Abstract
Male and female zebra finches are highly social and form pair bonds typically associated with reproduction. To determine how these bonds affect a female's behavioral response to future interactions, females were paired with a male for 2 weeks, separated for 48 h, and then exposed to the same or a novel male. Control females were left unpaired and introduced to a novel male. Behaviors, as well as neural ZENK expression, were quantified. Females displayed higher levels of behaviors associated with pair bonds (clumping and preening) toward their mates than novel males, and display of these behaviors was correlated with expression of the immediate early gene ZENK in the nucleus taeniae of one group of females, those interacting with their mates. Behaviors of the stimulus males were largely unaffected, but those interacting with an unpaired female attempted to mount more than those interacting with their mates. The results indicate that the nucleus taeniae may play some role in the maintenance of pair bonds in this species. Additionally, females may provide some signal to influence elements of the behavior of males.
Keywords: reproduction, amygdala, social behavior, songbird
The formation of social bonds is important to an individual's health and success in many social species, including humans (Lucas et al., 2003). In monogamous species, the reproductive sequence commonly involves formation of a pair bond. The neural mediation of these bonds has only been examined in a few model systems, including monkeys and prairie voles (see Mason and Mendoza, 1998; Wang and Aragona, 2004 for reviews). Zebra finches form strong pair bonds prior to reproduction. They are formed in 2-14 days, can be maintained through auditory contact alone (Silcox and Evans, 1982), and are characterized by close contact, allopreening, and synchronized behaviors (Zann, 1996). Appearance of these behaviors typically occurs shortly after the male and female are introduced, and they increase in frequency by the second day (Silcox and Evans, 1982). These behaviors are not typically displayed between unrelated single individuals (Zann, 1996; Butterfield, 1970). Females that have formed a pair bond react aggressively to new males, and close contact (clumping and preening) with them is inhibited (Silcox and Evans, 1982). Pair bonding seems to affect female behavior in two ways. It increases the incidence of pairing behaviors directed toward her mate and reduces the display of them toward other males. Studies designed to examine the responses of females to pairing manipulations are limited. Variations in female responses toward males that are observed following pair bonding are likely mediated by multiple neural systems, including those related to auditory perception, social behavior, and reward.
Responses to interactions with males have been investigated in several species using immediate early genes (IEGs; see Pfaus and Heeb, 1997; Meddle et al., 1999), although research on the neural correlates of behaviors associated with pairing in avian species is limited. Increased IEG expression is observed in female rodents following sexual interactions with males in regions in the social behavior network (defined by Newman, 1999 as brain areas involved in the mediation of social behaviors which are responsive to steroid hormones), including the ventromedial hypothalamus, bed nucleus of the stria terminalis, and amygdala (Pfaus and Heeb, 1997). In female Japanese quail, interaction with a male results in increased Fos expression in the ventromedial hypothalamus, intercollicular nucleus, and mesopallium (Meddle et al., 1999), at least some of which are involved in the control of reproductive and social behavior in birds (Gibson and Cheng, 1979; Cheng et al., 1999). The mesolimbic dopamine reward system is also implicated in the regulation of female reproductive behaviors in rodents (see Melis and Argiolas, 1995; Paredes and Agmo, 2004 for reviews). Dopamine levels increase following paced copulation in female rats (i.e. Becker et al., 2001; Mermelstein and Becker, 1995; Pfaus et al., 1995). Although work on the reward system in female birds is limited, singing behavior by male starlings results in increased IEG expression in the ventral tegmental area during the breeding season (Riters et al., 2004; Heimovics and Riters, 2005, 2006).
In the monogamous rodent, the prairie vole, IEG expression is seen in the bed nucleus of the stria terminalis and preoptic area when females cohabitate with a novel male (Cushing et al., 2003), and in the medial amygdala, preoptic area, and bed nucleus of the stria terminalis after 6 h of mating, which is typically sufficient for the formation of a pair bond in this species (Curtis and Wang, 2003). The reward system is also vital to the regulation and formation of the pair bond in these animals. Dopamine levels increase in the nucleus accumbens in response to mating in female prairie voles (Gingrich et al., 2000). Dopamine antagonists presented either systemically or directly into the nucleus accumbens block the formation of a pair bond following mating (Aragona et al., 2003). Similarly, pair bonds can be formed between a male and a female without mating by treating systemically or directly into the nucleus accumbens with a dopamine agonist (Aragona et al., 2003). Thus, regions in both the social behavior and mesolimbic reward system mediate pairing behaviors in the prairie vole.
Studies in birds have tended to focus on the role of auditory perception in female mate choice and pair formation. Females behaviorally discriminate between mate and stranger calls (Vignal et al., 2008), and auditory cues from males are critical for pair bond formation (Silcox and Evans, 1982; Tomaszycki and Adkins-Regan, 2005). Exposure to male song results in increased IEG expression in the auditory perception regions in numerous songbird species including zebra finches (Mello et al., 1992; Mello and Clayton, 1994; Bailey et al., 2002), and these regions display differential levels of IEG expression based on variations in male song (e.g. Gentner et al., 2001; Maney et al., 2003; Leitner et al., 2005; Sockman et al., 2005).
The present study was designed to examine the mediation of pair bonding behavior in the female zebra finch. Although studies have confirmed that females change their behavior as pair bonds are formed, the only study to examine how pairing affects responses to new individuals was conducted with male zebra finches (Caryl, 1976). To investigate whether forming a pair bond changes how a female behaves during subsequent social interactions with males, we evaluated behavioral responses in three groups of birds—females re-exposed to their partners after a brief break, and previously paired and unpaired females exposed to novel males. More importantly, to assess potential roles of the auditory perception, social behavior, and mesolimbic reward systems in the mediation of these behavioral responses, we examined neural correlates by measuring expression of the IEG, ZENK after these social interactions.
EXPERIMENTAL PROCEDURES
Animals
Adult female and male zebra finches were raised in our breeding colony at Michigan State University. Animals were kept on a 12-h light/dark cycle, and provided seed and water ad libitum. Their diets were enriched with orange and spinach and a hard-boiled egg/bread mixture provided once a week. Adults were raised in mixed-sex aviaries until they had reached adulthood (at least 100 days of age). They were then housed in unisex aviaries, in acoustic but not visual contact with the opposite sex, for at least 3 months prior to testing. All procedures were approved by the Michigan State University Animal Use and Care Committee and adhered to the guidelines of the National Institutes of Health. Number of birds used and their suffering were minimized as much as possible.
Behavior
Females were placed in an individual cage (30 cm×23×38 cm) either alone or with a sexually mature male zebra finch for 2 weeks. This time period is sufficient for the formation of a pair bond in this species (Silcox and Evans, 1982). A test was then conducted to confirm the formation of a pair bond. The cage containing two birds was taken to a separate testing room, and after one-half hour for acclimation, behaviors of both individuals were video-recorded for 1 h. Male and female behaviors (Table 1) were quantified by an observer blind to experimental group based on descriptions in Zann (1996) and Adkins-Regan and Ascenzi (1987). They were classified as bonded if they displayed either clumping or preening during the test. These criteria are routinely used to identify paired birds in this species (Butterfield, 1970; Silcox and Evans, 1982; Clayton, 1990; Zann, 1996; Adkins-Regan and Wade, 2001; Adkins-Regan, 2002) and are uncommon among unrelated, unpaired adult birds (Zann, 1996). Females housed alone were also taken to the testing room for the same time period, although their behavior was not videotaped. Following the test, cages were returned to the colony room, and males were removed and housed individually within visual and acoustic contact with the female for 48 h. Novel stimulus males were also placed in individual cages at this time, but they were housed out of visual contact with the experimental females. Birds that were not classified as bonded in this first test were not included in the study.
Table 1.
Descriptions of measured behaviors
| Behavior | Description |
|---|---|
| Pairing | |
| Clumping* | Male and female perch in physical contact, facing the same direction |
| Individual in proximity of another* | Male or female moves to perch less than a body width apart; they face the same direction |
| Preening* | One individual cleans another's feathers with beak |
| Reproductive | |
| Tail quiver | Rapid back and movement of the tail. In the female often associated with a bowed position on the perch, but also may be performed by males following copulation |
| Attempted mount | Male perches at least briefly on the back of the female, but no cloacal contact is observed |
| Copulation | Male perches on the back of the female and cloacal contact is observed |
| Beak wipe | Beak is moved back and forth in a rhythmic fashion along the perch |
| Other | |
| Directed singing* | Male produces song facing the female |
| Undirected singing* | Male produces the song, but is not facing the female |
| Beak fencing | Male and female swipe beaks with each other, can be an aggressive behavior |
| Approach | Direct movement toward the other individual with an upright posture (often followed by clumping or courtship behavior) |
Frequencies were assessed for all; durations were also measured for the behaviors with an asterisk.
After the 48-h separation, a second behavior test was conducted. Paired females were taken into the testing room in their individual cages. After 30 min of acclimation, they were exposed to either a familiar male (n=7) or a novel male (n=10). Individually housed (unpaired) females were exposed to a novel male (n=10). Behaviors were videotaped for 1 h, and those of both individuals were evaluated. A principal components (PC) analysis was conducted to determine associations among the behavioral variables. Several of the behaviors were infrequently displayed. If fewer than 35% of the individuals displayed a specific behavior (e.g. males preening the female, copulation, and producing undirected song), we excluded it from the PC analysis. This procedure allowed us to create groups of related variables that could then be examined as a whole to determine whether they were affected by our experimental manipulations. To do this, we compared the PC scores using one-way ANOVAs across exposure groups, followed by post hoc Tukey/Kramer tests where appropriate. Further analyses focused only on behaviors with high loadings for the PCs which were affected by group. Kruskal-Wallis tests were then used to compare the frequencies and durations of these specific behaviors among the three exposure groups. Pair-wise comparisons were conducted, as appropriate, with Mann-Whitney U-tests.
ZENK
The female was removed from the cage one-half hour after the conclusion of the second test, overdosed in the testing room with 0.12 cc equithesin, and perfused intracardially with 0.1 M phosphate-buffered saline (PBS) and 4% paraformaldehyde. The brain was removed, fixed in 4% paraformaldehyde for 15 min, embedded in gelatin, fixed for 1 h in 4% paraformaldehyde, and then placed in 30% sucrose overnight. It was then sagittally sectioned into four series at 30 μm on a freezing microtome. Tissue was stored in cryoprotectant at -20 °C until immunohistochemistry was performed.
The methods for visualizing ZENK were as described in Svec and Wade (in press; modified from Bailey et al. (2002) and Bailey and Wade (2003, 2005). Briefly, sections were rinsed in PBS, incubated in 0.5% hydrogen peroxide in PBS, rinsed, blocked in 5% normal donkey serum in phosphate-buffered saline with 0.3% Triton X-100 (PBST) for 1 h, and then incubated with primary antibody (Santa Cruz Biotech, Santa Cruz, CA; catalog no. sc-189, 0.1 μg/ml) in PBST overnight at 4 °C. After primary incubation, sections were rinsed, incubated in biotin-SP-conjugated donkey-antirabbit antibody (Jackson ImmunoResearch Laboratories; 1:500 dilution) in PBST for 1 h, exposed to ABC reagent (Elite kit, Vector Laboratories, Burlingame, CA, USA), and reacted with diaminobenzidine with 0.0075% hydrogen peroxide for 3 min. Tissue was then rinsed, mounted, dehydrated, cleared with xylenes, and coverslipped with DPX.
ZENK immunoreactivity was assessed using brightfield microscopy. Sampling regions were placed in brain areas within three neural systems: the auditory perception regions (caudomedial nidopallium and caudomedial mesopallium), the social behavior network (ventromedial hypothalamus, preoptic area, nucleus taeniae, and bed nucleus of the stria terminalis), and the reward system (ventral tegmental area and nucleus accumbens) as described and pictured in Svec and Wade (in press). A number of anatomical landmarks were utilized to identify these regions and place the boxes within the same portion of the region in each animal. Box sizes were as follows: caudomedial nidopallium (310×320 μm), caudomedial mesopallium (190×345 μm), ventromedial hypothalamus (190×290 μm), preoptic area (200×200 μm), nucleus taeniae (200×320 μm), bed nucleus of the stria terminalis (190×345 μm), nucleus accumbens (190×305 μm), and ventral tegmental area (210×368 μm). Immunoreactive cells were manually counted within each sampling region. The average number of immunoreactive cells was calculated from at least three sections per animal, and the density of ZENK immunoreactivity was calculated by dividing this average by the area of the sampling box. A mixed-model ANOVA was conducted to compare the density of ZENK immunoreactivity across brain regions (within animals) and groups (between animals), followed by Tukey post hoc tests where appropriate. To further investigate the relationship between behavior and ZENK expression, correlation analyses within each exposure group were used to determine associations between the densities of immunoreactive cells in each brain region with behaviors that differed among the groups.
An outlier was detected among the paired females interacting with their mates in the caudomedial mesopallium and one was also detected in the paired females interacting with novel males in the bed nucleus of the stria terminalis and ventral tegmental area using Dixon's test (Rohlf and Sokal, 1981); data reported exclude them.
The PC analysis and ANOVA of the PC scores were conducted in SPSS, while other statistical analyses were conducted with StatView (SAS Institute, Cary, NC, USA).
RESULTS
Behavior
PC analysis
Six PCs were revealed. The first two explained almost 50% of the variation in behavior. The first component (female PC) had high loadings for behaviors in which females increased proximity to or initiated contact with males. The second (PC2) involved reproductive behaviors and female beak wipes (Table 2). ANOVAs revealed a main effect of group on scores for the female PC (F=3.56, P=0.044), such that paired females interacting with their mates displayed higher PC scores than paired females exposed to novel males (Tukey HSD, P=0.037). A main effect of group on scores for the second PC was also detected (F=3.92, P=0.034), but post hoc tests revealed no significant differences between pairs of groups (all P>0.059). Scores for the other four PC did not differ among the groups (all F<1.78, P>0.190).
Table 2.
PC analysis
| Female PC | PC 2 | PC3 | PC4 | PC5 | PC6 | |
|---|---|---|---|---|---|---|
| Directed singing | ||||||
| Frequency | -0.401 | 0.759 | -0.331 | -0.187 | 0.187 | -0.038 |
| Duration | -0.395 | 0.693 | -0.412 | -0.186 | 0.071 | 0.067 |
| Beak wipe to female | -0.172 | 0.395 | 0.552 | 0.409 | 0.243 | 0.391 |
| Beak wipe away from female | -0.140 | 0.309 | 0.724 | 0.160 | -0.027 | 0.464 |
| Beak wipe toward male | 0.146 | 0.573 | 0.637 | -0.225 | 0.020 | -0.348 |
| Beak wipe away from male | 0.259 | 0.545 | 0.604 | -0.269 | 0.119 | -0.367 |
| Male approaching female | -0.192 | 0.745 | -0.386 | -0.030 | -0.170 | 0.142 |
| Female approaching male | 0.844 | 0.292 | -0.119 | -0.013 | -0.043 | 0.017 |
| Male in proximity of female | ||||||
| Frequency | 0.067 | 0.427 | -0.142 | 0.792 | 0.219 | -0.159 |
| Duration | -0.102 | 0.082 | -0.155 | 0.830 | 0.200 | -0.360 |
| Female-initiated clumping | ||||||
| Frequency | 0.866 | 0.052 | -0.151 | 0.062 | -0.069 | 0.183 |
| Duration | 0.560 | -0.066 | -0.105 | 0.238 | -0.098 | 0.296 |
| Female in proximity of male | ||||||
| Frequency | 0.725 | 0.267 | 0.020 | 0.123 | -0.375 | -0.126 |
| Duration | 0.722 | 0.142 | -0.068 | 0.053 | -0.358 | -0.124 |
| Female preening male | ||||||
| Frequency | 0.819 | 0.120 | -0.034 | -0.157 | 0.361 | 0.002 |
| Duration | 0.852 | 0.185 | -0.119 | -0.091 | 0.297 | 0.184 |
| Attempted mount | -0.263 | 0.780 | -0.242 | -0.072 | -0.277 | 0.128 |
| Beak fencing | 0.178 | -0.075 | -0.220 | -0.252 | 0.790 | 0.040 |
| Eigenvalues | 4.828 | 3.525 | 2.247 | 1.881 | 1.435 | 1.009 |
Loadings over 0.5 are indicated with bold type.
Female PC
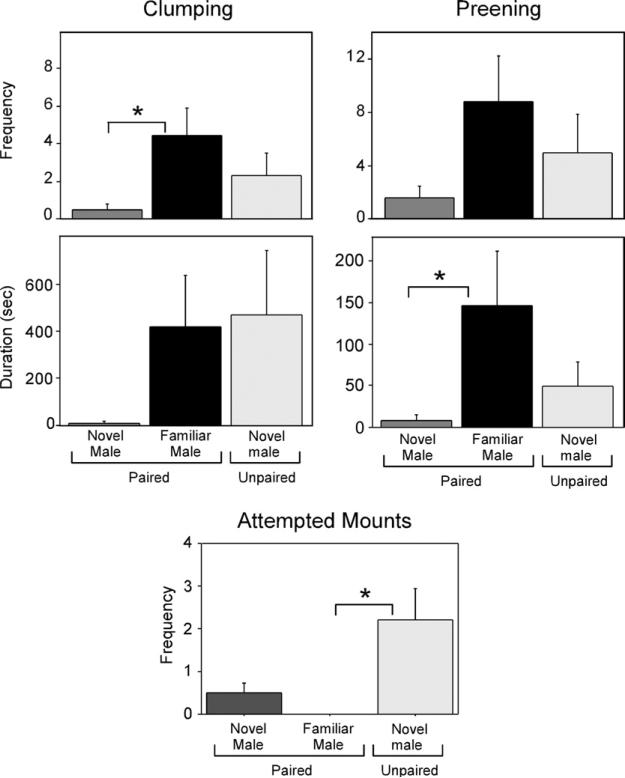
Among the individual behaviors with high loadings for the female PC, a significant effect of group was detected in the frequency of female-initiated clumping (Kruskal-Wallis: H=6.49, P=0.039) and the duration of females preening males (H=5.43, P=0.043). Both were higher in paired females interacting with their mates compared to novel males (Mann-Whitney U=12, P=0.012, Mann-Whitney U=11, P=0.015, respectively; Fig. 1). A trend also existed for paired females to spend more time preening their mates than unpaired females preened novel males (Mann-Whitney U=17, P=0.064). Finally, Kruskal-Wallis tests revealed trends for an effect of group on the duration of female-initiated clumping (H=4.74, P=0.057) and the frequency of females preening males (H=4.92, P=0.086; Fig. 1).
Fig. 1.
Female pairing behavior in the social interaction test. The two left panels depict the frequency (top) and duration (bottom) of clumping to the male. The right two panels depict the frequency (top) and duration (bottom) of preening the male. The bottom center panel depicts attempted mounts by the male. * Signifies P<0.05.
PC 2
From the analyses of behaviors with high loadings for the second PC, the only statistically significant effect detected was a difference among the groups on attempted mounts (H=5.429, P=0.027), such that novel males attempted to mount unpaired females more frequently than familiar males did their mates (Mann-Whitney U=14, P=0.016).
Neural responses
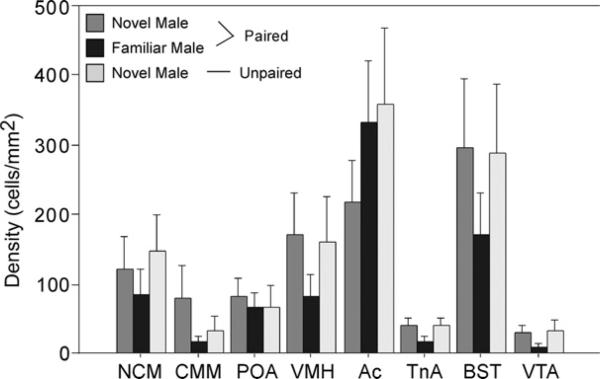
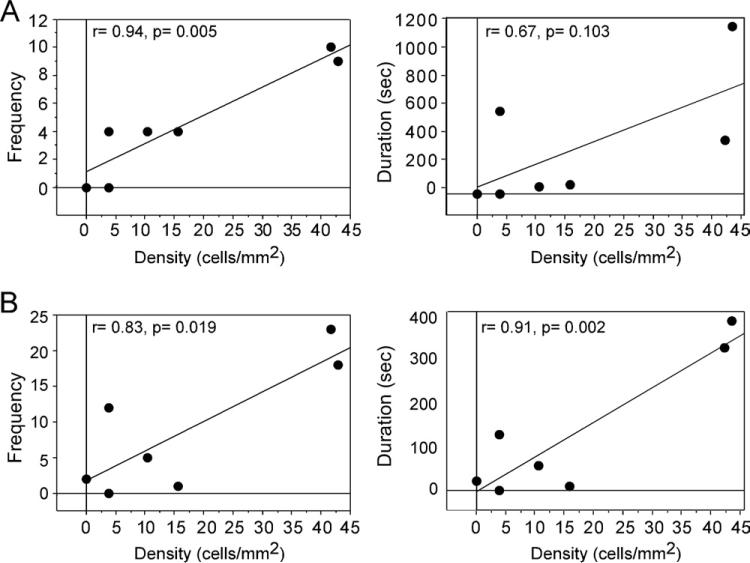
A main effect of brain region existed such that density of ZENK immunoreactive cells was higher in the nucleus accumbens and the bed nucleus of the stria terminalis than all of the other areas investigated (F=15.22, P<0.001, Fig. 2). No main effect of group or interaction between group and brain region was observed (F<0.73, P>0.700). Correlation analyses on the female behaviors that differed among the groups revealed associations in only the nucleus taeniae within only the group of paired females exposed to their mates. Positive correlations in this group existed in the nucleus taeniae between the density of ZENK immunoreactive nuclei and the frequency (r=0.94, P=0.005) of clumping, as well as the frequency (r=0.83, P=0.019) and duration (r=0.91, P=0.002) of preening (Figs. 3 and 4). The correlation between ZENK expression and duration of clumping was not statistically significant (r=0.67, P=0.103). No significant correlations were observed for these behaviors in any of the other groups (unpaired females or paired females interacting with novel males) or brain regions.
Fig. 2.
ZENK immunoreactivity across the brain regions investigated in the three groups of females (paired females exposed to novel males or their partner, and unpaired females exposed to novel males). A main effect of region was detected; the density of calls was highest in the Ac and BST. A main effect of group and interaction between group and region were not detected. Abbreviations: caudomedial nidopallium (NCM), caudomedial mesopallium (CMM), preoptic area (POA), ventromedial hypothalamus (VMH), nucleus accumbens (Ac), nucleus taeniae (TnA), bed nucleus of the stria terminalis (BST), ventral tegmental area (VTA).
Fig. 3.
Correlation analyses of the density of ZENK immunoreactive cells in the nucleus taeniae with female pairing behavior in the social interaction test. The figure includes only females interacting with their mates. (A) The frequency and duration of clumping to the male. (B) The frequency and duration of preening the male. Three of the four correlations are statistically significant.
Fig. 4.
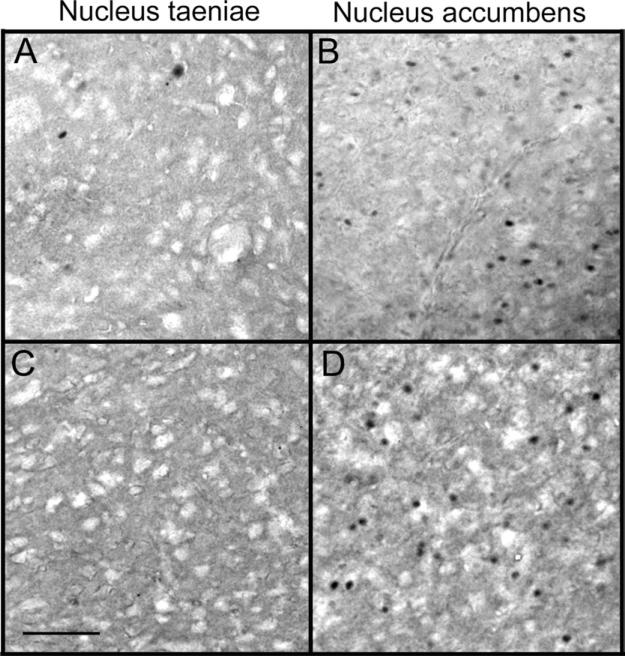
ZENK immunolabeling in the nucleus taeniae (A, C) in the nucleus accumbens (B, D) of females interacting with familiar males. The density of ZENK immunoreactivity was significantly higher in the nucleus accumbens than nucleus taeniae. Labeling within cells also appeared darker in the nucleus accumbens. ZENK immunoreactivity was more apparent in the caudal portion of nucleus taeniae (left side of photos), but was more homogenous in the nucleus accumbens. The top two panels (A, B) depict labeling from animals that displayed high levels of pairing behavior. The bottom two panels (C, D) depict labeling in animals that displayed low levels of pairing behaviors. Scale bar=50 μm.
DISCUSSION
In this study, we detected higher levels of behaviors indicative of a pair bond in females that interacted with their mates compared to novel males. While that is not particularly surprising, we were excited to observe specific correlations between those behaviors and ZENK immunoreactivity in the nucleus taeniae only in females interacting with their mates. These selective results suggest that the relationship between neural activity in nucleus taeniae and behavior differs among the groups, and that this particular brain region may play a role in maintaining pair bonds in zebra finches. Immunoreactivity was substantially higher in the bed nucleus of the stria terminalis and nucleus accumbens than nucleus taeniae, but the responses in those areas occurred with the introduction of any male stimuli, indicating that they were not specific to pair bonding; they might be important in more general responses to social interaction, however.
Behavior
The increased levels of female preening and clumping we observed in response to familiar males parallels the results from other studies examining pairing in zebra finches (Butterfield, 1970; Caryl, 1976; Silcox and Evans, 1982). Most of our data confirmed that females change their behaviors toward their partners after pairing, as occurs with male zebra finches (Caryl, 1976). We defined birds as partners if they presented clumping and/or preening behavior after an initial period of co-habitation. Other studies generally utilize one or both of these criteria when defining a bonded pair (Butterfield, 1970; Silcox and Evans, 1982; Clayton, 1990; Zann, 1996; Adkins-Regan and Wade, 2001; Adkins-Regan, 2002). However, if a more stringent method for classifying males and females as paired is used following the initial test (criteria involving a certain number of instances of proximity, clumping and preening, as in Ikebuchi and Okanoya, 2006), the sample sizes in the present study are reduced limiting statistical power, but the same pattern of results is detected. This fact suggests that the criteria commonly employed are appropriate for defining paired animals, and that the behavioral results we observed in the paired groups likely reflected the effects of a pair bond.
It is intriguing that the amount of clumping overall did not differ between unpaired and paired females in the final behavioral test. When the behaviors are more closely examined, the average latency for unpaired females to clump was two times greater than for the females interacting with their mates. In addition, the pattern throughout the course of the test differed. If the data from the hour-long test are examined in 10 min bins, it is clear that duration of clumping in females interacting with their mates is high in the first 20 min, but decreases over the next 40 min. In contrast, in unpaired females, the duration of clumping increases after 10 min and then remains consistent. The duration of preening was consistently higher in females interacting with their mates than that of unpaired females throughout the test period. Most pairing behaviors were initiated by females in all three groups, but males did display low levels of both clumping and preening. However, only males interacting with their mates clumped and preened for long durations.
All of these data lead to some interesting possibilities that need to be further explored. For example, the shorter latency compared to unpaired females and decrease in clumping over time in females interacting with their mates may indicate that initially the display of the behavior is important for re-establishment of the bond after a short absence, but is not required for continual maintenance. In contrast, previously unpaired females might be more gradually attempting to form new bonds when males are introduced. It is also plausible that the unpaired females displayed clumping behavior they might not otherwise simply due to a heightened need for social contact. This group was isolated 12 days longer prior to the test than the paired females. In addition, the fact that the pattern of clumping, but not preening, differs through the test seems to indicate that two behaviors may serve different functions. For example, clumping might be more important in the social aspects associated with re-establishing or forming a new bond, whereas preening may be vital to the maintenance of an established pair bond.
Pairing itself did not have a strong effect on a female's response to novel males, given that both clumping and preening were similar between paired and unpaired females interacting with novel males. This result corresponds with observations by Caryl (1976) that males courted novel females the same amount after pairing as they did before forming a bond. Finally, although clumping and preening are consistently used as indicators of pair bonding in this species (Butterfield, 1970; Silcox and Evans, 1982; Clayton, 1990; Zann, 1996; Adkins-Regan and Wade, 2001; Adkins-Regan, 2002), it is unknown whether additional levels or functions of these behaviors might exist.
Although the present experiment was designed to examine female behavior, we were also able to observe how manipulations of a female's pairing status affected male behavior. Females play a vital role in mate choice decisions in this species (Butterfield, 1970; Clayton, 1990; Zann, 1996), and in the present study they initiated 66% of the instances of clumping and 71% of the preening. This result is consistent with the idea that females control a large amount of the behavior associated with pair bonds. Male actions are likely influenced by signals from the females. This possibility is supported by the fact that unpaired males attempted more mounts with novel unpaired females than paired males did with their mates. Unpaired females may have provided different cues to the males. Of course, as the pairing status also differed between the males in these two groups, the fact that they had either formed a bond or had not been in contact with females recently could have contributed to the differential response.
Relationship between behavior and IEG expression in the nucleus taeniae
The relationship observed between clumping and preening and ZENK-immunoreactivity was highly specific. Correlations were observed only in one group, females interacting with their mates, and in only one brain region, the nucleus taeniae. This region is comparable to the mammalian amygdala in several ways. It contains some sensory inputs and sends projections to the hypothalamus and hippocampus (Cheng et al., 1999). It also expresses GAD 65, LAMP, and COUP-TF II like the mammalian amygdala (Yamamoto et al., 2005). Functionally, nucleus taeniae appears to mediate behaviors in birds similar to those initiated by the amygdala in mammals, such as social (Kollack-Walker and Newman, 1995; Numan and Sheehan, 1997; Cheng et al., 1999; Reiner et al., 2004) and reproductive behaviors (Pfaff et al., 1994; Thompson et al., 1998; Newman, 1999; Reiner et al., 2004). The mammalian amygdala has also been implicated in the mediation of interpretation of social cues, especially the reward and emotional significance of these cues (for review see Baxter and Murray, 2002; Phelps and LeDoux, 2005; Phelps, 2006; Murray, 2007).
In animals that form pair bonds, this region shows either increases (prairie vole; Curtis and Wang, 2003; Cushing et al., 2003) or decreases in activity (e.g. human; Bartels and Zeki, 2000, monkey; Bales et al., 2007) with regard to pairing. In the prairie vole, formation of a pair bond is correlated with an increase in Fos expression in the amygdala (Curtis and Wang, 2003), and lesions to the region decrease affiliative behaviors (Kirkpatrick et al., 1994). Neurochemical mediation of formation and maintenance of pair bonds differs, however. Formation involves dopamine D2 receptor binding (Wang et al., 1999; Gingrich et al., 2000), whereas bond maintenance involves altered dopamine D1 receptor density and binding in the nucleus accumbens (Aragona et al., 2006). It is possible that in the zebra finch, neurons within the nucleus taeniae are involved in the maintenance of a pair bond, whereas another system may be vital to the mediation of initial formation of a bond. It remains to be investigated whether similar relationships between neural activity in nucleus taeniae and behavior occur in other social situations.
Several potential explanations exist for the function of the relationship between bonding behaviors and ZENK induction in the nucleus taeniae of female zebra finches observed in the present study. First, this association between ZENK expression with clumping and preening may indicate that nucleus taeniae actively facilitates the production of these pairing behaviors or inhibits the presentation of other behaviors that are not related to bonding. The context of a specific behavior can affect IEG expression (as in singing behavior in starlings in breeding conditions vs. non-breeding conditions, i.e. Heimovics and Riters, 2005, 2006; Riters et al., 2004), so it is reasonable that this correlation was only detected when the female was interacting with her mate. However, it is also possible that the correlation might reflect sensory stimulation related to the behavior, perhaps affecting how sensory information is perceived when the female is in contact with an individual she remembers as her mate (see above references on mammals), or it might represent activity in cells that inhibit responses to less salient sensory stimuli. In either case, the increased neuronal response could serve to increase the focus of the female to the relevant cues and aid in maintaining the pair bond. These possibilities need to be explored further and could be partly elucidated by determining the neuronal subtypes of the responsive neurons within this brain region.
Neural response in other brain regions
Although no effects of our treatment groups were detected in any of the other brain regions, it is intriguing that we observed higher levels of ZENK immunoreactivity in the nucleus accumbens and bed nucleus of the stria terminalis compared to the other measured regions. There are at least two potential explanations for the higher level of immunoreactivity in these regions. First, it may have functional relevance, as females in all the groups interacted with a male during the behavior test. The relatively high neural activity in these brain regions may be associated with social interactions, whereas the nucleus taeniae might respond more specifically depending on whether or not a pair bond has been formed between the male and female that are interacting. Second, it is possible that these regions have a higher general level of activity than the other examined regions, resulting in greater baseline IEG expression. These ideas and the specific roles of the nucleus accumbens and bed nucleus of the stria terminalis in social situations warrant further investigation.
CONCLUSIONS
The present data provide information about both behavioral interactions and their neural mechanisms. Although pair bonding is generally identified and defined by the presence of specific behaviors (clumping and preening) in zebra finches, their functions appear to be more complex. Our results indicate that clumping and preening might serve different roles and can be displayed differentially. In addition, a region in the social behavior network (nucleus taeniae) was identified in which IEG expression is strongly correlated with the behaviors only in females interacting with their mates. Thus, the neural signal associated with the behaviors depends on the contexts in which they occur. Finally, the bed nucleus of the stria terminalis, a region in the social behavior network, and the nucleus accumbens, a region in the mesolimbic dopamine reward system, may be implicated in responses to female-male (or perhaps even other) social interactions in general. In contrast, the auditory perception regions investigated in this study appear to play little if any role in mediating the formation or maintenance of social bonds under the conditions tested here.
Acknowledgments
Research was supported through grants from the National Institutes of Health to Juli Wade (R01-MH55488 and K02-MH65907). We thank Michele Johnson and Deborah Kashy for advice and assistance with statistics
Abbreviations
- IEG
immediate early gene
- PBS
phosphate-buffered saline
- PBST
phosphate-buffered saline with 0.3% Triton X-100
- PC
principal components
REFERENCES
- Adkins-Regan E. Development of sexual partner preference in the zebra finch: a socially monogamous, pair-bonding animal. Arch Sex Behav. 2002;31:27–33. doi: 10.1023/a:1014023000117. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Ascenzi M. Social and sexual behaviour of male and female zebra finches treated with oestradiol during the nestling period. Anim Behav. 1987;35:1100–1112. [Google Scholar]
- Adkins-Regan E, Wade J. Masculinized sexual partner preference in female zebra finches with sex-reversed gonads. Horm Behav. 2001;39:22–28. doi: 10.1006/hbeh.2000.1627. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J Neurobiol. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. Differential expression of the immediate early genes FOS and ZENK following auditory stimulation in the juvenile male and female zebra finch. Mol Brain Res. 2003;116:147–154. doi: 10.1016/s0169-328x(03)00288-2. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. FOS and ZENK responses in 45-day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav Brain Res. 2005;162:108–115. doi: 10.1016/j.bbr.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield PA. The pair bond in the zebra finch. In: Crook JH, editor. Social behaviour in birds and mammals. Academic Press Inc; New York: 1970. pp. 249–278. [Google Scholar]
- Caryl PG. Sexual-behavior in zebra finch Taeniopygia-guttata—response to familiar and novel partners. Anim Behav. 1976;24:93–107. [Google Scholar]
- Cheng M, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain Behav Evol. 1999;53:243–270. doi: 10.1159/000006597. [DOI] [PubMed] [Google Scholar]
- Clayton NS. Mate choice and pair formation in Timor and Australian mainland zebra finches. Anim Behav. 1990;39:474–480. [Google Scholar]
- Curtis JT, Wang Z. Forebrain c-fos expression under conditions conducive to pair bonding in female prairie voles (Microtus ochrogaster) Physiol Behav. 2003;80:95–101. doi: 10.1016/s0031-9384(03)00226-9. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gibson MJ, Cheng MF. Neural mediation of estrogen-dependent courtship behavior in female ring doves. J Comp Physiol Psychol. 1979;93:855–867. [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav Neurosci. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50:726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi M, Okanoya K. Growth of pair bonding in zebra finches: physical and social factors. Ornithol Sci. 2006;5:65–75. [Google Scholar]
- Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav Neurosci. 1994;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64:275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Clark AE, Georgellis Y, Diener E. Reexamining adaptation and the set point model of happiness: reactions to changes in marital status. J Pers Soc Psychol. 2003;84:527–539. doi: 10.1037//0022-3514.84.3.527. [DOI] [PubMed] [Google Scholar]
- Maney DL, MacDougall-Shackleton EA, MacDougall-Shackleton SA, Ball GF, Hahn TP. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. J Comp Physiol A. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology. 1998;23:765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Foidart A, Wingfield JC, Ramenofsky M, Balthazart J. Effects of sexual interactions with a male on fos-like immunoreactivity in the female quail brain. J Neuroendocrinol. 1999;11:771–784. doi: 10.1046/j.1365-2826.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev. 1995;19:19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann N Y Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73:179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press, Ltd; New York: 1994. pp. 107–220. [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Sokal RR. Statistical tables. WH Freeman and Co; Stony Brook, NY: 1981. [Google Scholar]
- Silcox AP, Evans SM. Factors affecting the formation and maintenance of pair bonds in the zebra finch, Taeniopygia guttata. Anim Behav. 1982;30:1237–1243. [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Complementary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J Neurobiol. 2005;62:72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- Svec L, Wade J. Estradiol induces region-specific inhibition of ZENK but does not affect the behavioral preference for tutored song in adult female zebra finches. Behav Brain Res. doi: 10.1016/j.bbr.2008.12.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): a comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- Tomaszycki ML, Adkins-Regan E. Experimental alteration of male song quality and output affects female mate choice and pair bond formation in zebra finches. Anim Behav. 2005;70:785–794. [Google Scholar]
- Vignal C, Mathevon N, Mottin S. Mate recognition by female zebra finch: analysis of individuality in male call and first investigations on female decoding process. Behav Proc. 2008;77:191–198. doi: 10.1016/j.beproc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sun Z, Wang HB, Reiner A. Subpallial amygdala and nucleus taeniae in birds resemble extended amygdala and medial amygdala in mammals in their expression of markers of regional identity. Brain Res Bull. 2005;66:341–347. doi: 10.1016/j.brainresbull.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: A synthesis of field and laboratory studies. Oxford University Press; New York: 1996. [Google Scholar]