T, SCHULZ R, DENGLER TJ, LIAUDET L, JAGTAP PG, SOUTHAN GJ, VAHL CF, HAGL S, SZABO C. Poly(ADP-Ribose) polymerase inhibition reduces reperfusion injury after heart transplantation. Circ Res. 2002;90:100–6. doi: 10.1161/hh0102.102657. [DOI] [PubMed] [Google Scholar]
- SZABO G, BUHMANN V, ANDRASI T, STUMPF N, BAHRLE S, KEKESI V, HAGL S, SZABO C, JUHASZ-NAGY A. Poly-ADP-ribose polymerase inhibition protects against myocardial and endothelial reperfusion injury after hypothermic cardiac arrest. J Thorac Cardiovasc Surg. 2003;126:651–8. doi: 10.1016/s0022-5223(02)73235-2. [DOI] [PubMed] [Google Scholar]
- SZABO G, LIAUDET L, HAGL S, SZABO C. Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc Res. 2004a;61:471–80. doi: 10.1016/j.cardiores.2003.09.029. [DOI] [PubMed] [Google Scholar]
- SZABO G, SOOS P, MANDERA S, HEGER U, FLECHTENMACHER C, BAHRLE S, SERES L, CZIRAKI A, GRIES A, ZSENGELLER Z, VAHL CF, HAGL S, SZABO C. INO-1001 a novel poly(ADP-ribose) polymerase (PARP) inhibitor improves cardiac and pulmonary function after crystalloid cardioplegia and extracorporal circulation. Shock. 2004b;21:426–32. doi: 10.1097/00024382-200405000-00005. [DOI] [PubMed] [Google Scholar]
- TADDEI S, VIRDIS A, GHIADINI L, MAGAGNA A, SALVETTI A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–9. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- TSCHUDI MR, BARTON M, BERSINGER NA, MOREAU P, COSENTINO F, NOLL G, MALINSKI T, LUSCHER TF. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGVARI Z, GUPTE SA, RECCHIA FA, BATKAI S, PACHER P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–9. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIAO CY, CHEN M, ZSENGELLER Z, LI H, KISS L, KOLLAI M, SZABO C. Poly(ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–8. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- VIRAG L, SZABO C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]