Abstract
The question of what causes a male animal to seek out and choose a female as opposed to another male mating partner is unresolved and remains an issue of considerable debate. The most developed biologic theory is the perinatal organizational hypothesis, which states that perinatal hormone exposure mediates sexual differentiation of the brain. Numerous animal experiments have assessed the contribution of perinatal testosterone and/or estradiol exposure to the development of a male-typical mate preference, but almost all have used hormonally manipulated animals. In contrast, variations in sexual partner preferences occur spontaneously in domestic rams, with as many as 8% of the population exhibiting a preference for same-sex mating partners (male-oriented rams). Thus, the domestic ram is an excellent experimental model to study possible links between fetal neuroendocrine programming of neural mechanisms and adult sexual partner preferences. In this review, we present an overview of sexual differentiation in relation to sexual partner preferences. We then summarize results that test the relevance of the organizational hypothesis to expression of same-sex sexual partner preferences in rams. Finally, we demonstrate that the sexual differentiation of brain and behavior in sheep do not depend critically on aromatization of testosterone to estradiol.
Keywords: Sheep, rams, sexual partner preference, sexual orientation, aromatase, sexually dimorphic nucleus, preoptic area, anterior hypothalamus
Introduction
Sexual partner preferences are an essential part of the repertoire of an animal's sexual behavior that promotes initial physical contact, courtship, and mating. Obviously, sexual attraction between opposite-sex animals is essential to reproductive success and thus sexual partner preferences are typically highly sexually dimorphic; most males prefer to mate with females and most females prefer to mate with males. However, same-sex attraction, courtship, and genital contact are observed in many species (Bagemihl, 1999). Fifty years ago Phoenix, Goy, Gerall and Young (Phoenix et al., 1959) proposed that sexual differentiation of copulatory behavior resulted from organizing actions of sex hormones, namely, permanent actions of sex hormones occurring during a critical period in early development. Although their original hypothesis did not explore the scope of organizational effects on endpoints other than sex-typical motor patterns of sexual behavior, such as mounting and lordosis, it has nonetheless formed the predominant theoretic framework for testing the origins of other sexually dimorphic behaviors and brain functions. Today one of the most developed biologic theories pertaining to sexual partner preference or sexual orientation in animals is the organizational hypothesis. Numerous studies have been performed over the last several decades to assess the contribution of perinatal testosterone and/or estradiol exposure to the development of a male-typical preference to choose a female as opposed to a male mating partner. The animal literature on this topic has been extensively reviewed (Adkins-Regan, 1988; Wallen and Baum, 2002; Baum, 2006). Many of the animal models used in these studies (e.g., rats, ferrets, zebra finches) do not appear to exhibit spontaneous same-sex preferences as part of their species typical behavioral repertoires. Instead, same-sex mate preferences are the consequence of exposure to abnormally high or low hormone levels perinatally. These exogenous treatments alter genital sex as well as brain sex and are accompanied by same-sex attraction and partner preference. Although important insights have been gained from these studies, they are confounded by their inability to manipulate sexual partner preferences separately from genital anatomy and the other components of sexual behavior. Unlike these models, exclusive same-sex attraction occurs spontaneously in domestic ram populations. Rams that are attracted to other rams have normal male genitalia and show male-typical mounting not female-typical receptive behaviors. Sexual partner preferences in rams can be characterized and quantified by experimental observations using a choice test. Thus, the ram model offers an opportunity to test the prenatal organizational hypothesis in a hormonally and neurologically unmanipulated animal. The purpose of this review is to present an overview of current knowledge related to same- sex partner preferences in domestic rams.
Conventions and criteria used for determining sexual partner preferences in animals
By convention, sexual partner preference and sexual orientation are used interchangeably to describe an animal's mate selection. A male that directs his sexual activities toward females is classified as female-oriented or heterosexual. If on the other hand, these behaviors are directed toward other males, the subject is classified as male-oriented or homosexual. The use of this terminology is not meant to imply that sexual orientation as applied to animal behavior is identical to human sexual orientation, which represents a more complex set of feelings, fantasies, and erotic responses (Byne, 2007).
Criteria for assessing sexual partner preferences in animals were first articulated by Adkins-Regan (1988) and have been further elaborated by Baum (2006) and Vasey (2002). An animal's sexual partner preference can only be judged by administering `choice tests' that are designed to present both male and female incentive stimuli simultaneously. The conditions of this test are very important. The experimental subject must be able to choose between approaching and interacting sexually with either stimulus or to remain neutral. The stimulus animals should ideally be sexually proceptive vis- a- vis the experimental subject. These interactions must be uncoerced and culminate in actual sexual behavior between the subject and stimulus animal. Finally, the enduring quality of an animal's sexual partner preference should be tested by studying behavior over an extended period of the animal life. In rodents this may constitute repeat testing over several months, whereas in longer-lived animals testing should be repeated over longer periods, i.e., years.
The male-oriented ram model
In general sheep are polygamous and seasonally breeding animals that are most active during the autumn in the Northern Hemisphere. Extensive field studies have been performed in Bighorn mountain sheep (Ovis canadensis) that describe their social and sexual behavior (Geist, 1974). In the wild, mountain sheep are segregated by sex except during the breeding season or rut when rams join flocks of females in search of ewes in estrus. Mature rams congregate en masse in the fall before the rut and establish status, which for rams, is the main determinant of access to estrous ewes during the rut. In male groups (bands), dominant males often attempt to copulate with subordinate males. However, this homosexual behavior is situational because during the breeding season it is the top-ranking males that are most successful in breeding estrous ewes (Pelletier and Festa-Bianchet, 2006).
In domestic sheep breeds (Ovis aries), most rams are sexually attracted to and mate with estrous ewes, although like wild sheep, domestic rams also show non-exclusive male-male mounting, associated with social rank or induced by overcrowding. It is estimated that ~25% of domestic rams that are otherwise healthy show little or no sexual interest in receptive ewes. The rams have been called `asexual'(Price, 1987), `non-workers'(Lynch et al., 1992), or `low response rams' (Zenchak and Anderson, 1980) to distinguish them from rams with a more typical vigorous libido for ewes. Zencheck et al. (1981) first reported that some of these seemingly low libido rams actually show considerable sexual behavior directed towards other rams and concluded that their failure to breed was a consequence of their preference for rams as sexual partners. There are now a substantial number of reports documenting the occurrence of male-oriented preferences in rams (Price et al., 1988; Perkins and Fitzgerald, 1992a; Perkins et al., 1995; Resko et al., 1996; Pinckard et al., 1998; Alexander et al., 1999;Alexander et al., 2001a; Alexander et al, 2001b). Thus, four behavioral phenotypes have been described in rams: female-oriented, bisexual, asexual and male-oriented. The relative frequencies of each phenotype reported in three independent (Price et al. 2004; Perkins et al., 1992a; Roselli et al.,2004) studies show remarkable degree of similarity, see Table 1, with male-oriented rams accounting for ~8% of a given population of rams.
Table 1.
Frequencies (%) of different behavioral phenotypes in rams.
| Female-oriented | Bisexual | Male-oriented | Asexual | References |
|---|---|---|---|---|
| 55.6 | 18.4 | 7.4 | 18.5 | Price et al. (Price et al., 1988) |
| -----------74.4a---------- | 8.5 | 17.0 | Perkins et al. (Perkins et al., 1992a) | |
| 55.6 | 22.0 | 9.5 | 12.5 | Roselli et al. (Roselli et al., 2004b) |
Perkins et al. did not distinguish between female-oriented and bisexual rams.
Ram sexual preferences are characterized by extensive testing over a two to three year period. Initially a sexual behavior or performance test is administered during which 18-mo-old rams are exposed individually to 3 estrous ewes on 9 - 18 separate occasions during a two month period. Repeated exposure to ewes gives rams sufficient time to express sexual interest. The sexual performance test for rams was originally developed by Mattner et al. (1971) and modified by Perkins et al. (Perkins et al., 1992a). Each test lasts 30 min and the number of anogenital sniffs, foreleg kicks, flehmens (curling of the upper lip to facilitate pheromone detection by the vomeronasal organ), vocalizations, mounts, and ejaculations are recorded. Rams that do not copulate in this test have their chests marked with pigment and are placed overnight with ewes to see if they leave a color mark on the ewes as evidence of mating.
Following the last sexual performance test a group of rams that showed high libido and another group that did not show any sexual interest in females are then given preference tests. The sexual preference test was initially designed by Price (Price et al., 1988) and modified by Perkins and Fitzgerald (Perkins et al., 1992b). The rams are housed in separate pens for 7 to 9 days, and then are exposed to two restrained estrous ewes and two restrained rams in a standardized 30 min sexual partner preference test during which all behavioral interactions are recorded (Figure 1). Restraining stimulus animals assures that they will stand still and eliminates aggression (Zenchak et al., 1988). The immobile stance adopted by an estrous ewe is the single most important trigger for male mounting behavior (Signoret, 1976) and, thus, restraint simulates this sexual proceptivity in both stimulus animals. The ram being tested is free to interact and mate with each of the stimulus animals or remain neutral. The frequencies of pre-copulatory and copulatory behaviors are recorded as well as the sex of the animal to which these behaviors are directed. Sexual partner preference tests are administered twice when the rams are approximately 16 - 18 mo-old and twice again the following breeding season when they are 28 - 30 mo-old.
Figure 1.
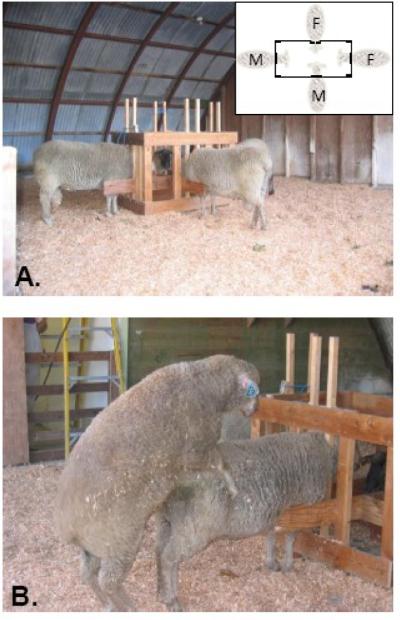
Photographs of the testing setup used to characterize sexual partner preferences in rams. A) Illustrates the position of the stimulus animals in a four-way stanchion (inset) placed in the center of a testing arena (10 m × 10 m). B) Shows a ram mounting a stimulus ram.
Following all testing, a male-oriented ram is operationally defined as a mature ram that was exposed to ewes; never expressed sexual interest or activity toward ewes; and when provided simultaneously with both ewes and rams as stimuli, exhibited sexual behaviors toward males only. Rams that showed sexual interest and copulated exclusively with ewes in both the performance and preference tests are classified as female-oriented rams. Rams that exhibited no sexual behavior after this extensive testing paradigm are considered to be asexual. Rams that copulated with both ewes and other rams in the sexual partner preference, regardless of the frequency of each, are classified as bisexual. In our studies so far, we have concentrated on comparisons between male- and female-oriented rams. Table 2 illustrates the differences in average behavioral responses exhibited by male- and female-oriented rams given sexual partner preference tests. Once established, sexual partner preferences appear to be stable throughout adulthood in rams suggesting that they are organized during an early period of life, probably during fetal development when sexual differentiation of the brain occurs.
Table 2.
Averages (±SE) of behaviors exhibited by female-oriented and male-oriented rams during exposure to two estrous ewes and two rams in four separate 30-min partner preference tests
| Female-oriented rams (n = 6) |
Male-oriented rams (n = 6) |
|||
|---|---|---|---|---|
| Behaviors | Estrous stimulus ewe | Ram stimulus | Estrous stimulus ewe | Ram stimulus |
| Precopulatory Behaviorsa | 33.4 ± 5.5 | 9.4 ± 1.9 | 2.6 ± 0.5 | 37.0 ± 10.4 |
| Mount attemptsb | 0.4 ± 0.2 | 0.1 ± 0.1 | 0 | 0.4 ± 0.1 |
| Mounts | 9.2 ± 1.2 | 0.5 ± 0.3 | 0 | 11.1 ± 3.3 |
| Ejaculations | 2.8 ± 0.3 | 0 | 0 | 0.6 ± 0.3 |
Before partner preference tests, rams were given performance tests with estrous ewes for a total of 9 h. Male-oriented rams did not mount ewes in any test.
Precopulatory behaviors include the sum of: genital sniffs, foreleg kicks, vocalizations and flehmen responses (lip curls).
Mount attempts signify unsuccessful mounts in which both front feet left the ground but the ram did not become firmly positioned on the ewe's rump.
Overview of sexual differentiation
The steps leading to sexual differentiation are remarkably consistent across all mammals and were first described by the elegant studies of Jost et al. (1973). Early in development the gonad is undifferentiated and bipotential and associated with two sets of paired urogenital ducts: the Wolffian and Mullerian ducts. If the fetus is male, it inherits a Y chromosome that contains the Sry gene that interacts with genes on the X chromosome and autosome to cause the undifferentiated gonad to become a testis instead of an ovary (Vilain, 2000). The testes then secrete hormones during a critical period of development that masculinize the genitalia. Mullerian Inhibitory Substance (MIS) causes the Mullerian ducts to degenerate assuring that the female reproductive tract will not develop. Testosterone, on the other hand, promotes the development of the Wolffian ducts into the epididymus, vas deferens and seminal vesicles. The female reproductive phenotype develops when the SRY gene is absent as in females or if androgen receptors are defective.
The brain can also be thought of as bipotential. The pioneering studies conducted by Phoenix et al. (1959) demonstrated that testosterone both masculinizes and defeminizes the brain. Testosterone acts only during a perinatal critical period that is specific for each species (MacLusky and Naftolin, 1981). Like the gonad and reproductive tract, the female brain will develop in the absence of testosterone exposure, although there is evidence to suggest that brain feminization may involve estrogens in mice (Bakker and Baum, 2008). Brain sex is defined by structural, neurochemical, and functional sex differences. The sexual differentiation of the brain can involve hormone-induced alterations in several processes including, the formation, migration, differentiation, and survival of neurons as well as the formation and stabilization of synapses (Wilson and Davies, 2007). Of these, the hormonal control of neuronal survival (i.e. cell death) is currently the best-established mechanism for creating sex differences in cell numbers in the brain and spinal cord (Forger, 2006).
In rats, the most studied aspect of brain masculinization is the development of the male-typical sexually dimorphic nucleus of the medial preoptic area, which is five larger in male rats than in females (Gorski et al., 1980). In addition, the number of dendritic spines and axospinous synapses in preoptic neurons is greater in male rats than in females (Larriva-Sahd, 1991; Amateau et al., 2004). Sexually dimorphic nuclei have also been described in the medial preoptic/anterior hypothalamus (MPOA/AH) of other vertebrate species, including humans (Cooke et al., 1998). Some sexually dimorphic nuclei are larger in females than in males (Cooke et al., 1998). In terms of reproductive function, brain masculinization programs adult male-typical copulatory behaviors and defeminization suppresses feminine receptive behaviors and the capacity for the surge gonadotropin response to estradiol.
Testosterone acts on the brain by two primary pathways: (1) an androgen pathway in which either testosterone or its active reduced metabolite 5α-dihydrotestosterone interact with androgen receptors on target cells, and (2) an estrogen pathway in which testosterone is converted to estradiol by cytochrome P450 aromatase and brain-derived estrogens activate estrogen receptors. Hormonal requirements for the sexual differentiation of the brain differ among species. For instance, in rats the aromatase pathway contributes to the defeminization of the brain and sexual behavior (McEwen et al., 1977), while androgen receptor mechanisms appear to be more important in guinea pigs, primates, and humans (Resko and Roselli, 1997; Grumbach and Auchus, 1999). It is apparent from animal work that brain sexual differentiation is not a singular event but a complex process that occurs during different periods of development in a sequence of temporally overlapping steps and changing hormonal requirements (Tobet and Schwarting, 2006).
Although hormones appear to play a dominant role, other observations suggest that genes residing on sex chromosomes that are asymmetrically inherited in males and females may also influence sexual differentiation of the brain. The recent development of the four core genotype (FCG) mouse model, in which chromosomal sex was engineered to be independent of gonadal sex, makes it possible to analyze the contribution that sex chromosome genes make to sexual differentiation. As recently reviewed by Arnold (Arnold and Chen, 2008), studies of the FCG model have uncovered sex differences in behaviors, gene expression, and susceptibility to disease that are not mediated by gonadal hormones but depend on chromosomal sex. It is expected that as research in this area continues, it will bring new understanding of the extent and specificity with which sex-linked genes and hormones act independently and in concert to define brain structure and function.
Finally, in addition to the physiological mechanisms that regulate brain sex, it is increasingly evident that exogenous hormones, nutrients, environmental endocrine disruptors, and other harmful chemical substances that enter the fetal circulation via the mother can produce permanent changes that alter sexual differentiation of brain structure and function (Gore, 2008).
Sexual differentiation in sheep
Gestation in the sheep lasts approximately 150 days. Gonadal differentiation occurs between gestational day 25 (GD25) and GD35 and the external genitalia begin to differentiate on GD45 (Clarke et al., 1976a). The fetal testes synthesize elevated levels of testosterone beginning around GD35 (Attal, 1969; Pomerantz and Nalbandov, 1975) and the testicular content of testosterone continues to increase from this time until birth. Fetal males have higher systemic concentrations of testosterone at GD65 to GD70 than do fetal females (Pomerantz et al., 1975). Concentrations of testosterone in males decline between GD70 and GD90, and are not significantly greater than in females until late in gestation and during postnatal life (Pomerantz et al., 1975) and Roselli et al. (unpublished data).
Clarke and colleagues (Clarke et al., 1976b; Clarke, 1977) assessed the effects of maternal testosterone treatment in sheep over several different gestational periods on estrous cyclicity and the display of male-typical mounting behavior. Results of their studies established that the critical period for behavioral masculinization/defeminization occurs slightly later than the sensitive period for genital masculinization. Furthermore, the period of maximum behavioral sensitivity to testosterone occurs prior to GD90. Extensive studies by Foster and colleagues (Foster et al., 2002) have also shown that exposure of ewe lamb fetuses to testosterone from GD30 - GD90 suppresses the LH surge mechanism, progesterone negative feedback on gonadotropin secretion, and masculinizes the control of tonic LH secretion resulting in the advancement of puberty. Although considerable information has been gathered relative to the effects of prenatal exposure to testosterone on neuroendocrine functions and behavior, less is known about the role of androgen and estrogen receptor mechanisms in the masculinization and defeminization of the sheep brain. The finding that prenatal exposure of fetal female lambs to either testosterone and dihydrotestosterone advances puberty, but that only testosterone disrupts the estradiol positive feedback response suggests that the former relies on androgen receptor activation while the latter requires aromatization prior to estrogen receptor activation (Masek, et al., 1999). However, as discussed later in this review, prenatal exposure of fetal ram fetuses to an aromatase inhibitor did not have the opposite effect of reprogramming genetic males to respond to estrogen treatment with an LH surge and display of female-typical receptivity.
Relationship between male-typical medial preoptic area/anterior hypothalamus and sexual partner preferences
Several studies have linked a male's preference for a female mate to the function of the sexually dimorphic MPOA/AH in rats and ferrets. Cherry and Baum (Cherry and Baum, 1990) demonstrated that bilateral lesions centered in the sexually dimorphic male nucleus (MN) of the MPOA/AH causes male ferrets to show female-like latencies in approach behavior to stud males in L-maze tests. Subsequently, these researchers demonstrated that either excitotoxic (Paredes and Baum, 1995) or electrolytic (Kindon et al., 1996) lesions of the MN caused males to exhibit female-like partner preferences in a T-maze test. The function of the MN was linked to male-typical processing of body odorant cues because bilaterally lesioned males resembled sham-operated females by preferring to approach body odors emitted from anesthetized male as opposed to female stimulus ferrets confined in the goal boxes of a Y-maze (Alekseyenko, et al., 2007). Lesioned males also showed a female-like Fos response to odors emitted from male soiled bedding. Similar to the ferret, male rats that were given bilateral MPOA/AH lesions showed a strong preference to approach a stud male versus an estrous female in a choice test (Paredes et al., 1998). Considered together with the studies that demonstrate that the volume of the MN and SDN-POA established by perinatal exposure to testosterone and estradiol (Dohler, et al., 1982; Tobet et al., 1986), these results suggests that the male's preference for an estrous female depends on the male-typical development of and functional integrity of neurons in the sexually dimorphic MPOA/AH. Destruction of these neurons in males or their absence in females leads to a female-typical sexual partner preference. Thus, given the adundance of sensory afferent input reaching the MPOA/AH (Simerly and Swanson, 1986), male-typical attraction for estrous females most likely depends on sexually dimorphic sensory processing.
In humans, the third interstitial nucleus of the anterior hypothalamus (INAH3) has been identified as a likely homologue of the rat SDN-POA. Three independent groups of investigators (Allen et al., 1989; LeVay, 1991; Byne et al., 2001) have reported that the volume of INAH3 is significantly larger in men presumed to be heterosexual than in women. Postmortem studies using men who died of AIDS and were presumed to be homosexual suggested that the volume of INAH3 was smaller in homosexual men than in heterosexual men, but the number of neurons in the nucleus does not vary with sexual orientation (LeVay, 1991;Byne et al., 2001).
Given the unavoidable technical difficulties associated with these human postmortem brain studies they cannot yet be viewed as conclusive. Nor is there available data to suggest that fetal differences in testosterone exposure lead to differences in the volume of INAH3 between heterosexual men, homosexual men, and women. Lesion studies in rats and ferrets functionally link the sexually dimorphic MPOA/AH with the expression of male-typical sexual partner preferences. However, lesion of MPOA/AH also increases the expression of female typical receptivity. Clearly, there is a complex and overlapping neural circuitry within the MPOA/AH given the diversity of functions regulated. This complexity makes it difficult to conclusively show that sexually dimorphic mate choice is due to sexual differentiation of MPOA/AH.
Testing the organizational hypothesis in relation to the sexual partner preferences of sheep
According to the organizational hypothesis of brain sexual differentiation, male-oriented rams are presumed to have experienced a subthreshold prenatal exposure to testosterone that is more typical of females. This leads, in turn, to incomplete masculinization and/or defeminization during development that is manifested as a sex-atypical sexual partner preference. The ability to identify and study a small population of rams that exhibit exclusive same-sex preferences has provided us with a unique opportunity to test this hypothesis in unmanipulated animals. Several approaches have been taken to test whether the prenatal organizational hypothesis applies to sexual partner preferences in rams. These primarily involve searches for correlations between sexual preferences, sexually differentiated endocrine responses, steroidogenic enzymes, receptor concentrations, and sexual dimorphic brain morphology.
Neither male- or female-oriented rams exhibit LH surge responses or female-typical sexual behavior
One well-studied sex difference in the sheep brain pertains to its role in regulating secretion of luteinizing hormone (LH) from the anterior pituitary gland (Karsch and Foster, 1974). In brief, the brain of a typical ewe responds to an injection of estradiol by signaling the pituitary gland to secrete a large amount or surge of LH. This positive feedback control of LH is the signal for ovulation and is accompanied by an increase in sexual receptivity. Usually rams are incapable of showing these responses to an injection of estradiol. This is because prenatal exposure to testosterone either from the fetal testis or exogenously administered, defeminizes the brain mechanisms controlling these responses (Foster et al., 2002). Tests show that the LH surge response is nonfunctional (defeminized) in male-oriented rams like it is in female-oriented rams (Perkins et al., 1995; Stormshak et al., 2008). Moreover, neither male- nor female-oriented rams express female-typical receptive behaviors in response to estradiol (Stormshak et al., 2008). Taken together, these data suggest that the neural mechanisms controlling the LH surge and female receptivity are defeminized regardless of their sexual partner preference.
Male- and female-oriented rams differ in their neural and endocrine response to female sexual cues
The presence of an estrous ewe induces LH and testosterone secretion in sexually experienced female-oriented rams (i.e., ewe effect) (Gonzalez et al., 1991; Booth and Signoret, 1992; Perkins et al., 1992b). Presumably this is a sexually differentiated response that is complementary to the phenomenon of male-induced LH secretion and ovulation in ewes (i.e., ram effect) (Gelez and Fabre-Nys, 2004). If same-sex behavior had the same physiological consequences as heterosexual behavior (with the only difference being the gender of the preferred partner), one would expect to find that male-oriented rams do not respond to females, but instead show elevations in LH when exposed to other males. In spite of apparent sexual arousal and clearly defined sexual activity directed at other males, no increases in pulse frequency or basal concentration of LH were observed when male-oriented rams were exposed to either male or female partners (Perkins et al., 1992a). This was shown not to be the result of a difference in pituitary sensitivity to GnRH between male- and female-oriented rams.
Alexander et al. (1999) demonstrated that sexual contact is not necessary to evoke a neuroendocrine response. Concentrations of LH increase after female-oriented rams are given fence line exposure to estrous ewes, but not to other rams. High libido female-oriented rams exhibit a high degree of investigatory behavior toward estrous ewes as well as other rams. As a consequence only female-oriented rams appear able to discriminate between stimulus animals suggesting that sensory signals provided by estrous females are either not detected by male-oriented rams or are not sufficiently provocative. Lending additional support to this explanation, Alexander et al. (2001a) found that more neurons stain positively for fos-related antigen in the MPOA and bed nucleus of the stria terminalis after exposure to estrous ewes and rams in female-oriented rams than in male-oriented rams. Taken together these results demonstrate that male and female-oriented rams have differential neural and endocrine response to salient sexual stimuli, but do not suggest that the response of the male-oriented ram is female-like.
Studies have not yet been performed to identify what sensory cues attract male-oriented rams to other rams. It seems likely that olfaction is involved, but may not be the only sensory modality involved. Lindsey demonstrated that rams rely very heavily on their sense of smell to detect females in estrus (Lindsay, 1965). Blissitt et al. (1994) found that rams can discriminate between urine from estrous and non-estrous ewes in an operant test. However, the ewe effect cannot be elicited in rams by the odors of estrous ewes alone, but appears to require both the odor and the visual stimulus of an estrous female (Gonzalez et al, 1991). Future studies are needed to explore what salient sensory cues are used for same-sex attraction in rams.
Neurobiological correlates of ram sexual partner preferences
Perkins et al. (Perkins et al., 1995) were first to suggest that male-oriented rams exhibit neurobiological attributes more typical of ewes than of female-oriented rams when they reported that the estrogen receptor content of the amygdala was significantly higher in female-oriented rams than in male-oriented rams and ewes. This observation is interesting because the amygdala, especially the medial amygdala, is a site for integrating chemosensory and hormonal cues important for the expression of opposite-sex odor preferences and copulatory behaviors in male Syrian hamsters (Wood, 1998; Maras and Petrulis, 2006). Estrogen receptors present in amygdala neurons that project to the medial preoptic area and anterior hypothalamus could, via aromatization (Roselli et al., 1998), translate information about peripheral concentrations of testosterone, thereby integrating the amygdala with other brain regions necessary for male sexual behavior.
Resko et al. (1996) reported that aromatase activity is significantly higher in the MPOA of female-oriented rams than of male-oriented rams; ewes were not used in this comparison study. As discussed already, several studies have linked the function of the sexually dimorphic MPOA/AH to the expression of male-typical sexual partner preferences (Cherry et al., 1990; Paredes et al., 1995; Kindon et al., 1996; Paredes et al., 1998). Previous studies in rats demonstrated that the capacity for aromatization in the MPOA is greater in males and females treated perinatally with testosterone than in control females, demonstrating that brain estrogen synthesis is sexually differentiated in rats (Roselli and Klosterman, 1998). Thus it was proposed that the lower levels of aromatase activity observed in male-oriented rams were the result of processes that occurred prenatally during the critical period for sexual differentiation (Resko et al., 1996). Resko also reported that systemic levels of testosterone and its synthesis in vitro by testes were significantly lower in male-oriented rams than in female-oriented rams. In a subsequent study, Roselli et al., (2002) found that this difference is related to a differential effect of anesthesia on male- and female-oriented rams in which the concentration of testosterone is inversely correlated with systemic levels of cortisol. Because the endocrine response to anesthesia is most likely coordinated through the central nervous system these results raise the question of whether functional differences that are unlikely to be directly involved in reproduction exist between the brains of male- and female-oriented rams.
Neuroanatomic correlates of ram sexual partner preferences
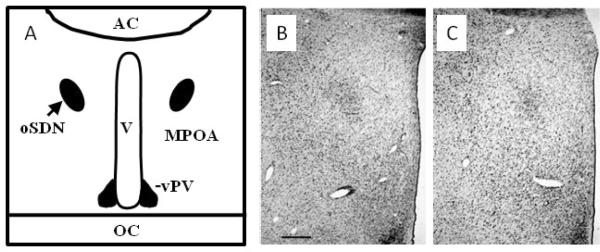
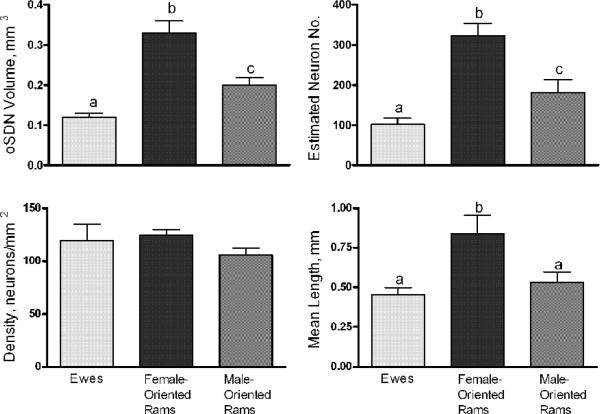
The evidence that the MPOA/AH participates in regulation of male-typical copulatory behavior and sexual partner preferences in rats and ferrets (Paredes et al., 1995; Paredes et al., 1998) led to the search for a sexually dimorphic nucleus in the sheep. A dense cell group was identified in the MPOA/AH of adult age matched adult sheep that is 2 to 3 times larger and contains more neurons in female-oriented rams than in ewes (Roselli et al., 2004). This cell group was named the ovine sexually dimorphic nucleus (oSDN) because it is neuroanatomically similar to the SDN-POA in rats. It is an oblong group of densely packed cells situated bilaterally adjacent to the third ventricle and comprises the central component of the medial preoptic nucleus (Figure 2). The oSDN is also significantly larger in female-oriented than in male-oriented rams and, in this aspect, is reminiscent of INAH3 in humans (Figure 2 and 3). This was the first demonstration of an association between naturally occurring individual variations in sexual preferences and brain structure in nonhuman male animals. The dense cluster of cells that comprise the oSDN exhibits a high level of aromatase mRNA expression. Aromatase mRNA levels in the oSDN are significantly greater in female-oriented rams than in male-oriented rams or ewes. Aromatase activity and mRNA expression is regulated by androgens in adults, but this does not explain the difference in expression because systemic testosterone concentrations are not different between male- and female-oriented rams (Roselli et al., 2004). How these differences in cell number and aromatase expression relate to the expression of sexual partner preferences is not yet understood. However, differences in the size of the oSDN among female-oriented rams, male-oriented rams, and ewes are not due to differences in adult exposure to testosterone because these differences persist even after adult sheep are gonadectomized and treated with physiological concentrations of testosterone (Roselli et al., 2008). Thus, it is plausible to suggest that the differences in the size of the oSDN result from the organizational effects of prenatal testosterone exposure.
Figure 2.
A)Schematic diagram of a coronal section through the sheep at the level of the optic chiasm (OC) and anterior commissure (AC). The position of the oSDN is shown bilateral to the third ventricle (V) in the central part of the medial preoptic nucleus (MPOA). The ventral paraventricular nucleus (vPV) is shown at the base of the ventricle. B) Micrograph of oSDN from the left hypothalamus of a female-oriented ram. The third ventricle is at the right of the figure. C) Section of a male-oriented ram comparable to that in (B). The photomicrograph are taken near the midpoint of the anterior - posterior extent of the oSDN. The scale bar is 1 mm.
Figure 3.
Differences of the oSDN among female-oriented rams (n=8), male-oriented rams (n=9), and mid-luteal phase ewes (n=10). A, Differences in oSDN volume; B, Differences in neuron counts; C, Differences in neuron density; and D, Differences in oSDN length. Data are presented as means ± SEM. Bars with different letters differ significantly, P < 0.05. Reprinted from Roselli et al. (Roselli et al., 2004).
Prenatal organization of the oSDN
The observation that rams which express partner preferences for other rams have a smaller oSDN than rams that are attracted to ewes does not establish whether the size of the oSDN is the cause or consequence of mate preference. Ideally, the volume of the oSDN would be measured sequentially, early in life to determine whether size predicts sexual preference and again, after sexual preferences are established, to determine whether social and/or sexual experiences affect oSDN size. Such a study is not yet technically feasible. But it is possible to determine when neurons composing oSDN differentiate into a dimorphic nucleus. In other long gestation species such as the ferret, the sexually dimorphic male nucleus in the MPOA/AH is identifiable as early as embryonic day 31 of a 41-day gestation (Cherry et al., 1990). Sexual differentiation occurs during midgestation in sheep at a time when there is a consistent sex difference in systemic concentrations of androgens (Pomerantz et al., 1975). A cluster of neurons in the MPOA/AH expressing high levels of aromatase mRNA is identifiable as early as day 60 of gestation in lamb fetuses and probably represents the nascent oSDN (Roselli unpublished observations). Later in gestation, ~days 130 -140 of a 150-day period of gestation, the oSDN is present and twice as large in male as in female fetuses (Figure 4) (Roselli et al., 2007). The volume of the nucleus and the magnitude of the sex difference approximate that observed in adults, suggesting that sexual differentiation is completed or nearly completed before birth in sheep. To determine whether the oSDN size was controlled by exposure to testosterone in utero, testosterone or oil vehicle was administered to pregnant ewes during the critical period (gestational day 30 to 90) and oSDN volumes in testosterone-exposed and control fetuses were measured on gestational day 135. The results showed that oSDN volume is significantly greater in testosterone-exposed females than in control females, and also larger than in control and testosterone-exposed males (Figure 5). These data indicate that sexual differentiation of the oSDN occurs prenatally. The larger volume of the oSDN in males and testosterone exposed females suggests that the numbers of neurons and/or volume of neuropil comprising the oSDN are established by androgen exposure during early development and may later predispose adult rams to express a sexual attraction to either ewes or other rams.
Figure 4.
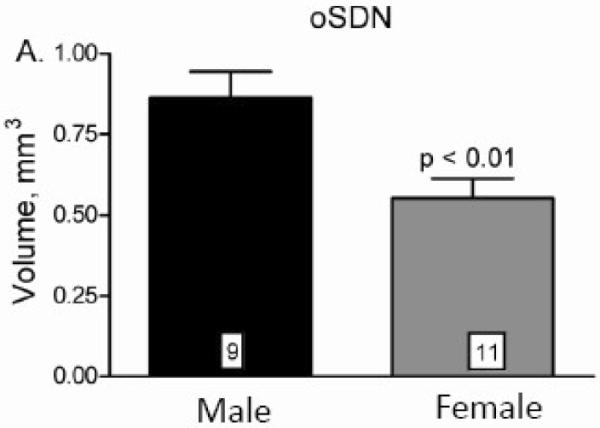
Sex comparisons of oSDN volumes in late gestational sheep fetuses (GD 135 ± 0.8 SEM). Data are presented as means ± SEM. Note that volume measurements were determined from autoradiograms from in situ hybridization for aromatase mRNA expression and correspond to similar measurements made in adults, see Fig. 3 in (Roselli et al., 2004). Reprinted from Roselli et al. (Roselli et al., 2007).
Figure 5.
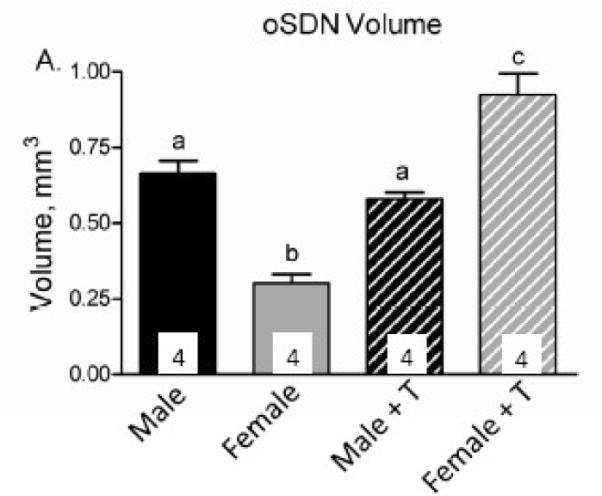
Effect of prenatal testosterone exposure on oSDN volume. Pregnant ewes received injections of testosterone propionate (100 mg) twice a week from days 30 to 90 of gestation so that their fetuses were exposed to elevated systemic levels of testosterone during the critical period for sexual differentiation. Control animals were not injected. Data are presented as means ± SEM, n = 4/group. Bars with different letters differ significantly, P < 0.05. Reprinted from Roselli et al. (Roselli et al., 2007).
Aromatization is not necessary for sexual differentiation of partner preferences in sheep
Most studies that have tested whether aromatase plays a role in the development of sexual partner preferences have been performed in short gestation mammals. In rats, the metabolism of endogenous testosterone to estradiol by cytochrome P450 aromatase is essential for organization of the SDN-POA as well as the expression of male-typical sexual partner preferences (Brand et al., 1991; Houtsmuller et al., 1994). Adult male rats treated perinatally with 1,4,6-androstatriene-3,17-dione (ATD), an aromatase inhibitor, exhibit very low or no preference for estrous females and a reduced SDN-POA volume compared to controls. However, studies using genetic mouse models have demonstrated roles for both aromatase and functional androgen receptors in the masculinization of sexual partner preferences (Bakker et al., 2004; Bodo and Rissman, 2007).
The abundant expression of aromatase mRNA in the oSDN raises the question of whether local conversion of testosterone to estradiol is required for organization of this nucleus. During development in sheep gonadal steroid secretion corresponds to the expression of high concentrations of estrogen and androgen receptors in MPOA/AH (Roselli et al., 2006). It has been suggested previously that aromatase is involved in defeminization of the sheep brain because prenatal treatment with testosterone, but not dihydrotestosterone, blocks the development of the LH surge-generating mechanism and decreases the capacity of females to show receptive behaviors (Masek et al., 1999). In contrast, both dihydrotestosterone and testosterone treatments in utero advance the onset of puberty in sheep indicating that hormonal requirements vary depending on the sexually dimorphic function being considered.
Recently a role for aromatase in sexual differentiation of sheep brain and behavior was tested directly by administering ATD to pregnant ewes from gestational days 50 to 80 so that aromatase activity was inhibited in the brains of exposed fetuses during the critical period (Roselli et al., 2006). Rams exposed to ATD prenatally from gestational days 50 to 80 exhibited male-typical sexual partner preferences, but showed a modest, yet significant, decrease in mounting behavior at 18 mo of age. No other aspect of brain masculinization/defeminization (i.e., age of puberty, LH surge response to estradiol, female-typical sexual behaviors, or oSDN volume) was altered by ATD exposure. Nor did prenatal ATD exposure interfere with normal development of female lambs. These results indicated that prenatal aromatization is necessary for complete behavioral masculinization in sheep similar to ferrets (Tobet and Baum, 1987).
This experiment has been repeated using higher doses of ATD given for longer periods of time. First, the dose of ATD was increased from 100 mg/day to 130 mg/day and pregnant ewes were treated from day 30 to 90 of gestation; the entire prenatal critical period. This dose of ATD inhibited aromatase in the fetal MPOA >90%, but failed to alter male-typical differentiation of brain and behavior. A third attempt was then initiated in which ATD (130 mg) was given twice a day at a 12 h interval from day 30 to 110 of gestation. This regimen was based on the observation that the elimination half-life of ATD in the pregnant sheep was 8.7 ± 1.2 h. Essentially the same results were obtained in all experimental iterations. Prenatal suppression of aromatase in the fetal lamb brain does not interfere substantially with masculinization and defeminization (Table 3). These results indicate that aromatization is not essential for the development of female-oriented sexual partner preferences in rams, nor is it critical for the defeminization of the LH surge mechanism and female-typical receptive behaviors. Rather, testosterone acting through androgen receptors, not its estrogenic metabolite, could be the agent responsible for masculine differentiation of the brain and behavior in sheep similar to what has been suggested for guinea pigs and nonhuman primates (Resko et al., 1997). Similarly, limited clinical information suggests that male-typical psychosexual differentiation does not rely on fetal aromatization and estrogen signaling in humans (Grumbach et al., 1999).
Table 3.
Summary of results from 3 experiments testing the effect of prenatal inhibition of aromatase on adult ram neuroendocrine function and behavior.
| Experiment 1 | Experiment 2 | Experiment 3 | |
|---|---|---|---|
| ATD Dose | 100 mg/day | 130 mg/day | 130 mg twice per day |
| Treatment Period | G50-G80 | G30-G90 | G30-G110 |
| Play Behavior | UM1 | UM | Nd4 |
| Growth Rate | UM | UM | UM |
| Sexual Maturation | UM | UM | UM |
| Copulatory Behavior | ↓M2 | UM | UM |
| Sexual Partner Preference | UM | UM | UM |
| LH Surge | UDF3 | UDF | UDF |
| Female-Typical Receptivity | UDF | UDF | UDF |
| oSDN Volume | UM | UM | ? |
| N | 6 controls; 8 ATD | 8 control; 10 ATD | 3 control; 2 ATD |
UM, unmodified masculinized
↓M, decreased masculinized
UDF, unmodified defeminized
nd, not determined
?, not yet tested
Fetal exposure to progesterone may also play a role in the development of sex differences in the brain and behavior of sheep. Roselli et al. (2006) found that, at day 65 of gestation, progesterone receptor mRNA is expressed in the MPOA and amygdala and is significantly greater in fetal male lambs than in females (Figure 6). Male rats are thought to be considerably more susceptible than females to the effects of maternal progesterone during development as a result of higher PR immunoreactivity in the medial preoptic area and other highly sexually dimorphic brain structures (Wagner et al., 1998; Quadros et al., 2002). Perinatal progesterone exposure suppresses copulatory behaviors in rats (Hull, 1981) and progesterone receptor ablation or pharmacologic blockade in mice enhances copulatory behavior (Schneider et al., 2005). However, it remains to be explored in any species whether progesterone is involved in the development of male-typical partner preferences.
Figure 6.
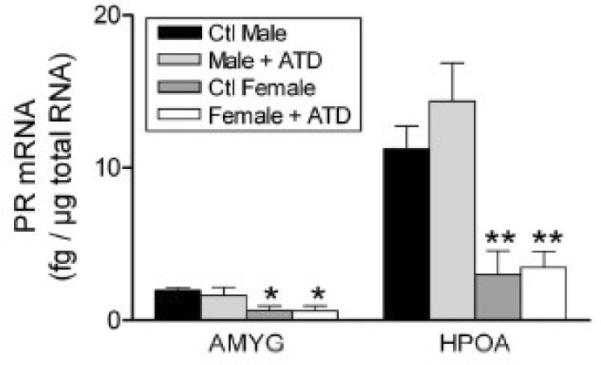
Sex differences in the expression of progesterone receptor mRNA in the amygdala (AMYG) and hypothalamus-preoptic area (HPOA) of gestational day 64 fetal lambs. The levels of progesterone receptor mRNA were measured using a RNase protection assay that measured mRNA concentration using a standard curve of progesterone receptor sense RNA and normalized values to levels of cyclophilin mRNA in each sample. Data are presented as means ± SEM, n =3- 4/group. *, P < 0.5; **, P < 01 male versus female. Some fetuses were exposed to the aromatase ATD given by Silastic implants to their mothers as described in (Roselli, Resko, and Stormshak, 2003). This treatment had no effect on progesterone receptor mRNA expression. Reprinted from Roselli et al. (Roselli et al., 2006).
Conclusions and future directions
The characterization of rams with distinct and verifiable sexual partner preferences provides a unique opportunity to test the organizational hypothesis of sexual differentiation in unmanipulated animals. Male-oriented rams actively court other rams using male-typical sexual behaviors, while completely ignoring estrous ewes. Yet, with respect to their responses to gonadal hormones and capacity to exhibit sex-typical consummatory behaviors, male-oriented rams show male-typical mounting, not estrogen-induced receptivity and LH surge secretion (Stormshak et al., 2008). These observations can be interpreted to suggest that male-oriented rams, like female-oriented rams, are masculinized and defeminized with respect to mounting, receptivity, and gonadotropin secretion, but not for sexual partner preferences. This is one of few examples, other than humans and nonhuman primates (Vasey, 2002), where sexual behaviors and sexual partner preferences are dissociated suggesting that these behaviors may be programmed differently. Together with their male-typical mate preference, male-oriented rams have a small, i.e., female-typical, oSDN. Sex differences in oSDN size are determined largely by prenatal exposure to testosterone. This observation reinforces the view that aspects of brain structure and function are not completely masculinized/defeminized in male-oriented rams and, thus, could have predisposed these animals to express sex-atypical mate preferences as adults. Although the exact function of the oSDN is not yet known, differences in volume and cell number could bias the processing of sexually relevant sensory cues involved in sexual partner choice.
Finally, despite abundant expression of aromatase mRNA in neurons of the oSDN, there is no evidence to suggest that aromatization plays an obligate role in male-typical development of oSDN or adult sexual partner preference. In this way, sheep appear similar to other long gestation mammals and may rely more on androgen receptor mediated mechanisms for sexual differentiation of reproductive behaviors. Nonetheless, this still leaves unanswered the question of what aromatase is doing in the oSDN. Thus, further investigation will be needed to understand the requirements and timing of oSDN development and ultimately the causal relationships between oSDN differentiation, adult function, and mate choice.
Acknowledgements
Preparation of this manuscript was supported by NCRR grant R01RR14270. The authors gratefully acknowledge their collaborators, students, and technicians for their many contributions to the work discussed in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adkins-Regan E. Sex hormones and sexual orientation in animals. Psychobiol. 1988;16:335–347. [Google Scholar]
- 2.Alekseyenko OV, Waters P, Zhou H, Baum MJ. Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. Physiol. Behav. 2007;2-3:438–449. doi: 10.1016/j.physbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander BM, Rose JD, Stellflug JN, Fitzgerald JA, Moss GE. Fos-like immunoreactivity in brain regions of domestic rams following exposure to rams or ewes. Physiol. Behav. 2001a;73:75–80. doi: 10.1016/s0031-9384(01)00441-3. [DOI] [PubMed] [Google Scholar]
- 4.Alexander BM, Rose JD, Stellflug JN, Fitzgerald JA, Moss GE. Low-sexually performing rams but not male-oriented rams can be discriminated by cell size in the amygdala and preoptic area: a morphometric study. Behav. Brain Res. 2001b;119:15–21. doi: 10.1016/s0166-4328(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 5.Alexander BM, Stellflug JN, Rose JD, Fitzgerald JA, Moss GE. Behavior and endocrine changes in high-performing, low-performing, and male-oriented domestic rams following exposure to rams and ewes in estrus when copulation is precluded. J. Anim. Sci. 1999;77:1869–1874. doi: 10.2527/1999.7771869x. [DOI] [PubMed] [Google Scholar]
- 6.Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J. Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amateau SK, Alt JJ, Stamps SL, McCarthy MM. Brain estradiol content in newborn rats: Sex differences, regional heterogeneity and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 8.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2008 doi: 10.1016/j.yfrne.2008.11.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attal J. Levels of testosterone, androstenedione, estrone and estradiol-17b in the testis of fetal sheep. Endocrinology. 1969;85:280–284. doi: 10.1210/endo-85-2-280. [DOI] [PubMed] [Google Scholar]
- 10.Bagemihl B. Biological exuberance: Animal homosexuality and natural diversity. St. Martin's Press; New York: 1999. [Google Scholar]
- 11.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front. Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm. Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Baum MJ. Mammalian animal models of psychosexual differentiation: When is `translation' to the human situation possible? Horm. Behav. 2006;50:579–588. doi: 10.1016/j.yhbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Blissitt MJ, Bland KP, Cottrell DF. Detection of oestrous-related odour in ewe urine by rams. J. Reprod. Fertil. 1994;101:189–191. doi: 10.1530/jrf.0.1010189. [DOI] [PubMed] [Google Scholar]
- 15.Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur. J. Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- 16.Booth WD, Signoret JP. Olfaction and reproduction in ungulates. Oxf Rev. Reprod. Biol. 1992;14:263–301. [PubMed] [Google Scholar]
- 17.Brand T, Kroonen J, Mos J, Slob AK. Adult partner preference and sexual behavior of male rats affected by perinatal endocrine manipulations. Horm. Behav. 1991;25:323–341. doi: 10.1016/0018-506x(91)90005-3. [DOI] [PubMed] [Google Scholar]
- 18.Byne W. Biology of Sexual Minority Status. In: Meyer IH, Northridge ME, editors. The Health of Sexual Minorities. Springer-Verlag; Berlin: 2007. pp. 65–90. [Google Scholar]
- 19.Byne W, Tobet S, Mattiace LA, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB. The interstitial nuclei of the human anterior hypothalamus: an investigation of variation with sex, sexual orientation, and HIV status. Horm. Behav. 2001;40:86–92. doi: 10.1006/hbeh.2001.1680. [DOI] [PubMed] [Google Scholar]
- 20.Cherry JA, Basham ME, Weaver CE, Krohmer RW, Baum MJ. Ontogeny of the sexually dimorphic male nucleus in the preoptic/anterior hypothalamus of ferrets and its manipulation by gonadal steroids. J. Neurobiol. 1990;21:844–857. doi: 10.1002/neu.480210603. [DOI] [PubMed] [Google Scholar]
- 21.Cherry JA, Baum MJ. Effects of lesions of a sexually dimorphic nucleus in the preoptic/anterior hypothalamic area on the expression of androgen- and estrogen-dependent sexual behaviors in male ferrets. Brain Res. 1990;522:191–203. doi: 10.1016/0006-8993(90)91461-o. [DOI] [PubMed] [Google Scholar]
- 22.Ciumas C, Hirschberg AL, Savic I. High fetal testosterone and sexually dimorphic cerebral networks in females. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn160. Advance Access published October 14, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Clarke IJ. The sexual behaviour of prenatally androgenized ewes observed in the field. J. Reprod. Fert. 1977;49:311–315. doi: 10.1530/jrf.0.0490311. [DOI] [PubMed] [Google Scholar]
- 24.Clarke IJ, Scaramuzzi RJ, Short RV. Effects of testosterone implants in pregnant ewes on their female offspring. J. Embryol. exp. Morph. 1976a;36:87–99. [PubMed] [Google Scholar]
- 25.Clarke IJ, Scaramuzzi RJ, Short RV. Sexual differentiation of the brain: endocrine and behavioral responses of androgenized ewes to oestrogen. J. Endocr. 1976b;71:175–176. doi: 10.1677/joe.0.0710175. [DOI] [PubMed] [Google Scholar]
- 26.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 27.Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is determined by the perinatal hormone environment. Neurosci. Lett. 1982;33:295–298. doi: 10.1016/0304-3940(82)90388-3. [DOI] [PubMed] [Google Scholar]
- 28.Foster DL, Padmanabhan V, Wood RI, Robinson JE. Sexual differentiation of the neuroendocrine control of gonadotrophin secretion: concepts derived from sheep models. Reprod. Suppl. 2002;59:83–99. [PubMed] [Google Scholar]
- 29.Geist V. Mountain Sheep A Study in Behavior and Evolution. The University of Chicago Press; Chicago: 1974. pp. 139–2554. [Google Scholar]
- 30.Gelez H, Fabre-Nys C. The “male effect” in sheep and goats: a review of the respective roles of the two olfactory systems. Horm. Behav. 2004;46:257–271. doi: 10.1016/j.yhbeh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez R, Levy F, Orgeur P, Poindron P, Signoret JP. Female effect in sheep. II. Role of volatile substances from the sexually receptive female; implication of the sense of smell. Reprod. Nutr. Dev. 1991;31:103–109. doi: 10.1051/rnd:19910110. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez R, Orgeur P, Poindron P, Signoret JP. Female effect in sheep. I. The effects of sexual receptivity of females and the sexual experience of rams. Reprod. Nutr. Dev. 1991;31:97–102. doi: 10.1051/rnd:19910109. [DOI] [PubMed] [Google Scholar]
- 33.Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front. Neuroendocrinol. 2008;29:358–374. doi: 10.1016/j.yfrne.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J. Comp. Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- 35.Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J. Clin. Endocrinol. Metab. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- 36.Houtsmuller EJ, Brand T, De Jonge FH, Joosten RNJMA, Van De Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol. Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 37.Hull EM. Effects of neonatal exposure to progesterone in sexual behavior of male and female rats. Physiol. Behav. 1981;26:401–405. doi: 10.1016/0031-9384(81)90166-9. [DOI] [PubMed] [Google Scholar]
- 38.Jost A, Vigier B, Prepin J, Perchellet JP. Studies on sex differentiation in mammals. Rec. Prog. Horm. Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- 39.Karsch FJ, Foster DL. Sexual differentiation of the mechanism controlling the preovulatory discharge of luteinizing hormone in sheep. Endocrinology. 1974;97:373–379. doi: 10.1210/endo-97-2-373. [DOI] [PubMed] [Google Scholar]
- 40.Kindon HA, Baum MJ, Paredes RG. Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm. Behav. 1996;30:514–527. doi: 10.1006/hbeh.1996.0055. [DOI] [PubMed] [Google Scholar]
- 41.Larriva-Sahd J. Ultrastructural evidence of a sexual dimorphism in the neuropil of the medial preoptic nucleus of the rat: A quantitative study. Neuroendocrinology. 1991;54:416–419. doi: 10.1159/000125923. [DOI] [PubMed] [Google Scholar]
- 42.LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- 43.Lindsay DR. The importance of olfactory stimuli in the mating behaviour of the ram. Anim.Behav. 1965;13:75–78. [Google Scholar]
- 44.Lynch JJ, Hinch GN, Adams DB. The Behaviour of Sheep: Biological Principles and Implications for Production. CSIRO Publications; East Melbourne: 1992. The reproductive behaviour of sheep; pp. 96–125. [Google Scholar]
- 45.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 46.Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur. J. Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- 47.Masek KS, Wood RI, Foster DL. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology. 1999;140:3459–3466. doi: 10.1210/endo.140.8.6913. [DOI] [PubMed] [Google Scholar]
- 48.Mattner PE, Braden AWH, George JM. The relation of libido tests to subsequent service activity of young rams. Aust. J. Exp. Agric. Anim. Husb. 1971;11:473. [Google Scholar]
- 49.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm. Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 50.Paredes RG, Baum MJ. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area anterior hypothalamus. J. Neurosci. 1995;15:6619–6630. doi: 10.1523/JNEUROSCI.15-10-06619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paredes RG, Nakagawa Y, Nakach N. Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res. 1998;813:1–8. doi: 10.1016/s0006-8993(98)00914-7. [DOI] [PubMed] [Google Scholar]
- 52.Pelletier F, Festa-Bianchet M. Sexual selection and social rank in bighorn rams. Anim. Behav. 2006;71:649–655. [Google Scholar]
- 53.Perkins A, Fitzgerald JA. Luteinizing hormone, testosterone, and behavioral response of male-oriented rams to estrous ewes and rams. J. Anim. Sci. 1992a;70:1787–1794. doi: 10.2527/1992.7061787x. [DOI] [PubMed] [Google Scholar]
- 54.Perkins A, Fitzgerald JA, Moss GE. A comparison of LH secretion and brain estradiol receptors in heterosexual and homosexual rams and female sheep. Horm. Behav. 1995;29:31–41. doi: 10.1006/hbeh.1995.1003. [DOI] [PubMed] [Google Scholar]
- 55.Perkins A, Fitzgerald JA, Price EO. Luteinizing hormone and testosterone response of sexually active and inactive rams. J. Anim. Sci. 1992b;70:2086–2093. doi: 10.2527/1992.7072086x. [DOI] [PubMed] [Google Scholar]
- 56.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 57.Pinckard K, Stellflug J, Williams M, Stormshak F. Influence of castration and estrogen replacement on sexual behavior of female-oriented, male-oriented, and asexual rams. J. Anim. Sci. 1998;78:1947–1953. doi: 10.2527/2000.7871947x. [DOI] [PubMed] [Google Scholar]
- 58.Pomerantz DK, Nalbandov AV. Androgen levels in the sheep fetus during gestation. Proc. Soc. Exp. Biol. Med. 1975;149:413–420. doi: 10.3181/00379727-149-38818. [DOI] [PubMed] [Google Scholar]
- 59.Price EO. Male sexual behavior. Vet. Clin. North Am. Food Anim Pract. 1987;3:405–422. doi: 10.1016/s0749-0720(15)31161-0. [DOI] [PubMed] [Google Scholar]
- 60.Price EO, Katz LS, Wallach SJR, Zenchak JJ. The relationship of male-male mounting to the sexual preferences of young rams. Appl. Anim. Behav. Sci. 1988;21:347–355. [Google Scholar]
- 61.Quadros PS, Lopez V, De Vries GJ, Chung WC, Wagner CK. Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J Neurobiol. 2002;51:24–32. doi: 10.1002/neu.10040. [DOI] [PubMed] [Google Scholar]
- 62.Resko JA, Perkins A, Roselli CE, Fitzgerald JA, Choate JVA, Stormshak F. Endocrine correlates of partner preference behavior in rams. Biol. Reprod. 1996;55:120–126. doi: 10.1095/biolreprod55.1.120. [DOI] [PubMed] [Google Scholar]
- 63.Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: Observations on guinea pigs and nonhuman primates. Cell. Mol. Neurobiol. 1997;17:627–648. doi: 10.1023/A:1022534019718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roselli CE, Estill CT, Stadelman HL, Stormshak F. The volume of the ovine sexually dimorphic nucleus of the preoptic area is independent of adult testosterone concentrations. Brain Res. 2008:1–7. doi: 10.1016/j.brainres.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roselli CE, Klosterman SA. Sexual differentiation of aromatase activity in the rat brain: Effects of perinatal steroid exposure. Endocrinology. 1998;139:3193–3201. doi: 10.1210/endo.139.7.6101. [DOI] [PubMed] [Google Scholar]
- 66.Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004a;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- 67.Roselli CE, Larkin K, Schrunk JM, Stormshak F. Sexual partner preference, hypothalamic morphology and aromatase in rams. Physiol Behav. 2004b;83:233–245. doi: 10.1016/j.physbeh.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 68.Roselli CE, Resko JA, Stormshak F. Estrogen synthesis in fetal sheep brain: effect of maternal treatment with an aromatase inhibitor. Biol. Reprod. 2003;68:370–374. doi: 10.1095/biolreprod.102.007633. [DOI] [PubMed] [Google Scholar]
- 69.Roselli CE, Resko JA, Stormshak F. Expression of steroid hormone receptors in the fetal sheep brain during the critical period for sexual differentiation. Brain Res. 2006;1110:76–80. doi: 10.1016/j.brainres.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 70.Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29:501–512. doi: 10.1385/ENDO:29:3:501. [DOI] [PubMed] [Google Scholar]
- 71.Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- 72.Roselli CE, Stormshak F, Resko JA. Distribution and regulation of aromatase activity in the ram hypothalamus and amygdala. Brain Res. 1998;811:105–110. doi: 10.1016/s0006-8993(98)00995-0. [DOI] [PubMed] [Google Scholar]
- 73.Roselli CE, Stormshak F, Stellflug JN, Resko JA. Relationship of serum testosterone concentrations to mate preferences in rams. Biol. Reprod. 2002;67:263–268. doi: 10.1095/biolreprod67.1.263. [DOI] [PubMed] [Google Scholar]
- 74.Savic I, Lindstrom P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proc. Natl. Acad. Sci. USA. 2008;105:9403–9408. doi: 10.1073/pnas.0801566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, Malley B, Levine JE. Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout (PRKO) mouse. Endocrinology. 2005;146:4340–4348. doi: 10.1210/en.2005-0490. [DOI] [PubMed] [Google Scholar]
- 76.Signoret JP. Chemical communication and reproduction in domestic mammals. In: Doty RL, editor. Mammalian Olfaction, Reproductive Processes, and Behaviour. Academic Press; New York: 1976. pp. 243–256. [Google Scholar]
- 77.Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J. Comp. Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- 78.Stormshak F, Estill CT, Resko JA, Roselli CE. Changes in LH secretion in response to an estradiol challenge in male- and female-oriented rams and in ewes. Reprod. 2008;135:733–738. doi: 10.1530/REP-07-0505. [DOI] [PubMed] [Google Scholar]
- 79.Tobet SA, Baum MJ. Role for prenatal estrogen in the development of masculine sexual behavior in the male ferret. Horm. Behav. 1987;21:419–429. doi: 10.1016/0018-506x(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 80.Tobet SA, Schwarting GA. Minireview: Recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology. 2006;147:1159–1165. doi: 10.1210/en.2005-1275. [DOI] [PubMed] [Google Scholar]
- 81.Tobet SA, Zahniser DJ, Baum MJ. Differentiation in male ferrets of a sexually dimorphic nucleus of the preoptic/anterior hypothalamic area requires prenatal estrogen. Neuroendocrinology. 1986;44:299–308. doi: 10.1159/000124660. [DOI] [PubMed] [Google Scholar]
- 82.Vasey PL. Same-sex sexual partner preference in hormonally and neurologically unmanipulated animals. Annu. Rev. Sex Res. 2002;13:141–179. [PubMed] [Google Scholar]
- 83.Vilain E. Genetics of sexual development. Annu. Rev. Sex Res. 2000;11:1–25. [PubMed] [Google Scholar]
- 84.Wagner CK, Nakayama AY, De Vries GJ. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology. 1998;139:3658–3661. doi: 10.1210/endo.139.8.6223. [DOI] [PubMed] [Google Scholar]
- 85.Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4. Elsevier Science; (USA), San Diego: 2002. pp. 385–423. [Google Scholar]
- 86.Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reprod. 2007;133:331–359. doi: 10.1530/REP-06-0078. [DOI] [PubMed] [Google Scholar]
- 87.Wood RI. Integration of chemosensory and hormonal input in the male Syrian hamster brain. Ann N. Y. Acad. Sci. 1998;855:362–372. doi: 10.1111/j.1749-6632.1998.tb10594.x. [DOI] [PubMed] [Google Scholar]
- 88.Zenchak JJ, Anderson GC. Sexual performance levels of rams (ovis aries) as affected by social experiences during rearing. J. Anim. Sci. 1980;50:167–174. [Google Scholar]
- 89.Zenchak JJ, Anderson GC, Schein MW. Sexual partner preferences of adult rams (ovis aries) as affected by social experiences during rearing. Appl. Anim. Ethol. 1981;7:157–167. [Google Scholar]
- 90.Zenchak JJ, Katz LS, Price EO, Wallach SJR. Sexual behavior of rams as influenced by the degree of restraining estrous ewes and by the additional presence of anestrous ewes. J. Anim. Sci. 1988;66:2851–2855. [Google Scholar]