Abstract
Phase I clinical trials are an essential step in the development of anticancer drugs. The main goal of these studies is to establish the recommended dose and/or schedule of new drugs or drug combinations for phase II trials. The guiding principle for dose escalation in phase I trials is to avoid exposing too many patients to subtherapeutic doses while preserving safety and maintaining rapid accrual. Here we review dose escalation methods for phase I trials, including the rule-based and model-based dose escalation methods that have been developed to evaluate new anticancer agents. Toxicity has traditionally been the primary endpoint for phase I trials involving cytotoxic agents. However, with the emergence of molecularly targeted anticancer agents, potential alternative endpoints to delineate optimal biological activity, such as plasma drug concentration and target inhibition in tumor or surrogate tissues, have been proposed along with new trial designs. We also describe specific methods for drug combinations as well as methods that use a time-to-event endpoint or both toxicity and efficacy as endpoints. Finally, we present the advantages and drawbacks of the various dose escalation methods and discuss specific applications of the methods in developmental oncotherapeutics.
Phase I trials represent the first application of a new drug or drug combination to humans and as such are the foundation of a successful clinical drug development process. Because the early clinical development of a novel agent may unduly influence its ultimate fate, a careful and thoughtful approach to the design of phase I trials is essential. Phase I clinical trials in oncology are typically small, single-arm, open-label, sequential studies that include patients with a good performance status whose cancers have progressed despite standard treatments. A principal goal of such trials is to establish the recommended dose and/or schedule of an experimental drug or drug combination for efficacy testing in phase II trials. A phase I trial design has many components, including starting dose, dose increment, dose escalation method, number of patients per dose level, specification of dose-limiting toxicity, target toxicity level, definition of the maximum tolerated dose (MTD) and recommended dose for phase II trials, patient selection, and number of participating centers (see definitions of basic concepts in Table 1). Although all of these components are relevant for the design of a phase I trial, this review will focus on selecting the dose escalation method that will yield an optimal balance of safety, efficiency, and ethical conduct.
Table 1.
Glossary of terms
| Term | Definition |
| Cohort | Group of patients treated at a dose level. |
| Starting dose | The dose chosen to treat the first cohort of patients in a phase I trial. |
| Dose increment (decrement) | The percent increase (or decrease) between dose levels. |
| Dose-limiting toxicity (DLT) | Toxic effects that are presumably related to the drugs that are considered unacceptable (because of their severity and/or irreversibility) and that limit further dose escalation. DLTs are defined before beginning the trial and are protocol specific. They are typically defined based on toxic effects seen in the first cycle and specified using a standardized grading criteria, for example, Common Terminology Criteria for Adverse Events. |
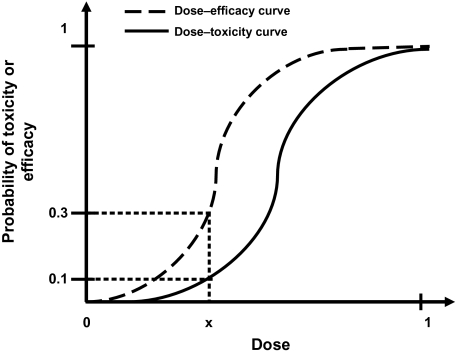
| Dose–efficacy curve | The dose–efficacy curve reflects the relationship between dose and probability of efficacy for an anticancer agent. A logistic function is commonly assumed to describe the dose–efficacy curve for cytotoxic agents and is characterized by a parameter, θ, which represents the slope of the dose–efficacy curve. Small values of θ indicate that the probability of efficacy increases very slowly with increasing dose levels, whereas large values of θ indicate a sharp increase in efficacy with increasing dose levels (seeFigure 1). |
| Dose–toxicity curve | The dose–toxicity curve reflects the relationship between dose and probability of toxicity for an anticancer agent. A logistic function is commonly assumed to describe the dose–toxicity curve for cytotoxic agents and is characterized by a parameter, θ, which represents the slope of the dose–toxicity curve. Small values of θ indicate that the probability of toxicity increases very slowly with increasing dose levels, whereas large values of θ indicate a sharp increase in toxicity with increasing dose levels (seeFigure 1). |
| Target toxicity level | The maximum probability of DLT that is considered acceptable in the trial. The target toxicity level in phase I trials is typically between 20% and 33%. |
| Maximum tolerated dose (MTD) | Phase I trials conducted in the United States: the highest dose level at which ≤33% of patients experience DLT. |
| Phase I trials conducted in Europe and Japan: the lowest dose level at which ≥33% of patients experience DLT (a misnomer in the sense that the MTD is actually not a tolerable dose). | |
| Phase I trials that use model-based methods: the dose that produces the target toxicity level. | |
| Optimal biological dose (OBD) | Dose associated with a prespecified most desirable effect on a biomarker among all doses studied (eg, inhibition of a key target in tumor or surrogate tissue or achievement of a prespecified immunologic parameter). |
| Recommended phase II dose | Phase I trials with a toxicity endpoint that are conducted in the United States: the MTD. |
| Phase I trials with a toxicity endpoint that are conducted in Europe and Japan: one dose level below the MTD. | |
| Phase I trials in which the endpoint is a prespecified biological endpoint: the OBD. | |
| Pharmacokinetics | Pharmacologic effects of the body on the drug (ie, the time course of drug absorption, distribution, metabolism, and excretion). |
| Pharmacodynamics | Pharmacologic effects of the drug on the body (eg, nadir neutrophil or platelet count, nonhematologic toxicity, molecular correlates, imaging endpoints). |
| Therapeutic index | The dosage or range of dosages of a drug that is required to produce a given level of damage to critical normal tissues (toxicity) divided by the dosage or range of dosages that yields a defined level of antitumor effect (efficacy) (seeFigure 1). |
The guiding principle for dose escalation in phase I trials is to avoid unnecessary exposure of patients to subtherapeutic doses of an agent (ie, to treat as many patients as possible within the therapeutic dose range) while preserving safety and maintaining rapid accrual. Dose escalation methods for phase I cancer clinical trials fall into two broad classes: the rule-based designs, which include the traditional 3+3 design and its variations, and the model-based designs. The rule-based designs assign patients to dose levels according to prespecified rules based on actual observations of target events (eg, the dose-limiting toxicity) from the clinical data. Typically, the MTD or recommended dose for phase II trials is determined by the prespecified rules as well. On the other hand, the model-based designs assign patients to dose levels and define the recommended dose for phase II trials based on the estimation of the target toxicity level by a model depicting the dose–toxicity relationship. However, because of safety concerns, most model-based designs are modified such that specific restrictions are set as safeguards for elements such as dose increments to avoid overshooting of the MTD and thus exposing patients to undue harm. All of these methods were developed in the era of cytotoxic drugs, during which time it was assumed that both efficacy and toxicity increase with dose. These relationships are typically represented by dose–toxicity and dose–efficacy curves in which toxicity and efficacy increase monotonically with increasing dose (Table 1 and Figure 1). Consequently, these methods have used toxicity as the primary endpoint. For molecularly targeted agents, the dose–efficacy and dose–toxicity curves may differ from those for cytotoxic agents, and efficacy may occur at doses that do not induce clinically significant toxicity (1–4). Thus, for trials involving these agents, the occurrence of drug-related biological effects has been suggested as an alternate primary endpoint besides toxicity (1–4).
Figure 1.
Typical dose–toxicity and dose–efficacy curves for cytotoxic agents. This example illustrates that at dose x, the probability of efficacy is 30% and the probability of toxicity is 10%; hence, the therapeutic index of the drug at dose x is 10% divided by 30% = 1/3.
Here we review the different dose escalation methods for phase I cancer clinical trials of single agents and drug combinations and discuss their pros and cons. Recent reviews (2,5) of phase I clinical trials including this update reveal that new dose escalation designs have been incorporated into phase I trials infrequently, and we explore the reasons for this disconnect. Finally, we recommend ways to assign dose escalation methods to evaluate new drugs or drug combinations. These recommendations are based on preclinical information, existing knowledge of agents that target the same or similar molecular pathways, and the availability of resources to execute such methods.
Rule-Based Designs
The main characteristic of rule-based designs is that they do not stipulate any prior assumption of the dose–toxicity curve. These designs comprise the so-called “up-and-down” designs because they allow dose escalation and de-escalation. The first up-and-down design was introduced in the late 1940s by Dixon and Mood (6), and Storer (7) described implementation of this design in clinical practice half a century later. The general principle of this design is to escalate or de-escalate the dose with diminishing fractions of the preceding dose depending on the absence or presence of severe toxicity in the previous cohort of treated patients (Figure 2, A). The simple up-and-down design converges to a dose that corresponds to a probability of severe toxicity of approximately 50%, which is higher than the 33% threshold commonly accepted in most phase I cancer clinical trials. Although variations of this up-and-down design have been developed in an attempt to increase patient safety and to use toxicity data collected in real time (8,9), these designs have not been used much in clinical practice because they risk exposing patients to unacceptable levels of toxicity. The first rule-based design to be used widely in clinical practice was the traditional 3+3 design. Variations of the traditional 3+3 design that have been put into clinical use include the accelerated titration designs and the pharmacologically guided dose escalation (PGDE) method.
Figure 2.
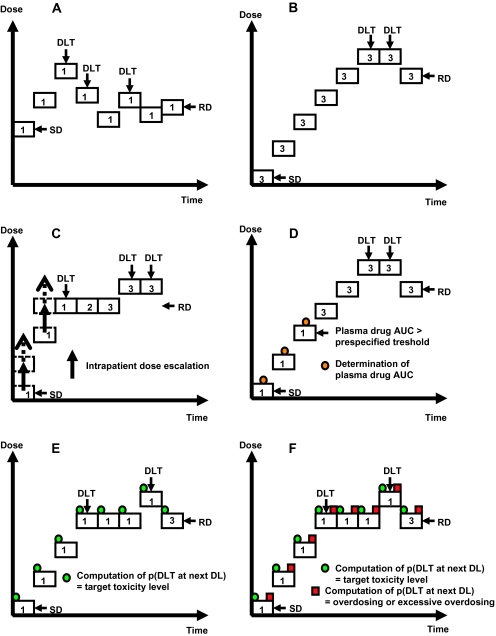
Graphical depiction of dose escalation methods for phase I cancer clinical trials. Each box represents a cohort comprising the indicated number of patients treated at a given dose level. A) Simple up-and-down design. B) Traditional 3+3 design. C) Accelerated titration design. Dashed arrows represent intrapatient dose escalation. D) Pharmacologically guided dose escalation. E) Modified continual reassessment method. F) Escalation with overdose control. “Overdosing or excessive overdosing” refers to doses that exceed the MTD. DLT = dose-limiting toxicity; SD = starting dose; RD = recommended dose; DL = dose level; AUC = area under the curve for drug concentration as a function of time; p(DLT at next DL) = probability of dose-limiting toxicity at the next dose level.
Traditional 3+3 Design
The traditional 3+3 design remains the prevailing method for conducting phase I cancer clinical trials (7). It requires no modeling of the dose–toxicity curve beyond the classical assumption for cytotoxic drugs that toxicity increases with dose. This rule-based design proceeds with cohorts of three patients; the first cohort is treated at a starting dose that is considered to be safe based on extrapolation from animal toxicological data, and the subsequent cohorts are treated at increasing dose levels that have been fixed in advance (Figure 2, B). Historically, dose escalation has followed a modified Fibonacci sequence in which the dose increments become smaller as the dose increases (eg, the dose first increases by 100% of the preceding dose, and thereafter by 67%, 50%, 40%, and 30%–35% of the preceding doses). In most cases, the prespecified dose levels do not fit the exact Fibonacci sequence as described in the 12th century (5). If none of the three patients in a cohort experiences a dose-limiting toxicity, another three patients will be treated at the next higher dose level. However, if one of the first three patients experiences a dose-limiting toxicity, three more patients will be treated at the same dose level. The dose escalation continues until at least two patients among a cohort of three to six patients experience dose-limiting toxicities (ie, ≥33% of patients with a dose-limiting toxicity at that dose level). The recommended dose for phase II trials is conventionally defined as the dose level just below this toxic dose level.
Alternative rules besides “3+3” have been proposed, including the “2+4,” “3+3+3,” and “3+1+1” (also referred as “best of five”) rules (10). In the “2+4” design, an additional cohort of four patients is added if one dose-limiting toxicity is observed in a first cohort of two patients. The stopping rule is the same as in the traditional 3+3 design. In the “3+3+3” design, a third cohort of three patients is added if two of six patients in the first two cohorts experience a dose-limiting toxicity at a certain dose level. The trial terminates if at least three of nine patients experience a dose-limiting toxicity. The “best of five” design is more aggressive than the traditional 3+3 design in that one additional patient is added if one or even two dose-limiting toxicities are observed among the first three patients. Another patient is added if two dose-limiting toxicities are observed among the four treated patients. Dose escalation is allowed if dose-limiting toxicities are observed among none of three, one of four, or two of five patients, but the trial will terminate if three or more dose-limiting toxicities are observed.
The main advantages of the traditional 3+3 design are that it is simple to implement and safe (Table 2). In addition, the accrual of three patients per dose level provides additional information about pharmacokinetic interpatient variability. However, a disadvantage of this design is that it involves an excessive number of escalation steps, which results in a large proportion of patients who are treated at low (ie, potentially subtherapeutic) doses while few patients actually receive doses at or near the recommended dose for phase II trials. This latter point is illustrated in Table 3, which presents the dose escalation method used as well as the number of dose levels in recent first-in-human single-agent phase I trials for anticancer agents that were eventually (1992–2008) approved by the US Food and Drug Administration (FDA) for the treatment of solid tumors. Among 21 trials that used the traditional 3+3 design, more than half involved six or more dose levels.
Table 2.
Theoretical main advantages and drawbacks of dose escalation methods for phase I cancer clinical trials*
| Dose escalation method | Advantages | Drawbacks |
| Rule-based designs | ||
| Traditional 3+3 design | Easy to implement and safe | Many patients treated at subtherapeutic doses |
| Slow dose escalation | ||
| Provide some data on PK interpatient variability | Uncertainty about the RP2D | |
| Only the result from the current dose is used for determining the dose of next cohort of patients. Information on other doses is ignored. | ||
| Accelerated titration designs | More rapid dose escalation | If model fitting is not performed (as is often the case in clinical practice): |
| May expose a greater proportion of patients at higher doses | Intrapatient dose escalation may mask cumulative or delayed toxicities | |
| Data from all patients, cumulative toxicity, and interpatient variability can be fit to a model to establish the RP2D | Difficult interpretation of the results when intrapatient dose escalation is allowed | |
| Uncertainty about the RP2D | ||
| Pharmacologically guided dose escalation | More rapid dose escalation | Need to obtain real-time PK results |
| Provide some data on PK interpatient variability | Interpatient variability may hamper dose escalation | |
| Model-based designs | ||
| Modified continual reassessment method, escalation with overdose control, time-to-event continual reassessment method, EffTox, TriCRM | Target toxicity level is explicitly defined | Need to have a prior guess of the RP2D |
| More rapid dose escalation | ||
| Use all available information from all patients | Computations after each patient or cohort of patients | |
| Estimate of the RP2D with a confidence interval | ||
| Take into account late-onset toxicities (time-to-event continual reassessment method) | Need real-time biostatistical support for dose escalation decisions (may also be an advantage) | |
| Take into account both toxicity and efficacy (EffTox TriCRM) |
PK = pharmacokinetic; RP2D = recommended phase II dose; EffTox = efficacy and toxicity method; TriCRM = an adaptative continual reassessment method that considers three potential trial outcomes: no efficacy and no toxicity, efficacy only, and toxicity only.
Table 3.
Characteristics of first-in-human phase I clinical trials for recent anticancer agents that were eventually approved by the US FDA*
| Agent | Class or mechanism of action | Year of FDA approval | Dose escalation method | Reason for stopping trial | No. of patients | No. of dose levels | Reference |
| Molecular targeted agents | |||||||
| Trastuzumab | Mab | 1998 | Traditional | PK | 18 | 4 | (11) |
| Imatinib | TKI | 2001 | Traditional | PK | 83 | 14 | (12) |
| Gefitinib | TKI | 2003 | Traditional | Toxicity | 64 | 8 | (13) |
| Erlotinib | TKI | 2004 | Traditional | Toxicity | 40 | 5 | (14) |
| Cetuximab | Mab | 2004 | Traditional | PK | 52 | 6 | (15) |
| Bevacizumab | Mab | 2004 | Traditional | Target inhibition | 25 | 5 | (16) |
| Sorafenib | TKI | 2005 | Traditional | Toxicity | 69 | >5 | (17) |
| Sunitinib | TKI | 2006 | Traditional | Toxicity | 28 | 6 | (18) |
| Panitumumab | Mab | 2006 | Traditional | PK | 96 | 13 | (19) |
| Lapatinib | TKI | 2007 | NR | NR | 81 | NR | (20) |
| Temsirolimus | STKI | 2007 | Modified CRM | Toxicity | 24 | 10 | (21) |
| Cytotoxic agents | |||||||
| Paclitaxel | Anti-tubulin | 1992 | Traditional | Toxicity | 34 | 11 | (22) |
| Vinorelbine | Alkaloid agent | 1994 | Traditional + IPDE | Toxicity | 20 | 7 | (23) |
| Docetaxel | Anti-tubulin | 1996 | ATD | Toxicity | 39 | 6 | (24) |
| Gemcitabine | Antimetabolite | 1996 | Traditional + IPDE | Toxicity | 47 | 12 | (25) |
| Topotecan | Anti–topoisomerase I | 1996 | Traditional | Toxicity | 28 | 5 | (26) |
| Irinotecan | Anti–topoisomerase I | 1998 | Traditional | Toxicity | 17 | 4 | (27) |
| Capecitabine | Antimetabolite | 1998 | Traditional | Toxicity | 34 | 5 | (28) |
| Liposomal doxorubicin | Anti–topoisomerase II | 1999 | Traditional | Toxicity | 26 | 4 | (29) |
| Temozolomide | DNA alkylating | 1999 | Traditional + IPDE | Toxicity | 51 | 15 | (30) |
| Oxaliplatin | DNA alkylating | 2002 | Traditional + IPDE | Toxicity | 23 | 9 | (31) |
| Pemetrexed | Antimetabolite | 2004 | Traditional | Toxicity | 38 | 10 | (32) |
| Trabectedin | Alkaloid agent | 2004 | Traditional | Toxicity | 21 | 4 | (33) |
| Albumin-bound paclitaxel | Anti-tubulin | 2005 | Traditional | Toxicity | 19 | 4 | (34) |
| Ixabepilone | Anti-tubulin | 2007 | ATD | Toxicity | 21 | 4 | (35) |
FDA = US Food and Drug Administration; Mab = monoclonal antibody; TKI = tyrosine kinase inhibitor; STKI = serine/threonine kinase inhibitor; NR = not reported; PK = pharmacokinetic data; CRM = continual reassessment method; IPDE = intrapatient dose escalation; ATD = accelerated titration design.
Accelerated Titration Designs
Accelerated titration designs combine features from variations of the traditional 3+3 design and the model-based design. Because the patient assignment to doses is based on prespecified rules, we classify accelerated titration designs as rule-based designs. Through simulations based on a stochastic model fit to data from 20 actual phase I trials of nine different drugs, Simon et al. (36) described one control design and three accelerated titration designs. The control design, design 1, is a standard 3+3 design with a 40% dose increment between successive cohorts of patients. Although the three accelerated titration designs, designs 2, 3, and 4, were created based on a statistical model as described (36), the assignment of patients to dose levels follows specific rules according to the observed toxicities at each dose level. Designs 2 and 3 allow 40% and 100% dose escalations, respectively, between single-patient cohorts until a dose-limiting toxicity or two moderate toxicities are observed during cycle 1, at which point dose escalation reverts to the more conservative one used in design 1. In design 4, the 100% dose escalation between single-patient cohorts in the accelerated phase reverts to design 1 when one dose-limiting toxicity or two moderate toxicities are observed during any cycle (not just during cycle 1). Intrapatient dose escalation is allowed during the accelerated phase of designs 2, 3, and 4 (Figure 2, C). In all three accelerated titration designs, the standard 3+3 design is used after the accelerated phase as a stopping rule, and then the described model is recommended to estimate the MTD with all toxicity data collected during the trial. In addition, the model recommended for use included a parameter for cumulative toxicity as well as a parameter for interpatient variability, such that the accelerated titration designs would provide information in these aspects. In practice, investigators often determine the MTD based on the conventional 3+3 escalation rule without fitting trial data to the model at the end of the trial. Consequently, the original model-based accelerated titration designs have been adapted primarily as rule-based designs in clinical practice.
The accelerated phase in accelerated titration designs—in which only one patient is included per dose level—along with the possibility of intrapatient dose escalation theoretically reduce the number of patients who are treated at subtherapeutic doses (Table 2). Permitting intrapatient dose escalation in accelerated titration designs is appealing because it gives some patients the opportunity to be treated at higher and presumably more effective doses. For example, in the first-in-human phase I trial of ixabepilone, which used an accelerated titration design with intrapatient dose escalation, all patients received the drug at the eventually established recommended dose for phase II trials (35). On the other hand, unless the model recommended in the original publication (36) using parameters for cumulative toxicity and interpatient variability is applied and fits the data well, one drawback of intrapatient dose escalation is that it may mask the cumulative effects of treatment or, at the very least, would make them harder to differentiate from chronic or delayed toxic effects. However, regardless of the trial design used, chronic, delayed, or cumulative toxic effects are generally not well captured by most phase I trials because most patients with advanced cancers do not remain on study for extended periods of time. Furthermore, it can be difficult to present and interpret results of trials that allow intrapatient dose escalations because a single patient may contribute data for several dose levels.
Pharmacologically Guided Dose Escalation
The PGDE method is another variation of the traditional 3+3 design that has not been widely used in clinical practice. This approach assumes that dose-limiting toxicities can be predicted by plasma drug concentrations and that animal models can accurately reflect this relationship in humans (37). The PGDE method has two stages. A prespecified plasma exposure defined by the area under the curve for drug concentration as a function of time (AUC) is extrapolated from preclinical data. Then, pharmacokinetic data are obtained for each patient in real time to determine the subsequent dose level. As long as the prespecified plasma exposure is not reached, dose escalation proceeds with one patient per dose level and typically at 100% dose increments (stage 1, Figure 2, D). When the target AUC is reached or if dose-limiting toxicities occur, dose escalation switches to the traditional 3+3 design with smaller (usually around 40%) dose increments (stage 2).
The PGDE method has not been widely adopted due to practical obstacles, including: 1) logistic difficulties in obtaining real-time pharmacokinetic results, which are required to determine the safety of the subsequent dose escalation; 2) problems in extrapolating preclinical pharmacokinetic data to phase I studies with different treatment schedules; and 3) risk of exposing the next patient to a highly toxic dose if the AUC obtained in the preceding patient was atypically low due to interpatient variability in drug metabolism. In clinical practice, the PGDE method has reliably defined the recommended dose for phase II trials for some cytotoxic agents such as certain anthracyclines and platinum compounds but has been found to be inappropriate for other classes of cytotoxic agents such as the antifolates, which display a high interpatient pharmacokinetic heterogeneity (38).
Other Rule-Based Designs
Several other rule-based designs have been proposed, including the isotonic regression model (39), the biased coin design (9) and its variations (40,41), and the “rolling six” design (42). The rolling six design was originally proposed as a way to shorten the timeline of pediatric phase I trials by reducing the number of times a study is suspended to accrual (42). This method allows accrual of two to six patients concurrently onto a dose level based on the numbers of patients who are currently enrolled and evaluable, who experience a dose-limiting toxicity and who remain at risk of developing a dose-limiting toxicity. Because pediatric trials are typically conducted only after completion of adult phase I trials, this design is intended to shorten the study duration in situations in which there is prior information about the dose range to be evaluated.
Ji et al. (43) developed a rule-based design in which subsequent patients are assigned to doses according to the toxicity outcome at the current dose by calculating the toxicity probability interval under the beta-binomial model. The authors also developed a freely available macro in Microsoft Office Excel software that can be downloaded to facilitate the study conduct. Simulations have shown that the performance of this dose-finding design is better than the traditional 3+3 design and comparable to some model-based designs.
Summary of Rule-Based Designs
The main advantages of rule-based methods are that they are easy to implement and do not require special software. However, their performance (operating characteristics) is not guaranteed and they have some drawbacks. For example, these designs may be inefficient in establishing the dose that meets a specific target toxicity level. In addition, the decision of dose allocation for future patients as well as the definition of the recommended dose for phase II trials rely on information from the current dose level and do not use all available information. As such, the recommended dose for phase II trials is then selected from the prespecified dose levels depending on which one best fits the definition of acceptable toxicity set a priori. However, although not ideal, the rule-based methods have been successful in establishing safe recommended doses for phase II trials during the past several decades for anticancer agents that were eventually used worldwide in clinical practice (Table 3).
Model-Based Designs
An alternative dose escalation method for phase I clinical trials is to use statistical models that actively seek a dose level that produces a prespecified probability of dose-limiting toxicity by using toxicity data from all enrolled patients to compute a more precise dose–toxicity curve. This method can be conveniently carried out using Bayesian models. Bayesian models require an initial estimation of θ (also called prior distribution of θ; Table 1), which characterizes the shape of the dose–toxicity curve. The occurrence of toxicity (or not) in patients enrolled at each dose level provides additional information for the statistical model and results in an adjustment of θ (also called posterior distribution of θ) according to Bayes’ theorem. The posterior distribution is then evaluated to identify the dose closest to the target toxicity level, and this dose is used to treat future patients and to set the recommended dose for phase II trials. These model-based designs use all of the available data to model the dose–toxicity curve, and they provide a confidence interval for the recommended dose for phase II trials at the end of the trial.
Continual Reassessment Method and Modifications
The continual reassessment method was the first Bayesian model–based method proposed for adoption in phase I trial designs (44). The initial estimate of θ required for this method is generally elicited from experts who are familiar with the preclinical data or who have experience with similar drugs if any exist. Although this initial estimate may not be accurate, it provides guidance about dose escalation. In the original description of the continual reassessment method (44), all patients are treated at the dose thought to be closest to the MTD, which corresponds to the dose at the target dose-limiting toxicity level. The estimation of the probability of encountering a dose-limiting toxicity is updated for each new patient who enters the study at any dose level until a prespecified condition is met, at which point the trial is stopped. Various stopping rules have been proposed, the simplest one requires that the trial be stopped when six patients are assigned to the same dose (45). Alternatively, the trial can be stopped if a certain precision in the probability of dose-limiting toxicity at the estimated MTD level is achieved. The original continual reassessment method allowed for multiple dose escalations and de-escalations.
The continual reassessment method as originally described (44) has not been well accepted because it can expose patients to unacceptably high (ie, toxic) doses if the prespecified model is incorrect. Consequently, several investigators have recommended modifications to the continual reassessment method, including: 1) treating the first patient at the lowest starting dose level, which is selected on the basis of animal toxicology and conventional criteria (45); 2) increasing the dose by only one prespecified level at a time (46–48); 3) not allowing dose escalation for the immediate next patient if a previous patient experienced a dose-limiting toxicity (thus hampering multiple dose escalations and de-escalations) (49); 4) treating several patients at the same dose level, especially for the higher dose levels (45,47,50); and 5) expanding the cohort of patients treated at the recommended dose for phase II trials (49,51). These modifications have been implemented in clinical practice, resulting in designs similar to the rule-based designs. For example, the trials start at the lowest dose level derived from animal data, a set of dose levels is prespecified, and up to three patients are included per dose level. After each cohort of patients completes the first treatment cycle (or whatever duration of time is specified for the evaluation of dose-limiting toxicity), a computation determines the next dose level that will be tested. A maximum dose increase for the next dose level is usually prespecified to prevent overdosing, regardless of the recommendation of the model (Figure 2, E). In some cases, the model may recommend a dose re-escalation after a dose de-escalation on the basis of the accumulated data on toxicity. A modified continual reassessment method was used in the first-in-human phase I trial of SAM486A, a polyamine biosynthesis inhibitor (52). The model was successful in that it allowed treatment of a single patient per dose level for six of the seven dose levels below the recommended dose for phase II trials.
Escalation With Overdose Control
Some investigators have claimed that the modified continual reassessment method can lead to exposure of patients to high toxic doses (53,54). To overcome this limitation, Babb et al. (53) and Rogatko et al. (54) suggested an alternative Bayesian approach called escalation with overdose control (EWOC). The EWOC method is essentially a modified continual reassessment method with additional safety measures put in place to avoid exposing patients to doses that are potentially too toxic. The only difference between the EWOC and the modified continual reassessment methods is that with the EWOC method, the probability of administering a dose that exceeds the MTD for each higher dose level is assessed after each patient, with an interdiction of dose escalation if this probability exceeds some critical prespecified value (Figure 2, F). For example, the EWOC method would restrict the dose escalation if the probabilities of overdosing and excessive overdosing exceed specified values (eg, 25% and 5%, respectively). Chu et al. (55) have shown that the continual reassessment method and the EWOC method can be unified in a hybrid model that seems to be able to determine the target dose more expeditiously than the EWOC method and results in smaller overdose proportions than the continual reassessment method.
Model-Based Designs That Use Time-to-Event Endpoints
Because most dose-limiting toxicities are acute events that occur soon after delivery of study drugs, phase I trials report dose-limiting toxicities that occur during the first one or two treatment cycles, generally over a period of a few weeks. However, if an agent causes late-onset or cumulative toxicities, an undesirably large number of patients may be treated at toxic doses before any toxicity is observed. It is impractical to mandate that phase I trial designs have an extended assessment period to monitor for late or cumulative toxicity, as this would result in prolonged delays between cohort openings and closures. To avoid this limitation, several model-based designs have been proposed that use time-to-event endpoints and do not mandate trial suspension while patients are being observed. Cheung and Chappell (56) developed a modification of the continual reassessment method known as the time-to-event continual reassessment method (TITE-CRM) that incorporates the time to the event (the event being toxicity) for each patient. Simulations suggest that for treatments with late-onset toxicity, the TITE-CRM is more efficient than the traditional 3+3 design or the continual reassessment method for determining the MTD and leads to shorter trial durations (57). However, in two clinical trials (58,59), this design led to the accrual of more patients to dose levels below the recommended dose for phase II trials than would have occurred with the traditional 3+3 design. A variation of the TITE-CRM has been proposed in which accrual is temporarily suspended if the risk of toxicity at proposed doses for future patients is unacceptably high (60). Although simulations suggest that this variation is safer than the originally proposed TITE-CRM design, on average, it tends to take a longer time to complete. In addition, designs that use time-to-event endpoints assume that the hazard of toxicity remains constant over time, which may not be the case.
Designs That Use Toxicity and Efficacy as Endpoints
Efficacy has not traditionally been the primary endpoint for phase I trials. However, some situations are particularly relevant for assessing both toxicity and efficacy, such as disease-specific phase I trials that are designed to examine the sensitivity of one or several tumor types to a drug or drug combination. Bayesian-based methods that incorporate both toxicity and efficacy in their designs have been developed under the assumption that efficacy can be accurately assessed with standard response criteria or with surrogate endpoints (61–63). For example, Thall and Cook (61) proposed the efficacy and toxicity method, which defines an acceptable dose combination based on trade-offs between the probabilities of treatment efficacy and toxicity. Zhang et al. (62) proposed an adaptive continual reassessment method called TriCRM that considers three potential trial outcomes: no efficacy and no toxicity, efficacy only, and toxicity only. Although these methods are appealing, they have not yet been widely adopted.
Summary of Model-Based Designs
Simulations have shown that model-based methods, which use all toxicity information accumulated during the trial, achieve good estimations of the target probability of dose-limiting toxicity at the recommended dose for phase II trials without treating too many patients at suboptimal doses (Table 2). Some of the challenges presented by model-based designs include the need for biostatistical expertise and available software on site to perform model fitting in real time, as well as an expedited collection of data from each cohort of patients to fit the model. As such, implementation of these designs may not be straightforward. In addition, the model may fail to reach the recommended dose for phase II trials if the prior distributions for the parameters of the dose–toxicity curve are inadequate, or conversely, if the prior assumptions are overbearing (64). Finally, inclusion of one patient per dose level may accelerate the dose escalation, but may also deprive the investigators of data on interpatient pharmacokinetic variability, although this limitation can be easily dealt with by expanding the cohort size if such data are needed.
Designs for Trials of Combinations of Agents
Many phase I studies are designed to investigate combinations of two or more agents. The combination of two or more agents in the clinic should be based on a strong scientific rationale rather than simple empiricism. Unfortunately, preclinical models that accurately predict synergism or even additivity are not well characterized, and existing preclinical models often focus on the antitumor effects of drug combinations while ignoring their potential for creating severe toxicities. Determining the recommended dose for phase II trials of agents to be administered in combination may appear easier than that for single agents, given that the recommended dose for phase II trials and the toxicity of each drug are already known. For this reason, phase I combination trials usually explore only a limited number of dose levels. Korn and Simon (65) developed a graphical method to define the MTDs of drugs to be used in combination that relies on the organ-specific toxicities of the drugs when given as single agents. However, this method was developed using cytotoxic drugs, which have a high likelihood of overlapping toxicities (in particular hematologic toxicities). By contrast, when drugs to be administered in combination have different mechanisms of action or nonoverlapping toxicities, the recommended dose for phase II trials for the drug combination is usually expected to be near the recommended dose for phase II trials of each drug given as a single agent. However, the biochemical and biological effects of the combination may be quite complex, and the dose–toxicity relationship may depend largely on unknown pharmacokinetic and/or pharmacodynamic interactions between the agents, which could limit the administration of all drugs at their recommended dose for phase II trials as single agents. In this case, it remains a challenge to determine which drugs should be administered at the full recommended dose for phase II trials and how to proceed with the dose escalation.
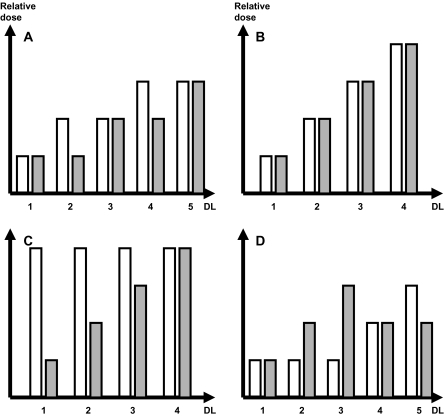
When a rule-based design is used for phase I trials of drug combinations, the dose levels must be chosen carefully so that the dose of each drug (or at least the ones felt to be most relevant) can be increased as close as possible to MTD. The choice of the dose levels to be tested may be based on several factors including preclinical data, the current standard treatments in tumor types for which the combination is intended, the expected control arm if the combination under evaluation is planned to be benchmarked in subsequent randomized trials, and/or sheer empiricism. Several strategies for dose escalation in phase I trials of two drugs are illustrated in Figure 3: 1) alternate escalation of the agents in the series of dose levels; 2) simultaneous escalation of both agents; 3) escalation of one agent to the recommended dose for phase II trials while holding the other agent at a fixed (generally high) dose; and 4) escalation of one agent to the recommended dose for phase II trials while holding the other agent at a low dose. In this latter case, the dose of the first agent is then reduced by one or two dose levels while the dose of the other agent is escalated to the recommended dose for phase II trials.
Figure 3.
Strategies for dose escalation in phase I trials testing combinations of two drugs. White bars represent drug 1, gray bars represent drug 2. A) Alternate dose escalation. B) Simultaneous dose escalation. C) Single-agent dose escalation. D) Compromised dose escalation with only one of the two agents achieving full dose escalation. DL = dose level.
In the above-mentioned rule-based strategies, although the dose escalation is preset, uncertainty may remain at trial completion about the optimal drug combination that yields the best therapeutic index (Table 1). Several Bayesian model–based designs specific for combination trials have been developed in an attempt to minimize this uncertainty. These designs do not require any prior assumptions about the best dose combination, and they aim to guide the dose escalation of the agents based on all toxicities observed (63,66–69). In these methods, the dose–toxicity probability curves are updated after each cohort of patients for all agents by using all available toxicity data so that the subsequent cohort of patients may be assigned to the most appropriate dose combination. The ultimate goal is to determine the most active drug combination among those also deemed to be safe. Some investigators have proposed methods for drug combination studies that use both toxicity and efficacy as endpoints (63,67). For example, in the design proposed by Yin et al. (63), patients are randomly assigned to one of several combinations that are selected by a statistical model to determine the most effective and least toxic combination. These methods, which incorporate both toxicity and efficacy as endpoints, may determine several MTDs, and the investigator may then choose the one with the best therapeutic index as the recommended dose for phase II trials.
Pharmacokinetic analyses may provide information about interactions between anticancer drugs administered in combination. To learn about potential drug interactions, one may initiate treatment with a run-in period by administering only one drug, followed by concurrent dosing of other drugs. This strategy would allow one to compare within the same patient the pharmacokinetic profile of the first drug given alone with that obtained in the presence of the other drugs. Alternatively, comparisons can be made with historical published pharmacokinetic data of the drugs, with the limitations of heterogeneity that may exist between different patient populations and variations in assay sensitivities.
Von Hoff et al. (70) have suggested a practical approach to conduct simultaneous independent parallel phase I combination studies that pair different chemotherapeutic agent or agents with a new drug, once the recommended dose of the new drug for phase II trials is known. This approach may expedite the accrual of patients because patients referred for a phase I trial may be eligible for more than one of the proposed combined regimens, if given the options.
Overall, the choice of the dose escalation scheme for drug combination trials is as relevant as it is for first-in-human monotherapy trials. The recommended dosages and schedules can have a critical role in the success or failure of a combination regimen during its subsequent clinical development.
Designs for Trials of Molecularly Targeted Agents
In phase I cancer clinical trials that involve cytotoxic agents, the conventional primary endpoint has been toxicity, which, with efficacy, is assumed to increase with the drug dose. Molecularly targeted agents modulate specific aberrant pathways in cancer cells while sparing normal tissues, such that the toxicity and efficacy of these novel agents may not be dose dependent. Alternative endpoints besides toxicity have been proposed for phase I trials that evaluate molecularly targeted agents, including target inhibition in tumors or surrogate tissues and/or detection of biologically relevant pharmacokinetic levels (1–4). The assessment of target inhibition may be one of the most challenging aspects of clinical trial designs for several reasons (71). First, tumor tissue or a valid surrogate tissue must be readily available and easily accessible. Second, there must be a reliable assay for measuring the effect of the drug on the target. Third, the optimal extent of target inhibition (ie, inhibition that yields a meaningful clinical benefit) must be known. Because these three conditions are rarely all met, a recommended dose for phase II trials that is established based solely on the measurement of target inhibition in a phase I trial may be suboptimal. Pharmacokinetic endpoints, such as the attainment of plasma drug concentrations that were shown to correlate with biological activity in preclinical studies, may enable dose selection of some molecularly targeted agents in the phase I setting. However, these endpoints are appropriate only if sufficient preclinical data exist that demonstrate a convincing pharmacokinetic–pharmacodynamic relationship.
In a limited number of reported clinical trials of molecularly targeted agents that fulfilled the above-mentioned conditions, specific designs were developed to define the recommended dose for phase II trials (72–75). For example, in a phase I trial of patients undergoing craniotomy for malignant glioma, Friedman et al. (72) evaluated the safety and biological activity of administering O6-benzylguanine to inhibit the DNA repair protein O6-alkylguanine-DNA alkyltransferase (AGT). The trial design stipulated that up to 13 patients could be accrued at a dose level and that dose escalation would stop if the biological endpoint of AGT inhibition in resected tumor was met in at least 11 of the 13 patients. In addition, Hunsberger et al. (73) proposed a design similar to that described by Friedman et al. except that it prespecifies the desired proportion of patients who will display a biological response at a given dose level, as well as the minimal threshold for biological response. Other proposals for phase I trial designs specific for molecularly targeted agents that have been described in the statistical literature await practical evaluations. For example, Mandrekar et al. (74) developed a Bayesian-based method based on an extension of TriCRM that incorporates toxicity and a biological endpoint for molecularly targeted agent combinations. Polley and Cheung (75) proposed a two-stage design for identifying the optimal biological dose that uses stepwise tests with a futility interim analysis.
To our knowledge, no specific clinical trial designs have been formulated to date for molecularly targeted agents that do not have a proven relevant target and a validated method for measuring target inhibition. The Task Force on Methodology for the Development of Innovative Cancer Therapies, which recently published its recommendations on phase I studies of targeted anticancer therapy (76), has not provided guidance on dose escalation methods specific for molecularly targeted compounds.
Dose Escalation Methods Used in Recent Clinical Practice
Despite the abundance of new dose escalation methods that have been published in the clinical and statistical literature, the application of these methods in practice has been limited. Rogatko et al. (5) found that only 1.6% of 1235 phase I cancer trials published between 1991 and 2006 used Bayesian adaptive designs such as the modified continual reassessment method or EWOC. To provide an update on dose escalation methods that have been used since that time period, we searched the SCOPUS database for all phase I cancer trials that were published between January 1, 2007, and December 1, 2008, by using the following search algorithm: TITLE(((phase i) OR (phase 1) OR (phase one)) AND ((study) OR (studies) OR (trial) OR (trials)) AND ((cancer) OR (malignancies) OR (carcinoma) OR (carcinomas)) AND NOT ((phase ii) OR (phase i/ii) OR (phase 2) OR (phase iii) OR (phase ii/iii) OR (phase 3))) AND PUBYEAR AFT 2006 AND (LIMIT-TO(DOCTYPE, “ar”) OR LIMIT-TO(DOCTYPE, “ip”) OR LIMIT-TO(DOCTYPE, “cp”)) AND (EXCLUDE(SUBJAREA, “PHYS”) OR EXCLUDE(SUBJAREA, “MATH”) OR EXCLUDE(SUBJAREA, “ENGI”)) AND (LIMIT-TO(LANGUAGE, “English”)).
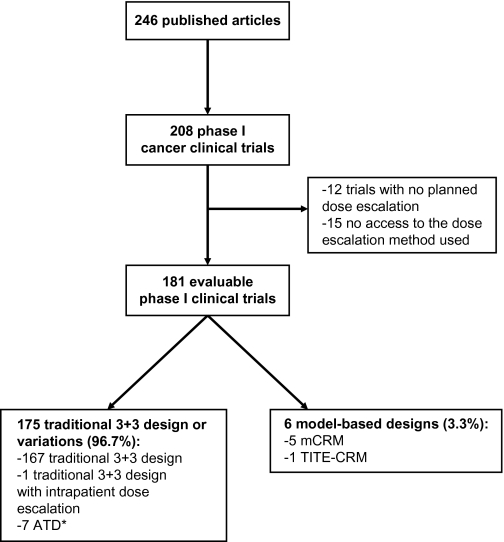
This search yielded 246 articles, of which 208 reported findings of a phase I trial (Figure 4). We excluded 27 trials because no dose escalation was planned in the study (n = 12 trials) or because the full article was not accessible to determine the dose escalation method (n = 15 trials). Of the remaining 181 trials, 175 (96.7%) used a traditional 3+3 design or variation and 6 (3.3%) used model-based designs. Among the 175 trials using a rule-based design, 167 used the traditional 3+3 design, one applied the 3+3 design but with intrapatient dose escalation, and the remaining seven trials used one accelerated titration design. However, none of the trials with an accelerated titration design reported model fitting for determining the recommended dose for phase II trials. Although the use of model-based designs was still very limited, this proportion has doubled compared with the 1991–2006 period (5), suggesting a trend toward a greater use of modern dose escalation methods. Among the 181 phase I trials reviewed, 32 involved molecularly targeted agents that were administered as single agents. Among these 32 trials, toxicity remained the most common reason for stopping dose escalation (63%); 13% were stopped on the basis of pharmacokinetic data before MTD was reached. These percentages are similar to those reported by Parulekar and Eisenhauer (60% and 13%, respectively) in their review of phase I trials involving molecularly targeted agents administered as monotherapy that were published through March 2003 (2). Toxicity remains the most widely used primary endpoint in phase I cancer clinical trials.
Figure 4.
Dose escalations methods used in phase I cancer clinical trials published between January 1, 2007, and December 1, 2008. Asterisk indicates that model fitting was not performed in any of the seven ATD trials to establish the recommended dose for phase II trials. ATD = accelerated titration design; mCRM = modified continual reassessment method; TITE-CRM = time-to-event continual reassessment method.
Discussion
A retrospective analysis of oncology phase I trials published between 1991 and 2002 has revealed that most of the objective tumor responses in phase I trials occurred when study drugs (primarily cytotoxic agents) were administered at 80% or more of their subsequent recommended dose for phase II trials (77). Evaluation of new dose escalation methods is needed to maximize the proportion of patients being treated at or near the recommended dose for phase II trials. Statistical simulations have demonstrated that new trials designs are frequently more efficient than the traditional 3+3 design in that they optimize the proportion of patients who are treated at or near the recommended dose for phase II trials and/or reduce the duration of time required to complete accrual. However, despite these putative advantages, most of the new methods have rarely been implemented in clinical practice (5,78,79). Our review also found that the modeling features in the accelerated titration design are rarely used in clinical practice. Some investigators have retrospectively assessed the efficiency, safety, and efficacy (eg, response rate) of different dose escalation methods used in phase I trials (80–83). Although these reports are quite heterogeneous in terms of time periods of the studies included, the nature of the studies (monotherapy trials or combination trials), and the type of anticancer agents (cytotoxic agents or molecularly targeted agents), they suggest that some of the newer methods, such as the modified continual reassessment method, expose fewer patients to subtherapeutic doses than the traditional 3+3 design but do not result in shorter trial durations (80,81). This latter point may reflect the fact that many other factors besides the speed of the dose escalation can slow the course of a trial, including amendment requirements, sluggish patient accrual, competing trials, and unexpected toxicities. Whether the use of new dose escalation methods results in improved safety and/or efficacy in phase I trials remains unclear (82,83). Whereas one review (82) claimed that the traditional 3+3 design is the safest in terms of grade 3 or 4 nonhematologic and grade 4 hematologic toxicities and had efficacy that is similar to that of more aggressive dose escalation methods, another review (83) found an increased response rate but no increased risk of toxicity when intrapatient dose escalation was allowed. Of note, the overall response rates reported by these two reviews were similar to those observed in other reviews of phase I trials (ie, 2%–11%) (79,84–86). Furthermore, it may not be appropriate to compare response rates across phase I studies because efficacy is not the primary objective, and response assessment in these trials is complicated by the heterogeneity of the patient population and variability in the biological activities of the agents being evaluated. It is yet to be determined by prospective clinical trials whether the new dose escalation methods are as safe as the traditional 3+3 design and whether they provide greater efficacy.
Clinicians may find it more informative to know appropriate settings in which a specific dose escalation method would be more efficient than the traditional 3+3 design while offering a similar degree of safety than to be given generalized recommendations on the application of phase I dose escalation methods. Preclinical data, including toxicology and pharmacology data, existing knowledge about the inhibition of the putative pathway and the resulting on-target and off-target effects, and available information about the drug or similar classes of drugs, should help formulate the trial design decision and may also provide valuable guidance on how best to proceed with a first-in-human phase I cancer clinical trial. If the preclinical data indicate a wide therapeutic window and little expected toxicity in human subjects, it is very reasonable to apply an aggressive dose titration (eg, by using an accelerated titration design or Bayesian-based methods), at least at the beginning of the phase I trial. However, if the preclinical data were less certain about how human subjects will tolerate the drug or predict a very narrow therapeutic window, then it would be prudent to choose a more conservative dose escalation scheme. For trials of drug combinations, the most challenging issue is likely to be the choice of the dose escalation scheme. The traditional 3+3 design is presumably a reasonable design if an expected optimal dose combination is specified before starting the trial. On the contrary, if the optimal dose combination is not prespecified, Bayesian model–based methods may help to determine the safest and most effective drug combination. Finally, specific dose escalation methods that incorporate time-to-event endpoints, such as the TITE-CRM, should be considered for drugs that are expected to produce delayed or cumulative toxicities.
It is unclear if the new dose escalation designs that have been proposed in the era of cytotoxic chemotherapy can be readily applied to molecularly targeted agents, or if different designs specific for such agents are needed (1). The main properties of molecularly targeted agents that distinguish them from cytotoxic agents include their allegedly superior therapeutic indices and the potential for identifying predictive biomarkers that correlate with clinical outcome and pharmacodynamic biomarkers that can confirm molecular mechanisms of activity. Whether the proposed differences between molecularly targeted agents and cytotoxic chemotherapy justify a need for distinctive phase I trial designs is debatable. In practice, however, toxicity has remained the primary endpoint of the vast majority of published phase I trials, as demonstrated by others previously (2) and by our own update in this review. Furthermore, as many as half of the molecularly targeted agents that were eventually approved by the US FDA underwent dose escalation in phase I trials until toxicity was observed (Table 3); the exceptions were monoclonal antibodies, for which dose escalations were stopped on the basis of favorable pharmacokinetic data, and agents such as imatinib, which demonstrated dramatic efficacy in the disease under investigation as well as a favorable pharmacokinetic profile before any dose-limiting toxicity was encountered. The identification of an optimal biological dose in phase I trials of molecularly targeted agents based on tissue-based biomarkers or plasma drug concentrations is challenging (87). The evaluation of predictive and/or pharmacodynamic biomarkers in phase I trials remains highly exploratory because of their small sample size, the heterogeneity of the patient population, and the lack of a control group. Only randomized controlled trials can validate relationships between these biomarkers and clinical outcome. Therefore, unless one is confident of the validity of the method of ascertaining biological activity of a new agent, toxicity should remain the most relevant reason to stop dose escalation in phase I trials (3).
Phase I trials in which a molecularly targeted agent is matched precisely with a population of patients whose tumors are driven predominantly by the target of interest, as in the cases of imatinib in chronic myelogenous leukemia and trastuzumab in HER2-amplified breast cancer, are rare. The vast majority of phase I trials evaluate new agents for which target tumor types have not yet been identified, and, as in phase I trials of cytotoxic compounds, toxicity is the primary endpoint and biomarker endpoints are only examined in exploratory analyses. Given these considerations, we presume that dose escalation methods developed in the era of cytotoxic agents are largely applicable to molecularly targeted agents. Continual efforts should be invested to evaluate the appropriateness of such applications and to explore new dose escalation methods that may better suit the properties of molecularly targeted agents. Deriving the optimal (ie, efficient and safe) dose escalation methods for anticancer therapies continues to be a challenge. However, there are many new designs proposed in recent literature. The use and evaluation of these new methods should be encouraged so that further improvements can be made to expedite the drug development process.
Funding
Christophe Le Tourneau is supported in part by a grant of the Fondation de France. J. Jack Lee is supported in part by a grant CA16672 from the National Cancer Institute.
Footnotes
The authors take full responsibility for the writing of the manuscript, its submission, and the analysis and interpretations presented.
References
- 1.Korn EL, Arbuck SG, Pluda JM, Simon R, Kaplan RS, Christian MC. Clinical trial designs for cytostatic agents: are new approaches needed? J Clin Oncol. 2001;19(1):265–272. doi: 10.1200/JCO.2001.19.1.265. [DOI] [PubMed] [Google Scholar]
- 2.Parulekar WR, Eisenhauer EA. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst. 2004;96(13):990–997. doi: 10.1093/jnci/djh182. [DOI] [PubMed] [Google Scholar]
- 3.Sleijfer S, Wiemer E. Dose selection in phase I studies: why we should always go for the top. J Clin Oncol. 2008;26(10):1576–1578. doi: 10.1200/JCO.2007.15.5192. [DOI] [PubMed] [Google Scholar]
- 4.Cannistra SA. Challenges and pitfalls of combining targeted agents in phase I studies. J Clin Oncol. 2008;26(22):3665–3667. doi: 10.1200/JCO.2008.17.2676. [DOI] [PubMed] [Google Scholar]
- 5.Rogatko A, Schoeneck D, Jonas W, Tighiouart M, Khuri FR, Porter A. Translation of innovative designs into phase I trials. J Clin Oncol. 2007;25(31):4982–4986. doi: 10.1200/JCO.2007.12.1012. [DOI] [PubMed] [Google Scholar]
- 6.Dixon WJ, Mood AM. A method for obtaining and analyzing sensitivity data. J Amer Stat Assoc. 1948;43:109–126. [Google Scholar]
- 7.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45(3):925–937. [PubMed] [Google Scholar]
- 8.Derman C. Nonparametric up and down experimentation. Ann Math Stat. 1957;28:795–798. [Google Scholar]
- 9.Durham SD, Flournoy N, Rosenberger WF. A random walk rule for phase I clinical trials. Biometrics. 1997;53(2):745–760. [PubMed] [Google Scholar]
- 10.Storer BE. An evaluation of phase I clinical trial designs in the continuous dose-response setting. Stat Med. 2001;20:2399–2408. doi: 10.1002/sim.903. [DOI] [PubMed] [Google Scholar]
- 11.Tokuda Y, Watanabe T, Omuro Y, et al. Dose escalation and pharmacokinetic study of a humanized anti-HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Br J Cancer. 1999;81(8):1419–1425. doi: 10.1038/sj.bjc.6690343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 13.Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002;20(9):2240–2250. doi: 10.1200/JCO.2002.10.112. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19(13):3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Pfister D, Cooper MR, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18(4):904–914. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- 16.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19(3):843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 17.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23(5):965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 18.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 19.Weiner LM, Belldegrun AS, Crawford J, et al. Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res. 2008;14(2):502–508. doi: 10.1158/1078-0432.CCR-07-1509. [DOI] [PubMed] [Google Scholar]
- 20.Pandite L, Burris HA, Jones S, et al. A safety, tolerability, and pharmacokinetic (PK) study of GW572016 in patients with solid tumors. J Clin Oncol. 2004;22:14s, 238. Abstract 3179. [Google Scholar]
- 21.Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22(12):2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 22.Wiernik PH, Schwartz EL, Strauman JJ, Dutcher JP, Lipton RB, Paietta E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47(9):2486–2493. [PubMed] [Google Scholar]
- 23.Mathé G, Reizenstein P. Phase I pharmacologic study of a new Vinca alkaloid: navelbine. Cancer Lett. 1985;27(2):285–293. doi: 10.1016/0304-3835(85)90186-7. [DOI] [PubMed] [Google Scholar]
- 24.Pazdur R, Newman RA, Newman BM, et al. Phase I trial of Taxotere: five-day schedule. J Natl Cancer Inst. 1992;84(23):1781–1788. doi: 10.1093/jnci/84.23.1781. [DOI] [PubMed] [Google Scholar]
- 25.Abbruzzese JL, Grunewald R, Weeks EA, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9(3):491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 26.Rowinsky EK, Grochow LB, Hendricks CB, et al. Phase I and pharmacologic study of topotecan: a novel topoisomerase I inhibitor. J Clin Oncol. 1992;10(4):647–656. doi: 10.1200/JCO.1992.10.4.647. [DOI] [PubMed] [Google Scholar]
- 27.Negoro S, Fukuoka M, Masuda N, et al. Phase I study of weekly intravenous infusions of CPT-11, a new derivative of camptothecin, in the treatment of advanced non-small-cell lung cancer. J Natl Cancer Inst. 1991;83(16):1164–1168. doi: 10.1093/jnci/83.16.1164. [DOI] [PubMed] [Google Scholar]
- 28.Mackean M, Planting A, Twelves C, et al. Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol. 1998;16(9):2977–2985. doi: 10.1200/JCO.1998.16.9.2977. [DOI] [PubMed] [Google Scholar]
- 29.Hong RL, Tseng YL. Phase I and pharmacokinetic study of a stable, polyethylene-glycolated liposomal doxorubicin in patients with solid tumors: the relation between pharmacokinetic property and toxicity. Cancer. 2001;91(9):1826–1833. [PubMed] [Google Scholar]
- 30.Newlands ES, Blackledge GR, Slack JA, et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856) Br J Cancer. 1992;65(2):287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathé G, Kidani Y, Triana K, et al. A phase I trial of trans-1-diaminocyclohexane oxalato-platinum (l-OHP) Biomed Pharmacother. 1986;40(10):372–376. [PubMed] [Google Scholar]
- 32.McDonald AC, Vasey PA, Adams L, et al. A phase I and pharmacokinetic study of LY231514, the multitargeted antifolate. Clin Cancer Res. 1998;4(3):605–610. [PubMed] [Google Scholar]
- 33.Ryan DP, Supko JG, Eder JP, et al. Phase I and pharmacokinetic study of ecteinascidin 743 administered as a 72-hour continuous intravenous infusion in patients with solid malignancies. Clin Cancer Res. 2001;7(2):231–242. [PubMed] [Google Scholar]
- 34.Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. [PubMed] [Google Scholar]
- 35.Abraham J, Agrawal M, Bakke S, et al. Phase I trial and pharmacokinetic study of BMS-247550, an epothilone B analog, administered intravenously on a daily schedule for five days. J Clin Oncol. 2003;21(9):1866–1873. doi: 10.1200/JCO.2003.03.063. [DOI] [PubMed] [Google Scholar]
- 36.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89(15):1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 37.Collins JM, Grieshaber CK, Chabner BA. Pharmacologically guided phase I clinical trials based upon preclinical drug development. J Natl Cancer Inst. 1990;82(16):1321–1326. doi: 10.1093/jnci/82.16.1321. [DOI] [PubMed] [Google Scholar]
- 38.Graham MA, Workman P. The impact of pharmacokinetically guided dose escalation strategies in phase I clinical trials: critical evaluation and recommendations for future studies. Ann Oncol. 1992;3(5):339–347. doi: 10.1093/oxfordjournals.annonc.a058203. [DOI] [PubMed] [Google Scholar]
- 39.Leung DH, Wang Y. Isotonic designs for phase I trials. Control Clin Trials. 2001;22(2):126–138. doi: 10.1016/s0197-2456(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 40.Ivanova A, Montazer-Haghighi A, Mohanty SG, Durham SD. Improved up-and-down designs for phase I trials. Stat Med. 2003;22(1):69–82. doi: 10.1002/sim.1336. [DOI] [PubMed] [Google Scholar]
- 41.Stylianou M, Follmann DA. The accelerated biased coin up-and-down design in phase I trials. J Biopharm Stat. 2004;14(1):249–260. doi: 10.1081/bip-120028518. [DOI] [PubMed] [Google Scholar]
- 42.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26(2):190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 43.Ji Y, Li Y, Bekele BN. Dose-finding in phase I clinical trials based on toxicity probability intervals. Clin Trials. 2007;4(3):235–244. doi: 10.1177/1740774507079442. [DOI] [PubMed] [Google Scholar]
- 44.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46(1):33–48. [PubMed] [Google Scholar]
- 45.Korn EL, Midthune D, Chen TT, Rubinstein LV, Christian MC, Simon RM. A comparison of two phase I trial designs. Stat Med. 1994;13(18):1799–1806. doi: 10.1002/sim.4780131802. [DOI] [PubMed] [Google Scholar]
- 46.O’Quigley J, Chevret S. Methods for dose finding studies in cancer clinical trials: a review and results of a Monte Carlo study. Stat Med. 1991;10(11):1647–1664. doi: 10.1002/sim.4780101104. [DOI] [PubMed] [Google Scholar]
- 47.Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995;14(11):1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- 48.Moller S. An extension of the continual reassessment methods using a preliminary up-and-down design in a dose finding study in cancer patients, in order to investigate a greater range of doses. Stat Med. 1995;14(9-10):911–922. doi: 10.1002/sim.4780140909. [DOI] [PubMed] [Google Scholar]
- 49.Faries D. Practical modifications of the continual reassessment method for phase I cancer clinical trials. J Biopharm Stat. 1994;4(2):147–164. doi: 10.1080/10543409408835079. [DOI] [PubMed] [Google Scholar]
- 50.Piantadosi S, Fisher JD, Grossman S. Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol. 1998;41(6):429–436. doi: 10.1007/s002800050763. [DOI] [PubMed] [Google Scholar]
- 51.Heyd JM, Carlin BP. Adaptive design improvements in the continual reassessment method for phase I studies. Stat Med. 1999;18(11):1307–1321. doi: 10.1002/(sici)1097-0258(19990615)18:11<1307::aid-sim128>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 52.Siu LL, Rowinsky EK, Hammond LA, et al. A phase I and pharmacokinetic study of SAM486A, a novel polyamine biosynthesis inhibitor, administered on a daily-times-five every-three-week schedule in patients with advanced solid malignancies. Clin Cancer Res. 2002;8(7):2157–2166. [PubMed] [Google Scholar]
- 53.Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17(10):1103–1120. doi: 10.1002/(sici)1097-0258(19980530)17:10<1103::aid-sim793>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Rogatko A, Babb JS, Tighiouart M, Khuri FR, Hudes G. New paradigm in dose-finding trials: patient-specific dosing and beyond phase I. Clin Cancer Res. 2005;11(15):5342–5346. doi: 10.1158/1078-0432.CCR-05-0458. [DOI] [PubMed] [Google Scholar]
- 55.Chu PL, Lin Y, Shih WJ, Unifying CRM. EWOC designs for phase I cancer clinical trials. J Stat Plan Inference. 2009;139:1146–1163. [Google Scholar]
- 56.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56(4):1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 57.Normolle D, Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J Clin Oncol. 2006;24(27):4426–4433. doi: 10.1200/JCO.2005.04.3844. [DOI] [PubMed] [Google Scholar]
- 58.Muler JH, McGinn CJ, Normolle D, et al. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22(2):238–243. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 59.Desai SP, Ben-Josef E, Normolle DP, et al. Phase I study of oxaliplatin, full-dose gemcitabine, and concurrent radiation therapy in pancreatic cancer. J Clin Oncol. 2007;25(29):4587–4592. doi: 10.1200/JCO.2007.12.0592. [DOI] [PubMed] [Google Scholar]
- 60.Bekele BN, Ji Y, Shen Y, Thall PF. Monitoring late-onset toxicities in phase I trials using predicted risks. Biostatistics. 2008;9(3):442–457. doi: 10.1093/biostatistics/kxm044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thall PF, Cook JD. Dose-finding based on efficacy-toxicity trade-offs. Biometrics. 2004;60(3):684–693. doi: 10.1111/j.0006-341X.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 62.Zhang W, Sargent DJ, Mandrekar S. An adaptive dose-finding design incorporating both toxicity and efficacy. Stat Med. 2006;25(14):2365–2383. doi: 10.1002/sim.2325. [DOI] [PubMed] [Google Scholar]
- 63.Yin G, Li Y, Ji Y. Bayesian dose-finding in phase I/II clinical trials using toxicity and efficacy odds ratios. Biometrics. 2006;62(3):777–787. doi: 10.1111/j.1541-0420.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 64.Paoletti X, Baron B, Schöffski P, et al. Using the continual reassessment method: lessons learned from an EORTC phase I dose finding study. Eur J Cancer. 2006;42(10):1362–1368. doi: 10.1016/j.ejca.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 65.Korn EL, Simon R. Using the tolerable-dose diagram in the design of phase I combination chemotherapy trials. J Clin Oncol. 1993;11(4):794–801. doi: 10.1200/JCO.1993.11.4.794. [DOI] [PubMed] [Google Scholar]
- 66.Thall PF, Millikan RE, Mueller P, Lee SJ. Dose-finding with two agents in Phase I oncology trials. Biometrics. 2003;59(3):487–496. doi: 10.1111/1541-0420.00058. [DOI] [PubMed] [Google Scholar]
- 67.Huang X, Biswas S, Oki Y, Issa JP, Berry DA. A parallel phase I/II clinical trial design for combination therapies. Biometrics. 2007;63(2):429–436. doi: 10.1111/j.1541-0420.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 68.Yuan Y, Yin G. Sequential continual reassessment method for two-dimensional dose finding. Stat Med. 2008;27(27):5664–5678. doi: 10.1002/sim.3372. [DOI] [PubMed] [Google Scholar]
- 69.Yin G, Yuan YA. Latent contingency table approach to dose finding for combinations of two agents [published online ahead of print August 28, 2008] Biometrics. 2008 doi: 10.1111/j.1541-0420.2008.01119.x. doi: 10.1111/j.1541-0420.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- 70.Von Hoff DD, Nieves JA, Vocila LK, Weitman SD, Cvitkovic E. The complete phase Ib clinical trial: a method to accelerate new agent development. J Clin Oncol. 2007;25(18s):112. Abstract 2562. [Google Scholar]
- 71.Schilsky RL. Phase I and II clinical trial design for targeted agents. Targ Oncol. 2006;1(4):220–227. [Google Scholar]
- 72.Friedman HS, Kokkinakis DM, Pluda J, et al. Phase I trial of O6-benzylguanine for patients undergoing surgery for malignant glioma. J Clin Oncol. 1998;16(11):3570–3575. doi: 10.1200/JCO.1998.16.11.3570. [DOI] [PubMed] [Google Scholar]
- 73.Hunsberger S, Rubinstein LV, Dancey J, Korn EL. Dose escalation trial designs based on a molecularly targeted endpoint. Stat Med. 2005;24(14):2171–2181. doi: 10.1002/sim.2102. [DOI] [PubMed] [Google Scholar]
- 74.Mandrekar SJ, Cui Y, Sargent DJ. An adaptive phase I design for identifying a biologically optimal dose for dual agent drug combinations. Stat Med. 2007;26(11):2317–2330. doi: 10.1002/sim.2707. [DOI] [PubMed] [Google Scholar]
- 75.Polley MY, Cheung YK. Two-stage designs for dose-finding trials with a biologic endpoint using stepwise tests. Biometrics. 2008;64(1):232–241. doi: 10.1111/j.1541-0420.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- 76.Booth CM, Calvert AH, Giaccone G, Lobbezoo MW, Seymour LK, Eisenhauer EA. Endpoints and other considerations in phase I studies of targeted anticancer therapy: recommendations from the Task Force on Methodology for the Development of Innovative Cancer Therapies (MDICT) Eur J Cancer. 2008;44(1):19–24. doi: 10.1016/j.ejca.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 77.Horstmann E, McCabe MS, Grochow L, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 78.Dent SF, Eisenhauer EA. Phase I trial design: are new methodologies being put into practice? Ann Oncol. 1996;7(6):561–566. doi: 10.1093/oxfordjournals.annonc.a010671. [DOI] [PubMed] [Google Scholar]
- 79.Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase I clinical trial design in cancer drug development. J Clin Oncol. 2000;18(3):684–692. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 80.Eckhardt SG, Siu LL, Clark G, DeMoor C, Von Hoff DD, Rowinsky EK. The continual reassessment method (CRM) for dose escalation in phase I trials in San Antonio does not result in more rapid study completion. Proc Am Soc Clin Oncol. 1999;18:163. Abstract 627. [Google Scholar]
- 81.Walling J, Zervos PH, McCarthy S, et al. Dose escalation methodology in phase I clinical trials: a comparison of the modified continual reassessment method (MCRM) and a traditional method. Experience with the multitargeted antifolate (MTA) Proc Am Soc Clin Oncol. 1997;16:209. Abstract 733. [Google Scholar]
- 82.Koyfman SA, Agrawal M, Garrett-Mayer E, et al. Risks and benefits associated with novel phase 1 oncology trial designs. Cancer. 2007;110(5):1115–1124. doi: 10.1002/cncr.22878. [DOI] [PubMed] [Google Scholar]
- 83.Roberts TG, Jr, Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292(17):2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 84.Estey E, Hoth D, Simon R, Marsoni S, Leyland-Jones B, Wittes R. Therapeutic response in phase 1 trials of antineoplastic agents. Cancer Treat Rep. 1986;70(9):1105–1115. [PubMed] [Google Scholar]
- 85.Von Hoff DD, Turner J. Response rates, duration of response, and dose response effects in phase 1 studies of antineoplastics. Invest New Drugs. 1991;9(1):115–122. doi: 10.1007/BF00194562. [DOI] [PubMed] [Google Scholar]
- 86.Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase-1 study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98(6):1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adjei AA. What is the right dose? The elusive optimal biologic dose in phase I clinical trials. J Clin Oncol. 2006;24(25):4054–4055. doi: 10.1200/JCO.2006.07.4658. [DOI] [PubMed] [Google Scholar]