Abstract
Object
Recent data from both experimental and clinical studies have supported the use of intravenous magnesium as a potential therapy in the setting of cerebral ischemia. This study assessed whether intraoperative magnesium therapy improves neuropsychometric testing (NPT) following carotid endarterectomy (CEA).
Methods
One hundred eight patients undergoing CEA were randomly assigned to receive placebo infusion or 1 of 3 magnesium-dosing protocols. Neuropsychometric testing was performed 1 day after surgery and compared with baseline performance. Assessment was also performed on a set of 35 patients concurrently undergoing lumbar laminectomy to serve as a control group for NPT. A forward stepwise logistic regression analysis was performed to evaluate the impact of magnesium therapy on NPT. A subgroup analysis was then performed, analyzing the impact of each intraoperative dose on NPT.
Results
Patients treated with intravenous magnesium infusion demonstrated less postoperative neurocognitive impairment than those treated with placebo (OR 0.27, 95% CI 0.10–0.74, p = 0.01). When stratified according to dosing bolus and intraoperative magnesium level, those who were treated with low-dose magnesium had less cognitive decline than those treated with placebo (OR 0.09, 95% CI 0.02-0.50, p < 0.01). Those in the high-dose magnesium group demonstrated no difference from the placebo-treated group.
Conclusions
Low-dose intraoperative magnesium therapy protects against neurocognitive decline following CEA.
Keywords: carotid endarterectomy, magnesium, neuroprotectant, stroke
Magnesium is an attractive neuroprotective agent; it is widely available, inexpensive, and has a well-established safety profile. Encouraging results from both animal and clinical studies have supported the use of intravenous magnesium as a potential therapy in the setting of cerebral ischemia. Middle cerebral artery occlusion models in rodents have demonstrated that magnesium reduces infarct volume, even when given up to 6 hours after the onset of ischemia.12,24 Several small clinical studies have reported reduced mortality and disability in stroke patients treated with magnesium.13,17 These results generated enthusiasm for several large scale trials. A randomized controlled study (IMAGES trial) examining the effect of intravenous magnesium administered within 12 hours of acute stroke, however, failed to show a clinically significant improvement in death or disability at 90 days.18 It was suggested that the 12-hour time window in the IMAGES study may have been too long.25 A large ongoing clinical trial, the FAST-MAG study attempts to address this shortcoming through the administration of intravenous magnesium by paramedics, often within 120 minutes of the onset of ischemic symptoms.19 Phase II results indicate safety and feasibility.
Initial data suggest a potential role for magnesium in the setting of cerebral ischemia; however, questions regarding the precise efficacy and necessary timeline for administration remain unanswered. Heterogeneous study populations and treatment regimens dissimilar to preclinical testing parameters have made it difficult to achieve therapeutic efficacy.
Carotid endarterectomy surgery affords a unique paradigm in which to evaluate the effects of potential neuroprotective agents in the setting of acute stroke. Although the risk of major morbidity is low, subtle neurocognitive decline occurs in a high percentage of cases. Cortical ischemia secondary to intraoperative hypoperfusion or microembolic events translates to clinical deficits evident on detailed neurocognitive testing.3,5,6,22 Standardized neuropsychometric evaluation has demonstrated cognitive decline in ∼ 25% of patients undergoing CEA in the early postoperative period.9,10 Scheduled treatment administration, coupled with standardized perioperative clinical assessment provides a controlled environment with predictable temporal associations between ischemic onset and neurologic sequelae. It is a clinical model that allows for preischemic dosing of potential therapeutic agents.
Data generated from prior magnesium stroke studies have suggested that early treatment may be critical to achieve clinical efficacy. To this end, our CEA model provides a controlled paradigm in which to assess the benefit of elevated serum magnesium levels at the onset of an ischemic insult.
Methods
Study Cohort
One hundred eight prospectively enrolled patients undergoing elective CEA for both symptomatic and asymptomatic carotid artery stenosis were enrolled in this institutional review board–approved study. All patients undergoing CEA had ≥ 60% stenosis of the operative carotid artery, and none had undergone previous ipsilateral CEA. Patients were randomly assigned to a placebo control group or to 1 of 3 intravenous magnesium protocols. Sixteen patients (6 in the treatment group and 10 in the placebo group) were excluded from analysis due to high postoperative pain levels (7 patients), patient refusal to complete testing (7), or postoperative complications (2). The postoperative complications both occurred in the placebo group and included a postoperative hemorrhage at the surgical site and a postoperative cerebrovascular accident resulting in death.
Operative Procedure
All patients received general anesthesia with routine hemodynamic and temperature monitoring as previously described. Patients undergoing CEA underwent continuous blood pressure monitoring with a radial artery catheter. An 8-channel electroencephalography monitor (Neurotrac II, Moberg Medical, Inc.) was used during surgery. Fentanyl and midazolam were administered for preinduction sedation. General anesthesia was induced with fentanyl, midazolam, and either vecuronium or rocuronium and maintained with isoflurane. The mean surgical time was 159 ± 38 minutes (± SD). All patients were extubated in the operating room and recovered in a postoperative care or neurological intensive care unit.
Magnesium Protocol
Patients were randomized by a research pharmacist to receive either normal saline (43 patients) or magnesium sulfate (49 patients) in identical, unlabeled, 100-ml intravenous infusion bags. After the administration of a loading dose consisting of 100 ml delivered in 25 minutes, a continuous infusion of 400 ml was given over the next 24 hours (16.6 ml/hour). The placebo group received normal saline. Patients receiving magnesium were assigned to 1 of 3 protocols that differed in the loading dose or the continuous infusion dose (Table 1). Baseline serum magnesium levels were obtained before induction, 15 minutes after the loading dose, and during the saline/magnesium infusion at 1, 2, 6, 12, and 24 hours.
Table 1. Magnesium protocol groups into which 92 patients were assigned.
| Group | No. of Patients | Magnesium Administered (g) | ||
|---|---|---|---|---|
| Loading Dose | At Infusion | Total Infused | ||
| placebo | 43 | 0 | 0 | 0 |
| Protocol I | 13 | 2 | 8 | 10 |
| Protocol II | 7 | 2 | 16 | 18 |
| Protocol III | 29 | 4 | 16 | 20 |
Neuropsychometric Evaluation
After obtaining informed consent, patients were evaluated using a battery of 5 NPTs before surgery and on postoperative Day 1. Neuropsychometric tests were chosen to represent a range of cognitive domains. All NPTs were administered by 1 of 3 research assistants, each trained and supervised by a neuropsychologist. The Boston Naming Test evaluated the patient's ability to verbally identify objects pictured on a series of cards. Halstead-Reitan Trails Parts A and B evaluated visual, conceptual, and visuomotor tracking by timing how long it took a patient to connect consecutively numbered circles with a single line (Part A) and then connect the same number of consecutively numbered and lettered circles by alternating between the 2 sequences (Part B).14 The Controlled Oral Word Association test assessed verbal fluency, providing information on dominant hemisphere function. Patients were asked to generate as many words as possible that begin with a certain letter within 60 seconds. Three separate trials were performed at each testing session, 1 each with the letters C, F, and L. The Copy Portion of Rey Complex Figure test evaluated visuospatial organization, providing insight into the function of the nondominant hemisphere. Patients were instructed to copy the figure, and a standardized scoring system was used to evaluate the presence of design-specific features and the accuracy of their locations.
As described previously, a control group of 35 contemporaneous patients undergoing lumbar laminectomy with a similar anesthetic regimen and similar operative times were included to account for effects of general anesthesia on NPT performance.10
All tests were performed > 3 hours after administration of any analgesic or sedative medication. Patients and controls who reported a pain score of > 5 (10-point scale) during testing were excluded from analysis, as pain has been shown to confound NPT performance.11 Seven patients were eliminated from analysis due to high postoperative pain scores—4 from the placebo group and 3 from the magnesium treatment group.
Each NPT was scored individually for patients undergoing CEA and control individuals as previously described.9 The change in individual test scores from baseline to postoperative Day 1 were converted into z-scores relative to change within the control group as follows: z-score = (change score – mean change in score/SD change in score). The z-scores were converted into a point system quantifying the degree of cognitive dysfunction associated with each NPT at postoperative Day 1. For each patient undergoing CEA, these deficit points were summed to generate a TDS that measures the global level of cognitive decline.
Statistical Analysis
Total Cohort Analysis
Magnesium levels (mean ± SD) were compared between the entire treatment and placebo groups before induction, 15 minutes after the loading dose, and during the magnesium infusion at 1, 2, 6, 12, and 24 hours using the Student t-test. A forward stepwise logistic regression analysis was performed to evaluate the impact of magnesium treatment and other variables that have previously been suggested to increase neurocognitive decline following CEA (age, presence diabetes, and previous CEA) on TDS. Variables found to be significant were included in a multivariate logistic regression analysis that was performed by dichotomizing the outcome variable, TDS, between 0 and > 0 to distinguish between patients who did and did not show relative neurocognitive decline.
Subgroup Analysis (Intraoperative Magnesium Levels)
Magnesium levels (mean ± standard error of the mean) were compared between each of the treatment (Protocols I, II, and III) and placebo groups before induction, 15 minutes after the loading dose, and during the magnesium infusion at 1, 2, 6, 12, and 24 hours. Analysis of variance was used for initial comparison with subgroup analysis determined by the Tukey test. Again, a forward stepwise logistic regression analysis was performed to evaluate the impact of magnesium treatment and other variables that have previously been suggested to increase neurocognitive decline following CEA. Variables found to be significant were included in a multivariate logistic regression analysis.
Results
Total Cohort Analysis
Magnesium Levels
Magnesium levels did not differ significantly between patients receiving placebo and those receiving magnesium treatment at the time of the baseline measurement. At all time points postinfusion, magnesium levels for patients treated with magnesium sulfate infusion were elevated when compared with those in the placebo group (p < 0.01 at all time points; Table 2).
Table 2. Blood magnesium levels in the different groups at different time points.
| Group | Mean Magnesium Level (mg/dl) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 15 Mins | 1 Hr | 2 Hrs | 6 Hrs | 12 Hrs | 24 Hrs | |
| placebo | 1.93 ± 0.17 | 1.84 ± 0.17 | 1.86 ± 0.18 | 1.84 ± 0.22 | 1.74 ± 0.19 | 1.73 ± 0.17 | 1.87 ± 0.38 |
| all treatment groups | 2.05 ± 0.53 | 4.20 ± 1.16 | 3.40 ± 0.71 | 3.27 ± 0.63 | 3.21 ± 0.63 | 3.63 ± 0.58 | 3.70 ± 0.78 |
| Protocol I | 1.94 ± 0.17 | 3.70 ± 0.86 | 3.07 ± 0.45 | 2.94 ± 0.33 | 2.86 ± 0.21 | 2.94 ± 0.32 | 2.75 ± 0.61 |
| Protocol II | 1.85 ± 0.21 | 3.33 ± 0.94 | 2.81 ± 0.41 | 2.84 ± 0.38 | 3.02 ± 0.52 | 3.68 ± 0.57 | 3.71 ± 0.77 |
| Protocol III | 2.17 ± 0.64 | 4.71 ± 1.04 | 3.73 ± 0.67 | 3.54 ± 0.75 | 3.40 ± 0.70 | 3.79 ± 0.52 | 3.94 ± 0.60 |
Neurocognitive Outcome: Multivariate Analysis
Demographic and intraoperative variables for all patients are shown in Table 3. In the forward stepwise logistic regression analysis, presence of diabetes and age were not found to impact NPTs (p = not significant), whereas prior CEA did (p < 0.05). Therefore, prior CEA was included in the logistic regression model (OR 5.56, 95% CI 1.40– 22.11, p = 0.01; Table 4). When compared with patients receiving placebo, those treated with magnesium sulfate showed improved neurocognitive outcome (OR 0.27, 95% CI 0.10–0.74, p = 0.01; Table 4).
Table 3. Demographics, intraoperative variables, and total deficit scores.
| Demographic/Variable | Group (no. of patients)* | |||
|---|---|---|---|---|
| Placebo (43) | Overall Treatment (49) | Low Dose (20) | High Dose (29) | |
| mean age (yrs) | 68.1 ± 9.2 | 68.8 ± 9.1 | 68.9 ± 9.7 | 70 ± 8.5 |
| sex (F/M) | 39/61 | 37/63 | 45/55 | 38/62 |
| handedness (rt/lt) | 100/0 | 99/1 | 100/0 | 97/3 |
| mean height (cm) | 171 ± 10 | 171 ± 10 | 172 ± 11 | 170 ± 10 |
| mean weight (kg) | 76 ± 16 | 77 ± 16 | 82 ± 19 | 76 ± 13 |
| mean education (yrs) | 15 ± 3.2 | 15 ± 2.9 | 14 ± 2.4 | 14.5 ± 2.9 |
| hypertension | 75 | 68 | 45 | 76 |
| diabetes mellitus | 25 | 25 | 25 | 24 |
| symptomatic stenosis | 56 | 47 | 50 | 45 |
| previous CEA | 11 | 14 | 20 | 14 |
| side (rt/lt) of CEA | 49/51 | 53/47 | 55/45 | 21/43 |
| mean duration of op (min) | 161 ± 33 | 159 ± 38 | 147 ± 41 | 163 ± 41 |
| mean duration of cross-clamping (min) | 41 ± 15 | 41 ± 16 | 37 ± 18 | 43 ± 17 |
| mean fentanyl dose (μg/kg) | 2.26 ± 1.15 | 2.12 ± 1.03 | 2.03 ± 1.06 | 1.96 ± 0.80 |
| mean midazolam dose (mg/kg) | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.03 ± 0.02 |
| mean total deficit score | 0.77 ± 1.87 | 0.10 ± 0.31 | 0.45 ± 0.78 | 0.53 ± 1.38 |
Numbers represent the percentage of patients unless otherwise indicated. The low-dose group comprises patients in Protocols I and II. The high-dose group comprises patients in Protocol III.
Table 4. Forward stepwise logistic regression analysis*.
| Type of Analysis | Value | |||
|---|---|---|---|---|
| TDS >0† | p Value | OR | 95% CI | |
| multivariate | ||||
| magnesium treatment | 44/22 | 0.01 | 0.27 | 0.10–0.74 |
| prior CEA | 29/54 | 0.01 | 5.56 | 1.40–22.11 |
| prior stroke or TIA | 40/25 | 0.02 | 0.30 | 0.11–0.84 |
| age >70 yrs | 26/38 | NS | ||
| DM | 33/30 | NS | ||
| HTN | 24/36 | NS | ||
| subgroup multivariate | ||||
| placebo vs low dose | 44/10 | 0.01 | 0.09 | 0.02–0.50 |
| placebo vs high dose | 44/31 | 0.15 | 0.45 | 0.16–1.33 |
| prior CEA | 29/54 | 0.01 | 6.78 | 1.56–29.45 |
| prior stroke or TIA | 40/25 | 0.02 | 0.30 | 0.11–0.84 |
DM = diabetes mellitus; HTN = hypertension; NS = not significant; TIA = transient ischemic attack.
Values in the TDS >0 column are expressed as percentage of patients with neurocognitive decline if variable is present/absent.
Subgroup Analysis (Intraoperative Magnesium Levels)
Magnesium Levels
Magnesium levels (mg/dl) up to 24 hours are shown in Table 5. Values did not differ significantly between patients receiving placebo treatment or any of the 3 magnesium protocols at the time of the baseline measurement. At 15 minutes, 1 hour, and 2 hours, magnesium levels in patients treated with magnesium Protocols I and II differed significantly from those treated with placebo and Protocol III (p < 0.05), but not from one another (p = not significant). At 12 and 24 hours postinfusion magnesium levels in patients treated with magnesium Protocols II and III differed significantly from those treated with placebo or magnesium Protocol I (p < 0.05), but not from one another (p = not significant).
Table 5. Blood magnesium levels as stratified by intraoperative group.
| Group | Mean Magnesium Level (mg/dl) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 15 Mins | 1 Hr | 2 Hrs | 6 Hrs | 12 Hrs | 24 Hrs | |
| placebo | 1.93 ± 0.17 | 1.84 ± 0.17 | 1.86 ± 0.18 | 1.84 ± 0.22 | 1.74 ± 0.19 | 1.73 ± 0.17 | 1.87 ± 0.38 |
| overall treatment | 2.05 ± 0.53 | 4.20 ± 1.16 | 3.40 ± 0.71 | 3.27 ± 0.63 | 3.21 ± 0.63 | 3.63 ± 0.58 | 3.70 ± 0.78 |
| low dose | 1.89 ± 0.20 | 3.46 ± 0.91 | 2.91 ± 0.43 | 2.88 ± 0.36 | 2.96 ± 0.43 | 3.38 ± 0.60 | 3.36 ± 0.87 |
| high dose | 2.17 ± 0.64 | 4.71 ± 1.04 | 3.73 ± 0.67 | 3.54 ± 0.75 | 3.40 ± 0.70 | 3.79 ± 0.52 | 3.94 ± 0.60 |
Neurocognitive Outcome: Multivariate Analysis
When evaluating for neurocognitive decline, logistic regression analysis was not possible using the original protocol groups because the Protocol I group did not have any patients that met injury criteria (0 of 13). As intraoperative magnesium levels (15 minutes, 1 hour, and 2 hours postbolus) were statistically equivalent between Protocols I and II (p < 0.05; Table 5), these 2 cohorts were analyzed collectively. This combined group was referred to as the low-dose group in subgroup analysis. Patients in Protocol III were referred to as the high-dose group in the intraoperative subgroup analysis. Patients in the placebo group, the low-dose group (Protocols I and II), and the high-dose group (Protocol III) had statistically different magnesium levels from one another at every time point up to and including 24 hours using analysis of variance and post hoc Tukey column comparison testing (p < 0.01; Table 5). Multivariate logistic regression analysis was performed on these groups and included all risk factors that showed significance in the total cohort analysis.
In our multivariate model, patients in the low-dose group had significantly less postoperative neurocognitive injury than those treated with placebo (OR 0.09, 95% CI 0.02–0.50, p = 0.01; Fig. 1 and Table 4). Those treated with high-dose magnesium demonstrated no difference in neurocognitive outcome when compared with patients given placebo (p = not significant). Prior CEA remained a risk factor for neurocognitive decline in our subgroup multivariate logistic regression model (OR 6.78, 95% CI 1.56–29.45, p = 0.01; Table 4).
Fig. 1.
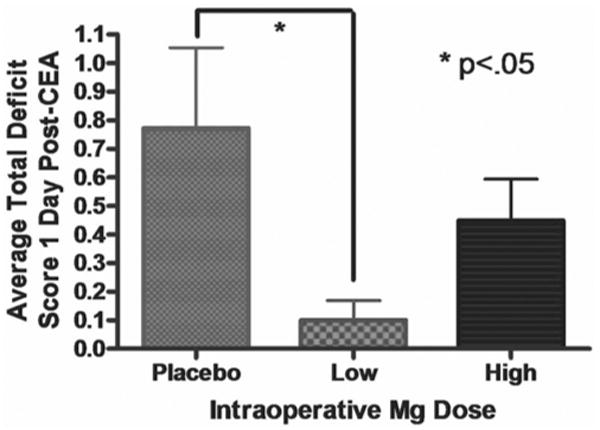
Graph showing that patients who received lower doses of magnesium had significantly lower TDSs than patients in the placebo group (p < 0.05).
Discussion
As is the case with many potential neuroprotective agents, preclinical and translational studies strongly support the efficacy of magnesium in the setting of cerebral ischemia. A well-conducted clinical trial, however, failed to demonstrate its usefulness in management of acute stroke. The IMAGES trial found that magnesium administration is safe, but that it did not reduce the incidence of death or disability following acute stroke. Several shortcomings have been postulated.8,25 Animal models have demonstrated that the time course for neuroprotection is relatively short. Only 1 preclinical study has suggested that magnesium administration 12 hours after vascular occlusion might translate to therapeutic efficacy.24 The median time to treatment was 7 hours in the IMAGES trial, with only 3% of patients receiving the therapy within 3 hours. To date, the only 2 intravenous therapies that have achieved significant results were administered within 3 hours of stroke onset.1,20 The heterogeneity of patients who have suffered stroke further differentiates the clinical study population from the controlled parameters used during preclinical assessment and renders the attainment of significant findings difficult.
Our study examined the efficacy of intravenous magnesium therapy in a smaller, more homogeneous patient population with parameters that more closely approximate those of the successful animal experiments and pertinent clinical data generated in related fields of study. Research on preischemic administration of magnesium in rodents showed significant reduction in infarct volume.15 A clinical investigation examining women at risk for preterm birth found that magnesium administration reduced gross motor dysfunction in newborns and suggested benefit in reducing mortality and rates of cerebral palsy in survivors.4 In addition, a recent clinical trial in which magnesium was given preoperatively to 350 patients having undergone cardiac surgery improved postoperative neurological and neuropsychometric examinations.2 These studies suggest that preischemic magnesium administration may affect neurological outcome in a wide range of cerebral ischemia models.
Carotid endarterectomy surgery affords an environment in which a subset of patients suffer cerebral ischemia with a predictable temporal onset. This model allows for administration of intravenous magnesium prior to the ischemic onset and a systematic assessment of biochemical, physiological, and functional outcome variables during and after the event.
We found that the total cohort of patients receiving intraoperative magnesium showed improved postoperative neurocognitive function when compared with those treated with placebo infusion. We also demonstrated a U-shaped dose response effect (low bolus dosing was beneficial, whereas placebo and high bolus dosing were not). Although 3 protocols were followed for magnesium administration, logistic regression analysis was not possible using the original groups because the lowest magnesium dosing protocol did not contain any patients that met injury criteria. Therefore, treatment groups were combined for post hoc subgroup analysis according to dosing bolus and intraoperative serum magnesium levels. With limited patient numbers, this method allowed us to reach significant power for statistical analysis of the subgroups. We segregated patients with similar intraoperative levels (significantly different from other groups, but the same as one another at all intraoperative time points) as we postulated that the magnesium values before and during the onset of cerebral ischemia, not those during the postoperative period, were the critical determinants of cerebroprotection. Prior studies in animals and the IMAGES trial have demonstrated that magnesium does not confer neuroprotection if provided at a significant delay following a neurological insult.18,24
We determined that low-dose intraoperative magnesium improves neuropsychometric testing on postoperative Day 1 following CEA. High-dose magnesium, however, demonstrated no benefit compared with placebo. Magnesium blood levels in the high dose group reached > 4.5 mg/dl at 15 minutes and remained > 3.5 mg/dl throughout the 24 hours. Previous literature has suggested that substantially elevated serum magnesium levels impair cognitive ability. Neuropsychometric testing done during magnesium infusion in obstetrics patients (whose blood levels ranged from 4.9 to 9.0 mg/dl) resulted in decreased attention and working memory.7 As blood levels remained elevated up to the time of neuropsychometric testing in our investigation, we are unable to determine whether the less favorable scores in the high-dose magnesium group were due to a lack of neuroprotection during CEA or impaired cognition at the time of testing.
In multivariate analysis, we found that having a prior CEA predicted cognitive decline on postoperative testing. Neurological injury in this patient population may have made these patients more susceptible to further intraoperative neurologic injury. That diabetes mellitus and age were not found to be predictive of poor outcome in our regression analysis may be related to the small sample size.16
This study is somewhat limited by its small patient population. The study is also limited by its inability to completely exclude alternative explanations for improved neurocognitive outcome in the low-dose magnesium group, such as the possibility of an interaction between magnesium and anesthesia. Our results, however, do suggest that intravenous magnesium, given prior to the onset of cerebral ischemia, may improve functional outcome in an applicable model of acute stroke. Furthermore, the data provide the first evidence that magnesium may decrease neurocognitive decline following CEA. Further study is needed.
Two potential theories may help explain why previous studies examining magnesium therapy in acute stroke have not succeeded. It may be that clinical trials, such as IMAGES, used primary outcome measures that were too broad to detect the subtle cognitive advantages conferred by magnesium therapy. It is also possible that serum magnesium levels must be elevated at the time of infarction rather than following an ischemic insult. This second issue may be addressed by the results of the FAST-MAG trial that is currently underway.19 If elevated magnesium levels at the time of infarction are critical for neuroprotection, magnesium would be especially suited to serve as a therapeutic agent during cardiac and vascular surgery, after which cognitive decline is a well-known phenomenon. It might also be possible to administer magnesium as a neuroprotectant following subarachnoid hemorrhage with focus on ameliorating symptomatic vasospasm. Current trials are underway that are designed to examine magnesium as both a neuroprotectant and a potential vasorelaxant in subarachnoid hemorrhage.21,23
Conclusions
This study provides preliminary evidence that low-dose magnesium therapy may protect against neurocognitive decline following CEA. High-dose therapy, however, does not seem to confer a benefit. There may be an ideal magnesium dose that could be identified in larger, future studies.
Acknowledgments
Disclosure: Financial support was provided by the Departments of Neurology and Radiology, New York University, New York, New York. Dr. Heyer received a grant from the National Institute on Aging (Grant No. R01 AG176.04). The Clinical Research Center at Columbia University Medical Center received a grant from the National Institutes of Health (Grant No. RR 00 645). Mr. Kellner was funded by the Doris Duke Clinical Research Foundation. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Abbreviations used in this paper
- CEA
carotid endarterectomy
- FAST-MAG
Field Administration of Stroke Therapy–Magnesium
- IMAGES
Intravenous Magnesium Efficacy in Stroke
- NPT
neuropsychometric test
- TDS
total deficit score
References
- 1.Anonymous. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Bhudia SK, Cosgrove DM, Naugle RI, Rajeswaran J, Lam BK, Walton E, et al. Magnesium as a neuroprotectant in cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2006;131:853–861. doi: 10.1016/j.jtcvs.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Brinkman SD, Braun P, Ganji S, Morrell RM, Jacobs LA. Neuropsychological performance one week after carotid endarterectomy reflects intra-operative ischemia. Stroke. 1984;15:497–503. doi: 10.1161/01.str.15.3.497. [DOI] [PubMed] [Google Scholar]
- 4.Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- 5.Cushman L, Brinkman SD, Ganji S, Jacobs LA. Neuropsychological impairment after carotid endarterectomy correlates with intraoperative ischemia. Cortex. 1984;20:403–412. doi: 10.1016/s0010-9452(84)80008-8. [DOI] [PubMed] [Google Scholar]
- 6.Gaunt ME, Martin PJ, Smith JL, Rimmer T, Cherryman G, Ratliff DA, et al. Clinical relevance of intraoperative embolization detected by transcranial Doppler ultrasonography during carotid endarterectomy: a prospective study of 100 patients. Br J Surg. 1994;81:1435–1439. doi: 10.1002/bjs.1800811009. [DOI] [PubMed] [Google Scholar]
- 7.Ghia N, Spong CY, Starbuck VN, Scialli AR, Ghidini A. Magnesium sulfate therapy affects attention and working memory in patients undergoing preterm labor. Am J Obstet Gynecol. 2000;183:940–944. doi: 10.1067/mob.2000.109045. [DOI] [PubMed] [Google Scholar]
- 8.Gorelick PB, Ruland S. IMAGES and FAST-MAG: magnesium for acute ischaemic stroke. Lancet Neurol. 2004;3:330. doi: 10.1016/S1474-4422(04)00762-8. [DOI] [PubMed] [Google Scholar]
- 9.Heyer EJ, Adams DC, Solomon RA, Todd GJ, Quest DO, McMahon DJ, et al. Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998;29:1110–1115. doi: 10.1161/01.str.29.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyer EJ, Sharma R, Rampersad A, Winfree CJ, Mack WJ, Solomon RA, et al. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heyer EJ, Sharma R, Winfree CJ, Mocco J, McMahon DJ, McCormick PA, et al. Severe pain confounds neuropsychological test performance. J Clin Exp Neuropsychol. 2000;22:633–639. doi: 10.1076/1380-3395(200010)22:5;1-9;FT633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumi Y, Roussel S, Pinard E, Seylaz J. Reduction of infarct volume by magnesium after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1991;11:1025–1030. doi: 10.1038/jcbfm.1991.170. [DOI] [PubMed] [Google Scholar]
- 13.Lampl Y, Gilad R, Geva D, Eshel Y, Sadeh M. Intravenous administration of magnesium sulfate in acute stroke: a randomized double-blind study. Clin Neuropharmacol. 2001;24:11–15. doi: 10.1097/00002826-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Lezak MD, Lezak MD. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 15.Marinov MB, Harbaugh KS, Hoopes PJ, Pikus HJ, Harbaugh RE. Neuroprotective effects of preischemia intraarterial magnesium sulfate in reversible focal cerebral ischemia. J Neurosurg. 1996;85:117–124. doi: 10.3171/jns.1996.85.1.0117. [DOI] [PubMed] [Google Scholar]
- 16.Mocco J, Wilson DA, Komotar RJ, Zurica J, Mack WJ, Halazun HJ, et al. Predictors of neurocognitive decline after carotid endarterectomy. Neurosurgery. 2006;58:844–850. doi: 10.1227/01.NEU.0000209638.62401.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muir KW, Lees KR. A randomized, double-blind, placebo-controlled pilot trial of intravenous magnesium sulfate in acute stroke. Ann N Y Acad Sci. 1995;765:315–316. doi: 10.1111/j.1749-6632.1995.tb16598.x. [DOI] [PubMed] [Google Scholar]
- 18.Muir KW, Lees KR, Ford I, Davis S. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet. 2004;363:439–445. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- 19.Saver JL, Kidwell C, Eckstein M, Starkman S. Prehospital neuroprotective therapy for acute stroke: results of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) pilot trial. Stroke. 2004;35:e106–e108. doi: 10.1161/01.STR.0000124458.98123.52. [DOI] [PubMed] [Google Scholar]
- 20.Sherman DG, Atkinson RP, Chippendale T, Levin KA, Ng K, Futrell N, et al. Intravenous ancrod for treatment of acute ischemic stroke: the STAT study: a randomized controlled trial. Stroke Treatment with Ancrod Trial. JAMA. 2000;283:2395–2403. doi: 10.1001/jama.283.18.2395. [DOI] [PubMed] [Google Scholar]
- 21.Stippler M, Crago E, Levy EI, Kerr ME, Yonas H, Horowitz MB, et al. Magnesium infusion for vasospasm prophylaxis after subarachnoid hemorrhage. J Neurosurg. 2006;105:723–729. doi: 10.3171/jns.2006.105.5.723. [DOI] [PubMed] [Google Scholar]
- 22.Townes BD, Dikmen SS, Bledsoe SW, Hornbein TF, Martin DC, Janesheski JA. Neuropsychological changes in a young, healthy population after controlled hypotensive anesthesia. Anesth Analg. 1986;65:955–959. [PubMed] [Google Scholar]
- 23.Wong GK, Chan MT, Boet R, Poon WS, Gin T. Intravenous magnesium sulfate after aneurysmal subarachnoid hemorrhage: a prospective randomized pilot study. J Neurosurg Anesthesiol. 2006;18:142–148. doi: 10.1097/00008506-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Li Q, Ahmad F, Shuaib A. Survival and histological evaluation of therapeutic window of post-ischemia treatment with magnesium sulfate in embolic stroke model of rat. Neurosci Lett. 2000;285:119–122. doi: 10.1016/s0304-3940(00)01048-x. [DOI] [PubMed] [Google Scholar]
- 25.Zivin JA. Slow-Mag. Stroke. 2004;35:1776–1777. doi: 10.1161/01.STR.0000132192.67192.44. [DOI] [PubMed] [Google Scholar]


