Abstract
Objectives To determine the quantitative efficacy of different classes of blood pressure lowering drugs in preventing coronary heart disease (CHD) and stroke, and who should receive treatment.
Design Meta-analysis.
Data source Medline (1966-2007).
Study selection Randomised trials of blood pressure lowering drugs recording CHD events and strokes. 108 trials studied differences in blood pressure between study drug and placebo (or control group not receiving the study drug) (“blood pressure difference trials”), and 46 trials compared drugs (“drug comparison trials”). Seven trials with three randomised groups fell into both categories. The results were interpreted in the context of those expected from the largest published meta-analysis of cohort studies, totalling 958 000 people.
Participants 464 000 people defined into three mutually exclusive categories: participants with no history of vascular disease, a history of CHD, or a history of stroke.
Results In the blood pressure difference trials β blockers had a special effect over and above that due to blood pressure reduction in preventing recurrent CHD events in people with a history of CHD: risk reduction 29% (95% confidence interval 22% to 34%) compared with 15% (11% to 19%) in trials of other drugs. The extra effect was limited to a few years after myocardial infarction, with a risk reduction of 31% compared with 13% in people with CHD with no recent infarct (P=0.04). In the other blood pressure difference trials (excluding CHD events in trials of β blockers in people with CHD), there was a 22% reduction in CHD events (17% to 27%) and a 41% (33% to 48%) reduction in stroke for a blood pressure reduction of 10 mm Hg systolic or 5 mm Hg diastolic, similar to the reductions of 25% (CHD) and 36% (stroke) expected for the same difference in blood pressure from the cohort study meta-analysis, indicating that the benefit is explained by blood pressure reduction itself. The five main classes of blood pressure lowering drugs (thiazides, β blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) were similarly effective (within a few percentage points) in preventing CHD events and strokes, with the exception that calcium channel blockers had a greater preventive effect on stroke (relative risk 0.92, 95% confidence interval 0.85 to 0.98). The percentage reductions in CHD events and stroke were similar in people with and without cardiovascular disease and regardless of blood pressure before treatment (down to 110 mm Hg systolic and 70 mm Hg diastolic). Combining our results with those from two other studies (the meta-analyses of blood pressure cohort studies and of trials determining the blood pressure lowering effects of drugs according to dose) showed that in people aged 60-69 with a diastolic blood pressure before treatment of 90 mm Hg, three drugs at half standard dose in combination reduced the risk of CHD by an estimated 46% and of stroke by 62%; one drug at standard dose had about half this effect. The present meta-analysis also showed that drugs other than calcium channel blockers (with the exception of non-cardioselective β blockers) reduced the incidence of heart failure by 24% (19% to 28%) and calcium channel blockers by 19% (6% to 31%).
Conclusions With the exception of the extra protective effect of β blockers given shortly after a myocardial infarction and the minor additional effect of calcium channel blockers in preventing stroke, all the classes of blood pressure lowering drugs have a similar effect in reducing CHD events and stroke for a given reduction in blood pressure so excluding material pleiotropic effects. The proportional reduction in cardiovascular disease events was the same or similar regardless of pretreatment blood pressure and the presence or absence of existing cardiovascular disease. Guidelines on the use of blood pressure lowering drugs can be simplified so that drugs are offered to people with all levels of blood pressure. Our results indicate the importance of lowering blood pressure in everyone over a certain age, rather than measuring it in everyone and treating it in some.
Introduction
Despite the widespread use of blood pressure lowering drugs and the results of many randomised trials,1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 w1-w162 uncertainty remains about which drugs to use and who to treat. Five questions encapsulate this uncertainty. Firstly, do β blockers have a special effect over and above lowering blood pressure in preventing coronary heart disease (CHD) events in people with a history of CHD? This view is widely held but such an effect has not been shown directly or quantified. We aimed to answer this question from an analysis of all relevant trials, and then to answer four further questions after excluding CHD events in trials of β blockers in people with a history of CHD if they did have a special effect. Secondly, does the effect of blood pressure lowering drugs in preventing CHD and stroke differ in people with and without a history of cardiovascular disease (that is, is there a different effect in secondary and primary prevention)? Thirdly, does blood pressure reduction alone explain the effect of blood pressure lowering drugs in preventing CHD and stroke? There are claims of additional non-blood pressure lowering (so called pleiotropic) effects of drugs.7 8 13 w135 w136 w139 Selected trial data have been used to suggest that each of the five main classes of blood pressure lowering drugs (thiazides, β blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) has a greater preventive effect,1 2 3 4 5 6 7 8 9 10 11 12 13 w126 w129 and each a lesser preventive effect,9 10 11 12 13 14 15 16 17 18 19 20 w126 w135 than other drugs. Clinical guidelines tend to reflect the view that differences in efficacy exist.21 22 23 Fourthly, should the use of blood pressure lowering drugs be limited to people with “high” blood pressure and not given to those at high risk of cardiovascular disease who have a lower blood pressure? A corollary is whether blood pressure should be reduced to a limited extent only, a treat to target approach.9 10 11 21 22 23 24 Although cohort (prospective observational) studies do not show a lower blood pressure limit below which risk ceases to decline (“the lower the better”),25 26 27 this has not been shown in randomised trials across a wide range of blood pressure. Finally, what is the quantitative effect of taking one or more blood pressure lowering drugs in lowering blood pressure and preventing CHD events and stroke according to dose, pretreatment blood pressure, and age? To date no such quantitative summary of effect, taking account of these determining factors, has been made.
We answered these questions using the results from 147 randomised trials of blood pressure lowering drugs and CHD events (n=22 000) and stroke (n=12 000), examined in the context of the results from the largest meta-analysis of epidemiological cohort studies of blood pressure and CHD and stroke.25 Previous meta-analyses of randomised trials of blood pressure lowering drugs and cardiovascular disease included fewer than 40 trials.1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 28 We also quantified the effect of blood pressure lowering drugs on the incidence of heart failure and on cancer mortality, other non-vascular mortality, and all cause mortality.
Methods
The database search (by MRL) used Medline (1966 to December 2007; any language) to identify randomised trials of blood pressure lowering drugs in which CHD events or strokes were recorded (irrespective of whether blood pressure reduction was considered the mechanism of action). Search terms were “anti-hypertensive agents” or “hypertension” or “diuretics, thiazide” or “adrenergic beta-antagonists” or “angiotensin-converting enzyme inhibitors” or “receptors, angiotensin/antagonists & inhibitors” or “tetrazoles” or “calcium channel blockers” or “vasodilator agents” or the names of all blood pressure lowering drugs listed in the British National Formulary as keywords or text words. Limits were Medline publication type “clinical trial” or “controlled clinical trial” or “randomized controlled trial” or “meta-analysis”. We also searched the Cochrane Collaboration and Web of Science databases and the citations in trials and previous meta-analysis and review articles.
We excluded non-randomised trials and trials in which treated groups but not control groups had other interventions as well as blood pressure reduction, such as cholesterol reduction. We excluded trials in patients with chronic renal failure because these patients typically have high blood pressure and high rates of cardiovascular disease and their response to standard blood pressure lowering therapy may differ from other people. We also excluded trials in which fewer than five CHD events and strokes were recorded or the duration of treatment was less than six months, as these data would contribute little to the overall results and substantially increase the complexity of the analyses. Randomised trials were otherwise included irrespective of participants’ age, disease status, blood pressure before treatment, or use of other drugs.
Data extraction
We recorded the numbers of participants having one or more CHD events (defined as fatal or non-fatal myocardial infarction or sudden cardiac death but excluding “silent” infarcts) and one or more strokes (haemorrhagic and ischaemic strokes could not be distinguished). We also recorded the numbers of participants with a new diagnosis of heart failure or an exacerbation of existing heart failure based on new hospital admissions or death from the disorder. Two authors (MRL and NJW) independently recorded data, with differences resolved by discussion. Outcomes were recorded regardless of whether participants took their allocated tablets (intention to treat analysis). Change in blood pressure in the trials (value on entry minus the average value during the trial in the treated group, minus the same change in the control group) was recorded on an intention to treat basis by determining the numbers of participants in the treated and in the control groups who stopped attending the clinics (so that their blood pressure reduction was no longer recorded) and taking the difference in blood pressure between them to be zero after they left the trial.
Categories of trial
The trials were divided into three predefined categories according to whether the recruitment was based on participants having no history of cardiovascular disease, a history of CHD (acute myocardial infarction, coronary artery disease without recent infarction, or heart failure), or a history of stroke (or other cerebrovascular disease). In the trials of participants with no history of vascular disease, blood pressure was usually high, variably defined, and a treat to target approach was used, typically based on one drug with the dose increased before the addition of other drugs to reach the target blood pressure. The control groups were allocated to usual care. In the trials of participants with a history of CHD there was generally no selection by blood pressure and no blood pressure target; treated patients were allocated a specified drug in fixed dose, varied only to avoid adverse effects. In the trials of participants with a history of stroke most followed the treat to target approach, some followed the specified drug approach. In trials of participants who had acute myocardial infarction on entry sudden deaths while in hospital were not recorded because it was not our objective to assess the efficacy of the drugs in reducing mortality in the period immediately after infarction; the CHD events and heart failure episodes we recorded were either those designated as reinfarction or those occurring after hospital discharge. Similarly in trials of participants who had heart failure on entry, sudden deaths were not recorded (as ischaemia and worsening heart failure could not be distinguished as causes). In trials of participants with CHD and who had acute myocardial infarction or heart failure on entry,29 30 many of the strokes recorded were likely to have been embolic (thrombus formation on an acute infarct or in a dilated left ventricle) and therefore not preventable by blood pressure reduction, so the estimate of the reduction in stroke was taken from the trials in which participants had coronary artery disease without recent infarction or heart failure.
We also categorised the trials into “blood pressure difference trials” and “drug comparison trials.” (Details of each trial are given in web extra tables 1i-iii and 2.) The blood pressure difference trials were those designed to achieve a difference in blood pressure between randomised groups who were given and were not given the study drugs to show the effect of this difference on the incidence of CHD events and stroke. Ninety two of the 108 trials in this category were placebo controlled, but in 16 the control group was not given a placebo. Additional blood pressure lowering drugs were commonly used in the different groups in each trial—for example, in trials comparing an angiotensin converting enzyme inhibitor with placebo in people with CHD, participants in both groups might also receive β blockers or calcium channel blockers, whereas in trials in which a treat to target approach was used, add-on drugs were given if necessary in both actively treated and placebo treated participants to reach their blood pressure targets (the target being lower for treated participants than for placebo participants). Through their design the blood pressure difference trials ensured that the intervention groups were more intensively treated. Trials were regarded as single drug trials if the difference between the groups in the mean number of drugs prescribed per participant (study drug included) was less than 1.5 (in the event 1.0 on average, and as combination drug trials if the mean number of drugs prescribed per participant was 1.5 or greater (2.0 on average).
Drug comparison trials were those that compared two blood pressure lowering drugs with each other. Although additional drugs could be used in either group there was no intention to achieve a different blood pressure reduction in one group compared with another. These trials therefore tested for effects of a drug that were unrelated to lowering blood pressure. In two drug comparison trials of three drugsw129 w147 each of the three pairwise comparisons was recorded separately. In both trial categories, additional drugs of a class allocated to one randomised group could not be used in the other.
Statistical analysis
All statistical analyses were done using Stata software. We combined relative risk estimates of disease events from individual trials using a random effects model31 (which avoids assuming that participants in the individual trials in the meta-analysis are sampled from populations in which the intervention has the same quantitative effect). Summary relative risk estimates from blood pressure difference trials were standardised to a blood pressure reduction of 10 mm Hg systolic or 5 mm Hg diastolic, by raising the relative risk estimate in each trial to the appropriate power (10 divided by the observed reduction in systolic blood pressure or 5 divided by the observed reduction in diastolic pressure)—for example, if the relative risk was 0.7 and the reduction in systolic blood pressure was 8 mm Hg, the standardised relative risk estimate was 0.64 (0.71.25, since 10/8=1.25). If reductions in both systolic and diastolic blood pressures were reported (as in most trials), we took the average of the two risk estimates (more strongly predictive than either alone25). As the reduction in blood pressure was not reported in most trials of people with a history of CHD, we estimated the average reduction from the average blood pressure before treatment and the average drug dose (as a multiple of standard dose32 33), using results from a meta-analysis in which the effect of pretreatment blood pressure and dose on blood pressure reduction was quantified.32 The estimated blood pressure reduction was 5.9 mm Hg systolic and 3.1 mm Hg diastolic, close to the median reduction in the 27 trials in which blood pressure reduction was reported, which was 6 mm Hg systolic and 3 mm Hg diastolic.
Predicting the trial results on CHD and stroke from epidemiological studies and trials of drugs on blood pressure
Effect of blood pressure lowering drugs in lowering blood pressure according to dose
These estimates are taken from a meta-analysis of 354 short term randomised placebo controlled trials of blood pressure lowering drugs in fixed dose,32 which showed that the five main classes of blood pressure lowering drugs produce similar reductions in blood pressure when taken at standard dose or at the same multiple of standard dose. It also showed that the blood pressure lowering effect of the drugs increased with dose and with pretreatment blood pressure, and reported regression equations that quantified the reduction in blood pressure from one drug according to pretreatment blood pressure. From the average blood pressure of 154 mm Hg systolic and 97 mm Hg diastolic one drug at standard dose lowered blood pressure by 9.1 mm Hg systolic and 5.5 mm Hg diastolic on average. At lower or higher pretreatment blood pressures the blood pressure reduction decreased (or increased) by 0.10 mm Hg systolic and 0.11 mm Hg diastolic per mm Hg decrease (or increase) in pretreatment blood pressure. The estimated effect of one drug at standard dose in lowering blood pressure from a pretreatment blood pressure P is therefore [9.1+0.10(P−154)] systolic and [5.5+0.11(P−97)] diastolic. So for example the reduction in blood pressure was 8.7 mm Hg systolic from a pretreatment value of 150 mm Hg, 4.7 mm Hg diastolic from a pretreatment value of 90 mm Hg. The estimated blood pressure reduction for two or three drugs at standard dose was calculated by applying these equations to each drug in turn, allowing for the effect of the first in lowering pretreatment blood pressure for the second, and the second for the third. In the above example the pretreatment blood pressure for the second drug would be 141.3 (150−8.7) mm Hg systolic and 85.3 (90−4.7) mm Hg diastolic.
Using drugs at half standard dose, taking dose and pretreatment blood pressure into account, it was estimated in the meta-analysis of 354 trials that one, two, and three drugs at half standard dose reduced a pretreatment systolic pressure of 150 mm Hg by 6.7 mm Hg, 13.3 mm Hg, and 19.9 mm Hg, respectively, and reduced a pretreatment diastolic pressure of 90 mm Hg by 3.7 mm Hg, 7.3 mm Hg, and 10.7 mm Hg, respectively (allowing for the effect of one drug in lowering pretreatment blood pressure for the next; table 4).32 These blood pressure reductions decreased (or increased) by an estimated 0.078 mm Hg systolic and 0.088 mm Hg diastolic, per mm Hg decrease (or increase) in pretreatment blood pressure per drug, 22% and 20% lower, respectively, than the changes at standard dose (0.10 mm Hg and 0.11 mm Hg.32 The blood pressure reductions from one, two, and three drugs at half standard dose were [R+n×0.078(P−150)] systolic and [R+n×0.088(P−90)] diastolic, where R is the blood pressure reduction at 150 mm Hg systolic or 90 mm Hg diastolic (given above), n is the number of drugs, and P is the pretreatment blood pressure
Expected reduction in disease events for a specified reduction in blood pressure
The associations between systolic and diastolic blood pressure and CHD events and stroke were taken from the largest published meta-analysis of 61 cohort (prospective observational) studies.25 This showed that in every age group cardiovascular mortality plotted on a logarithmic scale against blood pressure on an arithmetic scale is well fitted by straight lines, indicating a constant proportional change in risk for a specified change in blood pressure from any level of pretreatment blood pressure. Age specific slopes of the lines (regression coefficients) were published, permitting the calculation of the predicted proportional reduction in disease events for any age and blood pressure difference. For an age specific regression slope, S (see web extra table 3), and decrease in blood pressure, d, the relative risk is Sd/20 for systolic pressure and Sd/10 for diastolic pressure. The following examples illustrate the calculations. At age 60-69, the relative risk of stroke is 0.43 (57% decrease) for a 20 mm Hg decrease in systolic blood pressure. For a blood pressure decrease twice as great (40 mm Hg), the relative risk of 0.43 effectively applies twice (0.43×0.43, or 0.432), which is 0.18 (an 82% decrease). For a reduction in blood pressure half as great, by symmetry the relative risk is √0.43, or 0.431/2, which is 0.66 (a 34% decrease). For a 30 mm Hg decrease in blood pressure the relative risk is 0.431.5 (since 30/20=1.5), which is 0.28 (a 72% decrease). The sloping lines in the lower portion of figure 6 reflect these regression coefficients for stroke and CHD events in the age groups 50-59, 60-69, and 70-79 years.
The effect of blood pressure lowering drugs in reducing the risk of CHD events and stroke can therefore be estimated according to the reduction in systolic or diastolic blood pressure (or the average of the two), from the regression slope, S, and the decrease in blood pressure, d, from the above equations. As an example, the effect of three drugs at half standard dose in preventing stroke in people aged 60-69 with a pretreatment systolic blood pressure of 180 mm Hg systolic is estimated as: decrease in systolic blood pressure=[19.9+(3×0.078×(180−150))]=26.9 mm Hg, and relative risk of stroke=0.4326.9/20=0.32 (a 68% decrease).
Results
Overall, 147 trial reports were included in the analysis: 108 were blood pressure difference trials and 46 drug comparison trials (seven trial reports with two treatment groups and a placebo group fell into both categories, treatment versus placebo and one treatment versus the other). Table 1 summarises the trials (see web extra tables 1i-iii and 2 for individual data from the trials). Forest plots of individual trial results are presented in 55 web extra figures (available at www.wolfson.qmul.ac.uk/bptrial/) and the summary relative risk estimates and results for heterogeneity testing are shown in web extra table A. Results on CHD events and stroke are presented first, according to the five questions posed in the introduction, followed by results on heart failure and all cause mortality.
Table 1.
Randomised trials of blood pressure lowering drugs according to category of trial (see web extra tables 1i-iii and 2 for details of individual trials)
| Trial category and clinical history of participants on entry | No of trials | No of participants | Mean age on entry (years) | Mean duration (years) | No of disease events recorded | Range of mean pretreatment blood pressure in individual trials (mm Hg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coronary heart disease | Stroke | Heart failure | Systolic | Diastolic | |||||||
| Blood pressure difference trials | |||||||||||
| No vascular disease*w1-w33 | 27 | 108 297 | 62 | 4.5 | 3429 | 2843 | 582 | 132-186 | 72-119 | ||
| Coronary heart disease†: | |||||||||||
| Trials of β blockersw34-w72 | 37 | 38 892 | 57 | 1.7 | 2524 | 20 | 3198 | 112-149 | 72-92 | ||
| Trials of other drugsw73-w112 | 37 | 85 395 | 62 | 3.6 | 5815 | 964 | 6831 | 113-141 | 70-86 | ||
| Stroke‡w1 w7 w9 w29 w30 w113-w121 | 13* | 16 085 | 64 | 3.1 | 567 | 1593 | 13 | 132-186 | 72-115 | ||
| All blood pressure difference trialsw1-w121 | 108 | 248 445 | 62 | 3.5 | 12 324 | 5420 | 10 624 | 112-186 | 70-119 | ||
| Drug comparison trials | |||||||||||
| All trial categories¶w13-w17 w26 w34 w82 w122-w162 | 46 | 230 491 | 67 | 4.5 | 10 357 | 6862 | 7317 | 123-194 | 71-108 | ||
| All trials | 147§ | 464 164§ | 64 | 4.0 | 22 115§ | 12 034§ | 17 890§ | 112-194 | 70-119 | ||
*In the event 3% of participants had a history of myocardial infarction and 3% of stroke.
†In the event 90% of participants had a proved coronary heart disease, 8% had heart failure not caused by coronary heart disease, 1% had peripheral arterial or cerebrovascular disease, and 1% had no known vascular disease.
‡All participants had stroke or other cerebrovascular disease.
§“All trials” totals are less than column totals because six trials with two randomised treatment groups and one placebo group, included both as blood pressure difference trials and drug comparison trials,w11-w14 w29 w82 are counted twice; a seventh such trial is counted three times; and participants in five trials of stroke on entry were subgroups in five predominantly “no vascular disease” trialsw5 (see web extra table 1iii).
¶Participants had no vascular disease in most drug comparison trials; see web extra table 2.
Do β blockers have a special effect in preventing CHD events in people with a history of CHD?
Blood pressure difference trials
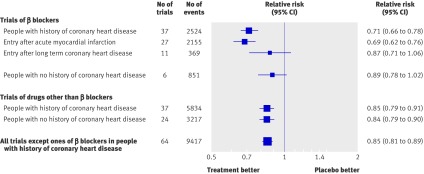
Figure 1 shows the reduction in CHD events in the 37 blood pressure difference trials of β blockers in people with a history of CHD, comparing β blockers with placebo (32 trials) or with an untreated control group (five trials). CHD events were, on average, reduced by 29% (relative risk 0.71, 95% confidence interval 0.66 to 0.78), significantly greater (P<0.001) than the 15% reduction in single drug trials of β blockers in people without a history of CHD and of other classes of drug in people with and without a history of CHD. The greater protective effect of β blockers in people with CHD was explained by a greater effect in the 27 trials that recruited participants at the time of an acute myocardial infarction (within a month in 25 trials and within four months in the other two). The risk reduction for recurrent CHD events in these 27 trials of people with an acute myocardial infarct was 31% (relative risk 0.69, 0.62 to 0.76); the duration of follow-up was short (77% of the events occurred in the first year and 94% in the first two years), so almost all the recurrent events occurred within one or two years of the infarct. Eleven trials remained (not 10 (37−27) because one trial recruited some participants with a recent infarct and some withoutw62); these recruited participants with a history of CHD but no recent infarct and in these the risk reduction was 13% (relative risk 0.87, 0.71 to 1.06; P=0.04 for the difference between the two groups of trials). In these 11 trials about 75% of the participants had had an infarct, but not within the last four months and typically several years before. The 13% risk reduction was similar to the 15% risk reductions in the other categories of single drug trials, whereas the 31% risk reduction after acute myocardial infarction was significantly greater (P<0.001). β blockers used for one or two years after an acute myocardial infarction were therefore about twice as effective as β blockers used in other circumstances and about twice as effective as other drugs used in any circumstances (see web figures 1a-e for forest plots of the individual trial results).
Fig 1 Relative risk estimates of coronary heart disease events in single drug blood pressure difference trials according to drug (β blockers or other), presence of CHD, and for β blockers according to acute myocardial infarction on entry. (Totals are less than the sum of the individual categories because some trials include more than one category; see web extra figures 1a-e for individual trial results and summary estimates)
Drug comparison trials
The four drug comparison trials of β blockers compared with other drugs in people with CHD but no recent infarct (see web extra table 2) confirmed the absence of a special effect of β blockers in the absence of a recent infarct; the summary relative risk of CHD events was 0.99 (0.82 to 1.20), a relative risk of 1.0 indicating the same risk reduction from β blockers and other drugs.
In view of the special effect of β blockers, CHD events (but not stroke or heart failure) in all 37 blood pressure difference trials and all four drug comparison trials of β blockers in people with CHD were excluded from subsequent analyses according to the prior stipulation that we would do so if a special effect was observed, even though post hoc the special effect was limited to a subset (those with acute infarction).
Does the preventive effect of drugs differ in people with and without a history of cardiovascular disease?
The summary relative risk estimates of CHD events and stroke in the blood pressure difference trials, observed and standardised for reduction in blood pressure, were similar in the three categories of trials (no vascular disease, history of CHD, and history of stroke), showing no difference in effect in people with or without vascular disease (table 2, also see web extra figures 2a-f for forest plots of individual trial results). There was no heterogeneity across the trials (table 2) and no special effect of drugs other than β blockers after acute myocardial infarction.
Table 2.
Summary relative risk estimates (95% confidence intervals) for coronary heart disease (CHD) events and stroke from randomised blood pressure difference trials observed and standardised to a blood pressure reduction of 10 mm Hg systolic and 5 mm Hg diastolic
| Clinical history of participants on entry | No of trials | Observed | Standardised for blood pressure reduction | |||
|---|---|---|---|---|---|---|
| CHD events | Stroke | CHD events | Stroke | |||
| No vascular disease | 27 | 0.84 (0.79 to 0.90) | 0.64 (0.56 to 0.73) | 0.79 (0.72 to 0.86) | 0.54 (0.45 to 0.65) | |
| CHD* | 37 | 0.85 (0.79 to 0.91) | 0.77 (0.68 to 0.87) | 0.76 (0.68 to 0.86) | 0.65 (0.53 to 0.80) | |
| Stroke | 13† | 0.85 (0.73 to 1.00) | 0.76 (0.68 to 0.85) | 0.79 (0.62 to 1.00) | 0.66 (0.56 to 0.79) | |
| All trials* | 72 | 0.84 (0.81 to 0.88) | 0.70 (0.65 to 0.76) | 0.78 (0.73 to 0.83) | 0.59 (0.52 to 0.67) | |
*Summary estimates omitting CHD events (but not strokes) in trials of β blockers in patients with a clinical history of CHD (heterogeneity for CHD, χ2=0.02, df=2, P=0.99; heterogeneity for stroke, χ2=2.0, df=2, P=0.37).
†Includes subgroups of participants with stroke on entry from five predominantly “no vascular disease” trials so total is less than the sum of the individual categoriesw5 (see web extra table 1iii).
Does blood pressure reduction alone explain the preventive effect of the drugs?
Blood pressure difference trials
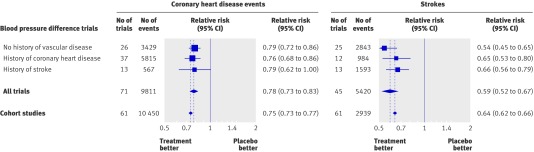
Figure 2 shows the relative risk estimates of CHD events and stroke in the blood pressure difference trials, standardised to a blood pressure reduction of 10 mm Hg systolic and 5 mm Hg diastolic, together with the corresponding relative risk estimates derived from the meta-analysis of cohort studies (Prospective Studies Collaboration analysis25), in the age group 60-69 years, the average age at the time of a cardiovascular event in the trials (table 1). The estimates from the trials meta-analysis were a 22% (95% confidence interval 17% to 27%) reduction in CHD events (relative risk 0.78) and a 41% (33% to 48%) reduction in stroke (relative risk 0.59). The cohort study meta-analysis showed a 25% decrease in CHD events (relative risk 0.75) and a 36% decrease in stroke (relative risk 0.64) for the same blood pressure difference of 10 mm Hg systolic, or 5 mm Hg diastolic (results from other cohort study meta-analyses were similar26 27). Thus the reductions in disease events in the trials were similar to those expected from the cohort study results for the same reduction in blood pressure.
Fig 2 Relative risk estimates of coronary heart disease events and stroke for a blood pressure reduction of 10 mm Hg systolic or 5 mm Hg diastolic in the blood pressure difference trials and in epidemiological cohort studies. (Total number of trials is fewer than the sum of the three categories as five included participants with and without vascular disease; see web extra figures 2a-f for individual trial results and summary estimates)
After only one year of follow-up (see web extra table 1) the reduction in CHD events was 20% (9% to 29%) and the reduction in stroke was 32% (18% to 44%) for a reduction of 10 mm Hg in systolic blood pressure and 5 mm Hg diastolic, similar to the long term trial results (22% and 41%) and similar to the results expected from the cohort studies (25% and 36%; see fig 2), indicating that the full potential effect of blood pressure reduction is achieved within a year.
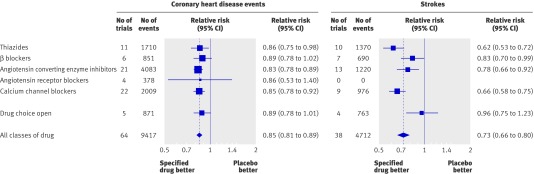
Figure 3 shows the reductions in CHD events and stroke in the single drug trials comparing a specified drug with placebo (or with a control group not receiving the study drug in nine trials), separately for each of the five main classes of drug (the only drugs tested in single drug trials). The five classes of drug produced reductions in CHD events and stroke that were similar in magnitude. All the reductions were statistically significant but for angiotensin receptor blockers there were only four trials and hence insufficient statistical power to show an effect. Average differences between the treated and control groups in use of add-on drugs were small (0.03 drugs per participant) and similar for the different classes of drug (see web extra figures 3a-i for forest plots of the results of individual trials for each class of drug). There was no statistically significant heterogeneity for CHD events across trials of the five classes of drug (χ2=2.0, df=5, P=0.86), but the reduction in incidence of stroke was smaller in trials of β blockers (17%) than in single drug trials of the other four classes of drug combined (29%; P=0.03).
Fig 3 Relative risk estimates of coronary heart disease events and stroke in single drug blood pressure difference trials according to class of drug (excluding CHD events in trials of β blockers in people with history of coronary heart disease). (Totals are less than the sum of the individual categories because some trials include more than one category; see web extra figures 3a-i for individual trial results and summary estimates)
Drug comparison trials
Figure 4 for CHD and web figures 4a-e show the results of the drug comparison trials comparing each of the five main classes of drug with drugs from the other classes. The summary relative risk estimates for CHD events were close to 1.0, indicating no advantage of any one drug over others in the prevention of CHD. The differences between classes of drug in average blood pressure reductions were close to zero (fig 4), and the differences in use of add-on drugs were negligible (0.03 or fewer drugs per participant).The different classes of drug therefore reduced blood pressure to about the same extent and reduced CHD to about the same extent, providing evidence of a lack of preventive effects attributable to mechanisms other than lowering blood pressure.
Fig 4 Relative risk estimates of coronary heart disease events and stroke in 46 drug comparison trials comparing each of the five classes of blood pressure lowering drug with any other class of drug (excluding CHD events in trials of β blockers in people with a history of coronary heart disease; see web extra figures 4a-j for individual trial results and summary estimates)
In the drug comparison trials the overall risk reduction in CHD events with thiazides was similar to that of other classes of drug (fig 4). There was, however, an increased risk of sudden cardiac death from using thiazides in very high dose, concealed in the summary results because few of the thiazide trials used very high doses and because sudden cardiac deaths were a small proportion of all CHD events. In trials in which the thiazide dose was high (≥4 times the standard dose33) there were 33 sudden cardiac deaths in participants allocated thiazides and 16 in those allocated other drugs (with similar numbers of participants in each group): relative risk 2.1 (P=0.01).w125 w163 In trials using doses between standard and twice standard, 57 and 40 sudden cardiac deaths occurred, respectively (relative risk 1.4),w15 w128 w130 w134 and in trials using around half standard dose there were 16 and 19 sudden cardiac deaths (relative risk 0.8;w127 w131 w133; P for trend 0.058). These results indicate that higher doses of thiazides probably cause sudden death, and prospective observational studies of people taking and not taking thiazides show the same (attributable to lower serum potassium levels causing ventricular arrhythmias).34 35 36 37 38 39 40 41
Figure 4 for stroke and web extra figures 4f-j show the corresponding drug comparison trial results on stroke. The summary relative risk estimates for stroke in the drug comparison trials were close to 1.0, with two exceptions. Figure 4 suggests a greater preventive effect of calcium channel blockers than other drugs, and a lesser effect of β blockers. The greater preventive effect of calcium channel blockers (relative risk 0.91, 95% confidence interval 0.84 to 0.98; P=0.01) was not materially altered after adjustment for the small difference in blood pressure reduction between the two groups (relative risk 0.92, 0.85 to 0.98), and is equivalent to a reduction in risk of stroke of 33% rather than 27% (the overall summary estimate), since 92% of 0.73 (the average relative risk in the single drug trials, fig 3) is 0.67 and 1.0−0.67 is 0.33. The lesser effect of β blockers in preventing stroke (fig 4; relative risk 1.18, 1.03 to 1.36; P=0.02) was not materially altered by adjusting for the small average difference in blood pressure reduction between the randomised groups and is equivalent to a 19% reduction in risk of stroke rather than 27% (since 1.11×0.73 (average relative risk from fig 3) is 0.81 and 1.00−0.81 is 0.19). The observed lesser effect of β blockers, however, rested on trials comparing calcium channel blockers with β blockers.w136-w140 Exclusion of the results of these trials weakened the evidence favouring a disadvantage of β blockers over the three other classes (relative risk 1.11, 0.86 to 1.44; P=0.40) but had little effect on the strength of evidence favouring an advantage of calcium channel blockers over the three other classes of drug (relative risk 0.93, 0.86 to 1.01; P=0.07).
The drug comparison trial results in figure 4 were similar and not significantly different when subdivided into the three prespecified groups (no vascular disease on entry (34 trials), history of CHD (10), and history of stroke (2)), but there were too few trial data to identify any but quite large differences in this respect.
Should the use of blood pressure lowering drugs be limited to people with “high” blood pressure?
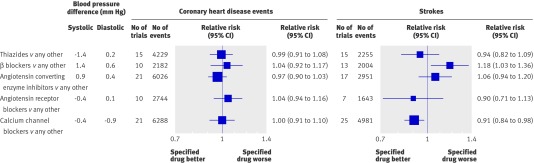
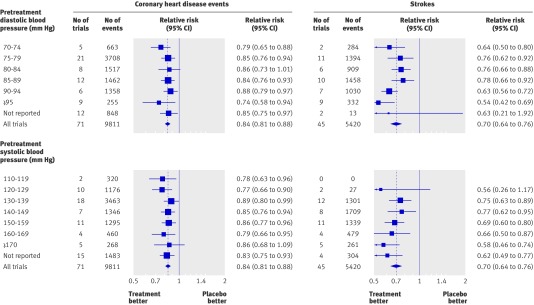
The relative risk estimates of CHD events and stroke in the blood pressure difference trials were similar across all levels of blood pressure before treatment down to 110 mm Hg systolic and 70 mm Hg diastolic, below which there were too few data (fig 5). At each blood pressure level the relative risk reductions were statistically significant and consistent with the summary relative risk estimates for all the trials of 0.84 for CHD events and 0.70 for stroke (table 2, also see web extra figures 5a-l and 6a-m for forest plots of the individual trial results). A meta-regression analysis showed no significant trend in proportional disease reduction with lower blood pressures before treatment indicating a constant proportional effect. The trial results mirror those in cohort studies,25 26 27 which show a proportional reduction in risk that is constant over all measured levels of blood pressure—that is, the same in people with lower and higher blood pressures.
Fig 5 Relative risk estimates of coronary heart disease events and stroke in blood pressure difference trials according to pretreatment diastolic and systolic blood pressures (taken as average in placebo group over course of trial). (Totals are less than the sum of the individual categories because some trials include more than one category; see web extra figures 5a-l and 6a-m for individual trial results and summary estimates)
There was no heterogeneity across the relative risk estimates for CHD according to pretreatment diastolic blood pressure (χ2=3.9, df=6, P=0.69; fig 5). There was heterogeneity for stroke (χ2=19, df=6, P=0.004), owing to a greater risk reduction in trials with the highest blood pressure before treatment (≥95 mm Hg), which arose because of more intensive treatment in these trials (average difference between treated and placebo groups of 1.7 blood pressure lowering drugs per participant, compared with 1.0 drugs per participant in the remaining trials with lower pretreatment blood pressure). The same applied to the analysis based on systolic blood pressure (CHD, χ2=3.7, df=7, P=0.82 and stroke χ2=12.24, df=6, P=0.06; fig 5).
What is the quantitative effect of one or more blood pressure lowering drugs in lowering blood pressure and in preventing CHD events and stroke?
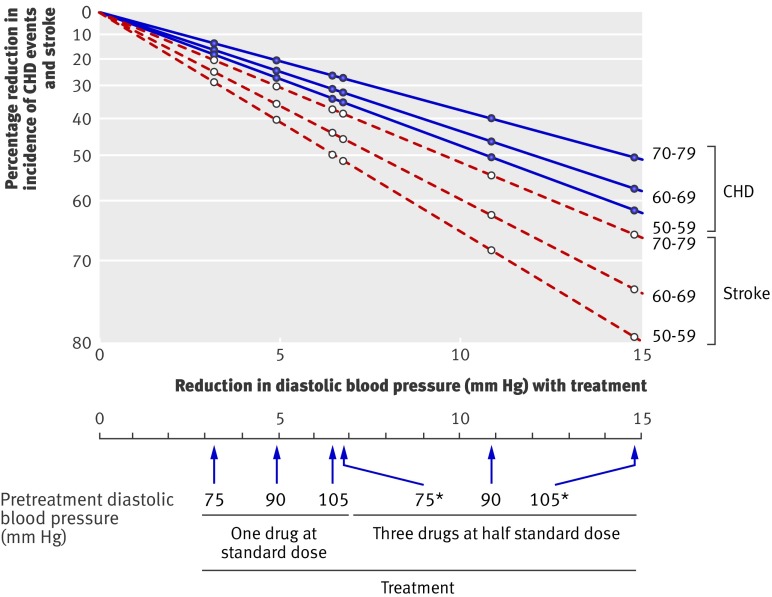
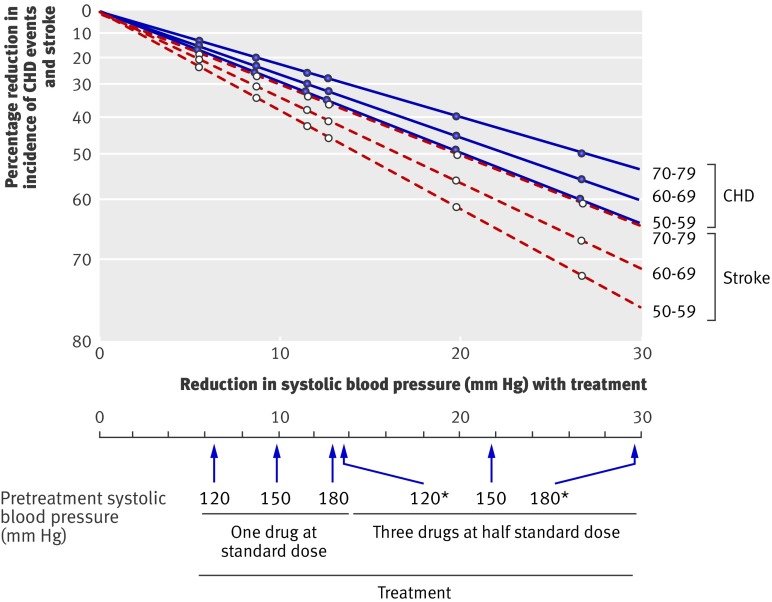
The effect of taking blood pressure lowering drugs in reducing the incidence of CHD and stroke according to number of drugs used, dose of drugs, and age cannot be estimated from the blood pressure differences trials alone. This is because a quarter of treated participants stopped taking their allocated drugs, individual trials used varying doses of drugs, trial data on combination drug therapy were limited, and the age range was relatively narrow. All this can be overcome by doing a two stage analysis, which is set out in figure 6 (based on diastolic blood pressure) and figure 7 (based on systolic blood pressure). The first stage (upper portion of figures) was to estimate the effect of one drug at standard dose in reducing blood pressure according to the level of blood pressure before treatment, which was done using results from a meta-analysis of short term (a few weeks) placebo controlled trials of the drugs in fixed dose.32 Because of their short duration these trial results have the advantage of not underestimating the effects of blood pressure reduction through treated participants dropping out or controls receiving treatment. The second stage (lower portion of figures) was to estimate the effect of these blood pressure reductions in preventing CHD and stroke. This was done using the results of the meta-analysis of cohort studies,25 rather than those from our meta-analysis of the trial results, because the cohort studies quantified the effects on disease reduction across a wide range of blood pressure reduction and age (which the trials cannot do), and the evidence presented here shows that the cohort study results reliably predict the results of randomised trials over the ranges of age and blood pressure reduction observed in the trials, so validating the use of the cohort study data in this way.
Fig 6 Reduction in incidence of coronary heart disease (CHD) events and stroke in relation to reduction in diastolic blood pressure according to drug dose, number of drugs, pretreatment diastolic blood pressure, and age. *Blood pressure reductions are more uncertain and hence also reductions in disease incidence
Fig 7 Reduction in incidence of coronary heart disease (CHD) events and stroke in relation to reduction in systolic blood pressure according to dose and combination of drugs, pretreatment systolic blood pressure, and age. *Blood pressure reductions are more uncertain and hence also reductions in disease incidence
Figures 6 and 7 show estimates of the effects of three drugs in combination at half standard dose. Tables 3 and 4 show the estimates of the effects of one, two, and three drugs at standard dose and one, two, and three drugs at half standard dose in preventing CHD and stroke according to pretreatment blood pressure and age.
Table 3.
Estimates of preventive effect of taking one or more blood pressure lowering drugs on coronary heart disease (CHD) events and stroke according to pretreatment systolic blood pressure, age, number of drugs, and dose (as multiple of standard33)
| Pretreatment systolic blood pressure (mm Hg) | Estimated reduction in systolic blood pressure (mm Hg)* | Relative risk of CHD events by age (years) | Relative risk of stroke by age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40-49 | 50-59 | 60-69 | 70-79 | 80-89 | 40-49 | 50-59 | 60-69 | 70-79 | 80-89 | |||
| One drug half standard dose: | ||||||||||||
| 180 | 9.0 | 0.72 | 0.73 | 0.76 | 0.79 | 0.83 | 0.63 | 0.65 | 0.68 | 0.73 | 0.83 | |
| 170 | 8.3 | 0.74 | 0.75 | 0.78 | 0.81 | 0.85 | 0.66 | 0.67 | 0.71 | 0.75 | 0.85 | |
| 160 | 7.5 | 0.77 | 0.77 | 0.79 | 0.83 | 0.86 | 0.68 | 0.70 | 0.73 | 0.77 | 0.86 | |
| 150 | 6.7 | 0.79 | 0.79 | 0.81 | 0.84 | 0.87 | 0.71 | 0.72 | 0.75 | 0.79 | 0.87 | |
| 140 | 5.9 | 0.81 | 0.81 | 0.83 | 0.86 | 0.89 | 0.74 | 0.75 | 0.78 | 0.81 | 0.89 | |
| 130 | 5.1 | 0.83 | 0.84 | 0.85 | 0.88 | 0.90 | 0.77 | 0.78 | 0.81 | 0.84 | 0.90 | |
| 120 | 4.4 | 0.86 | 0.86 | 0.87 | 0.89 | 0.92 | 0.80 | 0.81 | 0.83 | 0.86 | 0.92 | |
| One drug standard dose: | ||||||||||||
| 180 | 11.7 | 0.66 | 0.67 | 0.70 | 0.74 | 0.79 | 0.55 | 0.57 | 0.61 | 0.67 | 0.79 | |
| 170 | 10.7 | 0.68 | 0.69 | 0.72 | 0.76 | 0.81 | 0.58 | 0.60 | 0.64 | 0.69 | 0.81 | |
| 160 | 9.7 | 0.71 | 0.71 | 0.74 | 0.78 | 0.82 | 0.61 | 0.63 | 0.66 | 0.71 | 0.82 | |
| 150 | 8.7 | 0.73 | 0.74 | 0.76 | 0.80 | 0.84 | 0.64 | 0.66 | 0.69 | 0.74 | 0.84 | |
| 140 | 7.7 | 0.76 | 0.77 | 0.79 | 0.82 | 0.86 | 0.67 | 0.69 | 0.72 | 0.77 | 0.86 | |
| 130 | 6.7 | 0.79 | 0.79 | 0.81 | 0.84 | 0.87 | 0.71 | 0.72 | 0.75 | 0.79 | 0.87 | |
| 120 | 5.7 | 0.82 | 0.82 | 0.84 | 0.86 | 0.89 | 0.75 | 0.76 | 0.79 | 0.82 | 0.89 | |
| Two drugs half standard dose: | ||||||||||||
| 180 | 18.0 | 0.53 | 0.54 | 0.57 | 0.63 | 0.70 | 0.40 | 0.42 | 0.47 | 0.54 | 0.70 | |
| 170 | 16.4 | 0.56 | 0.57 | 0.60 | 0.66 | 0.72 | 0.43 | 0.45 | 0.50 | 0.57 | 0.72 | |
| 160 | 14.5 | 0.59 | 0.60 | 0.63 | 0.68 | 0.74 | 0.47 | 0.49 | 0.53 | 0.60 | 0.74 | |
| 150 | 13.3 | 0.62 | 0.63 | 0.66 | 0.71 | 0.77 | 0.51 | 0.53 | 0.57 | 0.63 | 0.77 | |
| 140 | 11.7 | 0.66 | 0.67 | 0.70 | 0.74 | 0.79 | 0.55 | 0.57 | 0.61 | 0.67 | 0.79 | |
| 130 | 10.2 | 0.70 | 0.70 | 0.73 | 0.77 | 0.82 | 0.59 | 0.61 | 0.65 | 0.70 | 0.82 | |
| 120 | 8.6 | 0.74 | 0.74 | 0.77 | 0.80 | 0.84 | 0.64 | 0.66 | 0.70 | 0.74 | 0.84 | |
| Two drugs standard dose: | ||||||||||||
| 180 | 22.2 | 0.45 | 0.46 | 0.50 | 0.57 | 0.64 | 0.32 | 0.34 | 0.39 | 0.46 | 0.64 | |
| 170 | 20.3 | 0.48 | 0.49 | 0.53 | 0.59 | 0.67 | 0.35 | 0.37 | 0.42 | 0.47 | 0.67 | |
| 160 | 18.4 | 0.52 | 0.53 | 0.57 | 0.62 | 0.69 | 0.39 | 0.41 | 0.46 | 0.53 | 0.69 | |
| 150 | 16.5 | 0.55 | 0.56 | 0.60 | 0.66 | 0.72 | 0.43 | 0.45 | 0.50 | 0.56 | 0.72 | |
| 140 | 14.6 | 0.59 | 0.60 | 0.64 | 0.69 | 0.75 | 0.47 | 0.49 | 0.54 | 0.60 | 0.75 | |
| 130 | 12.7 | 0.64 | 0.64 | 0.68 | 0.72 | 0.77 | 0.52 | 0.54 | 0.58 | 0.64 | 0.77 | |
| 120 | 10.8 | 0.68 | 0.69 | 0.72 | 0.76 | 0.81 | 0.58 | 0.59 | 0.63 | 0.69 | 0.81 | |
| Three drugs half standard dose: | ||||||||||||
| 180 | 26.9 | 0.38 | 0.39 | 0.44 | 0.50 | 0.58 | 0.25 | 0.27 | 0.32 | 0.39 | 0.58 | |
| 170 | 24.6 | 0.42 | 0.43 | 0.47 | 0.53 | 0.61 | 0.28 | 0.30 | 0.35 | 0.43 | 0.61 | |
| 160 | 22.2 | 0.45 | 0.46 | 0.50 | 0.57 | 0.64 | 0.32 | 0.34 | 0.39 | 0.46 | 0.64 | |
| 150 | 19.9 | 0.49 | 0.50 | 0.54 | 0.60 | 0.67 | 0.36 | 0.38 | 0.43 | 0.50 | 0.67 | |
| 140 | 17.6 | 0.50 | 0.51 | 0.55 | 0.61 | 0.68 | 0.37 | 0.39 | 0.44 | 0.54 | 0.68 | |
| 130 | 15.2 | 0.58 | 0.59 | 0.63 | 0.68 | 0.74 | 0.46 | 0.48 | 0.53 | 0.59 | 0.74 | |
| 120 | 12.9 | 0.63 | 0.64 | 0.67 | 0.72 | 0.77 | 0.52 | 0.54 | 0.58 | 0.64 | 0.77 | |
| Three drugs standard dose: | ||||||||||||
| 180 | 31.7 | 0.32 | 0.33 | 0.38 | 0.44 | 0.53 | 0.20 | 0.22 | 0.26 | 0.33 | 0.53 | |
| 170 | 29.0 | 0.36 | 0.37 | 0.41 | 0.48 | 0.56 | 0.23 | 0.25 | 0.29 | 0.37 | 0.56 | |
| 160 | 26.3 | 0.39 | 0.40 | 0.44 | 0.51 | 0.59 | 0.26 | 0.28 | 0.33 | 0.40 | 0.59 | |
| 150 | 23.6 | 0.43 | 0.44 | 0.48 | 0.55 | 0.62 | 0.30 | 0.32 | 0.37 | 0.44 | 0.62 | |
| 140 | 20.9 | 0.48 | 0.49 | 0.53 | 0.59 | 0.66 | 0.34 | 0.36 | 0.41 | 0.49 | 0.66 | |
| 130 | 18.2 | 0.52 | 0.53 | 0.57 | 0.63 | 0.70 | 0.40 | 0.42 | 0.46 | 0.53 | 0.70 | |
| 120 | 15.5 | 0.58 | 0.59 | 0.62 | 0.67 | 0.73 | 0.45 | 0.47 | 0.52 | 0.59 | 0.73 | |
This table is a numerical expansion of figure 7 (systolic blood pressure). Estimates calculated using a two stage procedure in which the effect of drug treatment in reducing systolic blood pressure was first estimated,32 then the effect of this blood pressure reduction on disease risk.25
*See Methods (section headed “Predicting the trial results.”)
Table 4.
Estimates of preventive effect of taking one or more blood pressure lowering drugs on coronary heart disease (CHD) events and stroke according to pretreatment diastolic blood pressure, age, number of drugs, and dose (as multiple of standard33)
| Pretreatment diastolic blood pressure (mm Hg) | Estimated reduction in diastolic blood pressure (mm Hg) | Relative risk of CHD events by age (years) | Relative risk of stroke by age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40-49 | 50-59 | 60-69 | 70-79 | 80-89 | 40-49 | 50-59 | 60-69 | 70-79 | 80-89 | |||
| One drug half standard dose: | ||||||||||||
| 110 | 5.5 | 0.66 | 0.70 | 0.73 | 0.77 | 0.82 | 0.56 | 0.55 | 0.61 | 0.67 | 0.78 | |
| 105 | 5 | 0.68 | 0.72 | 0.75 | 0.79 | 0.84 | 0.59 | 0.58 | 0.63 | 0.69 | 0.79 | |
| 100 | 4.6 | 0.71 | 0.74 | 0.77 | 0.80 | 0.85 | 0.62 | 0.61 | 0.66 | 0.71 | 0.81 | |
| 95 | 4.1 | 0.73 | 0.76 | 0.79 | 0.82 | 0.86 | 0.65 | 0.64 | 0.68 | 0.74 | 0.83 | |
| 90 | 3.7 | 0.76 | 0.79 | 0.81 | 0.84 | 0.88 | 0.68 | 0.67 | 0.71 | 0.76 | 0.84 | |
| 85 | 3.3 | 0.78 | 0.81 | 0.83 | 0.86 | 0.89 | 0.71 | 0.70 | 0.74 | 0.79 | 0.86 | |
| 80 | 2.8 | 0.81 | 0.83 | 0.85 | 0.87 | 0.90 | 0.74 | 0.74 | 0.77 | 0.81 | 0.88 | |
| 75 | 2.4 | 0.84 | 0.86 | 0.87 | 0.89 | 0.92 | 0.78 | 0.77 | 0.80 | 0.84 | 0.90 | |
| One drug standard dose: | ||||||||||||
| 110 | 6.9 | 0.59 | 0.64 | 0.67 | 0.72 | 0.78 | 0.48 | 0.47 | 0.53 | 0.60 | 0.73 | |
| 105 | 6.4 | 0.62 | 0.66 | 0.69 | 0.74 | 0.80 | 0.51 | 0.50 | 0.56 | 0.63 | 0.74 | |
| 100 | 5.8 | 0.64 | 0.68 | 0.71 | 0.76 | 0.81 | 0.54 | 0.53 | 0.59 | 0.65 | 0.76 | |
| 95 | 5.3 | 0.67 | 0.71 | 0.74 | 0.78 | 0.83 | 0.57 | 0.57 | 0.62 | 0.68 | 0.78 | |
| 90 | 4.7 | 0.70 | 0.73 | 0.76 | 0.80 | 0.84 | 0.61 | 0.60 | 0.65 | 0.71 | 0.80 | |
| 85 | 4.2 | 0.73 | 0.76 | 0.78 | 0.82 | 0.86 | 0.64 | 0.64 | 0.68 | 0.74 | 0.82 | |
| 80 | 3.6 | 0.76 | 0.79 | 0.81 | 0.84 | 0.88 | 0.68 | 0.68 | 0.72 | 0.77 | 0.85 | |
| 75 | 3.1 | 0.79 | 0.82 | 0.84 | 0.86 | 0.90 | 0.72 | 0.72 | 0.75 | 0.80 | 0.87 | |
| Two drugs half standard dose: | ||||||||||||
| 110 | 10.8 | 0.44 | 0.49 | 0.53 | 0.60 | 0.68 | 0.32 | 0.31 | 0.37 | 0.45 | 0.61 | |
| 105 | 9.9 | 0.47 | 0.52 | 0.56 | 0.62 | 0.70 | 0.35 | 0.34 | 0.40 | 0.48 | 0.63 | |
| 100 | 9.1 | 0.50 | 0.55 | 0.59 | 0.65 | 0.72 | 0.39 | 0.38 | 0.44 | 0.51 | 0.66 | |
| 95 | 8.2 | 0.54 | 0.59 | 0.62 | 0.68 | 0.75 | 0.42 | 0.41 | 0.47 | 0.55 | 0.69 | |
| 90 | 7.3 | 0.58 | 0.62 | 0.65 | 0.71 | 0.77 | 0.46 | 0.45 | 0.51 | 0.59 | 0.71 | |
| 85 | 6.4 | 0.62 | 0.66 | 0.69 | 0.74 | 0.80 | 0.51 | 0.50 | 0.56 | 0.62 | 0.74 | |
| 80 | 5.5 | 0.66 | 0.70 | 0.73 | 0.77 | 0.82 | 0.56 | 0.55 | 0.60 | 0.67 | 0.77 | |
| 75 | 4.7 | 0.70 | 0.74 | 0.76 | 0.80 | 0.85 | 0.61 | 0.60 | 0.65 | 0.71 | 0.81 | |
| Two drugs standard dose: | ||||||||||||
| 110 | 13.1 | 0.37 | 0.42 | 0.49 | 0.53 | 0.63 | 0.25 | 0.24 | 0.30 | 0.38 | 0.55 | |
| 105 | 12.1 | 0.40 | 0.45 | 0.50 | 0.56 | 0.65 | 0.28 | 0.27 | 0.33 | 0.41 | 0.57 | |
| 100 | 11.0 | 0.44 | 0.49 | 0.53 | 0.59 | 0.67 | 0.31 | 0.30 | 0.36 | 0.45 | 0.60 | |
| 95 | 10.0 | 0.47 | 0.52 | 0.56 | 0.62 | 0.70 | 0.35 | 0.34 | 0.40 | 0.48 | 0.63 | |
| 90 | 8.9 | 0.51 | 0.56 | 0.60 | 0.65 | 0.73 | 0.39 | 0.38 | 0.44 | 0.52 | 0.66 | |
| 85 | 7.9 | 0.55 | 0.60 | 0.63 | 0.69 | 0.75 | 0.44 | 0.43 | 0.48 | 0.56 | 0.69 | |
| 80 | 6.9 | 0.60 | 0.64 | 0.67 | 0.72 | 0.78 | 0.49 | 0.48 | 0.53 | 0.60 | 0.73 | |
| 75 | 5.8 | 0.64 | 0.68 | 0.71 | 0.76 | 0.81 | 0.54 | 0.53 | 0.59 | 0.65 | 0.76 | |
| Three drugs half standard dose: | ||||||||||||
| 110 | 16.0 | 0.30 | 0.35 | 0.40 | 0.47 | 0.57 | 0.19 | 0.18 | 0.23 | 0.31 | 0.48 | |
| 105 | 14.7 | 0.33 | 0.38 | 0.43 | 0.50 | 0.59 | 0.21 | 0.21 | 0.26 | 0.34 | 0.51 | |
| 100 | 13.3 | 0.37 | 0.42 | 0.46 | 0.53 | 0.62 | 0.25 | 0.24 | 0.29 | 0.38 | 0.54 | |
| 95 | 12.0 | 0.40 | 0.46 | 0.50 | 0.56 | 0.65 | 0.28 | 0.27 | 0.33 | 0.41 | 0.57 | |
| 90 | 10.7 | 0.45 | 0.50 | 0.54 | 0.60 | 0.68 | 0.33 | 0.32 | 0.38 | 0.46 | 0.61 | |
| 85 | 9.4 | 0.49 | 0.54 | 0.58 | 0.64 | 0.72 | 0.37 | 0.36 | 0.42 | 0.50 | 0.65 | |
| 80 | 8.1 | 0.54 | 0.59 | 0.63 | 0.68 | 0.75 | 0.43 | 0.42 | 0.48 | 0.55 | 0.69 | |
| 75 | 6.7 | 0.60 | 0.64 | 0.68 | 0.72 | 0.79 | 0.49 | 0.48 | 0.54 | 0.61 | 0.73 | |
| Three drugs standard dose: | ||||||||||||
| 110 | 18.6 | 0.25 | 0.30 | 0.34 | 0.41 | 0.52 | 0.14 | 0.13 | 0.18 | 0.26 | 0.42 | |
| 105 | 17.1 | 0.27 | 0.33 | 0.37 | 0.44 | 0.54 | 0.17 | 0.16 | 0.21 | 0.28 | 0.45 | |
| 100 | 15.6 | 0.31 | 0.36 | 0.40 | 0.47 | 0.57 | 0.19 | 0.19 | 0.24 | 0.32 | 0.49 | |
| 95 | 14.2 | 0.34 | 0.40 | 0.44 | 0.51 | 0.60 | 0.23 | 0.22 | 0.27 | 0.35 | 0.52 | |
| 90 | 12.7 | 0.38 | 0.44 | 0.48 | 0.55 | 0.64 | 0.26 | 0.25 | 0.31 | 0.39 | 0.56 | |
| 85 | 11.2 | 0.43 | 0.48 | 0.52 | 0.59 | 0.67 | 0.31 | 0.30 | 0.36 | 0.44 | 0.60 | |
| 80 | 9.7 | 0.48 | 0.53 | 0.57 | 0.63 | 0.71 | 0.36 | 0.35 | 0.41 | 0.49 | 0.64 | |
| 75 | 8.3 | 0.54 | 0.58 | 0.62 | 0.67 | 0.74 | 0.42 | 0.41 | 0.47 | 0.55 | 0.68 | |
This table is a numerical expansion of figure 6 (diastolic blood pressure). Estimates calculated using a two stage procedure in which the effect of drug treatment in reducing diastolic blood pressure was first estimated,32 then the effect of this blood pressure reduction on disease risk.25
*See Methods (section headed “Predicting the trial results.”)
These estimates of the preventive effect of blood pressure lowering drugs were calculated independently of the trials of blood pressure lowering drugs, so the trial results could be used to validate them. Table 5 shows the predictions of the reductions in CHD events and stroke in the blood pressure difference trials of single drug therapy (1.0 drug per participant on average) and of combination drug therapy (2.0 drugs per participant), calculated taking into account blood pressure before treatment, dose as a multiple of standard dose, and age and adjusted for the fact that a quarter of participants did not take their allocated treatment. Table 5 shows that the observed reductions in CHD events and stroke in the trials are similar to the predicted values, so the trial results validate the estimates.
Table 5.
Observed percentage reductions in coronary heart disease (CHD) events and stroke in single and combination drug treatment blood pressure difference trials compared with predicted reductions according to number of drugs, dose, pretreatment blood pressure, and age (60-69 years) (tables 3 and 4), adjusted for proportion of treated participants not taking their allocated tablets (25%)
| Category of drug trial† | No of trials* | Average No of drugs per participant | Mean dose (multiple of standard33) | Mean pretreatment blood pressure (mm Hg) | No of disease events | Percentage reduction in cardiovascular disease events | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHD | Stroke | |||||||||||||
| Systolic | Diastolic | CHD | Stroke | Observed in trials (95% CI) | Predicted based on systolic and diastolic | Observed in trials (95% CI) | Predicted based on systolic and diastolic | |||||||
| Single | 65 | 1.0 | 1.7 | 140 | 81 | 9417 | 4712 | 15 (11 to 19) | 19 and 17 | 27 (20 to 34) | 25 and 25 | |||
| Combination | 8 | 2.0 | 1.2 | 160 | 91 | 394 | 708 | 25 (9 to 38) | 36 and 34 | 41 (31 to 50) | 45 and 48 | |||
*One trial was part single drug and part combined drug therapy,w118 hence numbers sum to 73 when there were 72 trials.
†In “single” drug trials the difference between intervention and control groups in average number of drugs taken per participant, taking account of “add-on” therapy in individual trials, was <1.5, in combination drug trials ≥1.5.
Figures 6 and 7 show that one drug at standard dose reduced the incidence of CHD by about 24% and of stroke by 33% in people aged 60-69 with a systolic blood pressure of 150 mm Hg and a diastolic blood pressure of 90 mm Hg. Three drugs at half standard dose about doubled this effect, reducing the incidence of CHD by about 45% and of stroke by 60%. At higher blood pressure (180/105 mm Hg) and at lower blood pressure (120/75 mm Hg) the effect of one drug at standard dose was about 7-9 percentage points greater and smaller, respectively, and of three drugs at half standard dose about 12-14 percentage points greater and smaller. The proportional effect of age was relatively small; in people 10 years older the effect of one drug at standard dose was only 3 percentage points lower on average, and of three drugs at half standard dose 5 percentage points lower. Because mortality from CHD and stroke approximately trebles with each 10 year increase in age, the absolute gain from blood pressure reduction was greater at older ages.
If the drug treatment included a calcium channel blocker, and the greater effect of calcium channel blockers in preventing stroke (fig 4) was real and causal, the relative risk estimates for stroke in figures 6 and 7 and in tables 3 and 4 should be reduced by 8% (multiplied by 0.92).
Heart failure
Table 6 shows the results on heart failure (17 872 episodes), recorded in 64 blood pressure difference trials and 31 drug comparison trials. Heterogeneity existed within the results of the trials of β blockers and heart failure (P=0.008), explained by the observation that β blockers without cardioselective or α blocking (vasodilatory) properties (propranolol, oxprenolol, pindolol, and sotalol) lacked a preventive effect on heart failure (relative risk 1.01, 95% confidence interval 0.76 to1.35), but β blockers with one or other of these properties had a preventive effect (relative risk 0.77, 0.69 to 0.87; P=0.01 for difference). Data from trials of the first category of β blockers (seven trials, 385 episodes) were therefore excluded from table 6.
Table 6.
Results on heart failure in 64 blood pressure difference trials and 31 drug comparison trials of blood pressure lowering drugs, separately for calcium channel blockers and other drugs
| Class of drug | No of trials | No of episodes | Relative risk* (95% CI) |
|---|---|---|---|
| Blood pressure difference trials | |||
| Single drug therapy: | |||
| Calcium channel blockers | 13 | 1519 | 0.81 (0.69 to 0.94) |
| Thiazides | 5 | 222 | 0.59 (0.45 to 0.78) |
| β blockers | 13 | 2846 | 0.77 (0.69 to 0.87) |
| Angiotensin converting enzyme inhibitors | 16 | 3834 | 0.74 (0.68 to 0.81) |
| Angiotensin receptor blockers | 3 | 1675 | 0.82 (0.73 to 0.92) |
| All drug classes except calcium channel blockers | 36† | 8553† | 0.76 (0.72 to 0.81) |
| Combination drug therapy | 7 | 144 | 0.57 (0.36 to 0.92) |
| Drug comparison trials | |||
| Calcium channel blockers v any other drug class | 21 | 4572 | 1.22 (1.10 to 1.35) |
| Drug comparisons not involving calcium channel blockers: | |||
| Thiazides | 2 | 2335 | 0.91 (0.64 to 1.30) |
| β blockers | 2 | 335 | 1.04 (0.84 to 1.29) |
| Angiotensin converting enzyme inhibitors | 9 | 5063 | 0.98 (0.91 to 1.06) |
| Angiotensin receptor blockers | 7 | 2436 | 1.00 (0.93 to 1.08) |
*Relative risk <1.0 indicates specified drug class reduces risk of heart failure; >1.0 increases risk.
†All trials totals are less than column totals because one trial had two treated groups sharing same placebo group.
Calcium channel blockers reduced heart failure in the blood pressure difference trials by 19% (P=0.007), although the drug comparison trials showed that they were statistically significantly less effective in doing so than the other four classes of drugs (relative risk 1.22, 1.10 to 1.35; P<0.001). Each of the other four classes of drug significantly reduced the incidence of heart failure in the blood pressure difference trials (P<0.001) by 24% on average, with no significant differences in effect between them either in the blood pressure difference trials or the drug comparison trials. The effect of calcium channel blockers in reducing heart failure in the blood pressure difference trials (19%) was therefore not much less than that of the other classes of drug (24%). The effect of the drugs was similar in primary and secondary prevention (preventing new diagnoses of heart failure and preventing deterioration (hospital admission or death) in people with heart failure).
Non-vascular mortality and all cause mortality
In the blood pressure difference trials there was no increase in cancer mortality (relative risk 0.96, 0.85 to 1.09) or in non-vascular mortality (relative risk 1.00, 0.94 to 1.06). There were statistically significant reductions in all cause mortality in all the blood pressure difference trials (relative risk 0.87, 0.84 to 0.90; P=0.001) and in trials of people with no vascular disease on entry (0.89, 0.84 to 0.95) and with a history of CHD (0.86, 0.81 to 0.90) and stroke (0.91, 0.83 to 1.01).
Discussion
This, the largest meta-analysis of randomised trials of blood pressure reduction to date, shows that lowering systolic blood pressure by 10 mm Hg or diastolic blood pressure by 5 mm Hg using any of the main classes of blood pressure lowering drugs, reduces CHD events (fatal and non-fatal) by about a quarter and stroke by about a third, regardless of the presence or absence of vascular disease and of blood pressure before treatment, with no increase in non-vascular mortality. Heart failure is also reduced by about a quarter.
β blockers in people with CHD
Our results confirm that there is a special protective effect of β blockers in preventing CHD events in people with a clinical history of CHD over and above their blood pressure lowering effect. This special effect was limited to a few years after an acute myocardial infarction. The overall protective effect was about double that of β blockers in people with clinical CHD with no recent infarct or without CHD and that of other drugs regardless of history of CHD. This analysis and the consequent conclusion was possible because the trials in which participants were recruited immediately after an acute infarct had relatively short durations of follow-up (typically one or two years). The fortuitous dichotomy of the trial data on β blockers in CHD into short term trials in acute infarction and trials of non-acute CHD provided the opportunity to show that the special effect of β blockers was a short term effect, avoiding the dilution of effect that would have occurred had the acute infarct trials continued for many years after the infarct. The results confirm a suggestion made over 25 years ago from a non-significant difference between short term and long term prevention observed in one trial.w62
Preventive effect in people with and without cardiovascular disease
With the exception of the special short term effect of β blockers in acute myocardial infarction, our results show that the preventive effect of all classes of blood pressure lowering drugs is the same or similar in people with and without a history of cardiovascular disease (table 2), so there is no reason to use these drugs for secondary prevention but not for primary prevention. The trial results after one year of follow-up show that the preventive effect of blood pressure reduction is rapid, the full potential effect being achieved within a year, a result that differs from serum cholesterol reduction, which has little effect in the first year.42
Quantitative linking of blood pressure reduction and disease prevention
An important result from our analysis is that results from the meta-analysis of trials of drugs on blood pressure reduction linked to the cohort studies meta-analysis (differences in risk of CHD events and stroke for specified differences in blood pressure) accurately predict the results of the present trials meta-analysis, indicating that blood pressure reduction in itself explains the preventive effect of the drugs. With the possible minor additional effect of calcium channel blockers in preventing stroke the five classes of drugs were equally effective in lowering blood pressure (confirming previous work32) and equally effective in preventing CHD events and stroke (figs 3 and 4). A possible explanation for the greater effect of calcium channel blockers on the risk of stroke3 4 5 6 7 9 10 43 is the observation that although the different classes of blood pressure lowering drugs reduce peripheral arterial pressure to a similar extent,32 the reduction in central aortic pressure appears greater with calcium channel blockers and lower with β blockers than with the other three classes of drug.44 45 46 But it is not a persuasive argument because any additional reduction in central aortic pressure should also confer greater prevention against CHD than with other drugs but this was not observed (figs 3 and 4). Thus with the exception of β blockers after acute myocardial infarction and the minor additional effect of calcium channel blockers in reducing the risk of stroke, blood pressure reduction explains the action of the drugs in preventing CHD and stroke; the results thus exclude the blood pressure lowering drugs in general having material pleiotropic effects.
The assessment of β blockers as inferior drugs for lowering blood pressure14 15 16 17 18 was based on fewer trials than were considered here; they had a similar protective effect on CHD to other drugs (fig 3) and a greater protective effect after myocardial infarction (fig 1), supporting guidelines that do not discourage their use.24 Our results did not corroborate the suggested stronger protective effect of angiotensin converting enzyme inhibitors or weaker protective effect of angiotensin receptor blockers or calcium channel blockers against CHD.1 8 13 19 20 w155
Although our results do not exclude possible differences in efficacy between drugs within a class this is unlikely. Any such differences are likely to be small and clinically unimportant, however, because (β blockers and heart failure apart) for each class of drug there was no significant heterogeneity between trials of the individual drugs studied, either for blood pressure reduction32 or for reduction in disease events (see web extra table A). Trial results that suggest greater or lesser effects of some drugs can be explained by chance alone. One drug, atenolol, has been thought to be inferior to other β blockers,14 15 but as far as stroke prevention is concerned this result is probably secondary to the greater effect of calcium channel blockers in drug comparison trials. As far as prevention of CHD is concerned this was due to previous meta-analyses lacking statistical power. The relative risk estimate from a previous analysis of four blood pressure difference trials of atenolol of 0.99 (95% confidence interval 0.83 to 1.19)15 was revised to 0.93 (0.75 to 1.14) in our analysis with the inclusion of two additional atenolol trials,w64 w66 and both relative risk estimates are strongly influenced by the result of one trial, which implausibly suggests no effect at all (relative risk 1.01, 0.78 to 1.31w14). Without this one trial the relative risk estimate is 0.86 (0.64 to 1.16), close to the estimate of 0.85 from all single drug trials (fig 3). Given the available evidence, there is no reason to conclude that β blockers in general, or atenolol in particular, are less effective than other blood pressure lowering drugs.
In most of the blood pressure difference trials blood pressure was not monitored, but in about a third of these trials there was a treat to target policy applied to the treated groups. This was possible in double blind trials because the dispenser but not the investigator knew the allocated regimen and increased the dose of either the drug or the placebo as appropriate. In these trials the use of add-on treatment was the same on average in the treated and placebo groups (overall difference 0.3 drugs per participant). Over all the trials, 25% of participants allocated active treatment stopped taking their tablets; this non-adherence did not bias comparisons between the classes of drug because the proportions who stopped were similar for each class. The non-adherence underestimates the effect of taking the drugs on disease prevention but does not underestimate the effect of a specified blood pressure reduction from the drugs on disease prevention because the calculation of the difference in blood pressure took non-adherence into account. Thus the observations that a blood pressure reduction of 10 mm Hg systolic or 5 mm Hg diastolic, however achieved, reduced CHD events by 22% and stroke by 41% in the trials are unbiased estimates of efficacy.
A treat to target policy for lowering blood pressure was applied in most of the drug comparison trials; this was, from a scientific perspective, an advantage because it helped ensure that the differences in blood pressure between the arms of the trial were minimal (fig 3). The differences in use of additional drugs between the randomised groups were small (0.3 drugs per participant or fewer in trials comparing each class of drug with any other drug). The observation that there were no material differences in blood pressure between the groups and no material difference in the incidence of CHD or stroke (fig 4) permits the conclusion that the preventive effects of each class of drug are mediated through blood pressure reduction alone, corroborating the conclusion that the drugs did not have pleiotropic effects based on the similarity in predicted and observed results from the drug difference trials (fig 2).
Proportional disease reduction for a given blood pressure reduction independent of pretreatment blood pressure
Our results indicate that the use of blood pressure lowering drugs should not be limited to people with high blood pressure. The proportional reduction in disease events for a given blood pressure reduction was the same irrespective of blood pressure before treatment (fig 5), down to levels of 70 mm Hg (or lower) for diastolic blood pressure, as expected from the results of epidemiological cohort studies that showed a constant proportional change in risk for a specified change in blood pressure from any level of blood pressure before treatment.25 26 27 This result, and the previously published trials showing a greater risk reduction for a greater blood pressure reduction,4 5 6 support a “lower the better” approach to blood pressure reduction. It means that there is medical benefit in lowering a person’s blood pressure whatever the blood pressure, with the logically inescapable conclusion that there is then little or no gain in routinely measuring a person’s blood pressure—a conclusion that will undoubtedly stimulate discussion since it is at variance with 100 years of medical practice.
Our estimates of the proportional reduction in risk of CHD events and stroke vary according to age (figs 6 and 7 and tables 3 and 4). In a recent meta-analysis of 31 trials,47 using individual patient data or unpublished tabular data in prespecified categories, the authors concluded that age had no material influence on attenuating the effect of blood pressure reduction in preventing cardiovascular disease. However, their results did show an attenuating effect of age; the risk of cardiovascular disease was reduced by 24% per 5 mm Hg reduction in systolic blood pressure for a 15 year increase in age (11.9% cardiovascular disease prevention reduced to 9.1%), although this was not statistically significant.43 This estimate was close to the 20% expected decrease from the results of the cohort study meta-analysis we used.25 The 24% estimate from the trial meta-analysis was probably real but was not statistically significant because the blood pressure reductions observed in the trial were relatively small and the reductions in cardiovascular disease were therefore also small. The important conclusion is that the cohort studies and the trial data are entirely consistent in showing an age modifying effect on prevention of CHD events and stroke in relation to reductions in blood pressure.
From drugs to blood pressure reduction to disease prevention: a quantitative summary
Figures 6 and 7 provide an overall quantitative summary of the effect of blood pressure reducing drugs on blood pressure reduction according to blood pressure levels before treatment, dose of drugs, and number of drugs, and the corresponding reduction in CHD events and stroke according to age, derived from cohort studies, showing a reduced proportional effect with increasing age. The analysis of the randomised trials of blood pressure reduction presented here was not used to derive the estimates but provides important confirmation of them, both by showing that the cohort studies (on which these estimates were based) reliably predict the reduction in CHD events and stroke reduction in the randomised trials for the same difference in blood pressure (fig 2), and by showing that the estimates reliably predict the CHD events and stroke in the randomised trials according to number and dose of drugs without directly taking the blood pressure reduction into account (table 5). The estimates are therefore validated in that they predict trial data over the ranges of age and blood pressure reduction available in trials.
Tables 3 and 4 present a numerical expansion of figures 6 and 7. These results answer our fifth question, which enables a doctor prescribing blood pressure lowering treatment to know by how much that treatment is expected to reduce an individual’s risk of stroke and CHD in relation to the number and dose of drugs used, blood pressure level before treatment, and age. Meta-analyses of trials on disease events cannot, on their own, answer such questions. A synthesis of data from different sources, observational and experimental, is needed. Table 7 shows how the different pieces of the puzzle come from three large meta-analyses, the last one being the present study. Only such an integrative approach provides the quantitative summary set out in figures 6 and 7, which answers our fifth question.
Table 7.
Source of evidence from three large meta-analyses
| Drug effects | Meta-analysis of cohort studies of blood pressure and risk of CHD and stroke (2002)25 | Meta-analysis of trials of drugs by class and dose and blood pressure reduction (2003)32 | Meta-analysis of “event” trials of drugs by class and reduction in CHD and stroke (present analysis) | Comment on evidence from event trials (present analysis) |
|---|---|---|---|---|
| Effect of drug on blood pressure according to: | ||||
| Class of drug | Uninformative | Informative | Uninformative | Estimates diluted because 24% of participants stopped taking tablets |
| Dose of drug | Uninformative | Informative | Uninformative | Different participants took different doses; low dose not tested |
| No of drugs | Uninformative | Informative | Uninformative | Statistical power too low |
| Effect of blood pressure reduction on disease according to: | ||||
| Pretreatment blood pressure | Informative | Uninformative | Informative | Significant reduction in disease events even at lowest blood pressure |
| Class of drug | Uninformative | Uninformative | Informative | Extensive |
| Age | Informative | Uninformative | Uninformative | One narrow age range (60-69 years) |
CHD=coronary heart disease.
Assessing the need to tailor treatment
In clinical practice attention is often placed on tailoring blood pressure drug treatment to individual patients, on the basis of factors such as comorbidities, adverse effects, and cost.23 24
Comorbidities
The results of this analysis and of other studies on comorbidities indicate that the claimed advantages of one drug over another for an individual who has an existing disease are generally of minor importance. Our results show that all classes of drug are effective in heart failure (non-cardioselective β blockers apart) and after myocardial infarction (the greater effect of β blockers apart). Calcium channel blockers seem to be less effective than other drugs in heart failure but the difference in risk reduction is not large (19% v 24%; table 6) and they are no less effective after myocardial infarction. All classes of drug prevent headaches and migraine,48 49 50 although calcium channel blockers do so only in low (half standard) dose.51 There is no evidence to support recommendations for particular classes of drug in older or younger people.47 A relative contraindication of even cardioselective β blockers in people with airways obstruction or peripheral arterial disease is perceived,24 but a meta-analysis of trials has shown that cardioselective β blockers do not produce adverse respiratory effects in mild to moderate obstructive airways disease52; such considerations are not a reason to withhold a β blocker, but reinforce the principle that patients should be monitored for side effects of drugs and a drug should be withdrawn if it causes adverse effects. Most of the classes of drug have advantages in preventing non-vascular diseases. Thiazides prevent renal calculiw164 and may prevent hip fracture (they increase bone density in randomised trials and are associated with a reduced risk of hip fracture in prospective observational studies53 54 55). β blockers are advantageous in glaucoma.24 Angiotensin converting enzyme inhibitors and angiotensin receptor blockers reduce the incidence of diabetes and diabetic and non-diabetic nephropathy.24 Using the drugs in combination rather than singly therefore offers several medical benefits.
Adverse effects
Adverse effects are also a reason for seeking to individualise treatment. Tolerability, however, is minimised by using low dose combination therapy, as the prevalence of symptoms for three of the classes of drug is strongly related to dose,32 and low dose combination therapy can greatly improve safety. For example, the risk of sudden cardiac death was avoided using thiazides in low dose in the trials, probably because thiazides cause little reduction in serum potassium at half standard dose,32 33 and using thiazides in combination with angiotensin converting enzyme inhibitors or angiotensin receptor blockers, which increase serum potassium levels, counters the potential hazard.32 56 The diabetogenic effect of thiazides is strongly related to dose, and minor at low dose (the increase in blood glucose is 1% at half standard dose, 3% at standard dose, and 5% at twice standard dose32), and the effect is offset by angiotensin converting enzyme inhibitors and angiotensin receptor blockers.24 56 Thiazides may cause gout, but again the increase in serum uric acid is attenuated at low dose32 33 and opposed by angiotensin converting enzyme inhibitors and angiotensin receptor blockers.w165-w167 Angiotensin converting enzyme inhibitors and angiotensin receptor blockers are teratogenic and should be avoided in pregnancy.57 58 A randomised trial carried out to assess the effects of blood pressure lowering drugs on sexual function confirmed the recognised effects of thiazides but showed that other classes of blood pressure lowering drug (including β blockers) did not differ from placebo.59
Cost
Cost is another reason for selecting particular classes of drug but has become a minor consideration because suitable off-patent blood pressure lowering drugs in four of the five classes are available at low cost, and will be available for the fifth (angiotensin receptor blockers) later in 2009.
It is difficult to defend the widespread practice of tailoring treatment. The case for individualising blood pressure lowering therapy disappears with low dose combination therapy based on three drugs; the greater efficacy compared with selective monotherapy or dual therapy avoids the need to choose between drugs.
Strengths and limitations of the study
Potential limitations of this meta-analysis arise from not having individual patient data from the individual trials. While this would have provided more detail on the effects of blood pressure reduction in relation to pretreatment blood pressure and age it was not a serious limitation. The trials varied sufficiently for pretreatment blood pressure to be informative, so this was not a serious limitation. With regard to age, the observation that in the age group covered by the trials as a whole (60-69) the results were as expected from the cohort studies indicates that the synthesis of these two sources of data overcomes this limitation from the trial meta-analysis. The fact that our meta-analysis was based on trials that varied in many ways (for example, with respect to pretreatment blood pressure, previous disease, population sample) may be thought to be a limitation, possibly leading to random error obscuring real differences. This was not the case, however; the meta-analysis was sensitive enough to show that the trial results were as expected from cohort studies and it is therefore unlikely that random error or bias could have produced our results. It is also extremely unlikely that random or systematic error in the analysis would produce essentially identical quantitative results when dichotomised in different ways, such as with or without cardiovascular disease. Indeed, the consistency of our results in the face of such variable trial designs reinforces, not diminishes the validity of the conclusions. There are sparse direct data to show an additive effect of different combinations of three blood pressure lowering drugs on blood pressure, but it is reasonable to conclude that combinations of three are additive since this is true for combinations of two drugs,32 60 and the estimates of the efficacy of three drugs shown in figures 7 and 8 and in tables 3 and 4 are based on this inference.
The strength of our analyses is that, based as they are on relative reductions in risk, they are generally applicable irrespective of the incidence of cardiovascular disease. However the preventive potential needs to be assessed in terms of the absolute risk reduction. Our estimates of relative risk reduction may be converted to absolute risk reductions by multiplying them by the incidence in a specified population. For example at age 65 the 10 year risk of myocardial infarction (fatal or non-fatal) in England and Wales was estimated as being about 10% in men and 5% in women.33 Given an average blood pressure at that age of 150 mm Hg systolic and 90 mm Hg diastolic 33 the expected relative risk reduction using three drugs at half standard dose is 46% (tables 3 and 4), so the absolute risk reduction over 10 years in men is 4.6% (from 10% to 5.4%) and in women is 2.3% (from 5% to 2.7%). The corresponding absolute risk reduction for stroke is 2.9% in men and 2.3% in women, based on 10 year incidences of 5% in men and 4% in women.33 For myocardial infarction and stroke combined, therefore, the absolute risk reduction in men is 7.5% and in women is 4.6%.
Demonstrating the value of blood pressure lowering treatment to everyone in a population above a particular age might be perceived as “medicalising” a population. We disagree with this view. Identifying people with a relatively high blood pressure for their age, giving them the medical diagnosis “hypertension,” and treating this “disease” clearly medicalises the individuals concerned. Offering blood pressure lowering treatments to a population above a certain age, regardless of their blood pressure, on the basis that it would prevent a future heart attack and stroke with minimal adverse effects, in our view does not medicalise the population, unless the broad view is taken that anyone who takes a preventive agent regularly is medicalised. Such a broad view would mean that people receiving antimalarials, vaccines, or contraceptive pills are medicalised, which few would judge to be the case.
We conclude our analysis by summarising our answers to the five questions we initially posed (box). The preventive effect of lowering blood pressure is substantial and capable of reducing the incidence of CHD and stroke by at least half in all people at risk of CHD events or stroke whatever their blood pressure and whatever the basis of being at risk—for example, having had a cardiovascular disease event or simply being older. Our results support the view that blood pressure lowering drugs should no longer be regarded as treatment for hypertension in the same way that statins are now no longer regarded as treatment for hypercholesterolaemia. Consideration should be given to replacing current policies that focus on routinely measuring blood pressure with policies that focus on routinely lowering blood pressure.
What is already known on this topic
The different classes of blood pressure lowering drugs at standard doses, or the same multiple of standard dose, lower blood pressure to a similar extent
Blood pressure lowering drugs reduce the risk of coronary heart disease (CHD) events and stroke in people with a history of vascular disease and in those with high blood pressure
What this study adds
The effect of blood pressure lowering drugs in reducing the risk of disease is entirely or largely due to blood pressure reduction, with one main exception, a special extra effect of β blockers in people who have had a recent myocardial infarction
The proportional reduction in CHD events and stroke for a given reduction in blood pressure, an approximate halving in risk for each 10 mm Hg diastolic reduction, is the same in people with and without a history of vascular disease and in people without high blood pressure as well as in those with high blood pressure
There is benefit in lowering blood pressure in anyone at sufficient cardiovascular risk whatever their blood pressure, so avoiding the need to measure blood pressure routinely
Summary of answers to questions posed
Do β blockers have a special effect in preventing CHD events in people with a clinical history of CHD?
Yes. The effect is an approximate 30% reduction in CHD, present for a few years after the infarct. This risk reduction is about 15% thereafter, similar to that of other blood pressure lowering drugs
Does the preventive effect of drugs differ in people with and without a history of cardiovascular disease?
No. The percentage reduction in risk of CHD events and stroke is the same or similar. Since the absolute risk is higher in people with a history of cardiovascular disease, however, the absolute risk reduction is greater
Does blood pressure reduction alone explain the preventive effect of the drugs?
Yes, except for the special short term effect of β blockers
Should the use of blood pressure lowering drugs be limited to people with “high” blood pressure?
No. Blood pressure lowering drugs should be offered to anyone with a high enough risk to benefit from treatment whatever their reason for being at high risk, because a given blood pressure reduction lowers risk of CHD and stroke by a constant proportion irrespective of pretreatment blood pressure
What is the quantitative effect of taking one or more blood pressure lowering drugs on blood pressure and the risk of CHD events and stroke?
In people aged 60-69 with a diastolic blood pressure of 90 mm Hg (or systolic blood pressure of 150 mm Hg): one drug at standard dose lowers the risk of CHD by about 25% and of stroke by 35%; three drugs at half standard dose lower the risk of CHD by 45% and of stroke by 60%; the estimates are about 10 percentage points higher if blood pressure is higher by 30 mm Hg systolic or 15 mm Hg diastolic; the estimates are about 5 percentage points lower for a 10 year increase in age
We thank Mark Simmonds, Jon Bestwick, Neville Young, and Kate Walker for their help in preparing the figures, and Peter Whincup and Leo Kinlen for their comments on the manuscript.
Contributors: All authors participated in the design of the study, analysis of data, and preparation of the manuscript. JKM did the statistical analysis. MRL is guarantor.
Competing interests: MRL and NJW hold patents (granted and pending) on the formulation of a combined pill to simultaneously reduce four cardiovascular risk factors, including blood pressure.
Ethical approval: Not required.
Data sharing: An audit trail of the forest plots and related data is available at www.wolfson.qmul.ac.uk/bptrial/.
Cite this as: BMJ 2009;338:b1665
References
- 1.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003;289:2534-44. [DOI] [PubMed] [Google Scholar]
- 2.Kendall MJ, Lynch KP, Hjalmarson Å, Kjekshus J. β-blockers and sudden cardiac death. Ann Intern Med 1995;123:358-67. [DOI] [PubMed] [Google Scholar]
- 3.Elliott WJ, Bandari A. The role of calcium antagonists in stroke prevention. J Clin Hypertens 2005;7:5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus. Arch Intern Med 2005;165:1410-9. [DOI] [PubMed] [Google Scholar]
- 5.Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527-35. [DOI] [PubMed] [Google Scholar]
- 6.Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet 2000;355:1955-64. [DOI] [PubMed] [Google Scholar]
- 7.Chrysant SG, Chrysant GS. The pleiotropic effects of angiotensin receptor blockers. J Clin Hypertens 2006;8:261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens 2007;25:951-8. [DOI] [PubMed] [Google Scholar]
- 9.Staessen JA, Wang J-W, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet 2001;358:1305-15. [DOI] [PubMed] [Google Scholar]
- 10.Staessen JA, Wang J-G, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003;21:1055-76. [DOI] [PubMed] [Google Scholar]
- 11.Staessen JA, Li Y, Thijs L, Wang J-G. Blood pressure reduction and cardiovascular prevention: an update including the 2003-2004 secondary prevention trials. Hypertens Res 2005;28:385-407. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Thijs L, Staessen JA. Blood pressure lowering for primary and secondary prevention of stroke. Hypertension 2006;48:187-95. [DOI] [PubMed] [Google Scholar]
- 13.Verdecchia P, Reboldi G, Angeli F, Gattobigio R, Bentivoglio M, Thijs L, et al. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension 2005;46:386-92. [DOI] [PubMed] [Google Scholar]
- 14.Lindholm LH, Carlberg B, Samuelsson O. Should β blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005;366:1545-53. [DOI] [PubMed] [Google Scholar]
- 15.Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet 2004;364:1684-9. [DOI] [PubMed] [Google Scholar]
- 16.Wiysonge CS, Bradley H, Mayosi BM, Maroney R, Mbewu A, Opie LH, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev 2007;(1):CD002003. [DOI] [PubMed] [Google Scholar]
- 17.Bradley HA, Wiysonge CS, Volmink JA, Mayosi BM, Opie LH. How strong is the evidence of use of beta-blockers as first-line therapy for hypertension? Systematic review and meta-analysis. J Hypertens 2006;24:2131-41. [DOI] [PubMed] [Google Scholar]
- 18.Khan N, McAlister FA. Re-examining the efficacy of β-blockers for the treatment of hypertension: a meta-analysis. CMAJ 2006;12:1737-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma S, Strauss M. Angiotensin receptor blockers and myocardial infarction. BMJ 2004;329:1248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahor M, Psaty BM, Alderman MH, Applegate WB, Williamson JD, Cavazzini C, et al. Health outcomes associated with calcium antagonists compared with other first-line antihypertensive therapies: a meta-analysis of randomised controlled trials. Lancet 2000;356:1949-54. [DOI] [PubMed] [Google Scholar]
- 21.National Institute for Health and Clinical Excellence. Management of hypertension in adults in primary care. NICE clinical guideline 34. London; NICE, Jun 2006.
- 22.Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The seventh report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No 04-5230. Aug 2004. www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. 2007. [PubMed]
- 23.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004;328:634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2007 guidelines for the management of arterial hypertension. J Hypertens 2007;25:1105-87. [DOI] [PubMed] [Google Scholar]
- 25.Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. [DOI] [PubMed] [Google Scholar]
- 26.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765-74. [DOI] [PubMed] [Google Scholar]
- 27.Asia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003;21:707-16. [DOI] [PubMed] [Google Scholar]
- 28.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827-38. [DOI] [PubMed] [Google Scholar]
- 29.Witt BJ, Ballman KV, Brown RD, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006;119:354.e1-9. [DOI] [PubMed] [Google Scholar]
- 30.Witt BJ, Gami AS, Ballman KV, Brown RD, Meverden RA, Jacobsen SJ, et al. The incidence of ischemic stroke in chronic heart failure: a meta-analysis. J Cardiac Fail 2007;13:489-96. [DOI] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 32.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003;326:1427-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and strokes: a new preventive strategy. Health Technol Assess 2003;7(31). [DOI] [PubMed]
- 34.Duke M. Thiazide-induced hypokalemia association with acute myocardial infarction and ventricular fibrillation. JAMA 1978;239:43-5. [DOI] [PubMed] [Google Scholar]
- 35.Solomon RJ. Ventricular arrhythmias in patients with myocardial infarction and ischaemia. Drugs 1984;28(suppl 1):66-76. [DOI] [PubMed] [Google Scholar]
- 36.Ruder MA, Flaker GC, Alpert MA, Bertuso J. Hypokalemia as a cause of cardiac arrest: results of electrophysiologic testing and long-term follow-up. Am Heart J 1985;110:490-1. [DOI] [PubMed] [Google Scholar]
- 37.Dargie HJ, Cleland JGF, Leckie BJ, Inglis CG, East BW, Ford I. Relation of arrhythmias and electrolyte abnormalities to survival in patients with severe chronic heart failure. Circulation 1987;75(suppl IV):98-107. [PubMed] [Google Scholar]
- 38.Singh BN, Hollenberg NK, Poole-Wilson PA, Robertson JIS. Diuretic-induced potassium and magnesium deficiency: relation to drug-induced QT prolongation, cardiac arrhythmias and sudden death. J Hypertens 1992;10:301-16. [DOI] [PubMed] [Google Scholar]
- 39.Siscovick DS, Raghunathan TE, Psaty BM, Koepsell TD, Wicklund KG, Lin X, et al. Diuretic therapy for hypertension and the risk of primary cardiac arrest. N Engl J Med 1994;330:1852-7. [DOI] [PubMed] [Google Scholar]
- 40.Hoes AW, Grobbee ED, Lubsen J, Man in ‘t Veld AJ, van der Does E, Hofman A. Diuretics, β-blockers, and the risk for sudden cardiac death in hypertensive patients. Ann Intern Med 1995;123:481-7. [DOI] [PubMed] [Google Scholar]
- 41.Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation 1999;100:1311-5. [DOI] [PubMed] [Google Scholar]
- 42.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on LDL cholesterol, ischaemic heart disease and stroke: systematic review and meta-analysis. BMJ 2003;326:1423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamerson K, Weber MA, Bakrias GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high risk patients. N Engl J Med 2008;359:2417-28. [DOI] [PubMed] [Google Scholar]
- 44.Deary AJ, Schumann AL, Murfet H, Haydock S, Foo RS, Brown MJ. Influence of drugs and gender on the arterial pulse wave and natriuretic peptide secretion in untreated patients with essential hypertension. Clin Sci 2002;103:493-9. [DOI] [PubMed] [Google Scholar]
- 45.The CAFÉ Investigators, for the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Investigators. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcome. Principal results of the Conduit Artery Function Evaluation (CAFÉ) Study. Circulation 2006;113:1213-25. [DOI] [PubMed] [Google Scholar]
- 46.Asmar RG, London GM, O’Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patients: a comparison with atenolol. Hypertension 2001;38:922-6. [DOI] [PubMed] [Google Scholar]
- 47.Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008;336:1121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Law M, Morris JK, Jordan R, Wald N. Headaches and the treatment of blood pressure. Results from a meta-analysis of 94 randomized placebo-controlled trials with 24 000 participants. Circulation 2005;112:2301-6. [DOI] [PubMed] [Google Scholar]
- 49.Schrader H, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ 2001;322:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tronvik E, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with an angiotensin II receptor blocker. A randomized controlled trial. JAMA 2003;289:65-9. [DOI] [PubMed] [Google Scholar]
- 51.Law MR, Morris JK, Wald NJ. Calcium channel blockers and headache. Br J Clin Pharmacol 2007;63:157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salpeter SR, Ormiston TM, Salpeter EE, Wood-Baker R. Cardioselective beta-blockers for reversible airway disease. Cochrane Database Syst Rev 2002:(4);CD002992. [DOI] [PubMed]
- 53.LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. Ann Intern Med 2000;133:516-26. [DOI] [PubMed] [Google Scholar]
- 54.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. A prospective study of thiazide use and fractures in women. Osteoporosis Int 1977;7:79-84. [DOI] [PubMed] [Google Scholar]
- 55.Schoofs MWCJ, van der Klift M, Hofman A, de Laet CEDH, Herings RMC, Stijnen T, et al. Thiazide diuretics and the risk for hip fracture. Ann Intern Med 2003;139:476-82. [DOI] [PubMed] [Google Scholar]
- 56.Mann JFE, Yi Q-L, Sleight P, Dagenais GR, Gerstein HC, Lonn EM, et al. Serum potassium, cardiovascular risk, and effects of an ACE inhibitor: results of the HOPE study. Clin Nephrol 2005;63:181-7. [DOI] [PubMed] [Google Scholar]
- 57.Piper JM, Ray WA, Rosa FW. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors. Obstet Gynecol 1992;80:429-32. [PubMed] [Google Scholar]
- 58.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006;354:2443-51. [DOI] [PubMed] [Google Scholar]
- 59.Grimm RH, Grandits GA, Prineas RJ, McDonald RH, Lewis CE, Flack JM, et al. Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women. Treatment of Mild Hypertension Study (TOMHS). Hypertension 1997;29:8-14. [DOI] [PubMed] [Google Scholar]
- 60.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med 2009;122:290-300 [DOI] [PubMed] [Google Scholar]