Abstract
Purpose
Hypoxia preconditioning protects corneal stromal cells from stress-induced death. This study determined whether the transcription factor HIF-1α (Hypoxia Inducible Factor) is responsible and whether this is promulgated by VEGF (Vascular Endothelial Growth Factor).
Methods
Cultured bovine stromal cells were preconditioned with hypoxia in the presence of cadmium chloride, a chemical inhibitor of HIF-1α, and HIF-1α siRNA to test if HIF-1α activity is needed for hypoxia preconditioning protection from UV-irradiation induced cell death. TUNEL assay was used to detect cell apoptosis after UV-irradiation. RT-PCR and western blot were used to detect the presence of HIF-1α and VEGF in transcriptional and translational levels.
Results
During hypoxia (0.5% O2), 5 μM cadmium chloride completely inhibited HIF-1α expression and reversed the protection by hypoxia preconditioning. HIF-1α siRNA (15 nM) reduced HIF-1α expression by 90% and produced a complete loss of protection provided by hypoxia preconditioning. Since VEGF is induced by hypoxia, can be HIF-1α dependent, and is often protective, we examined the changes in transcription of VEGF and its receptors after 4 h of hypoxia preconditioning. VEGF and its receptors Flt-1 and Flk-1 are up-regulated after hypoxia preconditioning. However, the transcription and translation of VEGF were paradoxically increased by siHIF-1α, suggesting that VEGF expression in stromal cells is not down-stream of HIF-1α.
Conclusions
These findings demonstrate that hypoxia preconditioning protection in corneal stromal cells requires HIF-1α, but that VEGF is not a component of the protection.
Introduction
Keratocyte apoptosis is the earliest stromal event noted after corneal epithelial injury and has an important role in the overall wound healing response [1]. Keratocyte loss promotes the activation and proliferation of surrounding keratocytes which leads to a change in gene expression and matrix production that can affect cornea clarity [2-5]. Preventing keratocyte loss has been suggested as a possible approach to reduce keratocyte activation and possible subsequent myofibroblast formation [6]. Hypoxia preconditioning has been shown to be protective in brain [7], bladder [8], and retina [9]. We have shown that hypoxia preconditioning provides generalized protection to corneal stromal cells against induced apoptosis in vitro and in an ex vivo cornea model. Cobalt chloride, which is a chemical inducer of HIF-1α, provided protection to corneal stromal cells in the absence of hypoxia [10]. The nuclear transcription factor HIF-1α (hypoxia inducible factor) is induced by hypoxia in these cells and protection is also provided by an HIF-1α inducer, Cobalt chloride (CoCl2), suggesting that HIF-1α is a necessary component of hypoxia preconditioning protection [10].
HIF-1α is the major transcription factor that controls the expression of hypoxia-regulated genes. To activate transcription of target genes, HIF-1α dimerizes with ARNT (aryl hydrocarbon receptor nuclear translocator) and binds to the HRE (hypoxia responsive element ). ARNT is constitutively expressed so the hypoxic induction and modification of HIF-1α determines the transcriptional activity. Under normoxic conditions, HIF-1α is continuously degraded in proteasomes. Oxygen-dependent hydroxylation of proline residues in the ODD domain of HIF-1α leads to interaction with the VHL (von Hippel Lindau) ubiquitin ligase complex. Furthermore, oxygen-dependent hydroxylation of asparagine in the CAD domain prevents interaction of HIF-1α with the p300/CBP coactivator that is needed to induce transcription [11]. HIF-1α levels are inversely related to oxygen tension with a half-maximal response at 1.5-2% O2 and a maximal response at 0.5% O2 [12]. HIF-1α has been shown to be pro-apoptotic and anti-apoptotic. Hypoxia increases the expression of Nips, a pro-apoptotic member of the Bcl-1 family in human tumor cells [13]. Hypoxia preconditioning can be anti-apoptotic either by HIF-1α dependent or independent pathways. For example, up-regulation of the anti-apoptotic protein IAP-2 by hypoxia does not require HIF-1α and is regulated by the NFκb pathway [14]. However, protection of cortical neurons [15,16], pancreatic cancer cells [16], and retinal photoreceptors require HIF-1α, which is generally associated with upregulation of protective growth factors such as VEGF (vascular endothelial growth factor) and EPO (erythropoietin).
The VEGF gene has HREs and is a well-known target gene regulated by HIF-1α. VEGF expression can be increased by hypoxia preconditioning [17] or over-expression of HIF [18]. VEGF has been shown to prevent vascular endothelial cell death down stream of HIF-1α by at least two previous studies [19,20]. VEGF, down-stream of HIF-1α, also protects cardiomyocytes following ischemia [21]. VEGF and other tyrosine kinase activated receptors activate PI-3K and akt (Protein Kinase B) leading to phosphorylation of apoptotic factors that ultimately suppress release of cytochrome C and activation of caspases [22]. A recent study however, has shown that VEGF expression can be HIF-1α independent as shown in skeletal muscle cells where VEGF is regulated by PGC-1α ( peroxisome proliferator activated receptor gamma coactivator-1 alpha ) [23].
In this study, we found that siRNA knockdown of HIF-1α abrogated hypoxia dependent protection of corneal stromal cells. Because VEGF production is increased during corneal hypoxia and VEGF has very strong protective functions in many systems, we examined VEGF expression during HIF-1α knockdown. We found that VEGF expression was actually increased indicating that it is not a component of hypoxia dependent cell protection.
Methods
Cell culture
Corneal stromal cells were cultured as previously described [10]. Briefly, blocks of stroma were cut from fresh bovine cornea and cultured in DMEM (GIBCO) supplemented with 10% fetal bovine serum (FBS), 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 0.25 μg/ml of amphotericin B. Corneal stromal fibroblasts migrated from the stromal explants and grew exponentially at densities below 5×105 cells/ml. Second to third generation fibroblasts were seeded onto coverslips or petri-dishes and used in all cell culture experiments.
Induction of hypoxia
For hypoxia preconditioning, cells were placed in a hypoxia chamber (Coy Lab Products Inc., Grass Lake, MI) equilibrated with 5% CO2 and 0.5% oxygen-balance nitrogen for 4 h duration as indicated.
UV-irradiation
Corneal fibroblasts (5×104 cells) were sub-cultured to 25 mm coverslips in DMEM supplemented with 0.5% FBS for 2 days. Media was changed immediately before each experiment. This amount of serum was sufficient to prevent cell death, but does not promote proliferation. A germicidal lamp (TUV/30W/G30 T8; Philips Lighting Company, Somerset, NJ) that emitted radiation ranging from 230 to 400 nm was used as the UV source as previously described [10] Cells were irradiated for 2 min, which corresponds to 5.1 mJ/cm2. Cells were irradiated at 80-90% confluence. Culture media was removed and replaced with 2 ml of a balanced ringer’s solution to avoid variations in UV absorption from media components. After irradiation the ringer’s solution was discarded and replaced by fresh DMEM/0.5% FBS.
TUNEL assay and cell counting
Four hours after UV-irradiation, cells on coverslips were fixed in 4% formaldehyde/PBS at 4 °C for 25 min. Following fixation the cells were rinsed twice with PBS and permeabilized with prechilled 0.2% Triton X-100/PBS on ice for 5 min. A fluorescence-based TUNEL assay was used according to the manufacturer's instructions (ApoAlert; BD Biosciences, Palo Alto, CA). Cells were counterstained with DAPI and mounted with prolong antifade reagent (Molecular Probes, Eugene, OR). Images were obtained with a fluorescence microscope (Nikon E600; Nikon, Melville, NY) equipped with a charge coupled device camera with active cooling system. For fibroblasts cultured on coverslips, five random distinct 200X microscopic fields were photographed on each coverslip. DAPI(+) cells were counted to obtain the total cell count. DAPI(+) and TUNEL(+) cells were counted as apoptotic cells. DAPI(−) and TUNEL(+) areas were considered artifacts and excluded from the count. Data was collected from about 750 cells for each condition in each experiment. Experiments were repeated at least three times giving a total of at least 2,000 cells counted per condition.
RNA interference and cell transfection
Corneal fibroblasts (2×105 cells) were sub-cultured to 60 mm petri-dishes or 5×104 cells were sub-cultured to 25 mm coverslips in DMEM supplemented with 0.5% FBS for one day to reach 50% confluence. RNAi targeting HIF-1α was designed using Bos Taurus HIF-1α mRNA (GenBank NM_174339). The position for siRNA targeting starts at 1,450 of the HIF-1α mRNA. The sense 5’-AAG AAG GAG CCT GAT GCT TTA CCT GTC TC-3’ and antisense sequence 5’-AAT AAA GCA TCA GGC TCC TTC CCT GTC TC-3’, were synthesized and annealed as following the manufacturer’s protocol (Cat No. 1620; Ambion Inc., Austin, TX). Cells were transfected with the oligonucleotide duplexes for 6 h and then changed to regular medium. For mock transfection, cells were exposed to oligofectamine alone. A siRNA targeted to an irrelevant mRNA (Dharmacon Research, Chicago, IL) serves as non-targeting control.
RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). cDNA synthesis was performed using Invitrogen Superscript III (200 U μl−1), Oligo dT12–18 primer and 1 μg mRNA as manufacture’s instructions. VEGF, FLK-1, and Flt-1 primers were selected to amplify the 508 bp, 386 bp, and 334 bp fragments of mRNA respectively according to previous report [24]. Primer sequences: VEGF sense 5-TAC CTT CAC CAT GCA AG, VEGF antisense 5-CAC ATC TGC AAG TAC GTT CG; FLK-1 sense 5’-TTC TTG CCC AAC AAT CAG AG, FLK-1 antisense 5’-TAG CTG GGA ATA CTG AAG CC; Flt-1 sense 5’-TAT AGC ACC AAG AGC GAC, Flt-1 antisense 5’-GTG TCG AGT ACG TAA ACG. Each 25 ul of amplification reaction contains 0.4 μl Taq polymerase (cat. 92877933; Roche, Nutley, NJ), 2 μl of dNTP mix, 0.3 μm primers, 6 μl of cDNA for Flt-1 and 2 μl of cDNA for everything else. The PCR parameters are 40 cycles as follow: denaturation at 94 °C for 15 s, annealing at 55–60 °C for 25 s, extension at 72 °C for 35 s, according to a previous report [24]. The PCR products were separated on 1.7% agarose electrophoresis gels and stained with 0.5 μg/ml ethidium bromide and recorded for analysis.
Western blot analysis
Whole cell lysates were prepared as previously described [25]. Briefly, treated and untreated cells were extracted with lysis buffer (50 mmol/l Tris–HCl, pH 7.5, 5 mmol/l EDTA, 150 mmol/l NaCl, 0.5% Triton X-100, 10 mmol/l sodium fluoride, 20 mmol/l β-mercaptoethanol, 250 μmol/l sodium orthovanadate, 1 mmol/l PMSF, and complete protease inhibitor cocktail; Sigma, St Louis, MO), and incubated at 4 °C for 30 min. The lysates were sonicated and centrifuged at 14,000x g for 15 min. The supernatants were collected and stored at −80 °C. Protein concentrations were determined by the BCA method. Protein (50 μg) was separated on 8-12% polyacrylamide-SDS gel and electroblotted onto nitrocellulose membranes (Bio-Rad laboratories, Hercules, CA). After blocking with TBS/5% skim milk, the membrane was incubated overnight at 4 °C with primary antibodies against HIF-1α (Cat: MA1-516; ABR, Rockford, IL) at concentration of 1:2,000 or polyclonal antibody against VEGF (Cat: sc-507; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) , at 1:200 followed by peroxidase conjugated anti-mouse IgG or anti-rabbit IgG for 1 hr at room temperature. Signals were detected with ECL. Data was analyzed using Un-scan-it gel analysis software (Silk Scientific, Orem, UT). Relative increase in protein expression compared to its own control is calculated.
Statistical analysis
Data is presented as the mean±SE for at least three separate experiments. Student's t-test was employed for statistical analysis, with significant differences determined as p<0.05.
Results
Cadmium chloride prevents induction of HIF-1α and inhibits hypoxia preconditioning protection
Previously, we showed that 4 h of hypoxia preconditioning or application of CoCl2, a chemical HIF-1α inducer, provided protection against UV induced corneal stromal cell apoptosis [10], suggesting that HIF-1α has a role in preventing apoptosis. Conversely, treatment with low concentrations of cadmium chloride have been shown to inhibit the activation of HIF-1α by hypoxia [26-28]. Here we test whether cadmium chloride (5 μM) reduces HIF-1α activation by hypoxia in corneal stromal cells and whether it abrogates hypoxia preconditioning protection.
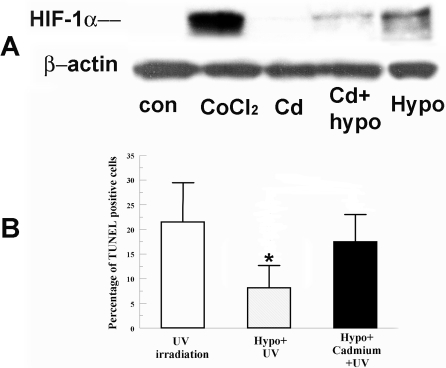
Figure 1A shows that cadmium chloride significantly reduces the induction of HIF-1α by hypoxia. Figure 1B shows that hypoxia preconditioning significantly protected cells from UV irradiation. However, the addition of cadmium eliminated this protection. Cadmium alone had no significant effect on HIF-1α (figure 1A) or apoptosis (data not shown). These results show that decreased HIF-1α levels lead to reduction of protection, which suggests that HIF-1α is involved in the hypoxia protection.
Figure 1.
Effect of Cadmium on HIF-1α accumulation and hypoxia preconditioning protection. A: Cells were incubated under normoxia with 200 μM CoCl2 (as a positive control), 5 μM CdCl2 alone, hypoxia (0.5% O2) alone, or hypoxia with CdCl2 for 4 h. Whole cell lysates were collected immediately after treatment, separated on SDS-PAGE gel and blotted for HIF-1α. β-actin was detected as a loading control. B: Cells were pretreated with hypoxia with or without CdCl2 for 4 h and irradiated 2 min with UV. Four hours after irradiation, cells were fixed and stained for TUNEL. Bar graph shows percentage of TUNEL positive cells in indicated groups. Error bars represent standard error of the mean (n=3); the asterisk indicates significantly different from UV control (p<0.05).
Characterization of RNAi targeting bovine HIF-1α in bovine corneal stromal cells
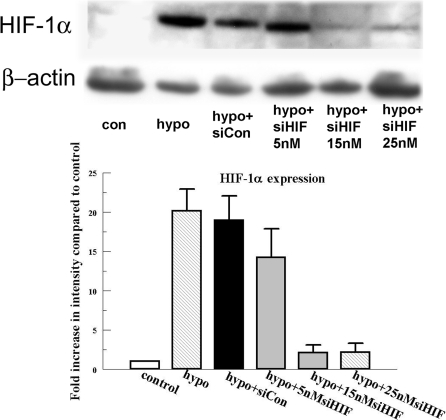
We designed a bovine specific HIF-1α small interference RNA. The efficiency and potency of this siRNA was tested in corneal stromal cells. Figure 2 shows that the HIF-1α siRNA produced a significant reduction in the hypoxia induced HIF-1α expression. The maximum effect (about 90% of reduction) of the siRNA could be achieved a concentration as low as 15 nM. Non targeting siRNA control did not significantly affect the HIF-1α level.
Figure 2.
Effect of HIF-1α siRNA on HIF-1α protein expression. Cells were transfected with 15 and 25 nM HIF-1α siRNA and an siRNA non targeting control as indicated for 6 h. Twenty-four hours after transfection, cells were exposed to 4 h of hypoxia. Protein was collected immediately after treatment and blotted for HIF-1α. Bar graph represents the band intensity relative to the control. Error bars represent the standard error of the mean (n=3). β-actin is detected as loading control.
Complete loss of hypoxia preconditioning protection by siHIF-1α
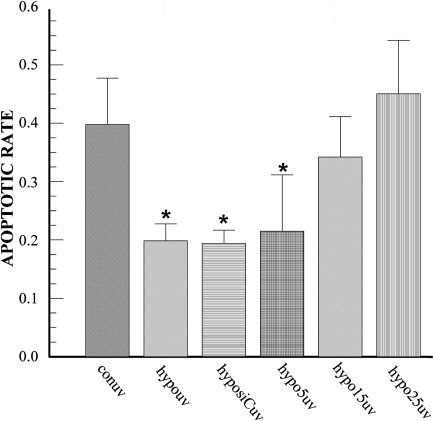
To definitively determine that HIF-1α is involved in hypoxia protection, we tested the effect of HIF-1α siRNA on hypoxia protection against UV-irradiation induced apoptosis in corneal stromal cells. Figure 3 shows that UV irradiation induced a 40±8.5% apoptotic rate whereas hypoxia preconditioning reduces this apoptotic rate to 20±3.0%. This is a similar protective effect to that reported previously [10]. Fifteen and 25nM siRNA targeting HIF-1α eliminates the hypoxia preconditioning protection, bringing the apoptotic rate back to 37.5±5.5% and 42±10.1%, respectively. These results demonstrate that hypoxia protection requires HIF-1α expression.
Figure 3.
Effect of HIF-1α siRNA on hypoxia preconditioning protection. Bovine stromal cells were transfected with the 15 nM (hypo15uv) and 25 nM (hypo25uv) HIF-1α siRNA and non targeting siRNA control (labeled as hyposiCuv) for 6 h. Twenty-four hours later, cells were exposed to hypoxia for 4 h and then stressed with UV-irradiation for 2 min. Cells were stained with TUNEL 4 h after irradiation. Error bars represent the standard error of the mean (n=3). The asterisk indicates statistically different from control UV irradiation.
VEGF is induced by hypoxia preconditioning in bovine corneal stromal cells
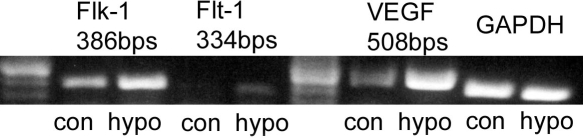
Genes prominently induced by hypoxia include growth factors like VEGF and EPO [29,30]. Among these growth factors, VEGF was found to be up-regulated in most cell types [31]. We tested here whether the transcription of VEGF and its receptors Flk-1 and Flt-1 are up-regulated in corneal stromal cells by hypoxia preconditioning. Figure 4 shows that VEGF is prominently up-regulated by hypoxia and both VEGF receptors Flk-1 and Flt-1 are also up-regulated. The transcription of receptor Flt-1 is significantly lower than Flk-1 in corneal stromal cells.
Figure 4.
Up-regulation of VEGF and its receptors by hypoxia in bovine keratocytes. Bovine stromal cells were treated with hypoxia for 4 h. Total RNA was collected immediately after treatment. Image shows RT-PCR analysis for VEGF, FLK-1, and Flt-1. GAPDH was detected as an internal control. Representative image of three experiments is shown.
Induction of VEGF by hypoxia preconditioning is not reduced by siHIF-1α
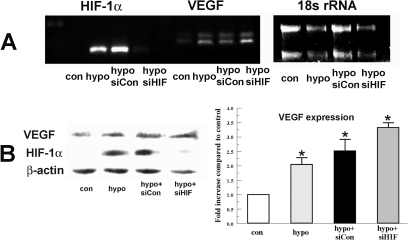
VEGF has been shown to be directly up-regulated by HIF-1α in vascular endothelial cells and kidney [32,33]. But recent evidence in skeletal muscle showed that VEGF expression is completely independent of HIF-1α [23]. We tested whether VEGF is down stream of HIF-1α in bovine corneal stromal cells. Figure 5A shows that VEGF mRNA is increased after hypoxia treatment but it is not reduced by HIF-1α siRNA. Figure 5B shows that HIF-1α siRNA significantly reduces the HIF-1α level, but it does not reduce VEGF expression. On the contrary, VEGF expression under hypoxia treatment with HIF-1α siRNA increases 3.3±0.1 fold compared to control which is significantly higher compared to hypoxia alone (2.1±0.3 fold increase).
Figure 5.
Effect of HIF-1α siRNA on hypoxia-induced VEGF expression. Bovine stromal cells were transfected with HIF-1α siRNA or non-targeting siRNA control for 6 h. Twenty-four hours after transfection cells were preconditioned with hypoxia for 4 h. A: Total RNA was collected immediately after treatment. Image shows RT-PCR analysis for HIF-1α and VEGF. 18s rRNA was detected as an internal control. B: Protein analysis of VEGF and HIF-1α. Whole cell lysates were collected immediately after treatment, separated by SDS PAGE and probed for VEGF and HIF-1α. β-actin was detected as a loading control. Bar graph shows VEGF expression in indicated groups relative to control. Error bars represent standard error of the mean (n=3).
Discussion
The concept of hypoxia preconditioning protection is well documented in many tissues and various factors are demonstrated to participate in the protection [9,34-37]. The production of EPO during whole body hypoxia protected photoreceptors from light induced cell death [9]. Hypoxia is also known to stimulate translocation of hsp27 and αB-crystallin from diffuse locations to defined structures, which is associated with a decrease in caspase-3 activity [38,39]. HIF-1α is a major modulator in the hypoxic environment. It is generally considered to play a protective role and induces the up-regulation of other protective factors like Hsp27 [40] and VEGF [32].
VEGF has been shown to be protective in several cell types such as skeletal muscle and kidney cells [23,32]. But whether the effect of VEGF on protection is dependent on HIF-1α is cell type specific. In this study, we used cadmium chloride to reduce the induction of HIF-1α. Cadmium chloride 5 μM, in the presence of hypoxia, completely blocks HIF-1α induction by hypoxia (Figure 1A). UV induced apoptosis of cells preconditioned by hypoxia in the presence of cadmium was not significantly different from UV alone, suggesting that prevention of HIF-1α induction is necessary for protection.
The HIF-1α specific siRNA efficiently reduces HIF-1α expression by 90% at 15 nM (Figure 2B). This reduction in HIF-1α completely eliminates the protection provided by hypoxia preconditioning (Figure 3) indicating that HIF-1α is essential for this protection. A similar conclusion has been drawn from a mouse study where complete loss of hypoxia protection was due to partial deficiency of HIF-1α [41].
The regulation of VEGF in response to hypoxia can be mediated by HIF-1α in a number of tissues [32,42]. Our result indicates that VEGF expression is up-regulated by hypoxia (figure 4), but that this increase in VEGF transcription and expression is independent of HIF-1α (figure 5). Therefore, protection by hypoxia depends on HIF-1α, but not VEGF in corneal stromal cells. A recent study on rat heart also showed that HIF-1α is protective, but is not VEGF or EPO dependent [43]. The regulation of VEGF expression must be cell type specific since in kidney [32] and vascular endothelial cells [33] VEGF, which is protective, is dependent on HIF-1α. On the other hand, VEGF expression is totally HIF independent in skeletal muscle cells where VEGF is regulated by peroxisome proliferator activated receptor gamma coactivator-1 alpha (PGC-1α) [23]. Interestingly, a recent study has shown that VEGF expression is regulated by HIF-2α in human lung endothelial cells [44]. Further studies are needed to determine the mechanism for VEGF regulation in corneal stromal cells.
Overall, the results from this study show that hypoxia preconditioning protection requires induction of HIF-1α. A likely protective factor, VEGF, is up-regulated by hypoxia preconditioning, but is not induced by HIF-1α, indicating that VEGF is not the protective factor during hypoxia preconditioning. We have preliminary evidence to suggest that the PI-3K and akt pathways are activated by hypoxia. This suggests that Receptor Tyrosine Kinase ligands other than VEGF, (e.g., EPO) are required for protection in corneal stromal cells. Further studies are needed to determine these factors induced by HIF-1α that may protect corneal stromal cells during hypoxia preconditioning.
Acknowledgments
This work was supported in part by NIH grant EY008834 (J.A.B.).
References
- 1.Wilson SE, Mohan RR, Mohan RR, Ambrosio J. Renato, Hong J, Lee J. The Corneal Wound Healing Response: Cytokine-mediated Interaction of the Epithelium, Stroma, and Inflammatory Cells. Prog Retin Eye Res. 2001;20:625–37. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 2.Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–92. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim WJ, Helena MC, Mohan RR, Wilson SE. Changes in corneal morphology associated with chronic epithelial injury. Invest Ophthalmol Vis Sci. 1999;40:35–42. [PubMed] [Google Scholar]
- 4.Soriano ES, Campos MS, Aguiar JA, Michelacci YM. Effect of epithelial debridement on human cornea proteoglycans. Braz J Med Biol Res. 2001;34:325–31. doi: 10.1590/s0100-879x2001000300005. [DOI] [PubMed] [Google Scholar]
- 5.Imayasu M, Petroll WM, Jester JV, Patel SK, Ohashi J, Cavanagh HD. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology. 1994;101:371–88. doi: 10.1016/s0161-6420(94)31326-1. [DOI] [PubMed] [Google Scholar]
- 6.Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–83. [PubMed] [Google Scholar]
- 7.Lin AM, Dung SW, Chen CF, Chen WH, Ho LT. Hypoxic preconditioning prevents cortical infarction by transient focal ischemia-reperfusion. Ann N Y Acad Sci. 2003;993:168–78. doi: 10.1111/j.1749-6632.2003.tb07527.x. [DOI] [PubMed] [Google Scholar]
- 8.Yu EZ, Li YY, Liu XH, Kagan E, McCarron RM. Antiapoptotic action of hypoxia-inducible factor-1 alpha in human endothelial cells. Lab Invest. 2004;84:553–61. doi: 10.1038/labinvest.3700071. [DOI] [PubMed] [Google Scholar]
- 9.Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–24. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 10.Xing D, Sun X, Li J, Cui M, Tan-Allen K, Bonanno JA. Hypoxia preconditioning protects corneal stromal cells against induced apoptosis. Exp Eye Res. 2006;82:780–7. doi: 10.1016/j.exer.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 12.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 13.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–73. [PubMed] [Google Scholar]
- 14.Dong Z, Venkatachalam MA, Wang J, Patel Y, Saikumar P, Semenza GL, Force T, Nishiyama J. Up-regulation of apoptosis inhibitory protein IAP-2 by hypoxia. Hif-1-independent mechanisms. J Biol Chem. 2001;276:18702–9. doi: 10.1074/jbc.M011774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaman K, Ryu H, Hall D, O'Donovan K, Lin KI, Miller MP, Marquis JC, Baraban JM, Semenza GL, Ratan RR. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19:9821–30. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura K, Hosokawa M, Asaka M. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548–54. [PubMed] [Google Scholar]
- 17.Tang Y, Pacary E, Freret T, Divoux D, Petit E, Schumann-Bard P, Bernaudin M. Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidates for stroke. Neurobiol Dis. 2006;21:18–28. doi: 10.1016/j.nbd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Date T, Mochizuki S, Belanger AJ, Yamakawa M, Luo Z, Vincent KA, Cheng SH, Gregory RJ, Jiang C. Expression of constitutively stable hybrid hypoxia-inducible factor-1alpha protects cultured rat cardiomyocytes against simulated ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;288:C314–20. doi: 10.1152/ajpcell.00374.2004. [DOI] [PubMed] [Google Scholar]
- 19.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–41. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 20.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–8. [PubMed] [Google Scholar]
- 21.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036–44. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20:1185–7. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- 23.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–12. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 24.Tscheudschilsuren G, Aust G, Nieber K, Schilling N, Spanel-Borowski K. Microvascular endothelial cells differ in basal and hypoxia-regulated expression of angiogenic factors and their receptors. Microvasc Res. 2002;63:243–51. doi: 10.1006/mvre.2001.2346. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Zhang ZF, Rao JY, Sato JD, Brown J, Messadi DV, Le AD. Treatment with siRNA and antisense oligonucleotides targeted to HIF-1alpha induced apoptosis in human tongue squamous cell carcinomas. Int J Cancer. 2004;111:849–57. doi: 10.1002/ijc.20334. [DOI] [PubMed] [Google Scholar]
- 26.Chun YS, Choi E, Kim GT, Choi H, Kim CH, Lee MJ, Kim MS, Park JW. Cadmium blocks hypoxia-inducible factor (HIF)-1-mediated response to hypoxia by stimulating the proteasome-dependent degradation of HIF-1alpha. Eur J Biochem. 2000;267:4198–204. doi: 10.1046/j.1432-1327.2000.01453.x. [DOI] [PubMed] [Google Scholar]
- 27.Horiguchi H, Kayama F, Oguma E, Willmore WG, Hradecky P, Bunn HF. Cadmium and platinum suppression of erythropoietin production in cell culture: clinical implications. Blood. 2000;96:3743–7. [PubMed] [Google Scholar]
- 28.Yang DI, Chen SD, Yang YT, Ju TC, Xu JM, Hsu CY. Carbamoylating chemoresistance induced by cobalt pretreatment in C6 glioma cells: putative roles of hypoxia-inducible factor-1. Br J Pharmacol. 2004;141:988–96. doi: 10.1038/sj.bjp.0705687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96:1173–7. doi: 10.1152/japplphysiol.00770.2003. [DOI] [PubMed] [Google Scholar]
- 30.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 31.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–81. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 32.Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, Sugimoto R, Choi AM, Stolz DB, Carrieri G, McCurry KR, Murase N, Nakao A. Carbon Monoxide Ameliorates Renal Cold Ischemia-Reperfusion Injury With an Upregulation of Vascular Endothelial Growth Factor by Activation of Hypoxia-Inducible Factor. Transplantation. 2008;85:1833–40. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- 33.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–69. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 34.Lin Anya MY, Dung S-W, Chen C-F, Chen W-H, Ho L-T. Hypoxic preconditioning prevents cortical infarction by transient focal ischemia-reperfusion. Ann N Y Acad Sci. 2003;993:168–78. doi: 10.1111/j.1749-6632.2003.tb07527.x. [DOI] [PubMed] [Google Scholar]
- 35.Yu HJ, Chien CT, Lai YJ, Lai MK, Chen CF, Levin RM, Hsu SM. Hypoxia preconditioning attenuates bladder overdistension-induced oxidative injury by up-regulation of Bcl-2 in the rat. J Physiol. 2004;554:815–28. doi: 10.1113/jphysiol.2003.056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 37.Piret JP, Lecocq C, Toffoli S, Ninane N, Raes M, Michiels C. Hypoxia and CoCl2 protect HepG2 cells against serum deprivation- and t-BHP-induced apoptosis: a possible anti-apoptotic role for HIF-1. Exp Cell Res. 2004;295:340–9. doi: 10.1016/j.yexcr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto K, Urushidani T, Nagao T. Translocation of HSP27 to cytoskeleton by repetitive hypoxia-reoxygenation in the rat myoblast cell line, H9c2. Biochem Biophys Res Commun. 1998;251:576–9. doi: 10.1006/bbrc.1998.9518. [DOI] [PubMed] [Google Scholar]
- 39.Webster KA. Serine phosphorylation and suppression of apoptosis by the small heat shock protein alphaB-crystallin. Circ Res. 2003;92:130–2. doi: 10.1161/01.res.0000056967.51841.21. [DOI] [PubMed] [Google Scholar]
- 40.Whitlock NA, Agarwal N, Ma JX, Crosson CE. Hsp27 upregulation by HIF-1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2005;46:1092–8. doi: 10.1167/iovs.04-0043. [DOI] [PubMed] [Google Scholar]
- 41.Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77:463–70. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 43.Wasserfuhr D, Cetin SM, Yang J, Freitag P, Frede S, Jakob H, Massoudy P. Protection of the right ventricle from ischemia and reperfusion by preceding hypoxia. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:27–32. doi: 10.1007/s00210-008-0303-x. [DOI] [PubMed] [Google Scholar]
- 44.Dutta D, Ray S, Vivian JL, Paul S. Activation of the VEGFR1 chromatin domain: An angiogenic signal-ETS1/HIF-2alpha regulatory axis. J Biol Chem. 2008;283:25404–13. doi: 10.1074/jbc.M804349200. [DOI] [PMC free article] [PubMed] [Google Scholar]