Abstract
Sex differences in motivation are apparent for the motivation to engage in sexual behavior, the motivation to take drugs of abuse, and the motivation to engage in parental behavior. In both males and females there is an increase in NAcc DA associated with motivated behaviors. Here it proposed that sex differences in the regulation of DA activity in the ascending mesolimbic projections may underlie sex differences in motivation. In particular, sex differences in the neuroendocrine regulation of this brain system plays a role in the expression of sex differences in motivated behaviors. Here it is proposed that sexual differentiation of motivation is mediated, at least in part, by a novel mechanism in which ovarian hormones secreted at puberty in the female actively feminize the DA system.
Keywords: sexual motivation, drug abuse, sex differences, nucleus accumbens, striatum, dopamine, maternal behavior
Introduction
In 1959, Phoenix and colleagues proposed that during the prenatal period gonadal hormones “organize” the development of brain regions important for mating behavior. In the adult, hormones were thought to “activate” functions that were established during development to allow mating behavior to occur. The authors concluded with a discussion of the concept that behavior could be treated as a dependent variable that can be described as having been shaped by the organizational effects of hormone exposure on the brain (Phoenix, et al., 1959). This was a novel concept at the time, but many behaviors are now known to be sexually dimorphic due to developmental exposure to gonadal hormones. As we learn more about sex differences in the brain we also begin to see that not all behaviors are “organized” in the same way. Here we discuss the idea that an important component of mating behavior, motivation, is organized through a mechanism different from the prenatal androgen-driven sexual differentiation of mating behavior.
Motivation is the internal state of an individual that induces or drives someone to engage in a specific behavior. Some behaviors are what we think of as “naturally motivated” because we engage in these behaviors in order to survive and reproduce, and the consequence of engaging in the behavior is intrinsically rewarding. This is true for eating, drinking, and engaging in sexual behavior, where the food, water or sexual encounter are considered ‘primary’ reinforcers. Motivation to engage in other behaviors is learned or acquired through experience with either primary reinforcers or ‘secondary’ reinforcers (items that have acquired value as a reinforcer through association with primary reinforcers). On the other hand, when individuals take drugs of abuse, it is thought that these drugs tap into the endogenous reward system. Sex differences in motivation could be due to learning differences, differences in the neural systems mediating reward mechanisms, or a combination of factors.
Sex differences in motivation are apparent for the motivation to engage in sexual behavior, the motivation to take drugs of abuse, and the motivation to engage in parental behavior. In a recent chapter, Jane Taylor and I proposed that the neural systems mediating the motivation to engage in behaviors that are essential for reproductive success are different for males and females (Becker and Taylor, 2008). For most mammals, reproductive success of males depends on a reproductive system that is always ready to reproduce: motivation and sperm production are ‘on’ all the time. On the other hand, motivation in females varies with reproductive status (i.e., estrous cycle or pregnancy) and is, therefore, modulated by gonadal, placental and lactational hormones. There are also neuroanatomical sex differences in the motivational systems important for attachment to offspring. We proposed that sex differences in motivation occur as a result of the neural system that mediates the rapid onset of maternal motivation, causing females to form rapid and strong attachments to their young, as well as rapid attachments to other desired items with enhanced motivation to obtain the item (Becker and Taylor, 2008). This brief review will begin with an overview of some of the evidence for sex differences in motivation, then follow with a proposed mechanism for sexual differentiation of the neural systems important for this sex difference.
The Neurobiology of Reward: The Role of Dopamine (DA)
Over 50 years ago, Olds and Milner (Olds and Milner, 1954) reported that rats would press a bar to receive electrical stimulation in certain areas of the brain. Studies that followed using ‘intracranial self-stimulation’ found that the medial forebrain bundle was critical for the reinforcing effects of electric shock (Olds and Olds, 1969). These studies lead to the idea that there are “rewarding” areas in the brain, and the concept of a ‘reward system’ was derived from this concept. Subsequent research found that it was stimulation of the DA axons in the medial forebrain bundle that was critical for rewarding effects of intracranial self-stimulation (Anden, et al., 1966; Ungerstedt, 1971; Ungerstedt, 1973; Ungerstedt, 1974; Ungerstedt, et al., 1974).
As the concept of a reward system became generally accepted, the evidence was accumulating that the reinforcing properties of intracranial self-stimulation, drugs of abuse, and natural rewards were mediated by activation of the ascending DA pathway, and in the absence of DA these things were not rewarding. As Wise summarized (Wise, 1984), “The notion that cocaine and amphetamine can centrally activate natural reward mechanisms and that they owe their reinforcing action to such activation … is consistent with the fact that these agents have the traditional properties of conventional reinforcers … In both cases the central reinforcer can be more powerful than more “natural” reinforcers, even in cases of acute need; access to psychomotor stimulant reinforcement … or to electrical brain stimulation …can cause self starvation to the point of severe weight loss.”
We know now that DA is not the only neurotransmitter involved in reward. As summarized in a recent review (Becker and Meisel, 2007), activation of DA, and nucleus accumbens (NAcc) DA in particular, is thought to be an internal cue that tells an animal that something is desirable, that something desired is available, or that something desirable is soon to be available. Glutamate and GABA neurons modulate DA release, providing input related to context and motivational state. GABA and endogenous opioids also provide qualitative information about the hedonic value of a stimulus.
Sex Differences in Sexual Motivation
Male Sexual Behavior
Sexual behavior has both appetitive (motivational) and consummatory components (sexual ability) as do many behaviors (Pfaus and Phillips, 1991b). This has been elegantly demonstrated in experiments from the Everitt laboratory with male rats (Everitt, 1990; Everitt and Stacey, 1987). Everitt and collaborators demonstrated that male rats could be trained to bar press for access to a sexually receptive female rat, with the number of bar presses made by the male used as an objective measure of sexual motivation. The ability to engage in sexual behavior was measured in terms of mounts by the male when the female was delivered into the testing chamber. In this model, castration reduced both bar pressing for the female (i.e., sexual motivation) and sexual behavior (i.e., sexual ability). As would be predicted from a large body of research (reviewed in (Hull and Dominguez, 2007)), lesions of the medial preoptic area (POA) resulted in a severe impairment of male copulatory behavior, but had little effect on operant responding for access to the female. On the other hand, lesions of the basolateral amygdala (blAMY) reduced bar pressing for access to the female rat (i.e., sexual motivation), but failed to affect sexual ability (Everitt, 1990; Everitt and Stacey, 1987).
The results of these experiments demonstrate that there are distinct neural substrates for sexual motivation vs. sexual ability in the male. Furthermore, when amphetamine (AMPH) was delivered to the NAcc of male rats with a blAMY lesion, bar pressing for access to the female was reinstated. (Everitt, 1990). Since there are projections from the blAMY to the NAcc, DA in the NAcc released by AMPH was implicated in sexual motivation. Subsequently, a number of investigators have demonstrated that DA increases in the NAcc of male rats in anticipation of gaining access to a sexually receptive female rat as well as during sexual behavior. (Damsma, et al., 1992; Pfaus, et al., 1990; Pfaus and Phillips, 1991a; Pleim, et al., 1990). Thus, DA in the NAcc is playing an important role in sexual motivation in males.
It should be noted that the medial POA has also been shown to contribute to sexual motivation in male rats, ferrets, and marmosets (Hull and Dominguez, 2007). DA increases in the POA in anticipation of sexual contact in male rats (Hull and Dominguez, 2007). In the Japanese quail, sub-regions of the medial POA are implicated in sexual motivation while other regions are associated with sexual ability (Balthazart and Ball, 2007).
Female Sexual Behavior
Both male and female rats exhibit an increase in extracellular concentrations of DA in the NAcc during sexual behavior (Mermelstein and Becker, 1995; Pfaus et al., 1990; Pfaus, et al., 1995). In the female, however, the increase in NAcc DA depends upon the context in which the sexual behavior occurs. Laboratory experiments on sexual behavior in rodents have historically been studied in a small chamber. The male initiates contacts and engages in a rapid series of mounts and intromissions that ultimately lead to ejaculation (Adler, 1969; Bermant, 1961; Bermant, 1967). These conditions are not rewarding for a female rat; for the female the context and timing of the sexual encounter is critical to whether sexual behavior is rewarding (Jenkins and Becker, 2003b; Martinez and Paredes, 2001; Oldenberger, et al., 1992; Paredes and Alonso, 1997; Paredes and Vazquez, 1999).
If sexual behavior takes place in a chamber where the female can escape from the male, she will establish and maintain longer latencies between sexual contacts (Adler, 1969; Adler, 1978; Erskine, et al., 1989; McClintock, 1984). For laboratory rodents, the female will remove herself from the presence of the male after an intromission and return to the male about 2 min later (Jenkins and Becker, 2003b). A similar behavior occurs in the wild, but with multiple partners both females and males are able to obtain their preferred pattern of contacts during the sexual encounter (McClintock, 1984). Achieving the preferred rate of copulation is important for the female rodent to optimize the rate of vaginocervical stimulation received from a male which activates a neuroendocrine reflex that is necessary for pregnancy to occur (Adler, 1969; Adler, 1978; Erskine, 1989; McClintock, 1984; McClintock and Adler, 1977).
The female’s repeated approach and withdrawal from the male during a sexual encounter is known as pacing behavior (Erskine et al., 1989). Pacing behavior allows the female to control both the rate and duration of the copulatory contacts during mating. Importantly, sexual behavior is rewarding to the female rat when she achieves her preferred rate of copulation (Jenkins and Becker, 2003b; Martinez and Paredes, 2001; Paredes and Alonso, 1997; Paredes and Vazquez, 1999), whether or not she is actively pacing the rate of copulation (Jenkins and Becker, 2003a).
In females it has not been as easy to dissociate sexual motivation from sexual ability, since a test comparable to the operant paradigm used by the Everitt laboratory in male rats (Everitt, 1990; Everitt and Stacey, 1987), has not yet been developed for the female rat. Studies of feminine sexual ability usually assess lordosis intensity and/or quotient as the dependent measure (McCarthy and Becker, 2002). In females, lordosis can be activated by implants of estradiol followed by progesterone into the ventromedial hypothalamus (VMH), where this hormonal activation of the VMH is necessary and sufficient for lordosis to be exhibited (Meisel, et al., 1987; Rubin and Barfield, 1983). Of course other areas of the brain contribute to feminine sexual ability (McCarthy and Becker, 2002; Pfaff, 1980; Pfaff and Schwartz-Giblin, 1988). Studies have assessed female motivation using conditioned place preference (CPP), partner preference tests, or tests of pacing behavior, but these different tests don’t always provide the same results about the neural basis of sexual motivation. In general, the NAcc and POA are both implicated in female sexual motivation, although there is some disagreement (Jenkins and Becker, 2001; Rivas and Mir, 1990; Xiao and Becker, 1997; Yang and Clemens, 2000). There is also some disagreement about the neurotransmitter systems mediating sexual motivation in females, as discussed below.
Within the NAcc, in support for a role for DA in sexual motivation in females, NAcc DA increases only when female rats are receiving copulatory stimulation at their preferred rate of intromissions, and not when they receive similar numbers of intromissions a rate faster or slower than their preferred rate (Becker, et al., 2001; Mermelstein and Becker, 1995). Female hamsters also exhibit an increase of DA in dialysate during sexual behavior (Meisel, et al., 1993). Increases in NAcc DA are not induced by coital stimulation, instead, the NAcc DA increases occur in anticipation of coital stimulation that occurs at a specific interval. These data support the hypothesis that DA increases in the NAcc signal the impending receipt of coital stimulation at the female’s preferred pacing interval, and that NAcc DA plays a role in sexual motivation.
The increase in DA in the NAcc is apparently not always necessary for a female to find sexual behavior rewarding, and may shed light on what it is that DA is doing in ‘reward’. Paredes and co-workers have found that pretreatment with the DA antagonists flupentixol or raclopride did not block conditioned place preference induced by paced mating in female rats (Garcia-Horsman and Paredes, 2004). In contrast the μ-opiate antagonist naloxone prevented establishment of conditioned place preference induced by paced mating (Paredes and Martinez, 2001). In these studies, animals are placed into the test apparatus for conditioned place preference training immediately after receiving an ejaculation, not during the paced mating. The rewarding value of the post-ejaculatory context is blocked by an opiate antagonist, but not by DA antagonists. On the other hand, pretreatment of female hamsters with D2-DA receptor antagonist raclopride blocked conditioned place preferences for the place in which mating occurred (Meisel, et al., 1996). So, DA may be important for development of conditioned place preferences when sexual behavior occurs in the testing environment being conditioned. This may reflect the role DA plays in signaling that a reward is imminent (Schultz, 2001; Schultz, 2004). Alternatively, the testing environment may develop increased saliency as a consequence of this DA release, and so testing in the environment is necessary to see the dependence on DA (Berridge, 2007). A role for the endogenous opiates in the reward system is also seen in other systems (Smith and Berridge, 2007). These results suggest that activation of D2 DA receptors is not necessary for all aspects of sexual behavior to be rewarding, and that μ-opioid receptor mediated activation is also important for sexual motivation in the female rat.
Sex that is rewarding has been shown to be associated with the triggering of a neuroendocrine reflex necessary for pregnancy (Adler, 1974; Erskine et al., 1989; Gilman, et al., 1979). One possibility is that the changes in DA observed during paced mating represent a coupling of the sexual interaction and its physiological consequences, both of which are necessary for sexual behavior to be rewarding in the female. In other words, increases in DA predict the receipt of coital stimulation, but DA only increases after the initiation of the sexual encounter when the coital stimulation is occuring at a rate that triggers the neuroendocrine reflex necessary for successful pregnancy to occur. Coital stimulation is also known to induce the release of oxytocin in rats and other species (Flanagan, et al., 1993), and we infer from the studies by Paredes and colleagues that the enkephalins are also released. The coordinated release of DA, enkephalins, and oxytocin during paced sexual behavior is postulated to mediate the rewarding value of this behavior in the female.
Summary
In both males and females there is an increase in NAcc DA associated with motivational components of sexual behavior. In females, context determines whether a sexual encounter is rewarding and neuroendocrine events may contribute to the determination of the rewarding nature of the context.
Maternal Motivation: a Sexually Dimorphic Behavior
The neuroendocrinology and neurochemistry of maternal motivation has recently been reviewed quite thoroughly (Lonstein and Morrell, 2007), so the reader is referred to this excellent review for additional details. The hormones of pregnancy and parturition prime the brain for the onset of maternal behaviors, which begin at parturition as a consequence of the exposure to pups. Once maternal behaviors have been induced by the presence of the pups, expression of maternal behavior continues to occur without additional hormones as long as the pups are present. Maternal behavior is also induced more rapidly by exposure to pups during subsequent pregnancies, indicating that establishment of maternal behaviors results in long term changes in the brain. As is true of sexual behavior, there are brain regions important for the ability to engage in the behaviors that comprise maternal behavior and other brain regions important for the motivation to engage in these behaviors.
Female rats will cross electrified grids to gain access to pups (Lonstein and Morrell, 2007), and recently parturient female rats will readily learn to bar-press for access to pups, bar-pressing for hours, retrieving hundreds of pups (reviewed in (Lonstein and Morrell, 2007)). Operant responding has also been used to identify the areas of the brain that are necessary for bar-pressing for access to pups. Lesions of the POA or blAMY reduced bar pressing for access to pups whereas lesions of the NAcc did not (Lee, et al., 2000). All lesions disrupted pup retrieval in the home cage (Lee et al., 2000). In other paradigms, lesion of the NAcc have been shown to decrease sensitivity to changes in the delivery of reinforcers (Acheson, et al., 2006), so it is possible that after NAcc lesions the bar pressing by parturient rats may reflect a learning deficit, rather than lack of involvement of NAcc in maternal motivation. The finding that lesions of the NAcc shell disrupt pup retrieval, but not locomotor activity, argues that the NAcc is involved in some aspect of maternal motivation (Li and Fleming, 2003).
Morrell and colleagues have shown that cues associated with pups in a conditioned place preference task result in activation of neurons the POA, prefrontal cortex, NAcc, and the blAMY, but not the dorsal striatum (Mattson and Morrell, 2005; Mattson, et al., 2003). Based on their analysis of the neural systems mediating maternal motivation, Lonstein and Morrell (Lonstein and Morrell, 2007) propose that increased DA activity in the ascending mesolimbic circuits is necessary for many of active components of maternal behavior.
What is unique about the motivation to retrieve pups in rats, and the positive valence of pup-associated cues, is that in adults this is primarily a trait of females. Adult male rats can be induced to exhibit parental behaviors, but it usually takes many more days to induce this response to pups in males, than it does for females (Lonstein and De Vries, 2000), and motivation to care for pups has not been examined. Sex differences in the regulation of DA activity in the ascending mesolimbic projections may underlie sex differences in motivation to engage in parental behavior, in addition to sex differences in the neuroendocrine regulation of this brain system.
Sex Differences in Drug Abuse
More men use drugs than women, but this sex difference is rapidly disappearing. In fact, the current sex difference may reflect historical differences in opportunity to experience drugs, rather than vulnerability to drug use (Van Etten and Anthony, 2001; Van Etten, et al., 1999). Use of cocaine and other stimulants in particular, have increased in the last decade among young girls so that more girls abuse stimulants in grades 8–12 than do boys (Johnston, et al., 2008). According to a recent report, among users 12–17 years old 51.5% are women, in the 18–25 age group 42.0% are women, and among cocaine users 26 years and older 38.8% are women (Johnston, et al., 2006). The use and dependence of women on stimulant drugs is steadily increasing and a growing public health concern (Carroll, et al., 2004; Lynch, et al., 2002; Wetherington and Roman, 1995).
Drug taking patterns are sexually dimorphic. Women tend to increase their rate of consumption of alcohol, marijuana, opioids and cocaine more rapidly than do men (Brady and Randall, 1999; Hernandez-Avila, et al., 2004; Lynch et al., 2002; Mann, et al., 2005; Randall, et al., 1999). Furthermore, once addicted to a drug, women can find it more difficult to quit than men do (Back, et al., 2005; Breese, et al., 2005; Carpenter, et al., 2006; Lynch et al., 2002). Among women in a residential treatment program, women tend to have greater lifetime use and greater current use of cocaine than their male counterparts (Lejuez, et al., 2007).
Of necessity, the information regarding drug use and addiction in men and women is observational or statistical in nature. Some investigators have been able to investigate the effect of ovarian hormones on the subjective effects of stimulants, which have been found to vary across the menstrual cycle (Justice and de Wit, 1999; Justice and de Wit, 2000; Justice and De Wit, 2000). Euphoria, desire, increased energy and intellectual efficiency induced by amphetmaine are enhanced during the follicular phase (when estradiol levels are low at first and rise slowly; progesterone levels are low) relative to the luteal phase (when estradiol levels are moderate and progesterone levels are high). If estradiol is administered during the follicular phase of the menstrual cycle the subjective effects of amphetamine are enhanced further (Justice and De Wit, 2000). In contrast, the subjective effects of psychomotor stimulant drugs are negatively correlated with salivary progesterone levels in women (White, 2002), and progesterone administered during the follicular phase has been reported to attenuate the subjective response to repeated self-administered cocaine (Evans and Foltin, 2006; Evans, et al., 2002; Sofuoglu, et al., 2002). Thus, ovarian hormones modulate the reward value of psychomotor stimulants in women.
Sex Differences in Response to Psychomotor Stimulants in Rats
Cocaine and other drugs of abuse act by increasing DA in the nucleus accumbens, so understanding sex differences in the effects of these drugs on the brain may provide insight into sex differences in the neural basis of reward. The acute behavioral response to psychomotor stimulants in rodents reflects both sex differences and the modulatory role of gonadal hormones in females. Research on rats and humans indicates that the behavioral effects of drugs of abuse, and the psychomotor stimulants in particular, are both sexually dimorphic and modulated by the gonadal steroid hormones (e.g., (Bazzett, et al., 2000; Becker and Beer, 1986; Becker and Ramirez, 1981b; Carroll et al., 2004; Di Paolo, et al., 1986; Di Paolo, et al., 1981; Dluzen and Ramirez, 1984; Dluzen and Ramirez, 1990; Gordon, 1980; Hruska, 1988; Hruska and Silbergeld, 1980; Joyce, et al., 1982; Lynch et al., 2002; Sell, et al., 2002; Van Hartesveldt, et al., 1989; Walker, et al., 2001).
Sensitization of AMPH or cocaine-induced psychomotor behavior can be defined as the absolute increase in the behavioral response exhibited when two tests are compared. Using this definition, gonad-intact females exhibit more robust sensitization than do intact males (Camp and Robinson, 1988a; Camp and Robinson, 1988b; Forgie and Stewart, 1994; Robinson, 1984; Robinson, et al., 1982; van Haaren and Meyer, 1991). Following ovariectomy (OVX) of female rats the expression of sensitization to AMPH is attenuated (Camp and Robinson, 1988a; Camp and Robinson, 1988b; Forgie and Stewart, 1994; Robinson, 1984; Robinson et al., 1982) or suppressed all together (Sircar and Kim, 1999; van Haaren and Meyer, 1991). Estradiol treatments in OVX rats enhance sensitization of locomotor activity induced by AMPH or cocaine (Forgie and Stewart, 1994; Peris, et al., 1991). These studies demonstrate that the neurobiological response to stimulant drugs is sexually dimorphic, but they do not address how this biological difference impacts sex differences in the motivation to take drugs.
Sex Differences in Stimulant Self-Administration in Animals
The animal model of human drug taking behavior that has the most face-validity is self-administration. In self-administration studies, animals are trained to bar-press or nose poke in order to receive access to a drug (usually by i.v. infusion). The animal’s pattern of drug taking can be studied during acquisition, maintenance and relapse. It is also possible to manipulate the schedule of reinforcement in order to determine motivation to take a drug.
Sex differences have been reported during all phases of the addiction process as assessed using various self-administration paradigms (see (Becker and Hu, 2008; Carroll et al., 2004; Lynch et al., 2002; Roth, et al., 2004). When a low dose of drug is used, female rats acquire cocaine self-administration at a faster rate than do males (Davis, et al., 2008; Hu, et al., 2004; Lynch, 2006; Lynch and Carroll, 1999; Lynch et al., 2002). Estradiol treatment enhances acquisition of cocaine self-administration in OVX female rats (Hu et al., 2004; Lynch, et al., 2001).
During maintenance conditions, when given a choice between two doses of cocaine, female rats in estrus preferred higher doses of cocaine compared with females in other phases of the estrous cycle or male rats (Lynch, 2006; Lynch, et al., 2000). When the role of estradiol in ‘binge’ cocaine intake and subsequent motivational changes is examined, estradiol benzoate treatment increases the initial binge length and total levels of cocaine self-administration (Lynch and Taylor, 2005). In another experiment, OVX rats treated with estradiol also consumed more cocaine than vehicle treated controls (Lynch, 2006). When responding for low doses of cocaine is assessed under a schedule in which the number of responses required in order to obtain a cocaine infusion progressively increases with each dose received, motivation for access to a drug can be assessed. Under this ‘progressive ratio schedule’ intact female rats reach much higher final ratios than do males, indicating that females are more motivated to obtain cocaine (Roberts, et al., 1989). Females also work harder for access to cocaine during the phase of the estrous cycle when estradiol is elevated, indicating that ovarian hormones modulate the motivation to obtain cocaine (Roberts et al., 1989). In fact, estradiol treatment enhances responding for cocaine on a progressive ratio schedule (Becker and Hu, 2008). These results show that estradiol influences both acquisition of cocaine self-administration and that there are motivational effects of estradiol on cocaine intake.
In contrast to estradiol, progesterone treatment given concurrently with estradiol counteracts the effect of estradiol on acquisition of cocaine self-administration behavior (Jackson, et al., 2006). We have recently confirmed this finding and find that progesterone alone does not affect cocaine self-administration, but that progesterone enhances cocaine intake in EB primed OVX rats (Yang, et al., 2007). Taken together, ovarian hormones contribute to sex differences in cocaine self-administration and estradiol in particular is a key factor influencing the reinforcing effects of cocaine in female rats. So, over the course of the estrous cycle, there are peaks and valleys during which females are more or less susceptible to the reinforcing properties of cocaine.
Castration (CAST) of males has been reported to enhance sensitization of AMPH- or cocaine-induced psychomotor behavior (e.g., (Camp and Robinson, 1988a; Camp and Robinson, 1988b; Robinson, 1984), although this result has not been found consistently (Forgie and Stewart, 1994; van Haaren and Meyer, 1991). It has been hypothesized that if CAST enhances the induction and/or expression of behavioral sensitization, that testosterone treatment should reverse this effect. This is not the case, however, as testosterone treatment has not been found to affect behavioral sensitization in CAST males (Forgie and Stewart, 1994). Furthermore, there is no effect of CAST on acquisition of cocaine self-administration behavior and a dose of estradiol that enhances self-administration in female rats has no effect on cocaine self- administration behavior in male (Jackson et al., 2006). Thus, testicular hormones do not affect self-administration in males, and the effects of estradiol on the acquisition of cocaine self-administration are sexually dimorphic.
Neural Mechanisms Mediating Sex Differences in the Ascending DA System
The sex differences in the motivation to take drugs of abuse and engage in sexual behavior or pup-related behaviors, discussed above, are most likely due at least in part to sex differences in the ascending mesotelencephalic DA systems. Basal extracellular concentrations of DA, as determined by the no net flux method, are twice as high in striatum of CAST males as in OVX females, and varies with the estrous cycle (Xiao and Becker, 1994). In experiments with in vivo voltammetry, cocaine or haloperidol induce a greater increase in electrical stimulation evoked extracellular DA in intact females than in males, possibly due to greater autoreceptor control of the DA transporter (DAT) (Walker, et al., 2006; Walker, et al., 2000). Following OVX, the AMPH-induced increase in striatal DA release is significantly less than the response of tissue from CAST (Becker, 1990b; Becker and Ramirez, 1981b), and estradiol treatment results in greater AMPH-induced DA in dialysate from OVX vs. CAST males (Castner, et al., 1993). A rapid effect of estradiol on the striatum of females is seen in vitro as well as in vivo (Becker, 1990b; Becker, 1990c). So, basal DA tone is chronically higher in males than in females, while the stimulated release of DA is modulated by estradiol in females, but not in males. In many systems an increase in tone is characteristic of greater basal activity within the system, but this does not appear to be the case here. In general, the male is less responsive than the female to drugs or other stimulation. This suggests that the chronic high DA signal results in down regulation of activity throughout the feedback network. Thus, when DA release is stimulated, the relative increase is less in males than in females and the behavioral response is proportionally less as well.
DA receptors are also sexually dimorphic. There are 10% more D1 DA receptors in the striatum and Nacc of male rats than in intact female rats (Andersen, et al., 1997; Hruska, et al., 1982). In one experiment intact female rats had fewer D2 receptors than males (Miller, 1983), while we have reported greater D2 binding in OVX vs. CAST in the dorsolateral striatum (Bazzett and Becker, 1994). Others have reported no sex differences in D2 binding when the entire striatum is considered (Andersen et al., 1997; Hruska et al., 1982). Additionally, in females, estradiol rapidly down-regulates D2 DA receptor binding in dorsolateral striatum of female rats (Bazzett and Becker, 1994).
Our knowledge of how estradiol acts in the striatum and NAcc of females to influence DA function is rapidly evolving. ERα and ERβ are thought to associate with chaperone proteins of the caveolin family to act in concert with metabotropic glutamate receptors (mGluR) to induce second messenger signaling (Boulware, et al., 2007; Dewing, et al., 2007; Micevych and Mermelstein, 2008). Depending on which caveolin protein (caveolin-1 chaperones ERα with mGluR5 and caveolin-3 chaperones ERα or ERβ with mGluR3), different intracellular signaling processes are activated (Boulware et al., 2007; Dewing et al., 2007; Micevych and Mermelstein, 2008). These effects are clearly “membrane” effects of estradiol, although the distinction between “rapid” indicating membrane effects and “slow” indicating nuclear ER activation is eroding the more we learn about the membrane effects. For example, in cells that have both membrane and nuclear ER receptors there is a synergistic interaction resulting in amplification of the effects of estradiol (Pedram, et al., 2002). Since there are some cells in the NAcc with nuclear ERs (ERα and ERβ) (Shughrue, et al., 1997) it is possible that some of the effects of estradiol on motivation are mediated by a combined effect of membrane and nuclear ERs.
There is, however, one functional difference in the membrane effects of ER activation and nuclear ER activation, at least in the striatum, and from behavioral data it is inferred that this is also the case in the NAcc. The membrane ER is activated most strongly by physiological concentrations of the hormone delivered intermittently. High concentrations of estradiol, or prolonged delivery of physiological concentrations of estradiol, shut down the rapid signaling response. This is seen behaviorally where physiological doses of estradiol enhance cocaine self-administration and high doses of estradiol do not (Hu and Becker, 2008). It is also seen in vitro where physiological concentrations of estradiol delivered in pulses induce DA release, physiological concentrations also enhance AMPH-induced DA release but high doses of estradiol do not have this effect (Becker, 1990a). This sensitivity of the ascending DA system to estradiol is sexually dimorphic, as discussed above, and is hypothesized to underlie the sex differences in motivation.
Ontogeny and Sexual Differentiation of the Ascending DA Sytem
As demonstrated by (Phoenix et al., 1959), the brains of mammals undergo sexual differentiation during sensitive periods of development. In rats this occurs during the perinatal period and again during the peripubertal period. Exposure to testosterone (which is converted to estradiol in some areas of the brain) during the perinatal period, influences neuronal survival, differentiation and connectivity resulting in masculinization (Becker, et al., 2005; Breedlove and Hampson, 2002). Additionally, during the peripubertal period, hormone exposure can influence neuronal and dendritic pruning (Zehr, et al., 2006), so that reorganization occurs when the brain is exposed to ovarian or testicular hormones (Sisk and Foster, 2004; Sisk and Zehr, 2005). Unlike its actions during the perinatal period, estradiol does not masculinize the female brain at puberty. As reviewed by Bakker and Baum (Bakker and Baum, 2008), evidence from the aromatase knockout mouse, taken with findings from previous research, indicates that estradiol exposure between birth and puberty is important for feminization of sexual behavior in females.
Phoenix et al., (1959) also noted that sex differences in brain function can be produced in response to exposure to gonadal hormones in the adult, or “activational” effects of hormones. This second type of sex difference can be due either to the effects of gonadal hormones acting on sexually dimorphic brain regions, or as a result of the different effects induced by ovarian vs. testicular hormones that in some neural regions may decrease differences between the sexes (McCarthy and Konkle, 2005). In the ascending DA system, it is hypothesized that both of these processes are involved, and there is sexual differentiation of the system during the perinatal period as well as an active feminization of the system by exposure to estradiol at puberty in females which induces sex differences in the activational effects of estradiol (Figure 1).
Fig 1. Schematic of hypothesized events determining sexual differentiation of motivation mediated by the ascending DA system.
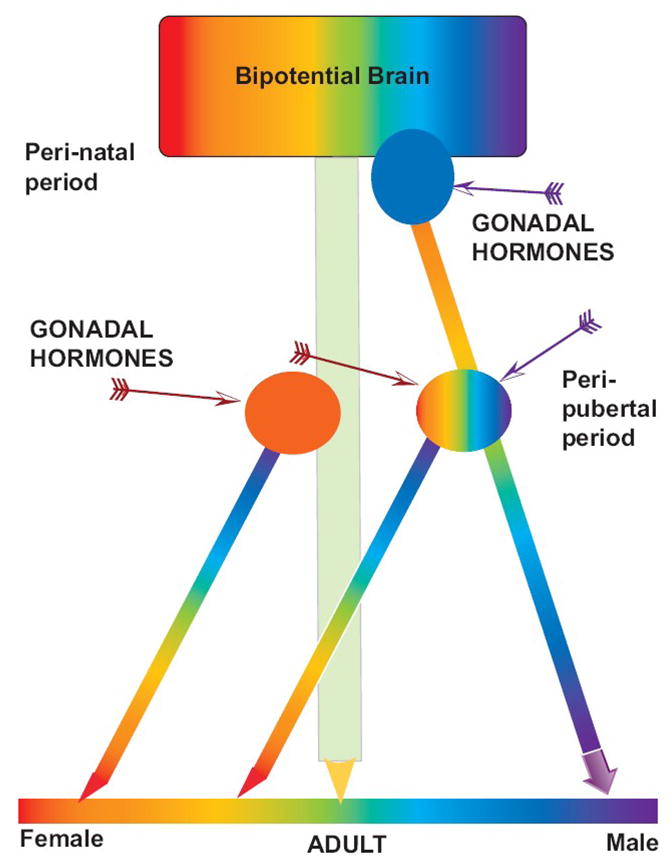
Gonadal hormones act in the rat brain during the sensitive perinatal period and again during the peripubertal period to result in adult sex differences.
During the peri-natal period in the rat, testicular hormones (Blue oval, purple arrow) act on the bipotential brain to induce masculinization of the brain (rainbow colored heavy arrow on the right). In the absence of testicular hormones the brain remains feminine (green arrow in center). During the peri-pubertal period, estradiol (red arrows), released from the ovary (orange oval) and possibly if given exogenously to a CAST male, permanently feminizes the neural response to estradiol. Testicular hormones may further masculinize the system at puberty or have no effect.
During late prenatal development of the rat, female striata are reported to display higher densities of both tyrosine hydroxylase immunoreactive (TH-IR) axons and GABA-IR cell body profiles than are seen in striata from male rats at embryonic days 16, 18, 20, and 21 (Ovtscharoff, et al., 1992). In the adult, if TH-IR in the substantia nigra (SN) and ventral tegmental area (VTA) are analyzed by stereotaxic level, the distribution of DA cells is sexually dimorphic and females have 15% more DA neurons than do males (McArthur, et al., 2007). Interestingly, if dams are given dexamethasone during the late prenatal or early postnatal period, the numbers of neurons and the 3-D architecture of the VTA and SN is de-masculinized in the male and the number of neurons is even greater in the female (McArthur et al., 2007). Since stress hormone exposure during this period of development inhibits the secretion of androgens by the male testes, these results may indicate the effects of perinatal androgen on sexual differentiation of these neurons.
Postnatally, it is known that in male rats there is over-production of D1 and D2 DA receptors in the striatum and NAcc of male rats, with a peak around postnatal day (PND) 40 with subsequent pruning (Andersen et al., 1997). In females, the over-production of DA receptors is attenuated, relative to males (Andersen et al., 1997). Gonadectomy of males or females prior to puberty does not alter the sex differences in pattern of DA receptor binding, indicating that neither the over-production or pruning are regulated by peri-pubertal hormones (Andersen, et al., 2002).
When DA concentrations in dialysate from NAcc were measured from males at PND 38 or 76 there was no difference between the ages in extracellular DA determined by the no net flux method, nor were there age differences in cocaine-induced increases in DA in NAcc (Frantz, et al., 2007). In another study there was a difference between PND35 males and adult males in cocaine-induced increases in DA in dialysate and DA reuptake in NAcc, with adolescent males exhibiting more rapid DA reuptake (Badanich, et al., 2006). If rats are gonadectomized at PND 15 (prior to puberty), males show a typical pattern of AMPH-induced DA release, while females show a more male-typical pattern of AMPH-induced DA release in vitro. From this it is inferred that exposure to ovarian hormones during the prepubertal period may play a role in feminizing the ascending DA system (Becker and Ramirez, 1981a).
The role of gonadal hormones in the ontogeny of sex differences in mesotelencephalic DA-mediated behaviors has not been investigated systematically. In one study, perinatal or prepubertal OVX partially masculinized dodging to protect a food item (Field, et al., 2004), a behavior that has been shown to be mediated by striatal DA activity (Field, et al., 2000). In another study, female rats exhibit greater exploratory activity in a large novel open-field than do males (Stewart and Cygan, 1980). This enhanced activity in the open-field is seen in females if animals are not exposed to gonadal hormones during the perinatal period and if they are exposed to estradiol during the period prior to weaning (days 10–20 when there is an endogenous surge of estradiol in females) or during the peripubertal period (days 30–40). Male rats gonadectomized at day 1 (GDXd1) and treated with estradiol on days 10–20 or 30–40 show higher levels of open-field activity than animals GDXd1 but not treated with estradiol (Stewart and Cygan, 1980). A model for this pattern of sexual differentiation is presented in Figure 1.
Enhanced activity in a novel-environment is a trait that predicts rapid acquisition of self-administration of cocaine or AMPH in rats and higher rates of AMPH- and cocaine-induced locomotor activity (Piazza, et al., 1998) It is possible, therefore, that the more rapid acquisition of drug taking seen in female rats is due to the absence of exposure to hormones perinatally plus exposure to estradiol prior to weaning and/or at puberty.
In male rats, prenatal stress during the third week of gestation attenuates testicular hormone release and masculinization of sexual behavior (Meisel, et al., 1979). Prenatal stress during the third week of gestation also enhances the acute response to AMPH and the amount of AMPH that animals will self-administer (Deminiere, et al., 1992). In pilot studies, prenatal stress enhances acquisition of cocaine-self administration in males but not females (Thomas, et al., submitted).
So, the available data suggest that feminization of the ascending midbrain DA neurons, novelty-induced locomotor behavior, and drug taking behavior, occurs if animals are NOT exposed to gonadal hormones during the perinatal period and are subsequently exposed to estradiol during the peripubertal period. In other words, it is hypothesized that there are at least two active processes working at cross-purposes during ontogeny of the ascending DA systems. Testicular hormones perinatally induce masculinization of neuronal architecture and neuronal survival in the VTA/SN and peripubertal estradiol feminizes the system, perhaps acting on GABAergic cells and/or DA terminals in the striatum and NAcc to change the basal set-point of the system and induce permanent sensitivity to estradiol. It is also possible that testicular hormones at puberty further reduce the sensitivity to estradiol in males (Figure 1).
Since this is a fairly new concept in the field of sexual differentiation of the brain, there are no data regarding apparently permanent changes in the brain induced by exposure to estradiol during the peripubertal period. From the breast cancer literature there are data that estrogenic exposure during the peripubertal period can result in epigenetic changes that result in reprogramming of the mitogen activated protein kinase (MAPK), and caveolin-1 genes in mammary tissue (De Assis and Hilakivi-Clarke, 2006) that have long term effects on the likelihood of a woman getting breast cancer due to enhanced sensitivity to estradiol exposure. Since estradiol’s effects in the striatum/NAcc are thought to be dependent on caveolin 1 to facilitate the association between ERα and mGluR5, one possibility is that estradiol at puberty is inducing epigenetic changes that result in a reprogramming of the striatal/NAcc response to estradiol. This is proposed to have functional consequences for the ascending DA system resulting in sexual differences in the motivational system.
Summary
Sex differences in the DA system of the NAcc and striatum are thought to mediate the sex differences in motivation. We have previously hypothesized that the presence of the neural circuits that mediate maternal motivation, and in particular the greater oxytocin projection to the NAcc in females, may contribute to this sex difference (Becker and Taylor, 2008). In addition, there are effects of gonadal hormones that modulate the reward system. Developmental events that lead to sex differences in motivation have not been investigated systematically, and may provide insight into the neurobiology of this system and lead to novel gender-specific approaches for treatment of drug abuse and other motivational disorders. Here it is proposed that sexual differentiation of the motivational system is mediated by a novel mechanism in which ovarian hormones secreted at puberty in the female can actively feminized the DA system.
Acknowledgments
DA12677 and NS048141 to JBB
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Acheson A, Farrar AM, Patak M, Hausknecht KA, Kieres AK, Choi S, de Wit H, Richards JB. Nucleus accumbens lesions decrease sensitivity to rapid changes in the delay to reinforcement. Behavioural Brain Research. 2006;173(2):217–28. doi: 10.1016/j.bbr.2006.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler NT. Effects of the male’s copulatory behavior on successful pregnancy of the female rat. J Comp Physiol Psychol. 1969;69(4):613–22. doi: 10.1037/h0028244. [DOI] [PubMed] [Google Scholar]
- Adler NT. The behavioral control of reproductive physiology. Adv Behav Biol. 1974;11(259):259–86. doi: 10.1007/978-1-4684-3069-1_12. [DOI] [PubMed] [Google Scholar]
- Adler NT. On the mechanisms of sexual behavior and their evolutionary constraints. In: Hutchison JB, editor. Biological Determinants of Sexual Behavior. Wiley and Sons, LTD; New York: 1978. pp. 657–694. [Google Scholar]
- Anden NE, Dahlstrom A, Fuxe K, Larsson K, Olson L, Ungerstedt U. Ascending monoamine neurons to the telencephalon and diencephalon. Act Physiol Scand. 1966;67:313–323. [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8(6):1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27(6):683–91. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180(1):169–76. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. European Journal of Pharmacology. 2006;550(1–3):95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Frontiers in Neuroendocrinology. 2008;29(1):1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Frontiers in Neuroendocrinology. 2007;28(4):161–78. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzett TJ, Albin RL, Becker JB. Malonic acid and the chronic administration model of excitotoxicity. Mitochondrial Inhibitors and Neurodegenerative Disorders. 2000:219–231. [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990a;5(2):157–64. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17β-estradiol on striatum: sex differences in dopamine release. Synapse. 1990b;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990c;118:169–71. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior.[see comment] Endocrinology. 2005;146(4):1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19(1):27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Meisel RL. Neurochemistry and Molecular Biology of Reward. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Neurobiology. Vol. 21. 2007. pp. 739–774. [Google Scholar]
- Becker JB, Ramirez VD. Experimental studies on the development of sex differences in the release of dopamine from striatal tissue fragments in vitro. Neuroendocrinol. 1981a;32(3):168–173. doi: 10.1159/000123151. [DOI] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981b;204:361–72. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. Journal of Neuroscience. 2001;21(9):3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Taylor JR. Sex differences in motivation. In: Becker JB, Berkley K, Geary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain: from Genes to Behavior. Oxford University Press; Oxford, UK: 2008. pp. 177–199. [Google Scholar]
- Bermant G. Response latencies of female rats during sexual intercourse. Science. 1961;133:1771–1773. doi: 10.1126/science.133.3466.1771. [DOI] [PubMed] [Google Scholar]
- Bermant G. Copulation in rats. Psychology Today. 1967;1:52–60. [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. Journal of Neuroscience. 2007;27(37):9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatric Clinics of North America. 1999;22(2):241–52. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Hampson E. Sexual differentiation of the brain and behavior. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. MIT Press; Cambridge, MA: 2002. pp. 75–115. [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcoholism: Clinical & Experimental Research. 2005;29(2):185–95. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res. 1988a;30(1):55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of D-amphetamine or restraint stress. Behav Brain Res. 1988b;30(1):69–88. doi: 10.1016/0166-4328(88)90009-5. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine & Tobacco Research. 2006;8(5):627–38. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25(5):273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behavioral Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Davis BA, Clinton SR, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively-bred high-responder and low-responder rats. Pharmacology, Biochemistry & Behavior. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Assis S, Hilakivi-Clarke L. Timing of Dietary Estrogenic Exposures and Breast Cancer Risk. Ann NY Acad Sci. 2006;1089:14–35. doi: 10.1196/annals.1386.039. [DOI] [PubMed] [Google Scholar]
- Deminiere JM, Piazza PV, Guegan G, Abrous N, Maccari S, Le Moal M, Simon H. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586:135–9. doi: 10.1016/0006-8993(92)91383-p. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. Journal of Neuroscience. 2007;27(35):9294–300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T, Levesque D, Daigle M. A physiological dose of progesterone affects rat striatum biogenic amine metabolism. Eur J Pharmacol. 1986;125(1):11–16. doi: 10.1016/0014-2999(86)90077-4. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Poyet P, Labrie F. Effect of chronic estradiol and haloperidol treatment on striatal dopamine receptors. Eur J Pharmacol. 1981;73(1):105–6. doi: 10.1016/0014-2999(81)90153-9. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinol. 1984;39(2):149–155. doi: 10.1159/000123971. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. In vitro progesterone modulation of amphetamine-stimulated dopamine release from the corpus striatum of ovariectomized estrogen-treated female rats: response characteristics. Brain Res. 1990;517:117–122. doi: 10.1016/0006-8993(90)91016-a. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: a review. Hormon Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Kornberg E, Cherry JA. Paced copulation in rats: effects of intromission frequency and duration on luteal activation and estrus length. Physiol Behav. 1989;45(1):33–9. doi: 10.1016/0031-9384(89)90163-7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31(3):659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14(2):217–32. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Stacey P. Studies of instrumental behaviour with sexual reinforcement in male rats (Rattus norvegicus): II Effects of preoptic area lesions, castration and testosterone. Journal of Comparative Psychology. 1987;101:407–419. [PubMed] [Google Scholar]
- Field EF, Whishaw IQ, Forgie ML, Pellis SM. Neonatal and pubertal, but not adult, ovarian steroids are necessary for the development of female-typical patterns of dodging to protect a food item. Behavioral Neuroscience. 2004;118(6):1293–304. doi: 10.1037/0735-7044.118.6.1293. [DOI] [PubMed] [Google Scholar]
- Field EF, Whishaw IQ, Pellis SM. Sex differences in catalepsy: evidence for hormone-dependent postural mechanisms in haloperidol-treated rats. Behavioural Brain Research. 2000;109(2):207–12. doi: 10.1016/s0166-4328(99)00174-6. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Pfaus JG, Pfaff DW, McEwen BS. Induction of FOS immunoreactivity in oxytocin neurons after sexual activity in female rats. Neuroendocrinology. 1993;58(3):352–8. doi: 10.1159/000126562. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Stewart J. Sex differenc in amphetamine-induced locomotor activity in adult rats: role of tetosterone exposure in the neonatal period. Pharmacol, Biochem, Behav. 1994;46:637–645. doi: 10.1016/0091-3057(93)90555-8. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32(3):625–37. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Garcia-Horsman P, Paredes RG. Dopamine antagonists do not block conditioned place preference induced by paced mating behavior in female rats. Behavioral Neuroscience. 2004;118:356–364. doi: 10.1037/0735-7044.118.2.356. [DOI] [PubMed] [Google Scholar]
- Gilman DP, Mercer LF, Hitt JC. Influence of female copulatory behavor on the induction of pseudopregnancy in the female rat. Physiol Behav. 1979;22:675–678. doi: 10.1016/0031-9384(79)90229-4. [DOI] [PubMed] [Google Scholar]
- Gordon JH. Modulation of apomorphine-induced stereotypy by estrogen: time course and dose response. Brain Res Bull. 1980;5:679–682. doi: 10.1016/0361-9230(80)90205-1. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug & Alcohol Dependence. 2004;74(3):265–72. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hruska RE. 17βEstradiol regulation of DA receptor interactions with G-proteins. Soc Neurosci Abstr. 1988;14:454. [Google Scholar]
- Hruska RE, Ludmer LM, Pitman KT, De Ryck M, Silbergeld EK. Effects of estrogen on striatal dopamine receptor function in male and female rats. Pharmacol Biochem Behav. 1982;16(2):285–291. doi: 10.1016/0091-3057(82)90162-9. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Silbergeld EK. Increased dopamine receptor sensitivity after estrogen treatment using the rat rotation model. Science. 1980;208:1466–1468. doi: 10.1126/science.7189902. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: Effect of estradiol dose or chronic estradiol administration. Drug and Alcohol Dependence. 2008;94:56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29(1):81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Hormones & Behavior. 2007;52(1):45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Role of the striatum and nucleus accumbens in paced copulatory behavior in the female rat. Behavioural Brain Research. 2001;121(1–2):119–128. doi: 10.1016/s0166-4328(00)00394-6. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. European Journal of Neuroscience. 2003a;18(7):1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Hormones and Behavior. 2003b;43(4):503–507. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2005: Volume I, Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2006. N. P. N. 06-5883. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE D. o. H. a. H. Services. Monitoring the Future national survey results on drug use, 1975–2007. Volume I: Secondary school students. Substance Abuse & Mental Health Services Administration; 2008. p. 707. Vol. NIH Publication No. 08-6418A. [Google Scholar]
- Joyce JN, Smith RL, Van Hartesveldt C. Estradiol suppresses then enhances intracaudate dopamine-induced contralateral deviation. Eur J Pharmacol. 1982;81(1):117–122. doi: 10.1016/0014-2999(82)90608-2. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145(1):67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000;71(1):51–9. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- Justice AJH, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacology Biochemistry and Behavior. 2000;66(3):509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement.[republished from Behav Brain Res. 1999 Apr;100(1–2):15–31; PMID: 10212050] Behavioural Brain Research. 2000;108(2):215–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Curtin JJ, Reynolds EK, Daughters SB. Risk Factors in the Relationship Between Gender and Crack/Cocaine. Experimental and Clinical Psychopharmacology. 2007;15:165–175. doi: 10.1037/1064-1297.15.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behavioral Neuroscience. 2003;117(3):426–45. doi: 10.1037/0735-7044.117.3.426. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000;24:669–686. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Morrell JI. Neuroendocrinology and neurochemistry of maternal motivation and behavior. In: Blaustein JA, editor. Behavioral Neurochemistry and Neuroendocrinology. XIV. Springer-Verlag; Berlin: 2007. p. 954. [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Experimental & Clinical Psychopharmacology. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152(2):132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68(4):641–6. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Decreased motivation following cocaine self-administration under extended access conditions: effects of sex and ovarian hormones. Neuropsychopharmacology. 2005;30(5):927–35. doi: 10.1038/sj.npp.1300656. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcoholism: Clinical & Experimental Research. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Martinez I, Paredes RG. Only self-paced mating is rewarding in rats of both sexes. Hormones and Behavior. 2001;40(4):510–517. doi: 10.1006/hbeh.2001.1712. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience. 2005;135(2):315–28. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology. 2003;167(1):1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies GE. The Size and Distribution of Midbrain Dopaminergic Populations are Permanently Altered by Perinatal Glucocorticoid Exposure in a Sex- Region- and Time-Specific Manner. Neuropsychopharmacology. 2007;32:1462–76. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Becker JB. Neuroendocrinology of sexual behavior in the female. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. MIT Press/Bradford Books; Cambridge, MA: 2002. pp. 117–151. [Google Scholar]
- McCarthy MM, Konkle ATM. When is a sex difference not a sex difference? Frontiers in Neuroendocrinology. 2005;26(2):85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McClintock MK. Group mating in the domestic rat as context for sexual selection: consequences for the analysis of sexual behavior and neuroendocrine responses. Adv Study of Behav. 1984;14:1–50. [Google Scholar]
- McClintock MK, Adler NT. The role of the female during copulation in wild and domestic norway rats. Behaviour. 1977;LXVII(1–2):67–96. [Google Scholar]
- Meisel RL, Camp DM, Robinson TE. A microdialysis study of ventral striatal dopamine during sexual behaivor in female Syrian hamsters. Behav Brain Res. 1993;55:151–157. doi: 10.1016/0166-4328(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Dohanich GP, McEwen BS, Pfaff DW. Antagonism of sexual behavior in female rats by ventromedial hypothalamic implants of antiestrogen. Neuroendocrinology. 1987;45(3):201–7. doi: 10.1159/000124726. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Dohanich GP, Ward IL. Effects of prenatal stress on avoidance acquisition, open-field performance and lordotic behavior in male rats. Physiology & Behavior. 1979;22(3):527–30. doi: 10.1016/0031-9384(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA, Rowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. European Journal of Pharmacology. 1996;309(1):21–24. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behavioral Neuroscience. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Molecular Neurobiology. 2008;38(1):66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC. Sex differences in dopaminergic and cholinergic activity and function in the nigrostriatal system of the rat. Psychneuroendocrinol. 1983;8:225–236. doi: 10.1016/0306-4530(83)90059-8. [DOI] [PubMed] [Google Scholar]
- Oldenberger WP, Everitt BJ, De Jonge FH. Conditioned Place Preference Induced by Sexual Interaction in Female Rats. Hormones and Behavior. 1992;26:214–228. doi: 10.1016/0018-506x(92)90043-u. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulationof septal area and other areas of the brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olds M, Olds J. Effects of lesions in the medial forebrain bundle on self-stimulation behavior. American Journal of Physiology. 1969;217(5):1253–1264. doi: 10.1152/ajplegacy.1969.217.5.1253. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Eusterschulte B, Zienecker R, Reisert I, Pilgrim C. Sex-Differences in Densities of Dopaminergic Fibers and Gabaergic Neurons in the Prenatal Rat Striatum. Journal of Comparative Neurology. 1992;323(2):299–304. doi: 10.1002/cne.903230212. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behavioral Neuroscience. 1997;111(1):123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Martinez I. Naloxone blocks place preference conditioning after paced mating in female rats. Behavioral Neuroscience. 2001;115(6):1363–1367. [PubMed] [Google Scholar]
- Paredes RG, Vazquez B. What do female rats like about sex? Paced mating. Behavioural Brain Research. 1999;105(1):117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CCW, Levin ER. Integration of the non-genomic and genomic actions of estrogen - Membrane-initiated signaling by steroid to transcription and cell biology. Journal of Biological Chemistry. 2002;277(52):50768–50775. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- Peris J, Decambre N, Coleman-Hardee M, Simpkins J. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated [3H]dopamine release. Brain Res. 1991;566:255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Neural Analysis of Hormone-Controlled Mammalian Reproductive Behavior. Springer-Verlag; New York: 1980. Estrogens and Brain Function. [Google Scholar]
- Pfaff DW, Schwartz-Giblin S. Cellular mechanisms of female reproductive behavior. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press, Ltd; New York: 1988. pp. 1487–1568. [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693(1–2):21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behavioral Neurosci. 1991a;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of Dopamine in Anticipatory and Consummatory Aspects of Sexual-Behavior in the Male-Rat. Behavioral Neuroscience. 1991b;105(5):727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Rouge-Pont F, Le Moal M. Behavioral and biological factors associated with individual vulnerability to psychostimulant abuse. NIDA Research Monograph. 1998;169:105–33. [PubMed] [Google Scholar]
- Pleim ET, Matochik JA, Barfield RJ, Auerbach SB. Correlation of dopamine release in the nucleus accumbens with masculine sexual behavior in rats. Brain Res. 1990;524:160–163. doi: 10.1016/0006-8993(90)90507-8. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. Journal of Studies on Alcohol. 1999;60(2):252–60. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Rivas FJ, Mir D. Effects of Nucleus-Accumbens Lesion on Female Rat Sexual Receptivity and Proceptivity in a Partner Preference Paradigm. Behavioural Brain Research. 1990;41(3):239–249. doi: 10.1016/0166-4328(90)90111-q. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berlin) 1984;84(4):466–75. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;253(1–2):231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Roth M, Cosgrove K, Carroll M. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neuroscience & Biobehavioral Reviews. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37(3):218–24. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7(4):293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics andbehavioral ecology. Current Opinion in Neurobiology. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of extrogen receptor-alpha and -beta mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis and Sprague-Dawley rats. J Pharmacol exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7(10):1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26(3–4):163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. Journal of Neuroscience. 2007;27(7):1594–605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacology, Biochemistry & Behavior. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Stewart J, Cygan D. Ovarian hormones act early in development to feminize adult open-field behavior in the rat. Horm Behav. 1980;14:20–32. doi: 10.1016/0018-506x(80)90012-4. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Hu M, Bhatnagar S, Lee TM, Becker JB. Sex-specific enhancement of cocaine self-administration and sensitization in rats with a history of prenatal stress submitted. [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367(95):95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Selective lesions of central catecholamine pathways: application in functional studies. Neurosci Res. 1973;5(0):73–96. doi: 10.1016/b978-0-12-512505-5.50010-0. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Functional dynamics of central monamine pathways. In: Schmitt FO, Worden FG, editors. The Neurosciences: Third Study Program. MIT Press; Cambridge, MA: 1974. pp. 979–988. [Google Scholar]
- Ungerstedt U, Ljungberg T, Steg G. Behavioral, physiological, and neurochemical changes after 6- hydroxydopamine-induced degeneration of the nigro-striatal dopamine neurons. Adv Neurol. 1974;5(421):421–6. [PubMed] [Google Scholar]
- Van Etten ML, Anthony JC. Male-female differences in transitions from first drug opportunity to first use: searching for subgroup variation by age, race, region, and urban status. Journal of Womens Health & Gender-Based Medicine. 2001;10(8):797–804. doi: 10.1089/15246090152636550. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. Addiction. 1999;94(9):1413–9. doi: 10.1046/j.1360-0443.1999.949141312.x. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer M. Sex differences in the locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Van Hartesveldt C, Cottrell GA, Meyer ME. Effects of intrastriatal hormones on the dorsal immobility response in male rats. Pharmacol Biochem Behav. 1989;35:307–310. doi: 10.1016/0091-3057(90)90160-j. [DOI] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: Disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25(1):118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31(6):1193–202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95(4):1061–70. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Wetherington CL, Roman AR, editors. Drug Addiction Research and the Health of Women. U. S. Department of Health and Human Services; Rockville, MD: 1995. [Google Scholar]
- White FJ. A behavioral/systems approach to the neuroscience of drug addiction. The Journal of Neuroscience. 2002;22(9):3303–5. doi: 10.1523/JNEUROSCI.22-09-03303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Neural mechanisms of the reinforcing action of cocaine. NIDA Research Monograph. 1984;50:15–33. [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentrations in male and female rats: effects of estrous cycle and gonadectomy. Neuroscience Letters. 1994;180:155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Hormones and Behavior. 1997;32:114–124. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behavioural Brain Research. 2007;184:174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LY, Clemens LG. MPOA lesions affect female pacing of copulation in rats. Behavioral Neuroscience. 2000;114(6):1191–1202. [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. Journal of Neurobiology. 2006;66(6):578–90. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]


