Abstract
With the recent resurgence of vector-borne diseases due to urbanization and development there is an urgent need to understand the dynamics of vector-borne diseases in rapidly changing urban environments. For example, many empirical studies have produced the disturbing finding that diseases continue to persist in modern city centers with zero or low rates of transmission. We develop spatial models of vector-borne disease dynamics on a network of patches to examine how the movement of humans in heterogeneous environments affects transmission. We show that the movement of humans between patches is sufficient to maintain disease persistence in patches with zero transmission. We construct two classes of models using different approaches: (i) Lagrangian models that mimic human commuting behavior and (ii) Eulerian models that mimic human migration. We determine the basic reproduction number R0 for both modeling approaches. We show that for both approaches that if the disease free equilibrium is stable (R0 < 1) then it is globally stable and if the disease free equilibrium is unstable (R0 > 1) then there exists a unique positive (endemic) equilibrium that is globally stable among positive solutions. Finally, we prove in general that Lagrangian and Eulerian modeling approaches are not equivalent. The modeling approaches presented provide a framework to explore spatial vector-borne disease dynamics and control in heterogeneous environments. As an example, we consider two patches in which the disease dies out in both patches when there is no movement between them. Numerical simulations demonstrate that the disease becomes endemic in both patches when humans move between the two patches.
Keywords: Vector-borne disease, human movement, discrete diffusion, basic reproduction number, disease free and endemic equilibria, stability
1 Introduction
Vector-borne diseases are a major public health problem (Gratz 1999). They include long-established scourges, such as malaria and dengue fever, as well as emerging and re-emerging diseases such as West Nile virus. The maintenance and resurgence of vector-borne diseases is related to ecological changes that favor increased vector densities or vector-host interactions, among other factors. There have been profound increases in the magnitude of vector-borne disease problems as the result of urbanization, deforestation, globalization, economic development, among other factors. Experts recognize urbanization as one of the most important drivers of global change, and predict that rapid increases in urban populations throughout the world will have major implications for human health in general and vector-borne diseases specifically (Sutherst 2004).
Travel and transport have also contributed to the spread of vector-borne diseases. There are reasons to believe that the spatial movement of humans may be important for the epidemiology of vector-borne diseases. One of the factors contributing to the reemergence of malaria is human migration (Martens and Hall 2000). Malaria remains surprisingly prevalent among residents of some urban areas where there are very few mosquitoes; however, many of those residents visit rural or periurban areas where the disease is much more prevalent, so those visits might make the persistence of malaria in the urban setting more likely. Empirical studies supporting the idea that travel outside urban areas is an important factor in maintaining malaria in urban areas where transmission is low are described by Osorio et al. (2004), Domarle et al. (2006), and Ronald et al. (2006). Ronald et al. (2006) also noted that lower socioeconomic status was correlated with increased risk of infection. The use of personal protection such as bednets may vary between locations or socio-economic classes; such an effect was explored using simple models by Kileen et al. (2003).
We use spatial models to examine how the movements of humans in heterogeneous environments affect the transmission of vector-borne diseases. Specifically, we study how diseases can be maintained in regions of low transmission by the movement of humans between regions of high and low transmission or the immigration of humans into regions of low transmission from regions of high transmission. Our study of this phenomenon is motivated by the specific case of malaria but may be relevant to other vector-borne diseases. Our analysis is based on spatial versions of the classical Ross-Macdonald model. A review of the derivation of Ross-Macdonald models is given by Smith and McKenzie (2004).
Although our goal is to understand spatial effects, our modeling approach could also be used to treat movement between different socio-economic classes or lifestyles. Because we want to consider the movement of humans we use the populations of infected humans and mosquitoes as state variables rather than the proportions of the human and mosquito populations that are infected. This is also how mosquito populations are treated by Smith et al. (2004), where mosquitoes are assumed to move but humans are not.
We model space as a network of patches and use two different sorts of descriptions of movement. One description identifies humans as resident in a given patch or belonging to a certain social group and assumes that they remain in that patch or group most of the time, but may visit other patches or groups often enough for pathogen transmission to occur there. In that case the infection rate for humans in a given class or location depends on the numbers of infectious vectors in other patches and the fraction of their time that individual humans spend in those patches but is not directly tied to an explicit description of human movement between classes or patches. This type of formulation has been used by Dye and Hasibeder (1986), Hasibeder and Dye (1988), Rodriguez and Torres-Sorando (2001), and Ruan et al. (2006). This approach is related to the Lagrangian approach in fluid dynamics because it in effect labels individuals (by patch or class) and tracks what happens to them. A type of movement we envision this modeling approach as describing is where people and/or vectors are commuting between locations (or changing their activities) on a regularly scheduled basis, so that there is a well defined fraction of time that any given individual spends in any given location or state of activity.
Another description assumes that pathogen transmission to humans in a given class or patch occurs only within that class or patch but there is mobility between classes or patches that can be explicitly described via something like discrete diffusion. This type of approach has been used by Allen et al. (2007), Arino and van den Driessche (2003), Arino et al. (2005), Dhirasakdanon et al. (2007), Hsieh et al. al. (2007), Liu et al. (2006), Salmani and van den Driessche (2006), Smith et al. (2004), Wang and Mulone (2003), and Wang and Zhao (2004). It is related to the Eulerian approach in fluid mechanics because it labels locations (or classes) and tracks what happens in them but does not distinguish individuals by residence, only by current location. We envision this modeling approach as describing migration from one location to another. Here discrete diffusion explicitly describes such movement and can result in changes in the total number of individuals in a given patch, at least until a population equilibrium is attained.
Sattenspiel and Dietz (1995) use a combined approach but do not consider vector-borne diseases. The models of Dye and Hasibeder (1986), Hasibeder and Dye (1988), Rodriguez and Torres-Sorando (2001), Smith et al. (2004), and Liu et al. (2006) describe various aspects of the transmission of vector-borne diseases in networks of patches or classes but are used to address specific questions that are different from those we consider here.
We would like to mention that the idea of using metapolulation models to describe spatial heterogeneities in disease transmission has been employed widely, see, for example, Bartlett, 1965; Lajmanovich and Yorke, 1976: Hethcote, 1978; Hethcote and Thieme, 1985; Rvachev and Longini, 1985; Dushoff and Levin, 1995; Sattenspiel and Dietz, 1995; Lloyd and May, 1996; Arino and van den Driessche, 2003. For more details and references on modeling infectious diseases in metapopulations, we refer to the survey articles of Wang (2007) and Arino (2009).
Another remark we would like to make is that, after the initial submission of our paper, the article of Auger et al. (2008) came to our attention. Auger et al. (2008) generalize the RossMacdonald malaria model to n patches and incorporated the fact that some patches can be vector free. They assume that the hosts can migrate between patches, but not the vectors. The susceptible and infectious individuals have the same dispersal rate. They compute the basic reproduction ratio and proved that if the basic reproduction ratio is less than or equal to the unity, then the disease-free equilibrium is globally asymptotically stable. When the basic reproduction ratio is greater than the unity, they prove that there exists a unique endemic equilibrium, which is globally asymptotically stable on the biological domain minus the disease-free equilibrium. Their model is similar to our second model, namely the patch model with migration using the Euler approach. While they assume that only hosts can migrate between patches, we consider both cases: (a) both hosts and vectors can migrate between patches, and (b) only hosts can migrate between patches.
2 Modeling Framework
2.1 A single-patch model
Within a single patch, we base our description of disease dynamics on the Ross-Macdonald type model of Smith and McKenzie (2004). Our notation is slightly different from theirs but our model is equivalent to theirs. The model assumes that human and mosquito populations are fixed but there is turnover in the mosquito population because of adult mortality. The state variables in the model are the proportions x(t) and y(t) of the human and mosquito populations respectively consisting of infectious individuals. The parameters in the model are as follows:
a– the human feeding rate of mosquitoes (number of bites on humans, per mosquito, per unit time),
b– the transmission efficiency from infected mosquitoes to humans,
c– the transmission efficiency from infected humans to mosquitoes,
μ– the mortality rate of mosquitoes,
r– the recovery rate of humans,
τ– the incubation period from the time a mosquito becomes infected until it becomes infectious,
M– the ratio of mosquitoes to humans.
In our notation the basic model is
| (2.1) |
A detailed derivation of the model and a discussion of how the parameters can be related to data and various indices such as the human blood index (HBI) and entomological inoculation rate (EIR) is given by Smith and MacKenzie (2004). The term e−μτ in the equation for the proportion of infectious mosquitoes arises because the rate of mosquito turnover due to adult mortality is typically high enough that a significant fraction of infected mosquitoes can be expected to die before they become infectious. Note that it is assumed that infected individuals become susceptible after they recovered from infection.
We need to rewrite (2.1) in terms of populations rather than fractions of populations for our derivation of spatial models. In parts of the derivation we want to consider the human and mosquito populations in each patch that can change due to the movement of humans or mosquitoes. Furthermore, we find it convenient to use the number of infected mosquitoes rather than the number of infectious mosquitoes as a state variable. To that end we introduce the following variables:
H– the total human population,
X– the number of infected humans,
V– the total mosquito population,
Y– the number of infected mosquitoes.
In a situation where H and V can vary, M will no longer be a constant parameter, but in any case M = V/H. In general, X = xH and e−μτ Y = yV. Using those relations we can rewrite (2.1) as
| (2.2) |
We use the formulation in (2.2) to build our spatial models. In those models we write parameters analogous to those appearing in (2.2) in condensed form, indexed by patch.
2.2 The spatial models
In our models we treat space as a network of connected patches. The patches (or nodes) typically represent different geographical locales such as rural areas, villages, or city districts, but the same modeling approach could be used to describe networks of different groups within a population (school children, factory workers, night watchmen, etc.) We examine models based on two different ways of describing the movement of humans and/or mosquitoes among the patches.
In the first type of model we label individuals as residents of a particular patch and describe their interactions with individuals from their own or other patches in terms of the rate of exposure to infection from residents of those patches. We assume that individuals do not move permanently from their patch of residence to another patch, but may visit other patches. The rate at which individuals become infected then depends upon the fraction of their time that they spend in each patch together with the transmission rates in those patches. We sometimes refer to this approach as Lagrangian in that it labels and in some sense tracks individual humans or mosquitoes. The Lagrangian approach has been used by Dye and Hasibeder (1986), Hasibeder and Dye (1988), Rodriguez and Torres-Sorando (2001), and Ruan et al. (2006).
In the second type of model we assume that humans and mosquitoes can migrate between patches and thus do not have a specified patch of residence. The rate at which individuals become infected depends only on the patch where they are located. We refer to this approach as Eulerian because we track what happens in a given location (patch) rather than what happens to labeled individuals. The Eulerian approach has been used by Allen et al. (2007), Arino and van den Driessche (2003), Arino et al. (2005), Dhirasakdanon et al. (2007), Hsieh et al. (2007), Liu et al. (2006), Salmani and van den Driessche (2006), Smith et al. (2004), Wang and Mulone (2003), and Wang and Zhao (2004). Models using a combination of these approaches have been used in Sattenspiel and Dietz (1995). Throughout our discussion we use the following notation:
N– the total number of patches in the network.
In reality it is plausible that humans may move longer distances than mosquitoes, so the patch networks for humans and mosquitoes might have different spatial scales. However, our models will incorporate coefficients describing the rate of movement between patches or the fraction of time an individual spends in patches other than his or her home patch. Those could be adjusted differently for humans and for mosquitoes. The coefficients of movement between distant patches could be taken to be small or zero for mosquitoes but large for humans. We will assume something of this sort in an important special case of the models that we will treat in section 3.2. Furthermore, since we are mainly interested in the effects of human movement, for our purpose the fine scale spatial structure of a mosquito metapopulation within a region that represents a single patch at the human scale can be aggregated over that patch. Hence, using the same patch networks for humans and mosquitoes is reasonable in the present context, although it might not be in others. It is worth noting that Smith et al. (2004) use models where mosquitoes move between patches but humans do not to study how spatial heterogeneity in mosquito populations can affect malaria transmission. We also assume that infection does not affect human movement greatly.
2.2.1 Lagrangian approach: Patch models with commuting
To formulate spatial models using the Lagrangian approach, we need to define transmission rates by averaging the rates across patches weighted by the fractions of their time that individuals spend in each patch. We denote those as follows:
pij– the fraction of time a human resident in patch i spends visiting patch j,
qij– the fraction of time a mosquito resident in patch i spends visiting patch j.
Note that
Let ai, bi, ci, μi, ri, τi, Hi, Vi denote the values of the parameters appearing in (2.2) in the case of the ith patch. Define
| (2.3) |
Our Lagrangian model then has the form
| (2.4) |
It is clear that the set {(X1,…, XN, Y1,…, YN): 0 ≤ Xi ≤ Hi, 0 ≤ Yi ≤ Vi, i = 1,…, N} is invariant for (2.4). We always assume that 0 ≤ Xi(0) ≤ Hi and 0 ≤ Yi(0) ≤ Vi for all i.
In some cases we may want to assume that the total vector populations in one or more of the patches are zero, so that the numbers of infected vectors in those patches are also zero (so there is no equation for the number of infected vectors in that patch) and thus some of the transmission terms in (2.4) are zero since some of the variables Yi are always zero. Such models can be cast in the form
| (2.4A) |
where N1 < N.
2.2.2 Eulerian approach: Patch models with migration
In deriving our Eulerian model we must address the issue that the total human and/or vector populations in a given patch might change sufficiently over time to affect the model. We start by formulating a model where those populations are viewed as dynamic variables, but then we make the assumption that those populations have come to the equilibrium predicted by the migration rates, at least relative to the time scale on which we want to study the system. That allows us to examine how vector-borne diseases might be propagated through populations that are distributed in space in situations where a migration pattern is relatively stable over time. It would be of interest to study transient effects, and even systems where migration rates can vary over time, but we do not do that in the present article.
To derive the Eulerian model we initially use Hi and Vi to denote human and vector populations on the ith patch, but we consider them as dynamic variables. We use Cij to denote the migration rate of humans from patch j to patch i and Dij to denote the corresponding rate for vectors:
Cij– the rate of human migration from patch j to patch i,
Dij– the rate of vector migration from patch j to patch i.
The movement model for migration then takes the form of a discrete diffusion:
| (2.5) |
Define
| (2.6) |
and
By summing up the equations for Hi in (2.5) we can see that , and similarly . Thus, H(t) = H(0) and V (t) = V (0). Also, (1,…, 1)((Cij)) = 0, so zero is an eigenvalue of ((Cij)), and similarly for ((Dij)). Under an additional assumption of irreducibility, zero can be seen to be principal eigenvalue of ((Cij)) and ((Dij)) by the Perron-Frobenius theorem (because it has a positive left eigenvector), so it is simple and any other eigenvalue has real part less than zero (see for example, Berman and Plemmons (1979) and Graham (1987)). Thus we have:
Lemma 1
Suppose that the matrix with off-diagonal entries Cij and diagonal entries equal to 0 is irreducible. If (H1(t),…, HN (t)) is a solution to the first system of equations in (2.5) with Hi(0) ≥0 for i = 1,…, N and Hi(0) > 0 for some i, then as t → ∞ for i = 1,…, N, where ( ) is the solution to
| (2.7) |
(In other words, is the right eigenvector of ((Cij)) corresponding to the eigenvalue 0 normalized so that its components sum to H(0).) Similarly, suppose that the matrix with off-diagonal entries Dij and diagonal entries equal to 0 is irreducible. If (V1(t),…, VN (t)) is a solution to the second system of equations in (2.5) with Vi(0) ≥ 0 for i = 1,…, N and Vi(0) > 0 for some i, then as t → ∞ for i = 1,…, N, where ( ) is the solution to
| (2.8) |
Proof
See Appendix.
In formulating our Eulerian model we assume that the migration process has reached a steady state, so that there may be exchange of individuals between patches but there is no net change in the total human or vector population in each patch. Thus, we assume that and with and are as in Lemma 1 for i = 1,…, N. We assume that disease transmission occurs only between individuals that are in the same patch at the same time. Let
| (2.9) |
Our Eulerian model with infected individuals present would take the form
| (2.10) |
It is clear from (2.7) and (2.8) that the set {(X1,…, XN, Y1,…, YN): , i = 1,…, N} is invariant for (2.10). We always assume that and for all i.
To address the issue of how diseases can be maintained in regions of low transmission by the movement of humans between regions of high and low transmission, we again want to consider cases where there are no vectors and thus no transmission in certain patches. Then (2.10) becomes
| (2.10A) |
where again as in (2.4A) we have N1 < N.
2.2.3 Relationship between Langrangian and Eulerian models
It is natural to ask whether it is possible to translate models between the forms (2.4) and (2.10). Suppose we denote the number of infected human residents of patch i in (2.4) as Xi, that is, let the variables Xi correspond to the state variables for humans in (2.4). Denote the number of infected humans currently located in patch i as X̂i, that is, let the variables X̂i correspond to the state variables for humans in (2.10). Similarly, denote the number of infected vector residents of patch i as Yi and the number of infected vectors currently located in patch i as Ŷi. Since the infected humans currently in patch i could be from any patch, but human residents of patch j spend a fraction pji of their time in patch i, and similarly for vectors with pji replaced by qji, we should have
Clearly we generally cannot solve this system unless the matrices ((pji)) and ((qij)) are invertible, but that need not be the case under the assumptions of our models. In cases where the matrices are invertible, the system resulting from translating the model (2.4) into a model with state variables Xi, Yi into a system in terms of X̂i, Ŷi is generally not of the form (2.10). Except in special cases where the amount of time individuals spend in patches other than their patch of residence is small, it is not even approximately of the form (2.10). Thus, the two modeling formulations are not equivalent, although in some cases they might both be reasonable as approximate descriptions of a given system. Hence, we want to analyze both types of models.
3 Analysis and Application of the Models
3.1 General properties
The models (2.4) and (2.10) are cooperative systems on the invariant sets {(X1,…, XN, Y1,…, YN): 0 ≤ Xi ≤ Hi, 0 ≤ Yi ≤ Vi, i = 1,…, N} and {(X1,…, XN, Y1,…, YN): , i = 1,…, N} respectively, so they generate flows that are order preserving on those sets; see for example Smith (1995). Since the models are epidemiological in character it is sensible to describe the stability or instability of the disease free equilibrium Xi = Yi = 0, i = 1,…, N in terms of a basic reproduction number R0. That number can be computed by the methods of van den Driessche and Watmough (2002). (Since the models describe vector-borne diseases that require the two-step process of a human transmitting the disease to a vector and the vector transmitting the disease to another human to achieve transmission from one human to another, some authors would consider the basic reproduction number for such models to be if R0 were the value computed as in van den Driessche and Watmough (2002). We use that convention here. In the case of (2.4), a formula for R0 and a description of the dynamics of the model were already obtained by Hasibeder and Dye (1988), partly on the basis of results of Lajmanovich and Yorke (1976). We consider that case first. Throughout our discussion we use ρ(M) to denote the spectral radius of the matrix M. In some cases, for example if M is primitive, ρ(M) is the principal eigenvalue of M.
Theorem 1 (Hasibeder and Dye 1988)
Let  = ((AijHi/μj)), ℬ = ((BijVi/rj)), where the entries in
= ((AijHi/μj)), ℬ = ((BijVi/rj)), where the entries in  and ℬ are taken from (2.4). Assume that the matrices
and ℬ are taken from (2.4). Assume that the matrices 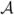 , ℬ are irreducible. Then for (2.4) we may take
. If R0 < 1 then the disease-free equilibrium in (2.4) is stable while if R0 > 1 it is unstable. If the disease-free equilibrium in (2.4) is stable then there is no positive equilibrium and the disease-free equilibrium is globally stable among nonnegative solutions. If the disease-free equilibrium is unstable there is a unique positive equilibrium which is globally stable among positive solutions.
, ℬ are irreducible. Then for (2.4) we may take
. If R0 < 1 then the disease-free equilibrium in (2.4) is stable while if R0 > 1 it is unstable. If the disease-free equilibrium in (2.4) is stable then there is no positive equilibrium and the disease-free equilibrium is globally stable among nonnegative solutions. If the disease-free equilibrium is unstable there is a unique positive equilibrium which is globally stable among positive solutions.
It follows from the theory of monotone dynamical systems that in the case of Theorem 1 where the disease-free equilibrium is unstable there is a monotone trajectory connecting the disease-free equilibrium to the positive equilibrium; see Smith (1995). Furthermore, (H1,…, HN, V1,…, VN) is a super-solution to the equilibrium problem for (2.4) so a solution of (2.4) with that initial data will decrease toward an equilibrium. Thus, when it exists, the positive equilibrium is globally stable in the set {(X1,…, XN, Y1,…, YN): 0 ≤ Xi ≤ Hi, 0 ≤ Yi ≤ Vi, i = 1,…, N}. It follows from the structure of 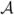 and ℬ that if one of the parameters Aij, Bij, Hi, or Vi is increased then R0 will increase but if ri or μi is increased then R0 will decrease. This is sensible biologically since increasing transmission rates or the initial number of susceptible individuals typically increase R0 while increasing recovery or mortality rates typically decrease it.
and ℬ that if one of the parameters Aij, Bij, Hi, or Vi is increased then R0 will increase but if ri or μi is increased then R0 will decrease. This is sensible biologically since increasing transmission rates or the initial number of susceptible individuals typically increase R0 while increasing recovery or mortality rates typically decrease it.
Theorem 2
Consider the system (2.10) restricted to the invariant region {(X1,…, XN, Y1,…, YN):
, i = 1,…, N}. Let C = ((Cij)) and D = ((Dij)). Let
, 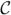 * = ((Cij − riδij)), and
* = ((Cij − riδij)), and 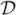 * = ((Dij − μiδij)), where δij is the Kronecker delta. Assume that the matrices C and D are irreducible. Then for (2.10) we may take
. If R0 < 1 then the disease-free equilibrium in (2.10) is stable while if R0 > 1 it is unstable. If the disease-free equilibrium in (2.10) is stable then there is no positive equilibrium and the disease-free equilibrium is globally stable among nonnegative solutions. If the disease-free equilibrium is unstable there is a unique positive equilibrium which is globally stable among positive solutions.
* = ((Dij − μiδij)), where δij is the Kronecker delta. Assume that the matrices C and D are irreducible. Then for (2.10) we may take
. If R0 < 1 then the disease-free equilibrium in (2.10) is stable while if R0 > 1 it is unstable. If the disease-free equilibrium in (2.10) is stable then there is no positive equilibrium and the disease-free equilibrium is globally stable among nonnegative solutions. If the disease-free equilibrium is unstable there is a unique positive equilibrium which is globally stable among positive solutions.
Proof
See Appendix.
The proof for Theorem 2 could be adapted to give an alternate proof of Theorem 1. A related result giving a similar formula for R0 in a discrete-diffusion type model for a disease with direct transmission in a patchy environment was obtained by Dhirasakdanon et al. (2007). The proof of Theorem 2 shows that the matrix 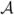 *
*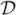 *−1ℬ*
*−1ℬ*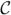 *−1 is nonnegative. Increasing the transmission rates and populations Ai, Bi,
or
will increase some of its entries and thus R0 will be monotone increasing in those parameters. In the proof of Theorem 2 it is also shown that the matrices −
*−1 is nonnegative. Increasing the transmission rates and populations Ai, Bi,
or
will increase some of its entries and thus R0 will be monotone increasing in those parameters. In the proof of Theorem 2 it is also shown that the matrices −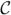 * = −C +((riδij)) and −
* = −C +((riδij)) and −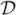 * = −D +((μiδij)) are nonsingular M-matrices. It follows that they are invertible with nonnegative inverses (see Berman and Plemmons 1979). To see how their entries depend on ri and μi, suppose that Ri > 0 for i = 1,…, N and observe that
* = −D +((μiδij)) are nonsingular M-matrices. It follows that they are invertible with nonnegative inverses (see Berman and Plemmons 1979). To see how their entries depend on ri and μi, suppose that Ri > 0 for i = 1,…, N and observe that
Hence, if ri ≥ Ri for all i then [−C + ((Riδij))]−1 − [−C + ((riδij))]−1 is nonnegative. Thus, the entries in 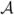 *
*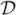 *−1ℬ*
*−1ℬ*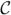 *−1 =
*−1 = 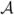 *(−
*(−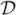 *−1)ℬ*(−
*−1)ℬ*(−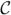 *−1) are monotone decreasing with respect to the recovery rates ri. Similarly, they are also monotone decreasing with respect to the mortality rates μi. It follows that ρ(
*−1) are monotone decreasing with respect to the recovery rates ri. Similarly, they are also monotone decreasing with respect to the mortality rates μi. It follows that ρ(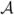 *
*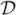 *−1ℬ*
*−1ℬ*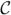 *−1) and hence R0 are monotone decreasing in those parameters. The dependence on the movement rates Cij, Dij is more subtle in general but sometimes can be determined in particular cases. We will return to that point later.
*−1) and hence R0 are monotone decreasing in those parameters. The dependence on the movement rates Cij, Dij is more subtle in general but sometimes can be determined in particular cases. We will return to that point later.
The analysis used to prove Theorems 1 and 2 also applies to models such as (2.4A) and (2.10A) where vectors are present only in some patches and the equations for the infected vectors in the patches where vectors are absent are dropped from the model. In such cases the dimensions of the matrices 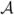 or
or 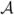 * are different from those of ℬ or ℬ* so the short formulations for R0 given in those theorems cannot be used; however, we can still compute R0 as the spectral radius of an appropriate matrix by using the methods of van den Driessche and Watmough (2002), or perhaps directly, and the arguments for the existence and uniqueness, or nonexistence, of a positive equilibrium are unchanged. In particular, for (2.4A) we can define the matrices
* are different from those of ℬ or ℬ* so the short formulations for R0 given in those theorems cannot be used; however, we can still compute R0 as the spectral radius of an appropriate matrix by using the methods of van den Driessche and Watmough (2002), or perhaps directly, and the arguments for the existence and uniqueness, or nonexistence, of a positive equilibrium are unchanged. In particular, for (2.4A) we can define the matrices 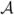 and ℬ as in Theorem 1, except that
and ℬ as in Theorem 1, except that 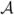 is N × N1 and ℬ is N1 × N; then the results of van den Driessche and Watmough (2002) imply that
is N × N1 and ℬ is N1 × N; then the results of van den Driessche and Watmough (2002) imply that
| (3.1) |
For (2.10A) we can define the entries in 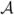 *, ℬ*,
*, ℬ*, 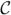 *, and
*, and 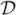 * as before, but with
* as before, but with 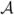 *, ℬ*, and
*, ℬ*, and 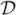 * now being N1 × N1 matrices. Define the N × N matrix
* now being N1 × N1 matrices. Define the N × N matrix 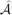 * by
* by
| (3.2) |
We can then compute R0 by the methods of van den Driessche and Watmough (2002) as
| (3.3) |
3.2 Two-patch models with no transmission in one patch
It is known (Carter et al., 2000) that malaria transmission is strongly associated with location in two main features. First, the disease is focused around specific mosquito breeding sites and can normally be transmitted only within certain distances from them: in Africa these are typically between a few hundred metres and a kilometre and rarely exceed 2–3 kilometres. Second, there is a marked clustering of persons with malaria parasites and clinical symptoms at particular sites, usually households. To understand how movement between patches might sustain infection in patches with no transmission we study models with two patches but with transmission only in one patch. We denote the patch with no transmission as patch number 2. We assume that there is no movement of vectors between patches, so that there are no infected vectors in patch number 2, that is, Y2 = 0. Since Y2 = 0 we omit the equation for Y2 from the models.
3.2.1 The Langrangian model
The first such model we consider has the form (2.4A) with N = 2 and N1 = 1, that is
| (3.4) |
Computing R0 by the method of van den Driessche and Watmough (2002) as described in the previous subsection yields
| (3.5) |
The first term on the right in (3.5) is the value of that would result if patch number 1 were isolated. Note that it is possible to have that value less than 1, so that the disease would not persist in patch number 1 in the absence of patch number 2, but still have in (3.5). If R0 > 1 in (3.5) then (3.4) has a unique positive equilibrium ( ) that is globally stable among positive solutions.
Suppose that R0 > 1 in (3.5). The components and satisfy
| (3.6) |
The component satisfies
| (3.7) |
It is possible to compute explicitly by solving (3.7), but that yields a quadratic equation with coefficients depending on the parameters of the model in a complicated way, so the result is not very illuminating. For our purposes we can obtain reasonably satisfactory results by making some simple observations and estimates.
If A11B11H1V1/r1μ1 > 1 so that the disease could persist in patch 1 if that patch were isolated, then it follows from the form of (3.7) that where is the equilibrium that would result if patch number 1 were isolated (equivalently if the second term on the left were dropped from (3.7)). We would then have
| (3.8) |
which yields a lower bound on in (3.6). However, our primary interest is in comparing and .
Suppose that p11/p21 ≥ r1/r2. (Recall that pij denotes the fraction of his or her time that a human resident of patch i spends in patch j, so if r1 = r2 this assumption would mean that residents of patch 1 spend a larger fraction of their time in patch 1 than do residents of patch 2, which is reasonable.) By (2.3) we then have . In that case it follows from (2.3) and (3.6) that
| (3.9) |
If the human populations and recovery rates are equal in the two patches then the last expression in (3.7) reduces to the ratio of the fractions of time spent in patch 1 by residents of patch 2 and patch 1 respectively. In any case, the model predicts that disease can indeed be maintained in patch 2 without transmission there, at a level that is proportional to the fraction of their time that residents of patch 2 spend in patch 1 relative to residents of patch 1.
3.2.2 The Eulerian model
Next we consider the case of models of the form (2.10A), again with transmission only in patch 1, and no movement of mosquitoes between patches, so that we do not include an equation for infected vectors in patch 2. This leads to models of the form
| (3.10) |
In this case R0 is given by
| (3.11) |
with coefficients as in (2.7)–(2.9). Note that where H(0) is the total initial human population in the two patches, so that if C21 is sufficiently large we have R0 < 1 in (3.11). Recall that the parameter C21 represents the rate of migration from the patch with transmission to the patch without transmission. Thus, a sufficiently high rate of migration from the patch with transmission into the patch without it can cause the disease to be eliminated. A similar observation was made by Hsieh et al. (2007) for diseases that are directly transmitted between humans.
For R0 > 1 in (3.11) the equilibrium ( ) of (3.10) satisfies
| (3.12) |
where
It is clear from the first equation in (3.12) that if the rates of migration as reflected by the size of the coefficients C12 and C21 are comparable to the recovery rate in patch 2 then disease can be sustained in patch 2 even though there is no transmission in that patch.
4 Numerical Simulations
To carry out numerical simulations, we consider the Langrangian model with two patches such as two villages. For simplicity, we use xi(t) and yi(t) to denote the fractions of infectious host and vector populations in Patch i(i = 1, 2). Assume that there is no vector movement between these two patches (i.e. b12 = b21 = 0), only humans can move between these two patches.
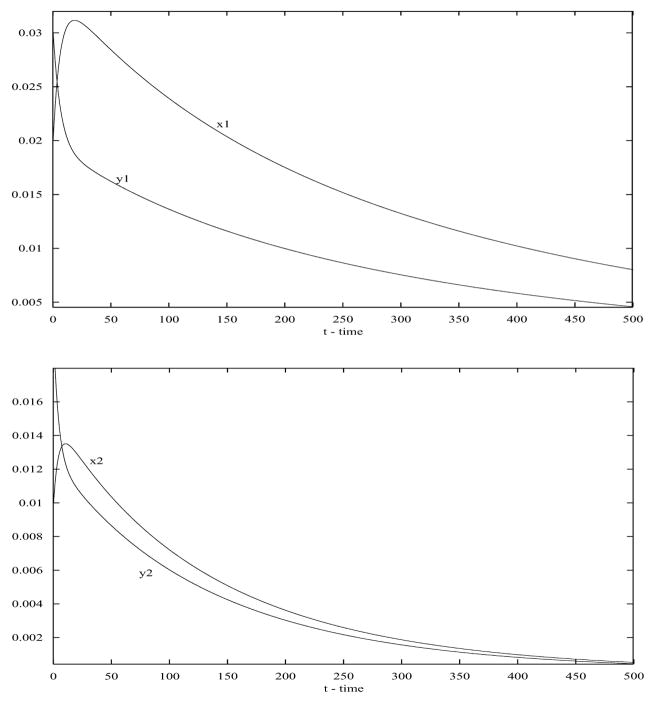
First, consider the case when there is no human movement between these two patches; that is, these two patches are isolated (a12 = a21 = 0). Choose parameters as follows: a11 = 0.12, r1 = 007., b11 = 0.05, μ1 = 0.09, a22 = 0.1, r2 = 0.09, b22 = 0.12, μ2 = 0.15. We can see the the basic reproduction numbers in Patches 1 and 2 are R1,0 = 09524 < 1 and R2,0 = 0.8888 < 1, respectively. So the disease dies out in both patches (see Figure 1).
Figure 1.
When there is no movement between the two patches, the disease dies out in both patches (top: Patch 1; bottom: Patch 2). Here a12 = a21 = 0.
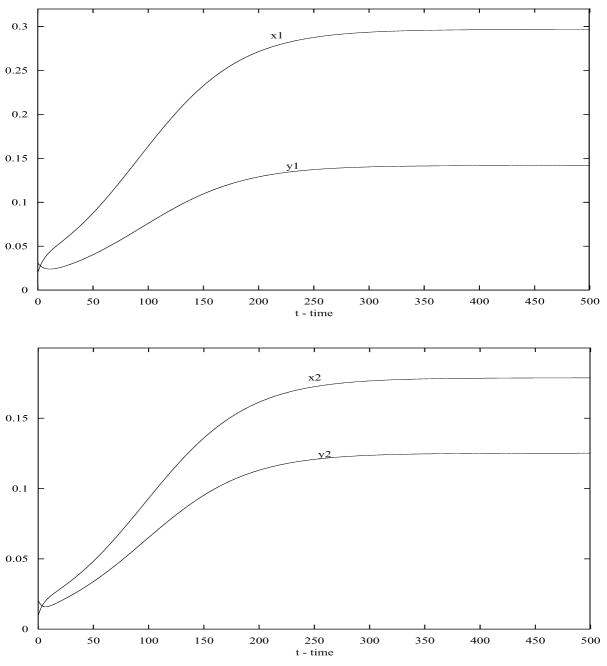
Now we want to see how the disease becomes endemic in both patches when humans move between these two patches. Notice that aij relates to the fraction of time a human resident in Patch i spends visiting Patch j, during that time he can be infected in Patch j and becomes infectious once he returns to Patch i. We can see that the disease becomes endemic in both patches (see Figure 2) when humans move back and forth between these two patches.
Figure 2.
When humans move between the two patches, the disease becomes endemic in both patches (top: Patch 1; bottom: Patch 2). Here a12 = 0.1, a21 = 0.05.
5 Conclusions
The models in (2.4), (2.4A), (2.10), (2.10A) describe vector borne disease systems on networks of patches. Those patches may reflect physical locations, socio-economic-behavioral classes, or other features that distinguish subpopulations of people or vectors. The models include terms describing the movement of humans and vectors between patches. The models can be parameterized in terms of coefficients that have clear biological interpretations and which in principle could be measured. The mathematical analysis shows that the models are cooperative systems with simple dynamics. They predict that either the disease will disappear or that it will become established at a unique stable equilibrium, depending on the parameters. Which of these two possibilities will actually occur will depend on the basic reproduction number R0, which is well defined for the models. The value of R0 for any of the models can be characterized as the spectral radius of an associated matrix and can be explicitly calculated in simple cases. Finally, since the modeling framework presented here is based on systems of coupled ordinary differential equations it may be easily expanded to explore optimal disease control in spatial environment using well established additional mathematically techniques.
Analysis of models with two patches but with pathogen transmission only in one patch shows that if there is sufficient movement of humans between patches the disease can be sustained in the patch with no transmission. This suggests that a possible explanation for observations that vector borne diseases persist in some patches where mosquito densities and hence disease transmission rates are very low is that there is either immigration of humans from patches with higher transmission or that humans residing in patches with low transmission commute to patches with high transmission. The strength of those effects depends on the rate of migration or the fraction of time spent by commuters in patches with high transmission rates.
Acknowledgments
We would like to thank the referee for his/her helpful comments.
Appendix
Proof of Lemma 1
Choose c0 > max{−Cii: i = 1 … N}. The matrix ((Cij)) + c0I is irreducible with positive diagonal elements, so it is primitive (see Graham 1987, pp. 137–138) and hence the Perron-Frobenius theorem applies to it. It follows that ((Cij))+ c0I has a principal eigenvalue characterized by having a positive eigen-vector, and all other eigenvalues have real parts smaller than that principal eigenvalue. By the definition of the entries Cii, the vector (1, …, 1) is a left eigenvector of ((Cij)) + c0I corresponding to the eigenvalue c0, so c0 must be the principal eigenvalue of ((Cij)) + c0I. Since every eigenvalue of ((Cij)) is equal to λ − c0, where λ is an eigenvalue of ((Cij)) + c0I, it follows that 0 is an eigenvalue of ((Cij)) with positive left and right eigenvectors and that all other eigenvalues of ((Cij))must have real parts less than zero. Any nonnegative nontrivial initial data (H1(0), …, HN (0)) has a positive component in the direction of the right eigenvector ( ) corresponding to the eigenvalue 0 of ((Cij)). Since all other eigenvalues of ((Cij)) have negative real parts and , the conclusion of the lemma follows for (H1, …, HN). The proof for (V1, …, VN) is the same.
Proof of Theorem 2
The proof will make use of results and ideas from van den Driessche and Watmough (2002) as well as some other results on matrices and monotone dynamical systems. We will briefly review the key ideas from van den Driessche and Watmough (2002) as they apply in this context. The models treated by van den Driessche and Watmough (2002) are formulated as
| (A.1) |
where x = (x1, …, xn), ℱi is the rate at which new infections occur in compartment i and −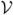 i is the rate of movement of individuals into or out of that compartment by other means. The rate
i is the rate of movement of individuals into or out of that compartment by other means. The rate 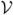 i is broken down further as
where
are rates of individuals entering and leaving compartment i respectively. The linearizations of ℱ and
i is broken down further as
where
are rates of individuals entering and leaving compartment i respectively. The linearizations of ℱ and 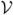 at the disease free equilibrium are denoted by F and V respectively. In our situation, n = 2N and each compartment describes the number of infected humans or vectors on one of the N patches. All compartments contain only infected individuals. We have x = (X1, …, XN, Y1, …YN). Then
for i = 1, …, N and
for i = N + 1, …, 2N;
for i = 1, …, N and
for i = N + 1, …, 2N; and
for i = 1…N and
for i = N + 1, …, 2N.
at the disease free equilibrium are denoted by F and V respectively. In our situation, n = 2N and each compartment describes the number of infected humans or vectors on one of the N patches. All compartments contain only infected individuals. We have x = (X1, …, XN, Y1, …YN). Then
for i = 1, …, N and
for i = N + 1, …, 2N;
for i = 1, …, N and
for i = N + 1, …, 2N; and
for i = 1…N and
for i = N + 1, …, 2N.
The disease-free equilibrium in our models is (0, …, 0). The hypotheses A1–A4 of van den Driessche and Watmough (2002) can be readily verified, at least for (X, Y) in the invariant region {(X1, …, XN, Y1, …, YN):
, i = 1, …, N}, from the forms of ℱ and 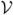 . The key hypothesis (A5) of van den Driessche and Watmough (2002) is that if ℱ is set to zero then all the eigenvalues of the Jacobian of what remains in f (x) evaluated at the disease-free equilibrium have negative real parts. In our case the eigenvalues in question are those of −V. The matrix V consists of two N × N blocks on the diagonal and zeroes elsewhere. The blocks are ((Cij − riδij)) and ((Dij − μiδij)) where δij is the Kronecker delta. Let C = ((Cij)). It follows as in the proof of Lemma 1 that C − ((riδij)) has an eigenvalue σ0 that is real, characterized by having a positive eigenvector φ→, and is larger than the real part of any other eigenvalue of C − ((riδij)). Let r0 = min{ri: i = 1, …, N}. We have ([C − ((riδij)] φ→)i = σ0φi so that (Cφ→)i = (ri + σ0) φ→, so componentwise Cφ→≥ (r0 + σ0) φ→. It follows from Lemma 2 of Cantrell et al. (2007) that C has a real eigenvalue greater than or equal to r0 + σ0 with non-negative nonzero eigenvector. If r0 + σ0 > 0 that would contradict the fact that 0 is the eigenvalue of C with largest real part, as established in the proof of Lemma 1. It follows that we must have σ0 ≤ −r0 < 0 so the eigenvalues of C − ((riδij)) must all have negative real parts, as required. (It then follows from Berman and Plemmons (1979, p.135, G20) that −C + ((riδij)) is a nonsingular M-matrix.)
. The key hypothesis (A5) of van den Driessche and Watmough (2002) is that if ℱ is set to zero then all the eigenvalues of the Jacobian of what remains in f (x) evaluated at the disease-free equilibrium have negative real parts. In our case the eigenvalues in question are those of −V. The matrix V consists of two N × N blocks on the diagonal and zeroes elsewhere. The blocks are ((Cij − riδij)) and ((Dij − μiδij)) where δij is the Kronecker delta. Let C = ((Cij)). It follows as in the proof of Lemma 1 that C − ((riδij)) has an eigenvalue σ0 that is real, characterized by having a positive eigenvector φ→, and is larger than the real part of any other eigenvalue of C − ((riδij)). Let r0 = min{ri: i = 1, …, N}. We have ([C − ((riδij)] φ→)i = σ0φi so that (Cφ→)i = (ri + σ0) φ→, so componentwise Cφ→≥ (r0 + σ0) φ→. It follows from Lemma 2 of Cantrell et al. (2007) that C has a real eigenvalue greater than or equal to r0 + σ0 with non-negative nonzero eigenvector. If r0 + σ0 > 0 that would contradict the fact that 0 is the eigenvalue of C with largest real part, as established in the proof of Lemma 1. It follows that we must have σ0 ≤ −r0 < 0 so the eigenvalues of C − ((riδij)) must all have negative real parts, as required. (It then follows from Berman and Plemmons (1979, p.135, G20) that −C + ((riδij)) is a nonsingular M-matrix.)
A similar analysis yields the corresponding conclusion for −D + ((μiδij)). Thus, Lemma 1 and Theorem 2 of van den Driessche and Watmough (2002) apply to our model (2.10). In particular, V is a nonsingular M-matrix, and the basic reproduction number is the spectral radius of FV −1, that is, R0 = ρ(FV −1). Using
, 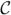 * = ((Cij − riδij)), and
* = ((Cij − riδij)), and 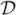 * = ((Dij − μiδij)), we have that
* = ((Dij − μiδij)), we have that
| (A.2) |
and
| (A.3) |
It follows that
| (A.4) |
To obtain a formulation analogous to that given by Hasibeder and Dye (1988) and quoted in Theorem 1, observe that
| (A.5) |
so that .
If R0 > 1 then the disease-free equilibrium is unstable. The Jacobian of linearization of the model (2.10) around the disease-free equilibrium is J = F − V. Again, the proof of Lemma 1 implies that F − V has a principal eigenvalue σ0 that is real, larger than the real part of any other eigenvalue, and which has a positive eigenvector. In the case where (0, …,0) is unstable, we have σ0 > 0. It is easy to see in that case that if ψ→ is a positive eigenvector for σ0 then for the model (2.10) written in the notation of (A.1) we have fi(εψ→) > 0 for all i as long as ε > 0 is sufficiently small. It then follows by the order preserving property of (2.10) that a solution to (2.10) with initial data εψ→ will increase componentwise toward an equilibrium of (2.10) that is the minimal positive equilibrium of (2.10) in the invariant set {(X1, …, XN, Y1, …, YN): , i = 1, …, N}. (See Cantrell and Cosner (2003, section 3.6) for further discussion and references.) Similarly, if we let we have fi(ξ→)< 0 for all i, so that the solution to (2.10) with initial data ξ→ will decrease componentwise toward an equilibrium (X**, Y**) that is the maximal equilibrium of (2.10) in the invariant set {(X1, …, XN, Y1, …, YN): , i = 1, …, N}.
The equilibrium (X*, Y*) (and any other positive equilibrium) must satisfy
| (A.6) |
In the invariant region for (2.10) the off-diagonal terms in the matrix in (A.6) are nonnegative, and the matrices 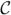 *,
*, 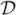 * are irreducible, so again as in the proof of Lemma 1 the matrix in (A.6) has a principal eigenvalue that is characterized by having a positive eigenvector. In this case (X*, Y*)T is the eigenvector and the eigenvalue is 0. For any other positive equilibrium (X***, Y***) the relation analogous to (A.6) with (X*, Y*) replaced by (X***, Y***) would necessarily hold, implying that the matrix
* are irreducible, so again as in the proof of Lemma 1 the matrix in (A.6) has a principal eigenvalue that is characterized by having a positive eigenvector. In this case (X*, Y*)T is the eigenvector and the eigenvalue is 0. For any other positive equilibrium (X***, Y***) the relation analogous to (A.6) with (X*, Y*) replaced by (X***, Y***) would necessarily hold, implying that the matrix
| (A.7) |
would also have principal eigenvalue 0. However, unless (X*, Y*) = (X***, Y***) that is impossible because the principal eigenvalue is increasing relative to the entries of the matrix. Hence the minimal equilibrium (X*, Y*) must be the unique equilibrium. (This proof is entirely analogous to that of the corresponding result in continuous space as in Cantrell and Cosner (2003, Proposition 3.3). In particular, the minimal and maximal equilibria must be the same, so that the unique positive equilibrium is globally stable for solutions of (2.10) with positive initial data in the invariant set {(X1, …, XN, Y1, …, YN): , i = 1, …, N}.
If R0 < 1 then the disease-free equilibrium is stable and the principal eigenvalue σ0 of the Jacobian of linearization of the model (2.10) around the disease-free equilibrium is negative. It follows that since the entries of the matrix in (A.7) at any positive equilibrium (X***, Y***) are less than or equal to those of the linearization around the disease free equilibrium (0, …, 0), the matrix in (A.7) also must have a principal eigenvalue that is negative. On the other hand, any positive equilibrium (X***, Y***) must satisfy (A.6) with (X*, Y*) replaced by (X***, Y***), so if such an equilibrium exists then the principal eigenvalue of the matrix in (A.7) must be zero, which is a contradiction. Thus, there can be no positive equilibrium, so the solution to (2.10) with initial data ξ→ will decrease toward the disease-free equilibrium. It then follows from the order preserving property of the system that the disease free equilibrium is globally stable in the invariant set {(X1, …, XN, Y1, …, YN): , i = 1, …, N}.
Footnotes
Research was partially supported by NIH grant P20-RR020770 (C.C., J.C.B., R.S.C., L.K., S.R.), NSF grants DMS-0514839 and DMS-0816068 (C.C., R.S.C.), and NSF grant DMS-0715772 (S.R.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen LJS, Bolker BM, Lou Y, Nevai AL. Asymptotic profiles of the steady states for an SIS epidemic patch model. SIAM Journal of Applied Mathematics. 2007;67:1283–1309. [Google Scholar]
- 2.Arino J. Diseases in metapopulations. In: Ma Z, Zhou Y, Wu J, editors. Modeling and Dynamics of Infectious Diseases. Series in Contemporary Applied Mathematics. Vol. 11. World Scientific; Singapore: 2009. pp. 65–123. [Google Scholar]
- 3.Arino J, van den Driessche P. A multi-city epidemic model. Mathematical Population Studies. 2003;10:175–193. [Google Scholar]
- 4.Arino J, Davis JR, Hartley D, Jordan R, Miller JM, van den Driessche P. A multi-species epidemic model with spatial dynamics. Mathematical Medicine and Biology. 2005;22:129–142. doi: 10.1093/imammb/dqi003. [DOI] [PubMed] [Google Scholar]
- 5.Auger P, Kouokam E, Sallet G, Tchuente M, Tsanou B. The Ross-Macdonald model in a patchy environment. Mathematical Biosciences. 2008;216:123–131. doi: 10.1016/j.mbs.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett MS. Deterministic and stochastic models for recurrent epidemics. Proceedings of the Third Berkeley Symposium on Mathematical Statistics and Probability; University of California Press; 1956. pp. 81–109. [Google Scholar]
- 7.Berman A, Plemmons J. Nonnegative Matrices in the Mathematical Sciences. Academic Press; New York: 1979. [Google Scholar]
- 8.Cantrell RS, Cosner C, DeAngelis D, Padron V. The ideal free distribution as an evolutionarily stable strategy. Journal of Biological Dynamics. 2007;1:249–271. doi: 10.1080/17513750701450227. [DOI] [PubMed] [Google Scholar]
- 9.Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bulletin of the World Health Organization. 2000;78(12):1401–1411. [PMC free article] [PubMed] [Google Scholar]
- 10.Dhirasakdanon T, Thieme H, van den Driessche P. A sharp threshold for disease persistence in host metapopulations. Journal of Biological Dynamics. 2007;1:363–378. doi: 10.1080/17513750701605465. [DOI] [PubMed] [Google Scholar]
- 11.Domarle O, Razakandrainibe R, Rakotomalala E, Jolivet L, Randremanana RV, Rakotomanana F, Ramarokoto CE, Soares JL, Ariey F. Seroprevalence of malaria in inhabitants of the urban zone of Antananarivo, Madagascar. Malaria Journal. 2006;5:106. doi: 10.1186/1475-2875-5-106. http://www.malariajournal.com/content/5/1/106. [DOI] [PMC free article] [PubMed]
- 12.Dushoff J, Levin SA. The effects of population heterogeneity on disease spread. Mathematical Biosciences. 1995;128:25–40. doi: 10.1016/0025-5564(94)00065-8. [DOI] [PubMed] [Google Scholar]
- 13.Dye C, Hasibeder G. Population dynamics of mosquito-borne disease: effects of flies which bite some people more frequently than others. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986;80:69–77. doi: 10.1016/0035-9203(86)90199-9. [DOI] [PubMed] [Google Scholar]
- 14.Graham A. Nonnegative Matrices and Applicable Topics in Linear Algebra. John Wiley and Sons; New York: 1987. [Google Scholar]
- 15.Gratz NG. Emerging and resurging vector-borne diseases. Annual Review of Entomology. 1999;44:5175. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 16.Hasibeder G, Dye C. Population dynamics of mosquito-borne disease: persistence in a completely heterogeneous environment. Theoretical Population Biology. 1988;33:31–53. doi: 10.1016/0040-5809(88)90003-2. [DOI] [PubMed] [Google Scholar]
- 17.Hethcote HW. An immunization model for a heterogeneous population. Theoretical Population Biology. 1978;14:338–349. doi: 10.1016/0040-5809(78)90011-4. [DOI] [PubMed] [Google Scholar]
- 18.Hethcote HW, Thieme HR. Stability of endemic equilibrium in epidemic models with subpopulations. Mathematical Biosciences. 1985;75:205–227. [Google Scholar]
- 19.Hsieh YH, van den Driessche P, Wang L. Impact of travel between patches for spatial spread of disease. Bulletin of Mathematical Biology. 2007;69:1355–1375. doi: 10.1007/s11538-006-9169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kileen GF, Knols BG, Gu W. Taking malaria transmission out of the bottle: implications of mosquito dispersal for vector-control interventions. The Lancet Infectious Diseases. 2003;3:297–303. doi: 10.1016/s1473-3099(03)00611-x. [DOI] [PubMed] [Google Scholar]
- 21.Lajmanovich A, Yorke JA. A deterministic model for gonorrhea in a nonhomogeneous population. Mathematical Biosciences. 1976;28:221–236. [Google Scholar]
- 22.Liu R, Shuai J, Wu J, Zhu H. Modeling spatial spread of West Nile virus and impact of directional dispersal of birds. Mathematical Biosciences and Engineering. 2006;3:145–160. doi: 10.3934/mbe.2006.3.145. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd AL, May RM. Spatial heterogeneity in epidemic models. Journal of Theoretical Biology. 1996;179:1–11. doi: 10.1006/jtbi.1996.0042. [DOI] [PubMed] [Google Scholar]
- 24.Martens P, Hall L. Malaria on the move: Human population movement and malaria transmission. Emerging Infectious Diseases. 2000;6:103–109. doi: 10.3201/eid0602.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osorio O, Todd J, Bradley DJ. Travel histories as risk factors in the analysis of urban malaria in Colombia. American Journal of Tropical Medicine and Hygiene. 2004;71:380–386. [PubMed] [Google Scholar]
- 26.Rodriguez DJ, Torres-Sorando L. Models for infectious diseases in spatially heterogeneous environments. Bulletin of Mathematical Biology. 2001;63:547–571. doi: 10.1006/bulm.2001.0231. [DOI] [PubMed] [Google Scholar]
- 27.Ronald LA, Kenny SL, Klinkenberg E, Akoto AO, Boakye I, Barnish G, Donnelly MJ. Malaria and anaemia among children in two communities of Kusami, Ghana: A cross-sectional survey. Malaria Journal. 2006;5:105. doi: 10.1186/1475-2875-5-105. http://www.malariajournal.com/content/5/1/105. [DOI] [PMC free article] [PubMed]
- 28.Ruan S, Wang W, Levin SA. The effect of global travel on the spread of SARS. Mathematical Biosciences and Engineering. 2006;3:205–218. doi: 10.3934/mbe.2006.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Rvachev LA, Longini IM. A mathematical model for the global spread of influenza. Mathematical Biosciences. 1985;75:3–22. [Google Scholar]
- 30.Salmani M, van den Driessche P. A model for disease transmission in a patchy environment. Discrete and Continuous Dynamical Systems - Series B. 2006;6:185–202. [Google Scholar]
- 31.Sattenspiel L, Dietz K. A structured epidemic model incorporating geographic mobility among regions. Mathematical Biosciences. 1995;128:71–91. doi: 10.1016/0025-5564(94)00068-b. [DOI] [PubMed] [Google Scholar]
- 32.Smith DL, McKenzie FE. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malaria Journal. 2004;3:13. doi: 10.1186/1475-2875-3-13. http://www.malariajournal.com/content/3/1/13. [DOI] [PMC free article] [PubMed]
- 33.Smith DL, Dushoff J, McKenzie FE. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biology. 2004;2(e368):1957–1963. doi: 10.1371/journal.pbio.0020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith HL. Monotone Dynamical Systems. American Mathematical Society; Providence, R.I: 1995. [Google Scholar]
- 35.Sutherst RW. Global change and human vulnerability to vector-borne disease. Clinical Microbiology Review. 2004;17:136–173. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Mathematical Biosciences. 2002;180:29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang W. Epidemic models with population dispersal. In: Takeuchi Y, Sato K, Iwasa Y, editors. Mathematics for Life Science and Medicine. Springer-Verlag; Berlin: 2007. pp. 67–95. [Google Scholar]
- 38.Wang W, Mulone G. Threshold of disease transmission in a patch environment. Journal of Mathematical Analysis and Applications. 2003;285:321–335. [Google Scholar]
- 39.Wang W, Zhao X-Q. An epidemic model in a patchy environment. Mathematical Biosciences. 2004;190:97–112. doi: 10.1016/j.mbs.2002.11.001. [DOI] [PubMed] [Google Scholar]